Abstract
Background: Angiopoietin-1 (Ang-1) and Ang-2 act selectively on endothelial cells by engaging the Tunica interna endothelial cell kinase-2 (Tie2) receptor. A soluble form of Tie2 (sTie2) blocks angiopoietin bioactivity.
Objective: The aim of the study was to characterize changes and expression patterns of Ang-1, Ang-2, and sTie2 in amniotic fluid (AF) and placenta during human pregnancy and intraamniotic inflammation (IAI)-induced preterm birth.
Design and Setting: We conducted a cross-sectional study at a tertiary university hospital.
Patients: AF levels of Ang-1, Ang-2, and sTie2 were evaluated in 176 women during second trimester (n = 40), third trimester (n = 37), and preterm labor (positive IAI, n = 50; negative IAI, n = 49). Placenta and cord blood of select women were analyzed.
Main Outcome Measures: Ang-1, Ang-2, sTie2, and IL-6 were evaluated by ELISA. Real-time PCR measured Ang-1, Ang-2, and Tie2 placental mRNA levels. Placenta was immunostained for Ang-1 and Ang-2. Placental explant cultures were stimulated with lipopolysaccharide, Pam3Cys, and modulators of protein synthesis/secretion (cycloheximide, monensin, and brefeldin A).
Results: In normal pregnancy, the levels and ratios of AF Ang-1, Ang-2, and sTie2 varied with gestational age (GA) (P < 0.001). PCR revealed corresponding changes in placental Ang-1 and Ang-2, but not Tie2, mRNA. IAI raised AF Ang-1, Ang-2, and sTie2 above the expected level for GA without affecting their placental mRNA. Ang-2 immunoreactivity appeared enhanced in areas of villous edema. AF Ang-2/Ang-1 ratio was an important determinant of cord blood IL-6 (P < 0.001). Ex-vivo, sTie2 release was increased by Golgi disrupting but not bacterial mimic agents.
Conclusions: Ang-1, Ang-2, and sTie2 are physiological constituents of AF that are GA and IAI regulated. Ang-2/Ang-1 ratio may play a role in modulating the fetal inflammatory response to IAI. Placental sTie2 shedding likely involves a Golgi-mediated mechanism.
Angiogenic cytokines Ang-1, Ang-2, and sTie2 are physiologic constituents of amniotic fluid; their relative ratios may play a role in modulating the fetal inflammatory response to intra-amniotic inflammation in the setting of preterm birth.
Placental vasculogenesis and angiogenesis are key elements required for normal development of the fetus (1). Angiopoietins, a family of recently identified proteins, are intimately involved in the process of placental maturation and growth (1). Specifically, angiopoietin-1 (Ang-1) and its counter-regulatory element Ang-2 influence the process of endothelial cell migration and increased vascular permeability attending formation of nascent placental vessels (2). Ang-1 and Ang-2 are both ligands for the extracellular domain of the tunica interna endothelial cell kinase-2 (Tie2) receptor (2). Binding of Ang-1 to Tie2 promotes endothelial cell migration and survival (3). Vascular endothelial growth factor (VEGF) has been shown to be responsible for initiation of vasculogenesis, a process that Ang-1 potentiates (4). Ang-1 seals the vasculature and augments VEGF-induced adhesion molecule expression (5). However, compelling data suggests that the functions of Ang-1 can be antagonized by a soluble form of the Tie2 receptor (sTie2) (6,7). Although many aspects related to the release of sTie2 are still unknown, it is currently thought that this antagonist results from proteolytic cleavage of the extracellular domain of the Tie2 receptor and that protein kinase C and phosphoinositide-3-kinase/AKt-dependent pathways can play a critical role for its processing (8).
The biological activity of Ang-2 is complex. Traditionally, it was described that Ang-2 functions as an Ang-1 antagonist for the Tie2 receptor (9). Yet, others have demonstrated that Ang-2 can mimic the actions of Ang-1 in human umbilical vein endothelial cells, concluding that Ang-2 can also act as a partial agonist (10). Recent studies showed that Ang-2 can act as an antagonist on vascular endothelium and as an agonist on lymphatic vessels (11). Given that Ang-1 and Ang-2 are competitive ligands, several reports have favored the Ang-1/Ang-2 ratio as a more optimal reflection of angiopoietin balance (12,13). A low Ang-1/Ang-2 ratio may possibly lead to vessel destabilization and a decrease in the angiogenic sprouting. This suggests that the interplay between angiogenic cytokines is more relevant than each measured alone (13).
The role of angiopoietins in inflammation has recently attracted much attention (14). Ang-1 carries a variety of antiinflammatory properties such as inhibition of nuclear factor-κB and a decrease in vascular permeability, neutrophil endothelial cell adhesion, and transvascular leukocyte migration (15). In contrast, Ang-2 has proinflammatory properties in vivo (16). In a mouse model of in vivo-induced inflammation, both Ang-1 and sTie2 inhibited edema formation in response to Ang-2 (13).
Little is known about the control of the angiopoietin system in amniotic fluid (AF) during normal gestation or in pregnancies complicated by intraamniotic inflammation (IAI). We postulate that the balance of Ang-1, Ang-2, and sTie2 correlates with gestational age (GA), reflecting the increasing need for vascular remodeling throughout pregnancy, and that inflammation destabilizes this equilibrium.
Patients and Methods
Patient population, procedures, and study design
In a cross-sectional study design, we analyzed AF samples retrieved by transabdominal amniocentesis from 176 women pregnant with singletons. A flowchart of enrolled women and biological samples used in this study is provided in the Supplemental Data (published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Patients were stratified in the following groups: 1) second trimester women who had a genetic amniocentesis for fetal karyotyping and delivered a healthy term baby [GA, median (interquartile range), 18 (17–20) wk; n = 40]; 2) third trimester women who had AF testing for fetal lung maturity before cesarean delivery [GA, 36 (35–37) wk; n = 37]; 3) women with preterm labor or preterm premature rupture of the membranes (PPROM) who had an amniocentesis to rule out infection/inflammation [GA, 27 (24–31) wk; n = 99]). Women in this last group were consecutive patients enrolled and followed prospectively at Yale-New Haven Hospital from February 2004 to September 2006. To study the relationship between Ang-1, Ang-2, sTie2, and IAI, we used surface-enhanced laser-desorption ionization time of flight mass spectrometry (17,18). The preterm labor group was divided in two subgroups: positive IAI [GA, 26 (24–29) wk; n = 50], and negative IAI [GA, 30 (25–32) wk; n = 49]. Of the 99 cases that were evaluated preterm, 27 women delivered a healthy baby at term. These cases were also used to determine GA regulation of Ang-1, Ang-2, and sTie2 in the AF. Information regarding the clinical indication for the second and third trimester amniocentesis procedures, exclusion criteria for the patients, and definition of preterm labor and PPROM is provided in the Supplemental Data.
Biochemical and microbiological studies of AF
The clinical tests employed for diagnosis of IAI/infection are presented in the Supplemental Data.
Umbilical cord blood
Cord blood serum was available for IL-6 analysis in eight of 49 (16%) of the negative IAI and 34 of 50 (68%) of the positive IAI patients who also provided AF samples. The protocol used for collection and storage of the cord blood serum is presented in the Supplemental Data.
Mass spectrometry analysis of the AF
We used surface-enhanced laser-desorption ionization time of flight because previous studies from our group determined that the mass restricted score was the most accurate test for diagnosing IAI and a better predictor of clinical outcome than microbiological and cytokine studies of the AF (18,19). For identity of the peak biomarkers comprising the mass restricted score, see the Supplemental Data.
Ang-1, Ang-2, sTie2, IL-6, and IL-8 immunoassays
ELISA assays for human Ang-1, Ang-2, sTie2, IL-6, and IL-8 were performed according to the manufacturer’s protocol (see Supplemental Data).
Histology and immunohistochemistry
Hematoxylin-eosin-stained tissue sections were available from 26 of 49 (53%) of the negative IAI and 47 of 50 (94%) of the positive IAI patients who also provided AF samples. Immunohistochemistry for placental villous Ang-1 and Ang-2 was performed as indicated in the Supplemental Data.
Quantitative real-time RT-PCR
Details regarding sample collection, RT-PCR methodology, and PCR primers are provided in the Supplemental Data.
Placental explant culture
To gain insight into the mechanisms responsible for the presence of Ang-1, Ang-2, and sTie2 in AF, we conducted ex vivo culture experiments in placental villous tissue from term elective cesarean deliveries (n = 10). Following 24 h of incubation, we evaluated Ang-1, Ang-2, and sTie2 levels in explant medium after exposure to Gram-negative [lipopolysaccharide (LPS), 1 μg/ml) or Gram-positive (Pam3Cys, 1 μg/ml) bacterial mimics. Explants were also incubated with cycloheximide (10 μg/ml; inhibitor of protein synthesis), brefeldin A (10 μm; protein secretion blocker through disruption of the Golgi complex), and monensin (20 μm; classical secretion blocker at the level of Golgi membrane). Details are presented in the Supplemental Data.
Statistical analysis
A two-step clustering method complemented by receiver operating curve (ROC) analysis was undertaken to identify an unbiased GA separation point that partitioned the levels of each angiogenic cytokine into two clusters (“low” vs.“high”) (20). Details regarding other statistical methods employed in this study are provided in the Supplemental Data.
Results
Characteristics of the patients at the time of AF sampling
The demographic, clinical, biochemical, and outcome characteristics of the cases enrolled in this study are provided in the Supplemental Data.
Gestational regulation of Ang-1, Ang-2, and sTie2 levels in AF
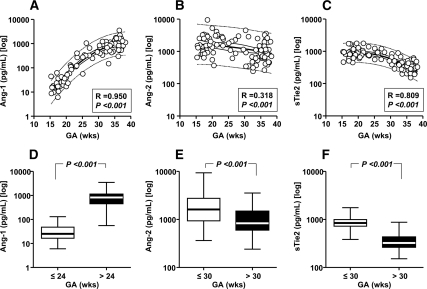
This analysis was restricted to the samples retrieved from healthy women during the second trimester (n = 40) and third trimester (n = 37) and women with symptoms of preterm labor, negative AF cultures, and who ultimately delivered at term (n = 27). The Ang-1, Ang-2, and sTie2 AF levels for the second and third trimester groups are provided in the Supplemental Data. Levels of angiogenic cytokines were analyzed first with GA as a continuous format variable. The best-fitted equations corresponded to second-order nonlinear interpolation of the log-transformed AF level of each analyte. We observed a significant direct correlation between Ang-1 and GA (Fig. 1A; P < 0.001). Conversely, there was an inverse correlation between GA, Ang-2 (Fig. 1B; P < 0.001), and sTie2 (Fig. 1C; P < 0.001). The transition point in GA from the “low” to the “high” level corresponded to 24 wk for Ang-1 [ROC area (95% confidence interval [CI], 0.992 (0.95–1.0), P < 0.001; Fig. 1D) and 30 wk for both Ang-2 [ROC area, 0.632 (0.532–0.725); P = 0.015; Fig. 1E] and sTie2 [ROC area, 0.927 (0.859–0.969); P < 0.001; Fig. 1F].
Figure 1.
AF levels of Ang-1, Ang-2, and sTie2 in pregnancies with normal outcomes (n = 104). A–C, The best-fitted equations corresponded to second-order nonlinear interpolations of log-transformed AF levels of each of the analytes, which resulted in significant direct (for Ang-1) (A) or indirect [for Ang-2 (B) and sTie2 (C)] correlations with GA. The two-step clustering analysis identified different GA cutoffs between samples with low vs. high analyte levels. D, Ang-1 levels were low before 24 wk gestation [median (interquartile range), 25 (17–46) pg/ml], increasing approximately 30-fold thereafter [>24 wk, 822 (464–1114) pg/ml]. E, Ang-2 levels were high before 30 wk gestation [1614 (949–2700) pg/ml], decreasing approximately 2-fold thereafter [>30 wk, 835 (607–1467) pg/ml]. F, sTie2 levels were also high before 30 wk [843 (728–991) pg/ml], decreasing approximately 2.5-fold thereafter [>30 wk, 324 (266–428) pg/ml]. Data are presented in logarithmic format. A–C, Thick lines, second-order regression line; thin continuous lines, 95% CI; dotted lines, 95% prediction intervals. D–F, Ends of the boxes, 25th and 75th percentiles; line inside the box, median; whiskers, largest and smallest values.
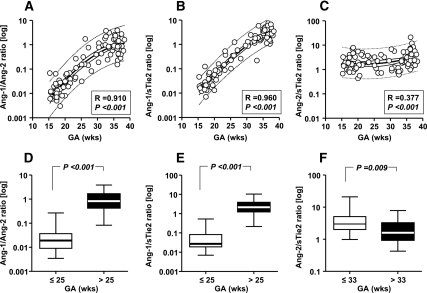
Changes in ratios of Ang-1, Ang-2, and sTie2 in AF across gestation
The Ang-1/Ang-2 ratio was increased significantly at the end compared with early gestation (Fig. 2A; P < 0.001). The Ang-1/sTie2 ratio was also significantly increased across normal pregnancy (Fig. 2B; P < 0.001). Figure 2C demonstrates that the Ang-2/sTie2 ratio increased as pregnancy progressed, albeit less prominently than the other two ratios. The separation point in GA from the low to the high AF Ang-1/Ang-2 ratio corresponded to 25 wk GA [ROC area, 0.996 (0.96–1.0); P < 0.001; Fig. 2D]. Similar results were seen for the Ang-1/sTie2 ratio [ROC area, 0.989 (0.95–1.0); P < 0.001; Fig. 2E]. In contrast, the transition between the low vs. the high levels for the Ang-2/sTie2 ratio occurred at 33 wk GA [ROC area, 0.711 (0.61–0.80); P < 0.001; Fig. 2F].
Figure 2.
Ratios of AF Ang-1/Ang-2, Ang-1/sTie2, and Ang-2/sTie2 in pregnancies with normal outcomes (n = 104). The best-fitted equations corresponded to second-order nonlinear interpolations of log-transformed AF levels of each ratio, which resulted in significant direct correlations with GA. A, Ang-1/Ang-2 ratio; B, Ang-1/sTie2 ratio; and C, Ang-2/sTie2. The two-step clustering analysis identified different GA cutoffs between samples with low vs. high ratio levels. Ang-1/Ang-2 (D) and Ang-1/sTie2 (E) ratios were low before 25 wk gestation, increasing thereafter. The GA at which the Ang-2/sTie2 ratio (F) decreases significantly was identified at 33 wk. Data are presented in logarithmic format. A–C, Thick lines, second-order regression line; thin continuous lines, 95% CI; dotted lines, 95% prediction intervals. D–F, Ends of the boxes, 25th and 75th percentiles; line inside the box, median; whiskers, largest and smallest values.
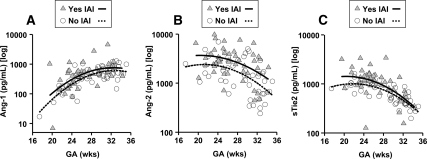
Effect of IAI on AF levels of Ang-1, Ang-2, and sTie2
The absolute levels of Ang-1, Ang-2, and sTie2 for the women enrolled in the preterm labor groups are presented in the Supplemental Data. The levels of Ang-1 were not significantly changed by IAI (P = 0.506). In contrast, women with positive IAI had increased AF levels of Ang-2 (P < 0.001) and sTie2 (P < 0.001). We further tested whether the observed differences remained after adjusting for GA at sampling. Using multivariate regression analysis, we determined that GA and IAI were independent covariates in modifying the AF levels of Ang-1 (GA, P < 0.001; IAI, P = 0.008; Fig. 3A), Ang-2 (GA, P < 0.001; IAI, P = 0.001; Fig. 3B), and sTie2 (GA, P < 0.001; IAI, P = 0.006; Fig. 3C), with inflammation causing an increase in the level of each of the analytes significantly above that expected for GA alone. After correction for GA, there was a significant correlation between AF IL-6 and Ang-2 (R = 0.301; P = 0.003) but not Ang-1 or sTie2 (P > 0.1 for both).
Figure 3.
Effect of IAI on AF levels of Ang-1, Ang-2, and sTie2 (n = 99). Differential multivariate regression analysis depicting the effect of IAI and GA on Ang-1 (A), Ang-2 (B), and sTie2 (C). IAI was associated with an increase in AF levels of Ang-1, Ang-2, and sTie2 independent of GA, as indicated by the shift in the second-order regression lines between the group with IAI (n = 50; open circles, continuous line) and without IAI (n = 49; gray triangles, dotted line).
IAI is a recognized risk factor for PPROM (18). We analyzed whether the relative concentration of AF Ang-1, Ang-2, and sTie2 was specifically altered in relationship to PPROM. Using multivariate logistic regression analysis with PPROM as the dependent variable and GA, IAI, maternal age, and smoking as independent variables, we determined that increased Ang-1 and Ang-2 and decreased sTie2 levels added to the presence of IAI as independent risk factors for PPROM [odds ratio (95% CI): Ang-1, 13.3 (2.3–75.0), P = 0.004; Ang-2, 7.8 (1.2–54.2), P = 0.038; sTie2, 0.028 (0.002–0.433), P = 0.011; positive IAI, 2.2 (1.4–3.3), P < 0.001]. The other variables were excluded from the model based on a P > 0.1. The level of AF Ang-2, but not Ang-1 or sTie2, appeared directly correlated with the presence and severity of choriodecidual inflammation, independent of GA at birth or amniocentesis-to-delivery interval (R = 0.460; P < 0.001). The degree of histological inflammation in the amnion or umbilical cord (funisitis) was not correlated with Ang-1, Ang-2, or sTie2 levels or their ratios (P > 0.1 for all).
Changes in placental expression of Ang-1, Ang-2, and Tie2 across gestation and in pregnancies complicated by IAI and histological chorioamnionitis
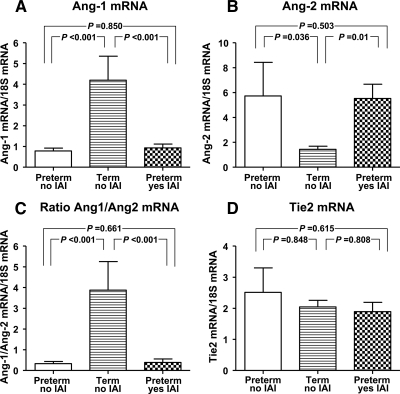
Our quantitative analysis of Ang-1, Ang-2, and Tie2 mRNA expression in placental villous samples from women who delivered preterm and at term in the absence or presence of IAI and histological chorioamnionitis is presented in Fig. 4. Compared with preterm placentas, at term Ang-1 mRNA was increased by 4-fold [Ang-1 preterm, 0.7 (0.5–1.0) vs. term, 2.7 (1.5–6.7) relative units; P < 0.001; Fig. 4A]. In women with preterm birth and IAI, Ang-1 mRNA [0.8 (0.5–1.1) relative units] was not significantly changed compared with women without IAI (P = 0.850), even after correction for GA (P = 0.089).
Figure 4.
mRNA expression levels of Ang-1, Ang-2, and their receptor Tie2 in placental tissue. Real-time quantitative PCR results showed that expression of Ang-1 (A) was increased, whereas Ang-2 (B) was decreased at term. The ratio of Ang-1/Ang-2 (C) was also increased at term. There were no GA changes noted in Tie2 expression (D). IAI was not associated with significant changes in mRNA expression for Ang-1, Ang-2, Tie2, or their ratios. Error bars show se.
At term, placental Ang-2 mRNA expression was decreased more than 2-fold compared with preterm gestation [Ang-2 preterm, 3.3 (1.4–5.0) vs. term, 1.5 (0.9–2.1) relative units; P = 0.036; Fig. 4B). Yet, similar to Ang-1, we saw no significant change in Ang-2 expression between preterm placentas with and without IAI (P = 0.503). The ratio between Ang-1/Ang-2 mRNA expression was significantly increased at term compared with preterm placentas (P < 0.001; Fig. 4C). This ratio did not change in relationship to either intraamniotic or histological inflammation (P = 0.661).
No differences were determined in placental Tie2 mRNA expression during gestation [preterm, 1.9 (0.9–3.1) vs. term, 2.0 (1.8–2.2) relative units; P = 0.848; Fig. 4D] or in relationship to histological chorioamnionitis (P = 0.615).
In the amniochorion, Ang-1, Ang-2, and Tie2 mRNA levels were not impacted by either GA at delivery or presence of IAI and/or histological inflammation (data not shown). Ang-2 transcript was expressed in the amniochorion biopsy at significantly lower levels [12 (6–29)-fold] than in the matched placental tissue (paired t test, P < 0.001 for each individual group). Ang-1 and Tie2 mRNA were amplified in amniochorion samples at levels similar to those found in preterm placentas.
Immunostaining of Ang-1 and Ang-2 in human placenta
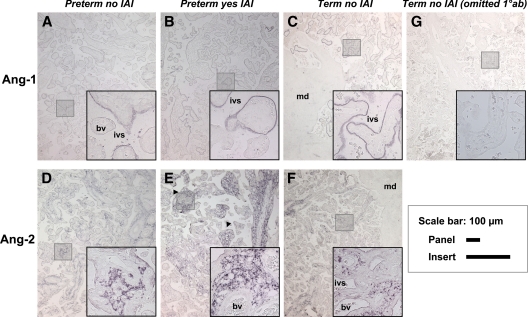
Because the notable changes in Ang-1 and Ang-2 mRNA expression were recorded in the placenta and not in the amniochorion, we searched for the cellular localization of these two angiogenic cytokines in sections of placental villous tissues. As seen in Fig. 5, there was a marked difference in the placental cells expressing each protein. Ang-1 appeared confined to syncytiotrophoblasts (Fig. 5, A–C) and more prominently to their apical pole in close contact with the maternal vascular spaces. The fetal vascular endothelium and the cells populating the maternal decidua were devoid of Ang-1. Overall, we noted a heterogeneous pattern of Ang-1 staining, with some villi exhibiting strong signal and adjacent villi lacking staining altogether (Fig. 5B, inset). Our analysis did not reveal a significant difference in Ang-1 immunostaining based on the presence or absence of IAI or histological chorioamnionitis (P = 0.902). Moreover, Ang-1 syncytiotrophoblast staining intensity appeared similar preterm (Fig. 5A) and term (Fig. 5C; P = 0.326).
Figure 5.
Representative placental sections demonstrating immunolocalization of Ang-1 and Ang-2 in preterm and term pregnancies. Ang-1 (A–C) immunoreactivity was primarily localized in syncytiotrophoblast in apposition to the maternal intervillous space (ivs) and was virtually absent in the villous stroma, blood vessel (bv) endothelium or maternal decidua (md). Ang-2 (D–F) was primarily localized in the villous stroma and staining intensity appeared decreased at term (P = 0.001). Ang-2 signal was more conspicuous in the regions affected by severe villous edema (E, arrowheads), G, Negative control [omitted primary antibody (10ab)]. Preterm placentas without IAI (A and D; n = 9), preterm placentas with IAI (B and E; n=14) and term placentas without IAI (C, F and G; n=10). The shaded squares are shown at a higher magnification in the right bottom corner of each panel. The sections were not counterstained. Scale bars for panels and insert are shown on the right.
In contrast to Ang-1, Ang-2 was predominantly present in cells populating the villous stroma (Fig. 5, D–F). Upon inspection of Ang-2 localization, we also noted a heterogeneous staining pattern among placental villi. Ang-2 staining was absent in the fetal vascular endothelium. In women with preterm birth and IAI, the Ang-2 signal was more conspicuous in the regions affected by severe villous edema (Fig. 5E, arrowheads). Analysis of the histological score demonstrated that Ang-2 immunostaining was increased in preterm villi (Fig. 5D) and decreased at term (Fig. 5F) (P = 0.001). Inflammatory status of the AF did not impact on Ang-2 immunostaining (P = 0.354).
Production of Ang-1, Ang-2, and sTie2 by placental explants
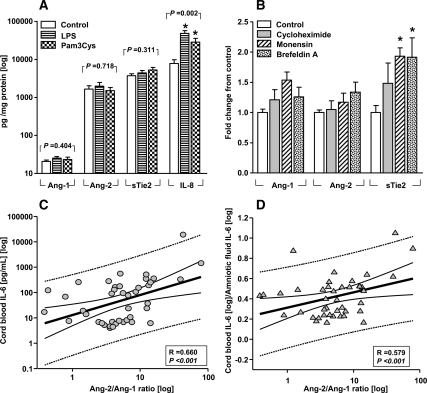
By 24 h of culture, placental villous explants produce significant amounts of Ang-2 and sTie2. In contrast, Ang-1 remained below the level of the lowest standard (Fig. 6A). The basal production of sTie2 from villous explants amounted to levels comparable to those of the chemokine IL-8 (P = 0.212). Exposure to LPS or Pam3Cys did not stimulate the release of Ang-1, Ang-2, or sTie2 in the explant medium, despite a significant increase in the IL-8 production (P = 0.002; Fig. 6A). Moreover, none of the disruptors of protein synthesis (cycloheximide) or Golgi-related secretion (monensin and brefeldin A) were able to inhibit the accumulation of Ang-1 and Ang-2. Interestingly, both monensin and brefeldin elevated sTie2 release in the explant media (P < 0.05; Fig. 6B). Similar production patterns were observed with amniochorion explants (data not shown).
Figure 6.
Ex-vivo production of angiogenic cytokines and relationships with cord blood to AF IL-6 ratio. A, Villous explants of term placentas (n = 10) were incubated for 24 hours in the absence (control) or presence of the Toll like receptor (TLR) agonists lipopolysaccharide LPS (1 μg/mL, TLR-4 ligand) or Pam3Cys-Ser-(Lys)4 hydrochloride (Pam3Cys, 1 μg/mL, synthetic TLR-2 ligand) which did not affect the production of Ang-1, Ang-2 and sTie2. Both bacterial mimics elevated levels of interleukin-8 (IL-8). *, P < 0.05 vs. untreated (one-way ANOVA, followed by Dunnett’s tests). B, Accumulation in placental explant medium of Ang-1, Ang-2 was not inhibited by incubation with protein synthesis (cycloheximide, 10 μg/mL) or secretion blockers (monensin, 2 μM and brefeldin A, 5 μg/mL). Monensin and brefeldin elevated sTie2 release in the explant media. *, P < 0.05 vs. untreated (one-way ANOVA followed by Dunnett’s tests). Incubations with the equivalent dose of vehicle alone had no effect on the release of angiogenic cytokines. Scattergrams of AF Ang-2/Ang-1 ratio on the x-axes and cord blood IL-6 (C) or cord blood to-AF IL-6 ratio (D), an indicator of the differential inflammatory response in the fetal vs. the intra-amniotic compartment. All axes are in logarithmic format. Thick lines, linear regression line; thin continuous lines, 95% CI; dotted lines, 95% prediction intervals. The correlations coefficients (R) and level of significance are presented after correction for GA at amniocentesis and delivery in multivariate regression.
Relationships of AF Ang-2/Ang-1 ratio with cord blood IL-6 and the differential inflammatory response in the fetal vs. the AF compartment
Cord blood IL-6 levels were significantly elevated in the context of IAI [positive IAI, 92.4 (14.0–248.5) vs. negative IAI, 6.3 (4.9–6.9) pg/ml; P = 0.002]. Consistent with the proinflammatory properties of Ang-2, we found that AF Ang-2/Ang-1 ratio was an independent predictor of cord blood IL-6 concentration which maintained its significance after correction for GA at amniocentesis and delivery and amniocentesis-to-delivery interval (F-ratio, 15.4; multivariate R = 0.700; P < 0.001; Fig. 6C). Next, we explored the possibility that an elevated Ang-2/Ang-1 ratio may act as a vascular destabilizing factor facilitating the transfer of inflammatory cytokines into the fetal circulation. Therefore, we analyzed the relationship between AF Ang-2/Ang-1 ratio and cord blood-to-AF IL-6 ratio (indicator of the differential inflammatory response in the fetal vs. the intraamniotic compartment) (21). There was a significant direct correlation between the Ang-2/Ang-1 and cord blood-to-AF IL-6 ratio (Fig. 6D). The combination of Ang-2/Ang-1 ratio (P = 0.002) and severity of placental histological inflammation (P = 0.014) was able to significantly predict the cord blood-to-AF IL-6 ratio, independent of GA at amniocentesis, GA at delivery, amniocentesis-to-delivery interval, and fetal acid-base status (F-ratio, 9.9; multivariate R = 0.585; P < 0.001).
Discussion
Here we demonstrate that Ang-1, Ang-2, and sTie2 are normal constituents of AF and that their levels and ratios change dynamically as pregnancy progresses. The human placenta is known to express Ang-1, Ang-2, and their receptor Tie2 (2,22,23). This study provides the first evidence that the changes in levels and ratios of Ang-1 and Ang-2 in AF across gestation concur with their mRNA expression in the villous trophoblast but not amniochorion.
A general discussion about the development of the fetoplacental vascular network that occurs through two distinct processes, vasculogenesis and angiogenesis, is provided in the Supplemental Data (24). In the Supplemental Data, we comment upon our findings regarding the presence, level, regulation, and possible origin of AF Ang-1, Ang-2, and sTie2 in physiological pregnancy.
Angiogenesis and inflammation exist in a mutually dependent association, but exactly how they are related is not well understood (14). In this study, IAI and histological chorioamnionitis modified the amount of AF Ang-1, Ang-2, and sTie2 significantly above the expected levels for GA. Yet, these changes were not accompanied by increases in the placental expression of Ang-1, Ang-2, or Tie2 receptor. The proinflammatory Ang-2 is constitutively present in the placenta and operates in an autocrine fashion by sensitizing endothelial cells to inflammatory mediators including endotoxin, TNF-α, and IL-6 (18,21). Endothelial activation launches an inflammatory cascade consisting of vascular barrier breakdown, edema, neutrophil recruitment, transmigration and molecular trafficking among different compartments. Based on the above findings, we propose that in the setting of IAI, the mechanisms responsible for the transfer and release of angiopoietins, sTie2, and cytokines from the placenta to the AF are amplified. This process may be further enhanced at an earlier GA when Ang-2 levels dominate and the incidence of chorioamnionitis is higher (21). Ang-1 is localized in the vascular pericytes but also in the cytosolic fraction of neutrophils, which are abundantly present in the AF and the placenta of pregnancies complicated by IAI (25). Ang-2 is stored in the Weibel-Palade bodies of the endothelial cells and possibly in the human neutrophils (14). Release of the presynthesized Ang-1 and Ang-2 or fetal contribution in response to IAI may explain why the AF angiopoietins are further elevated in the absence of an increased placental transcription level of these cytokines. As shown in vitro, after their release, Ang-1 and Ang-2 are capable of rebinding to fresh cells (26). This argues that these ligands can be recycled and reused by endothelial cells. Thus, for a sustainable effect an increase in their expression might not be necessary. This concurs with our placental tissue explant results, which showed that ex vivo inflammatory stimuli and cycloheximide failed to alter the production of angiopoietins.
Tie1 and Tie2 form a subfamily of tyrosine kinase receptors with important roles in the proliferation and differentiation of endothelial cells (27). Inflammatory cytokines stimulate the release of sTie1 via a transcription independent mechanism (27). Our in vitro data suggest that the same rules may not apply for the Tie2 receptor and that the mechanism responsible for the increased levels of AF sTie2 observed in pregnancies complicated by infection/IAI is not Toll-like receptor mediated. The observation that the LPS and Pam3Cys (inflammatory inducers) significantly elevated the levels of the proinflammatory cytokine IL-8 but failed to stimulate the release of sTie2 in the explant media supports the premise that cytokines may not be directly responsible for the “shedding” of the Tie2 receptor. Consequently, in the setting of IAI, the increased levels of AF sTie2 and that of the proinflammatory cytokines may be the result of two phenomena that are occurring in parallel but are not necessarily linked mechanistically. The lack of correlation between AF sTie2 and IL-6 supports this argument. Before our study, phorbol myristate acetate (via the protein kinase C pathway) and VEGF (via the phosphoinositide-3-kinase/AKt-dependent pathway) were the only two agents known to stimulate shedding of the sTie2 (7). The observation that in our ex vivo explant system, Golgi disrupting agents but not inhibitors of protein synthesis enhance the release of sTie2 is novel. Both brefeldin and monensin are known to reduce density of cell surface receptors (such as Tie2). An increase in the intracellular availability of the Tie2 for processing into sTie2 or blocking the Golgi transfer of proteins involved in inhibiting the pathways controlling shedding of the sTie2 may account for these observations. Increased amounts of AF Ang-1, Ang-2, low sTie2, and IAI were independent risk factors for PPROM. Molecular studies demonstrated that angiopoietins regulate, in part, activation of matrix metalloprotease-9 and, in concert with inflammatory cytokines, may facilitate PPROM (18,28).
We found a significant direct correlation between the Ang-2/Ang-1 and cord blood-to-AF IL-6 ratios. Histological inflammation of the placenta was also an important determinant of this relationship. Consistent with our previous study, we propose that an elevated Ang-2 level unbalanced by its Ang-1 or sTie2 counterparts augments the transfer of IL-6 through a disrupted maternal-fetal vascular barrier (21). Our observation that the Ang-2 signal was more conspicuous in the regions affected by severe villous edema in the placenta of women with preterm birth and IAI is in support of this concept. An unopposed Ang-2 signaling leading to placental inflammation and edema may contribute to an increased cytokine transfer to the fetus, which may result in an increased risk for adverse neonatal outcome and cerebral palsy (21). This paradigm is supported by the evidence linking Ang-2 to edema formation in fetal organ systems (lung, bowel, brain) as well as placental vascular lesions and villous edema with neurological impairment and cerebral palsy (29). Thus, two events, namely unopposed Ang-2 release and cytokine outpouring, may account for a redundant cycle that furthers pathological angiogenesis and prematurity-related sequelae.
Supplementary Material
Acknowledgments
We are indebted to the nurses, residents, and fellows from the Department of Obstetrics, Gynecology and Reproductive Sciences, and the Department of Pediatrics, Division of Perinatal Medicine at Yale-New Haven Hospital, and to all patients who participated in the study.
Footnotes
This work was supported by National Institutes of Health Grant RO1 HD 047321 (to I.A.B.) and funds from the Yale University Department of Obstetrics, Gynecology and Reproductive Sciences.
Contributions to Authorship: C.S.B., V.B., and I.A.B. formulated the hypothesis, designed the study, and drafted the manuscript. C.S.B., A.T.D., C.S.H., U.A.A., and I.A.B. collected, analyzed, and interpreted the demographic data. A.T.D., C.S.B., and I.A.B. designed and performed the placental explant experiments. G.Z. conducted the ELISA assays and the immunohistochemistry studies of the placenta. E.Z. examined the placental specimens. C.S.B., A.T.D., S.T., S.A.-R., V.R., C.S.H., E.Z., E.F.F., and U.A.A. recruited patients, collected biological specimens, and followed the patients prospectively to the point of delivery. All the coauthors participated with aspects of study design and critical interpretation of the data, contributed to writing of the paper, and have reviewed and approved the final version.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 21, 2010
Abbreviations: AF, Amniotic fluid; Ang-1, angiopoietin-1; CI, confidence interval; GA, gestational age; IAI, intraamniotic inflammation; LPS, lipopolysaccharide; PPROM, preterm premature rupture of the membranes; ROC, receiver operating curve; sTie2, soluble Tie2; Tie2, Tunica interna endothelial cell kinase-2; VEGF, vascular endothelial growth factor.
References
- Folkman J, Klagsbrun M 1987 Angiogenic factors. Science 235:442–447 [DOI] [PubMed] [Google Scholar]
- Zhang EG, Smith SK, Baker PN, Charnock-Jones DS 2001 The regulation and localization of angiopietin-1, -2, and their receptor Tie2 in normal and pathologic human placentae. Mol Med 7:624–635 [PMC free article] [PubMed] [Google Scholar]
- Peters KG, Kontos CD, Lin PC, Wong AL, Rao P, Huang L, Dewhirst MW, Sankar S 2004 Functional significance of Tie2 signaling in the adult vasculature. Recent Prog Horm Res 59:51–71 [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y 1995 Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376:70–74 [DOI] [PubMed] [Google Scholar]
- Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM 1998 Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEG-induced postnatal neovascularization. Circ Res 83:233–240 [DOI] [PubMed] [Google Scholar]
- Singh N, Macnamara E, Rashid S, Ambati J, Kontos CD, Higgins E, Ambati BK 2005 Systemic soluble Tie2 expression inhibits and regresses corneal neovascularization. Biochem Biophys Res Commun 332:194–199 [DOI] [PubMed] [Google Scholar]
- Reusch P, Barleon B, Weindel K, Martiny-Baron G, Gödde A, Siemeister G, Marmé D 2001 Identification of a soluble form of the angiopoietin receptor TIE-2 released from endothelial cells and present in human blood. Angiogenesis 4:123–131 [DOI] [PubMed] [Google Scholar]
- Findley CM, Cudmore MJ, Ahmed A, Kontos CD 2007 VEGF induces Tie2 shedding via a phosphoinositide 3-kinase/Akt dependent pathway to modulate Tie2 signaling. Arterioscler Thromb Vasc Biol 27:2619–2626 [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD 1997 Angiopoietin-2, a natural antagonist for the Tie2 that disrupts in vivo angiogenesis. Science 277:55–60 [DOI] [PubMed] [Google Scholar]
- Kim I, Kim JH, Moon SO, Kwak HJ, Kim NG, Koh GY 2000 Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Oncogene 19:4549–4552 [DOI] [PubMed] [Google Scholar]
- Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD 2002 Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning and only the latter role is rescued by angiopoietin-1. Dev Cell 3:411–423 [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos A, Eleftherakis-Papaiakovou V, Kastritis E, Tsionos K, Bamias A, Meletis J, Dimopoulos MA, Terpos E 2007 Serum concentrations of angiogenic cytokines in Waldenstrom macroglobulinaemia: the ration of angiopoietin-1 to angiopoietin-2 and angiogenin correlate with disease severity. Br J Haematol 137:560–568 [DOI] [PubMed] [Google Scholar]
- Roviezzo F, Tsigkos S, Kotanidou A, Bucci M, Brancaleone V, Cirino G, Papapetropoulos A 2005 Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther 314:738–744 [DOI] [PubMed] [Google Scholar]
- Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG 2006 Angiopoietin-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation. Nat Med 12:235–239 [DOI] [PubMed] [Google Scholar]
- Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, Sotiropoulou C, Zakynthinos S, Armaganidis A, Papapetropoulos A, Roussos C 2007 Angiopoietin-2 is increased in severe sepsis. Correlation with inflammatory mediators. Crit Care Med 35:199–206 [DOI] [PubMed] [Google Scholar]
- Lemieux C, Maliba R, Favier J, Théorêt JF, Merhi Y, Sirois MG 2005 Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood 105:1523–1530 [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Christner R, Buhimschi CS 2005 Proteomic biomarker analysis of AF for identification of intra-amniotic inflammation. BJOG 112:173–181 [DOI] [PubMed] [Google Scholar]
- Buhimschi CS, Bhandari V, Hamar BD, Bahtiyar MO, Zhao G, Sfakianaki AK, Pettker CM, Magloire L, Funai E, Norwitz ER, Paidas M, Copel JA, Weiner CP, Lockwood CJ, Buhimschi IA 2007 Proteomic profiling of the AF to detect inflammation, infection, and neonatal sepsis. PLoS Med 4:e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS 2009 Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol 47:38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mansouri L, Bahiri R, Abourazzak FE, Abouqal R, Hajjaj- Hassouni N 2009 Two distinct patterns of ankylosing spondylitis in Moroccan patients. Rheumatol Int 29:1423–1429 [DOI] [PubMed] [Google Scholar]
- Buhimschi CS, Dulay AT, Abdel-Razeq S, Zhao G, Lee S, Hodgson EJ, Bhandari V, Buhimschi IA 2009 Fetal inflammatory response in women with proteomic biomarkers characteristic of intra-amniotic inflammation and preterm birth. BJOG 116:257–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiessl B, Innes BA, Bulmer JN, Otun HA, Chadwick TJ, Robson SC, Lash GE 2009 Localization of angiogenic growth factors and their receptors in the human placental bed throughout normal human pregnancy. Placenta 30:79–87 [DOI] [PubMed] [Google Scholar]
- Geva E, Ginzinger DG, Zaloudek CJ, Moore DH, Byrne A, Jaffe RB 2002 Human placental vascular development: vasculogenic and angiogenic (branching and nonbranching) transformation is regulated by vascular endothelial growth factor-A, angiopoietin-1, and angiopoietin-2. J Clin Endocrinol Metab 87:4213–4224 [DOI] [PubMed] [Google Scholar]
- Demir R, Seval Y, Huppertz B 2007 Vasculogenesis and angiogenesis in the early human placenta. Acta Histochem 109:257–265 [DOI] [PubMed] [Google Scholar]
- Neagoe PE, Brkovic A, Hajjar F, Sirois MG 2009 Expression and release of angiopoietin-1 from human neutrophils: intracellular mechanisms. Growth Factors 27:335–344 [DOI] [PubMed] [Google Scholar]
- Bogdanovic E, Nguyen VP, Dumont DJ 2006 Activation of Tie2 by angiopoietin-1 and angiopoietin-2 results in their release and receptor internalization. J Cell Sci 119:3551–3560 [DOI] [PubMed] [Google Scholar]
- Yabkowitz R, Meyer S, Black T, Elliott G, Merewether LA, Yamane HK 1999 Inflammatory cytokines and vascular endothelial growth factor stimulate the release of soluble tie receptor from human endothelial cells via metalloprotease activation. Blood 93:1969–1979 [PubMed] [Google Scholar]
- Das A, Fanslow W, Cerretti D, Warren E, Talarico N, McGuire P 2003 Angiopoietin/Tek interactions regulate mmp-9 expression and retinal neovascularization. Lab Invest 83:1637–1645 [DOI] [PubMed] [Google Scholar]
- Redline RW, Wilson-Costello D, Borawski E, Fanaroff AA, Hack M 2000 The relationship between placental and other perinatal risk factors for neurologic impairment in very low birth weight children. Pediatr Res 47:721–726 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.