Abstract
Context: Phase response curves (PRCs) to melatonin exist, but none compare different doses of melatonin using the same protocol.
Objective: The aim was to generate a PRC to 0.5 mg of oral melatonin and compare it to our previously published 3.0 mg PRC generated using the same protocol.
Design and Setting: The study included two 5-d sessions in the laboratory, each preceded by 7–9 d of fixed sleep times. Each session started and ended with a phase assessment to measure the dim light melatonin onset (DLMO). In between were 3 d in an ultradian dim light (<150 lux)/dark cycle (light:dark, 2.5:1.5).
Participants: Healthy adults (16 men, 18 women) between the ages of 18 and 42 yr participated in the study.
Interventions: During the ultradian days of the laboratory sessions, each participant took one pill per day at the same clock time (0.5 mg melatonin or placebo, double blind, counterbalanced).
Main Outcome Measure: Phase shifts to melatonin were derived by subtracting the phase shift to placebo. A PRC with time of pill administration relative to baseline DLMO and a PRC relative to midpoint of home sleep were generated.
Results: Maximum advances occurred when 0.5 mg melatonin was taken in the afternoon, 2–4 h before the DLMO, or 9–11 h before sleep midpoint. The time for maximum phase delays was not as distinct, but a fitted curve peaked soon after wake time.
Conclusions: The optimal administration time for advances and delays is later for the lower dose of melatonin. When each dose of melatonin is given at its optimal time, both yield similarly sized advances and delays.
Melatonin (0.5 mg and 3.0 mg) produces similarly sized circadian phase shifts, but the lower dose should be taken at a later time.
It was first shown that exogenous melatonin can phase-shift the circadian clock in rodents more than 25 yr ago (1). Later, it was shown that exogenous melatonin can also shift the timing of the circadian clock in humans (2) and according to a phase response curve (PRC) (3). However, questions still remain on the optimal dose and timing of melatonin administration to produce desired phase advance shifts (reset the circadian clock to an earlier time) or phase delay shifts (reset the circadian clock to a later time) in humans.
We recently generated a PRC to 3.0 mg of exogenous melatonin (4). Subjects took melatonin while free-running through an ultradian light/dark cycle to avoid confounding phase shifts due to abrupt shifts of sleep/dark and light exposure. Because different durations of light produce differently shaped PRCs (5), different doses of melatonin may produce differently shaped PRCs. Thus, the times to take a dose of melatonin for maximal advances or maximal delays may differ depending on the dose, as may the magnitude of the maximum advances and delays. Therefore, we generated a PRC to a 0.5 mg dose of melatonin using the same protocol as we used previously to compare it directly to our 3.0 mg melatonin PRC. Both PRCs showed the time of melatonin administration relative to each individual’s dim light melatonin onset (DLMO). We also created novel PRCs by calculating the time of melatonin administration relative to each individual’s baseline home sleep midpoint, to use when the DLMO is not known.
Subjects and Methods
Ethical approval
The protocol conformed to the standards set by the Declaration of Helsinki and was approved by the Rush University Medical Center Institutional Review Board. All subjects gave written informed consent and were reimbursed for their participation.
Subjects
Subjects were healthy [16 men, 18 women; all ≤100 kg; age (mean ± sd), 25.3 ± 4.8 yr; body mass index, 23.9 ± 2.9 kg/m2). Subjects were screened as in our previous study (4). All subjects were free of prescription medication, except five women who used hormonal birth control. There were nine moderate morning types, 21 neither types, and four moderate evening types (6). Subjects were assigned to the next available group with a preset clock time of pill administration. There was no attempt to balance groups for sex.
Protocol
The methods (Fig. 1) were identical to those used to generate our 3.0 mg PRC (4). Analyses of samples of the 0.5 mg pills showed that they varied from 0.5 to 0.8 mg, and samples of the 3.0 mg pills varied from 2.4 to 7.8 mg (Stockgrand Ltd., University of Surrey, Surrey, UK). The pills were dissolved in absolute ethanol and diluted numerous times, which can increase the error of measurement. Circadian phase assessments were as previously described (4). The final phase assessments started at least 8.5 h after the last pill administration to allow for pill “wash out” before saliva collection. The samples were later shipped in dry ice to Pharmasan Laboratories (Osceola, WI) and were radioimmunoassayed for endogenous melatonin levels. All samples from an individual subject were assayed in the same batch. The sensitivity of the assay was 0.7 pg/ml, and intra- and interassay coefficient of variabilities were 12.1 and 13.2%, respectively.
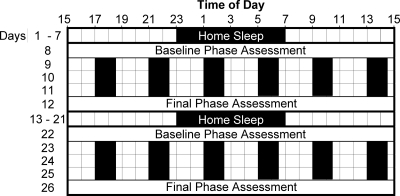
Figure 1.
Diagram of the protocol. Subjects slept on a fixed sleep schedule (8 to 9 h per night) tailored to their habitual sleep times for 7 d (example of 2300 to 0700 h shown in figure), and then participated in a laboratory session for 5 d. Subjects returned to sleeping on the fixed sleep schedule for 9 d before returning for a second laboratory session. During the phase assessments, on d 8, 12, 22, and 26, saliva was sampled every 30 min in dim light (<5 lux) to measure the entire melatonin profile and calculate the DLMOs. There were 3 d of an ultradian light/dark cycle during each laboratory session in which subjects lay on cots in the dark (0 lux) for 1.5 h, were permitted to sleep (represented by black bars), and were kept awake for 2.5 h in room light [<150 lux, 37.3 ± 13.8 lux (mean ± sd)] and were permitted to eat and drink ad libitum. For each of the 3 ultradian days, subjects were given a pill at the start of one of the wake episodes, thus at the same clock time each day. During one laboratory session, the pill was melatonin (0.5 mg immediate release), and during the other session it was placebo, counterbalanced, double-blind. Different groups of three to four subjects received the pills at the start of different awake episodes to cover the entire 24-h day. The phase shift during each laboratory session was the baseline DLMO minus the final DLMO. The phase shift (free-run) during the placebo session was subtracted from the phase shift during the melatonin session and plotted against the time of melatonin administration relative to baseline DLMO or relative to home sleep midpoint to generate the PRCs.
Data analysis
The melatonin profiles were smoothed as previously described (4). The threshold for each melatonin profile was the mean of five low consecutive raw daytime values + 2 sd values (7). The average threshold from each laboratory session was used for both profiles. This method yields low thresholds that are typically close to the physiological onset of endogenous melatonin secretion. The mean ± sd threshold was 3.3 ± 1.8 pg/ml. The thresholds for the 3.0 mg PRC were also recalculated this way so that the PRCs for these two different doses could be more exactly compared. The DLMO for each profile was the point in time (as determined with linear interpolation) when the smoothed melatonin curve exceeded the threshold and remained above the threshold for at least 4 h. The dim light melatonin offset (DLMOff) for each profile was the point in time when the smoothed melatonin curve fell below the threshold.
Each individual’s phase shift during the melatonin session was corrected by subtracting their phase shift during the placebo session (effect of laboratory session or free-run) to yield the phase shift due to exogenous melatonin alone. This net shift was plotted against the time of the melatonin administration relative to the individual’s baseline DLMO. We also plotted PRCs with the time of melatonin administration relative to the midpoint of each individual’s baseline assigned home sleep schedule from before the laboratory sessions. A dual harmonic sinusoidal regression was fit to the raw data of each PRC.
Statistical analysis
The times of the maximum fitted advances in the 3.0 and 0.5 mg PRCs were compared by calculating the difference between the curve fits at the time of the 3.0 mg PRC peak advance and dividing by the se of the predicted value. In this case, a one-tailed test was considered appropriate due to our a priori prediction that PRCs to smaller doses of melatonin would result in later peak advances (4). The timing of the maximum delays in each PRC was compared in the same manner at the time of the 3.0 mg PRC peak delay, but with a two-tailed test. This was due to our a priori prediction that there would be no difference in the timing of peak delays to different doses of melatonin (4). The amplitudes of the maximum advance (or maximum delay) in each PRC were compared by calculating the difference in maximum advances (or delays) and dividing by the se of the predicted value. In these cases, two-tailed tests were calculated. In all cases, an α of 0.05 was used to determine statistical significance.
Results
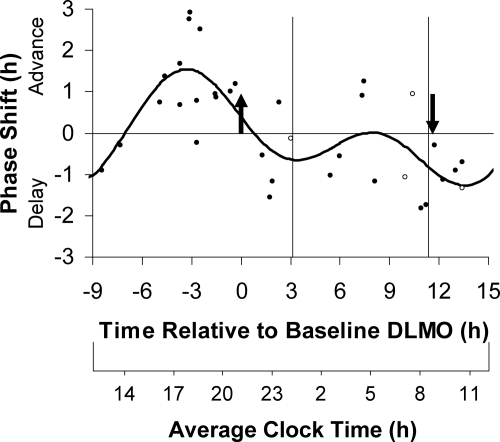
Of the five women who used hormonal birth control, four had varying hormone levels during the study. Including these four women in the PRCs did not significantly change the resulting curve fits, and these women are represented by open circles in all PRC figures. The PRC to 0.5 mg of melatonin plotted relative to the DLMO (Fig. 2) had an advance peak 3.3 h before the DLMO, in the afternoon. The delay portion was not as distinct, but the trough of the fitted curve occurred 13.6 h after the DLMO. The fitted curve had a maximum advance of 1.5 h and a maximum delay of 1.3 h, although of course, larger phase shifts (as much as 2.9 h for advances) occurred in some individuals. In general, the scatter of points across the 24 h shows more variability, coalescing into less clear advance and delay portions than the corresponding PRC to 3.0 mg of melatonin (Fig. 2 in Ref. 4).
Figure 2.
The three-pulse PRC to 0.5 mg of exogenous oral melatonin generated from subjects free-running during 3 d of an ultradian light/dark cycle (LD 2.5:1.5). Phase shifts of the circadian clock, measured by changes in the timing of the DLMO, are plotted against the time of administration of the melatonin pill relative to the baseline DLMO (top x-axis). The average baseline DLMO is represented by the upward arrow, the average baseline DLMOff by the downward arrow, and the average assigned baseline sleep times at home from before the laboratory sessions are enclosed by the vertical lines. Each dot represents the phase shift of an individual subject, calculated by subtracting the phase shift during the placebo session (free-run) from the phase shift during the melatonin session. The open circles represent the four women who had varying hormone levels during the study due to the use of hormonal birth control. The curved line illustrates the dual harmonic curve fit. The average clock time axis (bottom x-axis) corresponds to the average baseline sleep times at home.
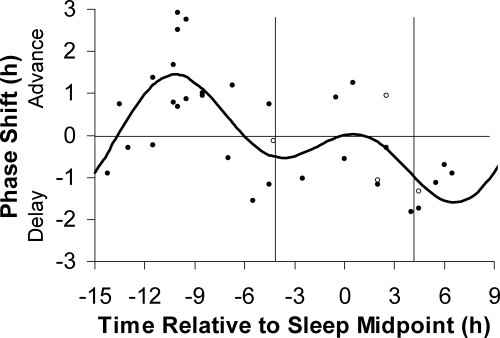
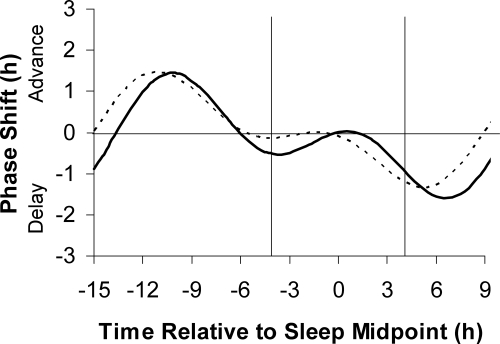
Figure 3 shows the 0.5 mg PRC plotted using the midpoint of the assigned baseline sleep schedule at home. Note that the magnitude of the phase shift for each individual is the same as in Fig. 2, but the placement along the x-axis differs. Again, there is a cluster of points for advances that form a distinct peak, whereas the cluster of points for delays is less distinct. The curve peaked 10.2 h before sleep midpoint for advances and 6.6 h after sleep midpoint for delays. The fitted curve had a maximum advance shift of 1.5 h and a maximum delay shift of 1.6 h.
Figure 3.
The three-pulse PRC to 0.5 mg of exogenous oral melatonin generated from subjects free-running during 3 d of an ultradian light/dark cycle (LD 2.5:1.5). Phase shifts of the circadian clock, measured by the DLMO, are plotted against the time of administration of the melatonin pill relative to the midpoint of each individual’s assigned baseline sleep schedule at home from before the laboratory sessions. The vertical lines represent the average assigned baseline bedtime and wake time. Each dot represents the phase shift of an individual subject, calculated by subtracting the phase shift during the placebo session (free-run) from the phase shift during the melatonin session. The open circles represent the four women who had varying hormone levels during the study due to the use of hormonal birth control. The curved line illustrates the dual harmonic curve fit.
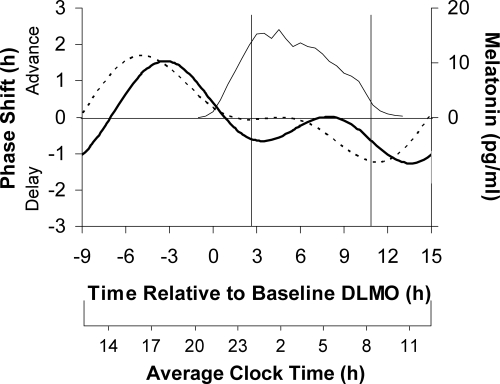
A comparison of the 0.5 and 3.0 mg PRCs plotted relative to the DLMO (Fig. 4) reveals that the timing of maximum advances and maximum delays was slightly later for the 0.5 mg dose. The difference in the advances was statistically significant (z = 1.76; one-tailed P = 0.039). The delays were also statistically different (z = 2.00; two-tailed P = 0.046). The peak-to-trough amplitude of the 0.5 mg PRC was 2.8 h, with a 95% confidence interval that did not include zero, indicating statistical significance. This is similar to the peak-to-trough amplitude of our previously published 3.0 mg PRC that was 2.9 h, also with a 95% confidence interval that did not include zero. Both doses of melatonin produced similarly sized advances and similarly sized delays (z = 0.51; z = 0.18; both two-tailed P > 0.60).
Figure 4.
The dual harmonic curve fits to the three-pulse PRC generated to 0.5 mg of exogenous oral melatonin (solid curve) and to 3.0 mg of exogenous oral melatonin (dashed curve). Phase shifts of the DLMO (left y-axis) are plotted against the time of administration of the melatonin pill relative to the baseline DLMO (top x-axis). The average baseline melatonin profile (thin line, right y-axis) and the average assigned baseline sleep times from before the laboratory sessions (vertical lines) for both groups are shown. The average clock time axis (bottom x-axis) corresponds to the average baseline sleep times at home.
The 0.5 mg PRC shows minimal phase shifts from about the time of the baseline DLMO to about the time of habitual wake, an interval corresponding to the time of the endogenous melatonin profile (see average melatonin profile in Fig. 4). The 3.0 mg PRC shows a more distinct dead zone from about the time of the baseline DLMO to about halfway through the time corresponding to the home sleep episode.
We also superimposed the two PRCs with the time of melatonin administration relative to home sleep midpoint (Fig. 5). The shapes were similar to the PRCs plotted relative to the DLMO, but the differences between the times of the peaks and troughs were slightly less.
Figure 5.
The three-pulse PRC to 0.5 mg (solid curve) and 3.0 mg (dashed curve) of exogenous oral melatonin generated from subjects free-running during 3 d of an ultradian light/dark cycle (LD 2.5:1.5). Phase shifts of the circadian clock, measured by the DLMO, are plotted against the time of administration of the melatonin pill relative to the midpoint of each individual’s assigned baseline sleep schedule at home before the laboratory sessions. The vertical lines show the average assigned baseline bedtime and wake time.
For the 0.5 mg subjects, the average assigned home baseline bedtime and wake times were 0015 and 0832 h. The average baseline DLMO was 2110 h in the melatonin sessions and 2058 h in the placebo sessions. The difference between the baseline DLMOs in the placebo vs. melatonin session ranged from 0.0 to 1.7 h, with only three subjects having a difference of more than 1 h. In the melatonin session, the average baseline DLMO to home bedtime interval was 3.1 h, and the average baseline DLMO to DLMOff interval was 11.7 ± 1.3 h. The average phase shift during the 5-d laboratory sessions with placebo was a delay of 1.11 ± 0.8 h, which corresponds to a free-running period of 24.28 h. These values are similar to those reported for the 3.0 mg PRC (4).
Discussion
We have generated a new PRC to 0.5 mg of exogenous oral melatonin and compared it to our previously published PRC to 3.0 mg of melatonin (4). Both PRCs were generated with the same protocol: a single daily dose was given at the same clock time to free-running subjects for 3 consecutive days. The pills were administered at various times of day to different subjects to generate these three-pulse PRCs. Thus, this is the first direct comparison of the phase shifting effects of two doses of melatonin when given at various times across the 24-h day. The PRCs to 0.5 mg of melatonin show that maximum advances occur when it is taken about 2–4 h before the DLMO or about 9–11 h before sleep midpoint. The fitted curves show that maximal delays occur when it is taken about 12–15 h after the DLMO or within 4 h or so after wake time (Figs. 2 and 3). The fitted curves show maximum advances and delays of similar magnitudes, about 1.5 h over 3 d (Figs. 2 and 3).
The peak advance and delay occurred about 2 h later relative to the DLMO with the lower dose (Fig. 4). However, the magnitude of the maximum advances and the magnitude of the maximum delays were similar with both doses. Both PRCs show a region of minimal sensitivity starting from about the time of the DLMO and continuing through most of the home sleep episode. The fitted curve to the 0.5 mg dose shows a small delay around bedtime, but this delay dip is much smaller than the major advance and delay portions. Both PRCs show that taking melatonin at bedtime, as a sleep aid, will have a relatively minor effect on circadian phase. On the other hand, both PRCs (especially the 3.0 mg PRC) indicate that if exogenous melatonin is taken near the end of sleep it will coincide with the beginning of the delay portion and could inadvertently delay the circadian clock. Both PRCs (Fig. 4) also indicate that the largest advance shifts to exogenous melatonin occur when circulating levels of endogenous melatonin are low. Phase shifts diminish around the time that endogenous melatonin appears in the circulation and remain minimal until melatonin levels start to decrease, perhaps around the time that the pineal gland has dramatically reduced its production of melatonin [sometimes called the “SynOff” (8)]).
The difference in the timing of the peak advance portions in our 0.5 and 3.0 mg PRCs can be explained by the concept of overlap. Lewy et al. (3) proposed that the largest phase shifts in humans occur when exogenous melatonin in the circulation overlaps with the endogenous melatonin profile. Thus, exogenous melatonin taken in the afternoon/evening should overlap with the beginning of the endogenous melatonin profile to simulate an earlier dusk and produce an advance (cf. Fig. 4). The smaller 0.5 mg dose of melatonin needs to be taken later in the afternoon/evening than the larger 3.0 mg dose to ensure that it will remain it the circulation long enough to overlap with the endogenous melatonin profile and produce a continuous melatonin signal. Thus, a later peak advance portion is predicted for the smaller melatonin dose, which is what we observed. Similarly, exogenous melatonin given in the morning should overlap with the end of the endogenous melatonin profile to simulate a later dawn and produce a delay. In this case, both doses given at the same time should extend the duration of melatonin, and thus the times of the peak delay portions should not be different. However, the fitted trough of the delay portion occurred slightly later in the 3.0 mg PRC. Nevertheless, the largest individual delays of both PRCs clustered around the same time (∼11 h after the DLMO).
Previous work has identified a clear dose-response relationship for physiological doses of exogenous melatonin, such as from 0.02 to 0.3 mg (9). Our PRCs to supraphysiological doses of melatonin indicate that both doses produced similarly sized advances and delays. Previously, we had suggested that the smaller advances to lower doses of melatonin that we and others observed (10,11) could have been due to the higher doses being administered at more optimal times. For example, in one study 0.05, 0.5, and 5.0 mg of melatonin were all administered 7 h before bedtime (approximately 5 h before the DLMO), resulting in advances of 0.4, 0.7, and 1.4 h, respectively (10). Figure 4 illustrates that this apparent dose-response relationship may simply be due to this particular time of administration being more optimal for the 5.0 mg dose and progressively less optimal for the 0.5 and 0.05 mg doses. Indeed, it may be that supraphysiological melatonin produces phase shifts in an all-or-none fashion and any dose greater than 0.5 mg, if administered at the optimal time for each respective dose, elicits the same-sized phase shift.
Alternatively, it is possible that by timing the administration of a single melatonin pill per day, at the same clock time, we may have not observed the maximum advance and delay that each melatonin dose can produce. This is because, after the first melatonin pill, the circadian clock and associated melatonin PRC likely shifted, meaning that the second and third pills fell on slightly different parts of the melatonin PRC. Thus, a fixed administration time would exclude the possibility of all three melatonin pills falling exactly on the peak advance or peak delay portions of the melatonin PRCs. Nonetheless, in 3 d we did observe larger maximum advances than those seen after a single melatonin dose of 0.5 mg (0.7 h in Ref. 10) and 5 mg (0.4 h in Ref. 12; 0.7 h in Ref. 13). Future studies that incorporate such a gradually shifting time of melatonin administration (e.g. Ref. 14) are needed to determine whether in optimal circumstances larger supraphysiological doses of exogenous melatonin can in fact produce larger phase shifts.
Although the DLMO is widely regarded as the most reliable circadian phase marker currently available (8,15), it is not yet readily accessible to the general public. Thus, when the timing of an individual’s DLMO is not known, PRCs that are plotted relative to the DLMO (3,4,16) must be translated to be relative to sleep times. For example, because the Lewy 0.5 mg PRC indicates that peak advances occur about 3 h before the DLMO (see curve fit in Fig. 1 in Ref. 17), we previously administered 0.5 mg of melatonin 5 h before bedtime, assuming that there was 2 h between the individual’s DLMO and bedtime (18). However, there is variability in the DLMO-to-bedtime interval. In a large sample, we found that the mean DLMO-to-bedtime interval was 2.7 h, and the range varied from −0.3 to 5.8 h (19). Indeed, 60% of the sample had a DLMO-to-bedtime interval outside of 2–3 h. Therefore, to increase the practicality of our PRCs, we have replotted them relative to sleep midpoint (Figs. 3 and 5). These PRCs show that maximum advances occur when 0.5 mg is taken about 10 h before sleep midpoint and when 3.0 mg of melatonin is taken about 11 h before sleep midpoint. Maximal delays occur when it is taken near the end of sleep and in the morning.
We recommend that when the DLMO is known, PRCs plotted relative to the DLMO should be used instead of PRCs plotted relative to sleep midpoint. This is because bed and wake times are more readily influenced by social factors and can vary greatly between weekends and weekdays. Sleep midpoint may approximate circadian phase better than bedtime or wake time, but probably not as well as the DLMO.
Our 0.5 mg PRC is partly consistent with the most widely cited PRC to melatonin, the PRC of Lewy et al. (3). The Lewy PRC was generated by administering 0.5 mg of melatonin per day for 4 consecutive days to entrained subjects. Each subject maintained his or her habitual sleep schedule at home for 2 wk, and three times at weekly intervals they visited the laboratory to have their DLMO assessed. Subjects were instructed to take a pill once per day, at the same time of day when they slept at home. In 1 wk, the pill was always placebo, in the other week, the last four pills were melatonin. Both our 0.5 mg PRC and the Lewy PRC indicate that advances result when melatonin is taken in the afternoon and early evening. More specifically, both PRCs indicate that peak advances occur 3–3.5 h before the DLMO. Both PRCs also show a broad range of delays. These similarities are quite remarkable, considering the two PRCs were generated with markedly different protocols (although with similar DLMO thresholds of approximately 3 pg/ml) and that the Lewy PRC was generated in the field, whereas our PRC was generated in the laboratory under more controlled conditions.
By contrast, despite the Lewy PRC having an additional day of melatonin administration, our PRC shows larger maximum phase shifts of about 2–3 h, whereas the Lewy PRC showed maximum phase shifts of about 1.5 h. As we have previously described (4), the smaller phase shifts in the Lewy PRC are likely due to the fixed sleep-wake schedule, the fixed light/dark cycle, and/or other 24-h zeitgebers opposing or constraining the phase shifts to melatonin. Our subjects were free-running and not constrained by 24-h zeitgebers.
Both of our melatonin PRCs and the Lewy PRC show some individual variability in the phase shift response to exogenous melatonin. In addition to the variability in the amount of melatonin in pills sold over the counter, like ours, individual differences in the bioavailability of melatonin are a likely contributor to the variability (20). Similar variability has also been observed in animal studies. In one such study, 90 μg of melatonin was injected once per day for 3 d into mice free running in constant dark (21). Many mice showed no shift in their wheel running activity, whereas others phase advanced more than 1.5 h.
A PRC is classically generated from a single exposure to a zeitgeber or stimulus in free-running animals. Although we did apply exogenous melatonin to free-running subjects, the dose of exogenous melatonin was repeated across 3 d instead of 1 d. We did this to produce larger, more detectable phase shifts and also because multiple doses of exogenous melatonin are more typically used in the field. Thus, we call our PRC a three-pulse PRC. Others have used the term “PRC” for graphs generated from the administration of multiple pulses of a zeitgeber (3,22), from entrained humans (3) and even from reentrainment protocols (16,22).
We have tested protocols designed to shift human circadian rhythms using a gradually shifting sleep schedule plus bright light and/or melatonin at the appropriate circadian times (14,18,23,24,25,26). This approach can phase shift the clock without causing extreme circadian misalignment between the clock and the sleep/wake schedule, and thus avoids the associated jet-lag type symptoms and negative consequences of abrupt shifts of sleep. By gradually changing the times of administration, the bright light and melatonin are more likely to hit the optimal times on their PRCs as they shift. We have observed that on average, the combination of afternoon melatonin and morning bright light are additive and produce the largest advances (18). Whether morning melatonin can enhance delays to evening bright light remains to be determined.
Our recommendations on what dose of melatonin to use to phase shift vary according to each individual’s circumstance. The 3.0 mg dose is more likely to produce a substantial phase shift than the lower 0.5 mg dose. However, if the potential soporific effect of the 3.0 mg dose (18) needs to be avoided, then the 0.5 mg dose would be more appropriate.
Acknowledgments
We thank the following people for their assistance with data collection: Daniel Alderson, Elisabeth Beam, Jillian Canton, Erin Cullnan, Corrine Eckstein, Heather Holly, Jacqueline Munoz, Meredith Rathert, Mark Smith, Christina Suh, and Nicole Woodrick. We thank Lou Fogg, Ph.D., for his statistical advice. We thank our medical director Margaret Park, M.D. We also thank Ecological Formulas (Concord, CA) for donating the melatonin and matching placebo pills. We thank Drs. Debra Skene and Benita Middleton at the University of Surrey (Surrey, UK) for their time and effort in analyzing the pills.
Footnotes
This work was supported by Grant R01 HL086934 from the National Institutes of Health (to C.I.E.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. V.L.R. is currently supported by the 6th Framework project EUCLOCK (018741).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 21, 2010
Abbreviations: DLMO, Dim light melatonin onset; DLMOff, dim light melatonin offset; PRC, phase response curve.
References
- Redman J, Armstrong S, Ng KT 1983 Free-running activity rhythms in the rat: entrainment by melatonin. Science 219:1089–1091 [DOI] [PubMed] [Google Scholar]
- Arendt J, Bojkowski C, Folkard S, Franey C, Marks V, Minors D, Waterhouse J, Wever RA, Wildgruber C, Wright J 1985 Some effects of melatonin and the control of its secretion in humans. In: Evered D, Clark S, eds. Photoperiodism, melatonin, and the pineal. London: Pitman; 266–283 [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, Moffit MT, Sack RL 1998 The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int 15:71–83 [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Revell VL, Eastman CI 2008 A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol 586:639–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas M, Beersma DG, Spoelstra K, Daan S 2006 Phase and period responses of the circadian system of mice (Mus musculus) to light stimuli of different duration. J Biol Rhythms 21:362–372 [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O 1976 A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 4:97–110 [PubMed] [Google Scholar]
- Voultsios A, Kennaway DJ, Dawson D 1997 Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms 12:457–466 [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Cutler NL, Sack RL 1999 The endogenous melatonin profile as a marker of circadian phase position. J Biol Rhythms 14:227–236 [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Emens JS, Lefler BJ, Yuhas K, Jackman AR 2005 Melatonin entrains free-running blind people according to a physiological dose-response curve. Chronobiol Int 22:1093–1106 [DOI] [PubMed] [Google Scholar]
- Deacon S, Arendt J 1995 Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res 688:77–85 [DOI] [PubMed] [Google Scholar]
- Sharkey KM, Eastman CI 2002 Melatonin phase shifts human circadian rhythms in a placebo-controlled simulated night-work study. Am J Physiol Regul Integr Comp Physiol 282:R454–R463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A, Kräuchi K, Cajochen C, Danilenko KV, Renz C, Weber JM 2004 Evening melatonin and bright light administration induce additive phase shifts in dim light melatonin onset. J Pineal Res 36:192–194 [DOI] [PubMed] [Google Scholar]
- Kräuchi K, Cajochen C, Möri D, Graw P, Wirz-Justice A 1997 Early evening melatonin and S-20098 advance circadian phase and nocturnal regulation of core body temperature. Am J Physiol 272:R1178–R1188 [DOI] [PubMed] [Google Scholar]
- Crowley S, Smith M, Munoz J, Eastman C Advancing circadian rhythms with afternoon melatonin and a gradually advancing sleep/dark schedule. Proc 24th Annual Meeting of the Associated Professional Sleep Societies, San Antonio, TX, 2010 (Abstract 0183) [Google Scholar]
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE 2002 Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms 17:181–193 [DOI] [PubMed] [Google Scholar]
- Khalsa SB, Jewett ME, Cajochen C, Czeisler CA 2003 A phase response curve to single bright light pulses in human subjects. J Physiol 549:945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell VL, Eastman CI 2005 How to trick Mother Nature into letting you fly around or stay up all night. J Biol Rhythms 20:353–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell VL, Burgess HJ, Gazda CJ, Smith MR, Fogg LF, Eastman CI 2006 Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. J Clin Endocrinol Metab 91:54–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Fogg LF 2008 Individual differences in the amount and timing of salivary melatonin secretion. PLoS One 3:e3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMuro RL, Nafziger AN, Blask DE, Menhinick AM, Bertino Jr JS 2000 The absolute bioavailability of oral melatonin. J Clin Pharmacol 40:781–784 [DOI] [PubMed] [Google Scholar]
- Benloucif S, Dubocovich ML 1996 Melatonin and light induce phase shifts of circadian activity rhythms in the C3H/HeN mouse. J Biol Rhythms 11:113–125 [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM 1989 Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science 244:1328–1333 [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI 2003 Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms 18:318–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Gazda CJ, Burgess HJ, Crowley SJ, Fogg LF 2005 Advancing circadian rhythms before eastward flight: a strategy to prevent or reduce jet lag. Sleep 28:33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Eastman CI 2009 Phase delaying the human circadian clock with blue-enriched polychromatic light. Chronobiol Int 26:709–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Revell VL, Eastman CI 2009 Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med 10:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]