Abstract
Context: Brain-derived neurotrophic factor (BDNF) haploinsufficiency is associated with hyperphagia and obesity in both animals and humans. BDNF appears to function downstream of the leptin-melanocortin signaling pathway to control energy balance. The potential role of BDNF in the etiology of the severe hyperphagia associated with PWS has not been previously explored.
Objective: The aim was to compare BDNF concentrations in subjects with PWS and obese controls (OC) and lean controls (LC).
Design and Setting: We conducted a cross-sectional study at an outpatient clinical research center.
Participants: We studied 13 subjects with PWS [five females and eight males; mean ± sd: age, 11.0 ± 4.1 yr; body mass index (BMI)-Z, 2.05 ± 0.78], 13 OC (eight females, five males; age, 12.3 ± 2.7 yr; BMI-Z, 2.18 ± 0.61), and 13 LC (six females, seven males; age, 12.4 ± 2.6 yr; BMI-Z, −0.57 ± 0.73).
Main Outcome Measure: BDNF was measured in serum and plasma by ELISA. Analysis of covariance adjusted for age, sex, and BMI-Z.
Results: All groups were comparable for age (P = 0.50) and sex distribution (P = 0.49). BMI-Z was comparable between PWS and OC (P = 0.89) and lower in LC (P < 0.001). Adjusted serum BDNF was comparable (P = 0.35) in OC (mean ± sem: 13.5 ± 1.2 ng/ml) and LC (19.2 ± 1.3 ng/ml), but lower in PWS (8.3 ± 1.2 ng/ml; P = 0.01 vs. OC; P = 0.03 vs. LC). Adjusted plasma BDNF in PWS (217 ± 130 pg/ml) was lower than OC (422 ± 126 pg/ml; P = 0.02), but statistically comparable with LC (540 ± 143 pg/ml; P = 0.10).
Conclusions: Lower BDNF in PWS suggests insufficient central BDNF production because BDNF in peripheral circulation is believed to reflect cerebral BDNF output. Decreased BDNF may be a potential cause for the disordered satiety and morbid obesity associated with PWS. Further studies are needed to confirm this preliminary pilot study in a larger cohort of patients with PWS.
Patients with Prader-Willi syndrome have lower brain-derived neurotrophic factor concentrations compared to obese and lean control subjects.
Prader-Willi syndrome (PWS) is caused by the lack of expression of paternally derived genes on chromosome 15q11–q13 and is characterized by hypotonia, poor suck, and feeding difficulties during infancy, followed by rapid onset of weight gain and severe hyperphagia between ages 1 and 5 yr. The etiology of this striking dysfunction in energy homeostasis remains unknown. Hyperghrelinemia has been observed in PWS, but its role remains unclear (1,2). Similar to nonsyndromic obesity, leptin concentrations in PWS are elevated in proportion to adiposity (3), and immediate downstream mediators of leptin signaling also appear to be preserved because expression of the orexigenic peptides, neuropeptide Y and agouti-related protein, appear to be appropriately decreased in obese patients with PWS (4). However, possible disruption of the leptin signaling pathway further downstream of these mediators has not been explored.
Brain-derived neurotrophic factor (BDNF) is believed to function downstream of leptin-melanocortin signaling and to play an important role in regulating energy homeostasis (5). Hypothalamic Bdnf mRNA expression is increased in the fed vs. the fasted state in normal mice (5), and selective deletion of Bdnf in this key satiety region of the brain causes hyperphagia and obesity (6). Heterozygous Bdnf knockout mice also exhibit hyperphagia and obesity, reversible with BDNF administration (7). Similarly, in humans, BDNF haploinsufficiency, due to heterozygous deletion of BDNF in patients with WAGR (Wilms tumor, aniridia, genitourinary anomalies, mental retardation) syndrome (8) or disruption of BDNF expression caused by interstitial 11p inversion (9), is associated with decreased serum BDNF concentrations, hyperphagia, and obesity. We examined serum and plasma BDNF concentrations in subjects with PWS compared with obese and lean controls to explore the potential role of BDNF in the pathophysiology of PWS.
Subjects and Methods
The study protocol was approved by the Institutional Review Board of the Duke University Medical Center. Informed written consent from the parents/guardians and assent from each child were obtained.
Subjects
Subjects with PWS were recruited from local pediatric endocrinology clinics and advertisements through PWS organizations. Control subjects were recruited from local community pediatricians and insulin resistance and obesity clinics at Duke University Medical Center. Subjects with diabetes mellitus, other chronic illnesses, or those taking investigational drugs were excluded.
Anthropometric measures
Weight was measured in subjects wearing light clothing using the same calibrated digital scale. Height was measured in triplicate using a Harpenden stadiometer. Body mass index (BMI)-Z scores were calculated using EpiInfo version 3.3.2 (Centers for Disease Control and Prevention, Atlanta, GA).
Assays
Venous whole blood was collected from subjects after a 10-h fast. Serum separator tubes (after 30-min clotting) and plasma EDTA tubes were centrifuged at 4 C for 15 min at 1500 × g. Serum and plasma were stored at −70 C. Serum leptin (Linco/Millipore, Billerica, MA) and plasma glucose and insulin were measured at the Sarah W. Stedman Center for Nutrition and Metabolism Core Laboratory, Duke University. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as [glucose (mmol/liter) × insulin (mU/liter)] ÷ 22.5 (10). BDNF was measured in duplicate by ELISA (Quantikine; R&D Systems, Inc., Minneapolis, MN; interassay coefficient of variation, 7.6–11.3%; intraassay coefficient of variation, 3.8–6.2%). Before assay, all serum samples were diluted 21-fold to permit values to fall within the standard curve range. All samples were assayed simultaneously to minimize interassay variability.
Statistical analyses
Skewed data were normalized by log-transformation. χ2 tests and one-way ANOVA with post hoc Tukey honestly significant difference compared groups. One-way analyses of covariance with post hoc least significant difference compared variables among groups with age, sex, and BMI-Z as covariates. Leptin and HOMA-IR were also examined as covariates for BDNF because BDNF is believed to act downstream of leptin signaling (5) and because BDNF has been associated with impaired glucose tolerance (11). Bivariate Pearson correlation and partial correlation tests adjusting for age, sex, and BMI-Z were used to examine the association between leptin and BDNF within each group and for all subjects combined.
Results
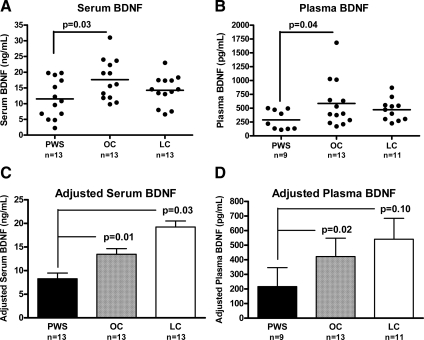
We studied 13 subjects with PWS, 13 obese controls (OC), and 13 lean controls (LC). Subject characteristics and comparisons are shown in Table 1. Groups were comparable for age and sex distribution. BMI-Z was comparable between PWS and OC and lower in LC. Unadjusted serum and plasma BDNF in PWS were lower compared with OC, but comparable with LC. There was overlap in BDNF values between the three groups compared (Fig. 1), but nearly half (six of 13 serum and four of nine plasma) of the values in the PWS children were lower than the lowest value in OC. Even after excluding the highest BDNF value in the OC group, PWS remained statistically lower (serum, P = 0.047; plasma, P = 0.043). We compared PWS subjects with BDNF values in the upper half (n = 7) vs. the lower half (n = 6) and found no differences in age (P = 0.52), BMI-Z (P = 0.84), or HOMA-IR (P = 0.92). After correction for age, sex, and BMI-Z, adjusted serum BDNF concentrations were comparable for OC and LC, but lower in PWS compared with both OC and LC; similarly, adjusted plasma BDNF concentrations were comparable for OC and LC, but lower in PWS compared with OC (Fig. 1). Inclusion of leptin and HOMA-IR as covariates resulted in similar results, with lower serum BDNF in PWS compared with OC and LC (P = 0.008 for overall ANOVA; P = 0.008 for PWS vs. OC; P = 0.01 for PWS vs. LC; P = 0.17 for OC vs. LC). However, for plasma BDNF, of which fewer samples were available for analysis, overall ANOVA was nonsignificant when leptin and HOMA-IR were included in the model, with P = 0.09. No significant correlations between leptin and BDNF were observed (all P > 0.22).
Table 1.
Clinical characteristics of PWS and OC and LC subjects
| PWS | OC | LC | ANOVA or χ2 3-groups P value | Post hoc PWS vs. OC | Post hoc PWS vs. LC | |
|---|---|---|---|---|---|---|
| Age (yr) | 11.0 ± 4.1 (5.3–17.4) | 12.3 ± 2.7 (8.2–16.6) | 12.4 ± 2.6 (8.1–16.9) | 0.50 | n/a | n/a |
| Sex (% female) | 38.5 | 61.5 | 46.2 | 0.49 | n/a | n/a |
| BMI (kg/m2) | 30.5 ± 14.3 (17.0–65.0) | 33.1 ± 9.2 (20.0–48.0) | 17.2 ± 2.0 (14.6–21.8) | <0.001 | 0.78 | 0.004 |
| BMI-Z | 2.05 ± 0.78 (0.65–3.46) | 2.18 ± 0.61 (0.67–2.77) | −0.57 ± 0.73 (−1.81 to 0.60) | <0.001 | 0.89 | <0.001 |
| Leptin (ng/ml) | 32.1 ± 23.7 (2.8–78.0) | 37.8 ± 23.2 (9.5–82.4) | 3.5 ± 2.5 (1.3–9.8) | <0.001 | 0.63 | <0.001 |
| HOMA-IR (mU · mmol/liter2) | 3.72 ± 2.19 (1.35–9.27) | 6.22 ± 2.75 (2.67–10.28) | 2.36 ± 0.94 (0.28–3.57) | <0.001 | 0.04 | 0.12 |
| Serum BDNF (ng/ml) | 11.5 ± 6.2 (2.3–19.8) | 17.6 ± 6.4 (9.8–31.0) | 14.2 ± 4.7 (6.6–23.0) | 0.04 | 0.03 | 0.47 |
| Plasma BDNF (pg/ml) | 289 ± 175 (111–501) | 586 ± 432 (172–1684) | 473 ± 197 (228–871) | 0.04 | 0.04 | 0.08 |
Data are expressed as mean ± sd (range). Groups were compared using χ2 test for sex distribution and one-way ANOVA with post hoc Tukey HSD for between-group comparisons of continuous variables after log-transformation of skewed variables (back-transformed values are shown for ease of interpretation). BMI-Z, BMI sd Z score; n/a, not applicable; HSD, honestly significant difference.
Figure 1.
BDNF concentrations in patients with PWS compared with OC and LC. Fasting serum and plasma BDNF concentrations were measured by ELISA and compared among groups. Individual subjects’ BDNF concentrations in serum (A) and plasma (B) are shown as black circles, and the mean is depicted as a horizontal line. BDNF concentration adjusted for age, sex, and BMI-Z is shown as mean ± sem for serum (C) and plasma (D). Back-transformed values of adjusted means are shown for ease of interpretation. Overall ANOVA P = 0.02 for serum and P = 0.04 for plasma. P values represent post hoc least significant difference pairwise comparisons between PWS and OC or LC. P value for comparison between OC and LC not shown in figure (P = 0.35 for serum and P = 0.65 for plasma).
Discussion
In this pilot study, we observed lower serum and plasma BDNF concentrations in a small cohort of patients with PWS compared with OC. BDNF concentrations in PWS were comparable to that of lean controls, who would be appropriately expected to have lower BDNF levels than OC because BDNF is believed to play a role in regulating energy homeostasis by functioning as a satiety signal downstream of the leptin pathway (5). In fact, after correction for BMI-Z, adjusted BDNF concentrations were comparable for OC and LC, but still remained lower in PWS. Thus, our findings suggest that patients with PWS lack appropriate up-regulation of BDNF in response to adiposity.
A limitation of our study is the lack of platelet count values for our subjects. Because BDNF is stored in and released from platelets in peripheral circulation (12), platelet levels ideally should be considered as a covariate in analyses of BDNF concentrations. Nine PWS subjects were receiving growth hormone therapy; the effect of growth hormone on BDNF is uncertain, and further studies are needed to address this. Another limitation is lack of data regarding body composition and body fat distribution, both of which could contribute to differences in metabolic parameters, including insulin sensitivity and local BDNF production in skeletal muscle (13). Our study is also limited by the small sample sizes, and further studies are needed to confirm these findings in a larger cohort of patients with adjustment for platelet levels and body fat, but these initial observations still point to an interesting possibility that BDNF may play a role in the pathophysiology of the hyperphagia and obesity of PWS.
The primary source of BDNF in peripheral circulation is believed to be the central nervous system. Platelets serve as a reservoir, rather than as a site of BDNF production because BDNF mRNA is not detectable in either platelets or megakaryocytes (12). Thus, peripheral BDNF concentrations may serve as a proxy of cerebral output of BDNF (11), and therefore, the lower peripheral BDNF concentrations in our patients with PWS may reflect insufficient central BDNF production. Our observation that serum [which would contain a large portion of the BDNF content contained in platelets (12)] and platelet-poor plasma are both decreased in PWS suggests that overall stores of BDNF as well as available free BDNF in circulation are diminished. Because BDNF appears to be an important regulator of satiety, BDNF insufficiency may be a potential cause for the disordered appetite regulation seen in PWS, which would be consistent with previous studies showing an association between low serum BDNF and hyperphagia and severe obesity (8,9). Furthermore, examination of changes in BDNF concentration with age could yield insight into the progression from failure-to-thrive in infancy to hyperphagia and obesity during childhood in PWS.
Because leptin levels were similar between PWS and OC, a finding that has been previously observed (14), the lack of association between BDNF and leptin was not surprising. PWS subjects appear to have lower BDNF despite having similar BMI-Z and leptin levels as OC. This would suggest that the defect in energy homeostasis lies distal to leptin production by the adipocytes—possible sites could include decreased leptin transport into the central nervous system, defects in leptin receptor signaling, proopiomelanocortin neuronal dysfunction, defects in melanocortin-4 receptor signaling, or primary defects in BDNF expression.
BDNF, acting through tropomyosin-related kinase B (TrkB), a receptor tyrosine kinase that regulates the development, differentiation, and survival of neurons and is critical in embryological nervous system development and postnatal synaptic plasticity (15). Thus, relative BDNF reduction in PWS may potentially contribute to the complex neurobehavioral phenotype seen with this disorder, which includes variable degrees of cognitive impairment, maladaptive behaviors (obsessive-compulsiveness, temper tantrums, skin picking, rigidity of routine, high pain tolerance, attention deficit, and hyperactivity), and language difficulties (16). In our study, approximately half of PWS subjects had BDNF concentrations that were below the lowest value observed in the OC, but there was still considerable overlap in BDNF concentrations between the PWS and control groups, suggesting interindividual variability in BDNF production. Larger studies are needed to determine whether such variations could account for differences in neurocognitive function among patients with PWS.
Interestingly, necdin, a signaling protein encoded by a gene within the PWS locus at chromosome 15q11-q12, has been shown to mediate the development and survival of sensory and autonomic neurons through facilitation of TrkA signaling (17,18). Due to the sequence homology of the Trk family of receptor tyrosine kinases, necdin also binds to the intracellular domain of TrkB, the receptor for BDNF (17). Therefore, it is plausible that patients with PWS may have impaired BDNF/TrkB signaling due to necdin insufficiency. Furthermore, because BDNF and TrkB are coexpressed within the hypothalamus, diminished BDNF may be a consequence of and subsequently a perpetuating cause of reduced BDNF/TrkB signaling. Moreover, BDNF levels in PWS may be reduced due to genetic and other factors, and even mild decreases in BDNF may have a more robust effect in PWS due to the possibility that TrkB signaling may already be defective in this disorder. Of note, heterozygous Bdnf knockout mice display hyperphagia, diminished pain sensitivity, and behavioral disturbances that phenotypically resemble the abnormal behaviors of PWS (7,19). If larger studies confirm a connection between BDNF and PWS, therapeutic interventions aimed at modulating BDNF/TrkB signaling may ameliorate some of the behavioral abnormalities. For example, TrkB agonists may be of benefit, as may early behavioral therapy, because early-life environmental factors have been shown in rodents to alter Bdnf promoter DNA methylation and cause lifelong changes in Bdnf expression (20).
In summary, our observation of lower BDNF in patients with PWS compared with OC and LC sheds insight into the potential role of BDNF in the pathophysiology of the altered satiety and neurocognitive abnormalities associated with PWS.
Acknowledgments
The authors are grateful to Dr. Michael Freemark and Ms. Juanita Cuffee for additional help in conducting this study. We also acknowledge support from the Sarah W. Stedman Center for Nutrition and Metabolism, Duke University Medical Center and the Duke General Clinical Research Center (MO1-RR-30, National Center for Research Resources, Clinical Research Centers Program, National Institutes of Health).
Footnotes
This work was supported by Grant 1K23-RR-021979 from the National Center for Research Resources, National Institutes of Health (to A.M.H.). M.J.M., H.N.C., and C.B.N. are supported by the Sarah W. Stedman Nutrition and Metabolism Center. J.C.H. is a commissioned officer of the U.S. Public Health Services and is supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Disclosure Summary: The authors have nothing to declare.
First Published Online April 28, 2010
Abbreviations: BDNF, Brain-derived neurotrophic factor; BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance; LC, lean control subjects; OC, obese control subjects; PWS, Prader-Willi syndrome; Trk, tropomyosin-related kinase.
References
- Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Schwartz MW, Basdevant A, Weigle DS 2002 Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med 8:643–644 [DOI] [PubMed] [Google Scholar]
- Haqq AM, Farooqi IS, O'Rahilly S, Stadler DD, Rosenfeld RG, Pratt KL, LaFranchi SH, Purnell JQ 2003 Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. J Clin Endocrinol Metab 88:174–178 [DOI] [PubMed] [Google Scholar]
- Lindgren AC, Marcus C, Skwirut C, Elimam A, Hagenäs L, Schalling M, Anvret M, Lönnqvist F 1997 Increased leptin messenger RNA and serum leptin levels in children with Prader-Willi syndrome and nonsyndromal obesity. Pediatr Res 42:593–596 [DOI] [PubMed] [Google Scholar]
- Goldstone AP, Unmehopa UA, Bloom SR, Swaab DF 2002 Hypothalamic NPY and agouti-related protein are increased in human illness but not in Prader-Willi syndrome and other obese subjects. J Clin Endocrinol Metab 87:927–937 [DOI] [PubMed] [Google Scholar]
- Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF 2003 Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci 6:736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M 2007 Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci 27:14265–14274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF 2000 BDNF regulates eating behavior and locomotor activity in mice. EMBO J 19:1290–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, Adler-Wailes DC, Sanford EL, Lacbawan FL, Uhl GR, Rennert OM, Yanovski JA 2008 Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med 359:918–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, Yeo GS, Cox JJ, Morton J, Adlam AL, Keogh JM, Yanovski JA, El Gharbawy A, Han JC, Tung YC, Hodges JR, Raymond FL, O'rahilly S, Farooqi IS 2006 Hyperphagia, severe obesity, impaired cognitive function and hyperactivity associated with functional loss of one copy of the BDNF gene. Diabetes 55:3366–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, Fischer CP, Lindegaard B, Petersen AM, Taudorf S, Secher NH, Pilegaard H, Bruunsgaard H, Pedersen BK 2007 Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 50:431–438 [DOI] [PubMed] [Google Scholar]
- Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN 2002 Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost 87:728–734 [PubMed] [Google Scholar]
- Pedersen BK, Pedersen M, Krabbe KS, Bruunsgaard H, Matthews VB, Febbraio MA 2009 Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp Physiol 94:1153–1160 [DOI] [PubMed] [Google Scholar]
- Haqq AM, Muehlbauer M, Svetkey LP, Newgard CB, Purnell JQ, Grambow SC, Freemark MS 2007 Altered distribution of adiponectin isoforms in children with Prader-Willi syndrome (PWS): association with insulin sensitivity and circulating satiety peptide hormones. Clin Endocrinol (Oxf) 67:944–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL 2009 New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci 29:12764–12767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy SB, Driscoll DJ 2009 Prader-Willi syndrome. Eur J Hum Genet 17:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwako K, Hosokawa A, Nishimura I, Uetsuki T, Yamada M, Nada S, Okada M, Yoshikawa K 2005 Disruption of the paternal necdin gene diminishes TrkA signaling for sensory neuron survival. J Neurosci 25:7090–7099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennese AA, Gee CB, Wevrick R 2008 Loss of the Prader-Willi syndrome protein necdin causes defective migration, axonal outgrowth, and survival of embryonic sympathetic neurons. Dev Dyn 237:1935–1943 [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, Young LT, Fahnestock M 2001 Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci 115:1145–1153 [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD 2009 Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry 65:760–769 [DOI] [PMC free article] [PubMed] [Google Scholar]