Abstract
Context: Nonclassic congenital lipoid adrenal hyperplasia (lipoid CAH) is a recently recognized disorder caused by mutations in the steroidogenic acute regulatory protein (StAR) that retain partial function. Affected individuals can present with a phenotype of late onset adrenal insufficiency with only mild or minimally disordered sexual development.
Objectives: The aim was to delineate the clinical spectrum of StAR mutations and correlate phenotype with StAR activity.
Patients: Four patients had nonclassic/atypical lipoid CAH. Adrenal insufficiency was manifested at birth in two patients and at 11 months and 4 yr in the other two. Three were 46,XY with underdeveloped genitalia.
Methods: The StAR gene was sequenced, mutations were recreated in expression vectors, and StAR activity was measured as pregnenolone production in COS-1 cells cotransfected with the cholesterol side-chain cleavage system. StAR mutants were expressed as N-62 StAR in bacteria, and purified proteins were tested for activity with isolated steroidogenic mitochondria and for cholesterol-binding capacity.
Results: DNA sequencing identified mutations on all alleles. Missense mutations were R188C, G221D, L260P, and F267S; we also tested R192C described by others. The respective activities of R188C, R192C, G221D, L260P, and F267S were 8.0, 39.4, 2.4, 3.1, and 6.1% of wild-type in transfected cells, and 12.8, 54.8, 6.3, 1.8, and 9.5% with isolated mitochondria. Cholesterol binding capacities of R188C, R192C, G221D, L260P, and F267S were 6.7, 55.3, 10.2, 4.6, and 20.9%. These data are correlated to the three-dimensional structure of StAR.
Conclusions: There is a broad clinical spectrum of StAR mutations; StAR activities in vitro correlate well with clinical phenotypes.
Additional StAR mutations are identified that cause nonclassical congenital lipoid adrenal hyperplasia and correlate clinical findings, mutations, and in vitro activity.
Congenital lipoid adrenal hyperplasia (lipoid CAH) is the most severe form of congenital adrenal hyperplasia (CAH) in which the synthesis of all adrenal and gonadal steroid hormones is impaired. Early clinical hormonal studies and incubations of affected tissue in vitro with various precursors identified a defect in the conversion of cholesterol to pregnenolone, so that the disorder was initially called “20,22 desmolase deficiency” because it was thought to result from a defect in the enzyme system converting cholesterol to pregnenolone. This enzyme was later identified as mitochondrial P450scc. For a review of the early history of lipoid CAH, see Ref. 1. However, the P450scc gene is normal in lipoid CAH (2,3), and in 1995 it was found that lipoid CAH results from mutations in the gene encoding the steroidogenic acute regulatory protein (StAR) (4,5). StAR facilitates the movement of cholesterol into mitochondria, where it is converted to pregnenolone by P450scc (6). Patients with classic lipoid CAH usually present with adrenal failure and salt wasting, beginning within the first few months of life, and have female external genitalia irrespective of genetic sex (7). Until the description of nonclassic lipoid CAH in 2006, only one minimally virilized 46,XY female had been reported (8). Nonclassic lipoid CAH is a recently recognized disorder caused by StAR mutations that retain partial activity (9). Affected individuals can present with adrenal insufficiency resembling nonautoimmune Addison disease with only mildly disordered sexual development or normal development with hypergonadotropic hypogonadism (9,10). More than 40 StAR mutations causing classic lipoid CAH have been described (4,7,8,11), but very few partial loss-of-function mutations have been reported (8,9,10). We describe four patients with nonclassic or atypical lipoid CAH, identify their StAR mutations, and characterize the activities of the mutants to explore genotype/phenotype correlations.
Patients and Methods
Patient 1
A 46,XY phenotypic male from a consanguineous Thai family had an uneventful birth and had two febrile seizures during his first 2 yr of life, but no laboratory data are available. He was first evaluated at Chulalongkorn University Hospital at age 4 yr for progressive pigmentation. His older brother had had progressive hyperpigmentation and at 3 yr had fever, convulsions, and sudden unexplained death. The patient had descended testes, a stretched penile length of 3.5 cm and glandular hypospadias. Hormonal data (Table 1) showed unmeasurable cortisol unresponsive to cosyntropin, low dehydroepiandrosterone (DHEA) sulfate, and grossly elevated ACTH. Plasma renin activity (PRA) was 9.62 ng/ml/h (normal range, 1.0–6.5), aldosterone was 25.5 ng/dl (normal range, 3–35), and the electrolytes were normal, indicating a subtle defect in mineralocorticoid secretion. Abdominal ultrasound showed a normal-size right adrenal, but the left adrenal was not seen. The patient was treated with prednisone in doses equivalent to hydrocortisone 11–14 mg/m2/d and remained well with normal electrolytes. Sequencing of the ACTH receptor (MC2R), DAX1, and HSD3B2 genes was normal.
Table 1.
Clinical and biochemical characteristics of patients
| Patient 1 R188C | Patient 2 L260P/F267S | Patient 3 R188C/W250X | Patient 4 G221D | Patient 5 (Ref. 10) R192C | |
|---|---|---|---|---|---|
| Country of origin | Thailand | United States | Switzerland | Nepal | Lebanon |
| Karyotype | 46,XY | 46,XY | 46,XY | 46,XX | 46,XY |
| Age at investigation | 4 yr | Newborn | 3.5 months | 12 yr | 5 yr |
| Genitalia | Normal male, glandular hypospadias | Micropenis, small testes, severe hypospadias | Micropenis, cryptorchidism | Normal female | Normal male |
| Presentation | Pigmentation | Ambiguous genitalia | Pigmentation, ambiguous genitalia | Adrenal crisis | Pigmentation |
| Adrenal imaging | Normal US | Normal CT | ND | Normal MRI | ND |
| ACTH (pg/ml) | >1250 | 560 | 5497 | 2070 | >225 |
| Cortisol (μg/dl) | |||||
| Basal | 0.1 | 8 | 5.3 | <1 | 0.6 |
| Peak | 0.1 | 11 | ND | ND | ND |
| PRA (ng/ml/h) | 9.62 | 95 | ND | 15 mU/liter (3–40) | 5.82 |
| Aldosterone (ng/dl) | 25.5 | 14 | ND | <2.5 | 1.2 |
| LH (IU/liter) | ND | 15.2 | 7.6 | ND | 21.9 |
| FSH (IU/liter) | ND | 16.7 | 5.0 | ND | 50.4 |
| Treatment | Prednisone | Hydrocortisone, fludrocortisone | Hydrocortisone, fludrocortisone | Hydrocortisone, fludrocortisone | Hydrocortisone |
CT, Computed tomography; MRI, magnetic resonance imaging; ND, not done; US, ultrasound. Conversion to System International units: ACTH, picograms per milliliter × 0.22 for picomoles per liter; cortisol, micrograms per deciliter × 27.6 for nanomoles per liter; PRA, nanograms per milliliter per hour × 0.77 for picomoles per milliliter per hour; aldosterone, nanograms per deciliter × 27.7 for picomoles per liter.
Patient 2
A 27-yr-old 46,XY male was born at term with micropenis, third-degree hypospadias, severe chordee, a hypoplastic scrotum, and palpable testes. A human chorionic gonadotropin stimulation test at 3–7 d of life failed to increase progesterone, 17-hydroxyprogesterone (17OHP), DHEA, androstenedione, and testosterone. His electrolytes were normal, and he did not require supplemental steroids during the neonatal period. At age 10 wk, cortisol was 8.5 μg/dl, but his ACTH was 560 pg/ml (normal, 10–50), indicating partially compensated adrenal insufficiency. His aldosterone was 14 ng/dl (normal, 5–90), and his PRA was 95 ng/ml/h (normal, 2.3–37). During an insulin tolerance test, his basal cortisol was 21 μg/dl, but it failed to rise further; an ACTH stimulation test failed to increase urinary 17-ketosteroids or serum levels of progesterone, 17OHP, cortisol, and DHEA. An abdominal computed tomography scan at age 12 wk did not show adrenal enlargement. The patient was diagnosed with “partial 20,22 desmolase deficiency” and started on lifelong glucocorticoid and mineralocorticoid replacement therapy. During his first 2 yr, he had several adrenal crises, including at least one with documented salt-wasting (Na, 124 mEq/liter; and K, 6.1 mEq/liter), associated with respiratory, gastrointestinal, or skin infections and requiring brief hospitalizations for parenteral glucocorticoids. He had normal growth and development and underwent a two-stage genital repair at age 2. He did not undergo puberty, and testosterone treatment was initiated during adolescence. When seen at the University of Pennsylvania at age 25, he was 185 cm tall, weighed 157 kg, and had hyperpigmented skin, 5-ml testes bilaterally, and a penile length of 4 cm. His 0800 h cortisol before taking hydrocortisone was less than 5 μg/dl, with ACTH 5911 pg/ml, total testosterone 78 ng/dl, LH 15.2 IU/liter, and FH 16.7 IU/liter. The parents declined genetic testing. His sister is unaffected, and her carrier status is unknown.
Patient 3
A 46,XY Serbian male infant presented at age 3.5 months having had prolonged neonatal jaundice. He had severe generalized hyperpigmentation, cryptorchidism, micropenis, but no hypospadias. When evaluated at University Children’s Hospital, Zurich, Switzerland, his sodium and potassium were normal, cortisol was 5.3 μg/dl, and ACTH was 5497 pg/ml. Aldosterone and PRA were not measured; testosterone was 29 ng/dl (normal up to 232 ng/dl). He was treated with hydrocortisone and fludrocortisone and received three testosterone injections (25 mg) between 4 and 6 months of age with good penile response to 3.5 cm. He underwent right orchiopexy at 2 yr. When he was 8.5 yr old, his height was 134.5 cm (50th–75th percentile), and weight was 39.8 kg (>97th percentile) while receiving hydrocortisone 20.8 mg/m2/d and fludrocortisone 0.1 mg/d. He remained well with normal electrolytes but had a history of salt-craving. Sequencing of DAX1 and SF1 was normal.
Patient 4
A 15-yr-old, 46,XX Nepalese girl had recently moved to the United Kingdom and was referred to the Portsmouth Hospital with a diagnosis of “CAH”. She had first presented at 11 months of age with adrenal crisis including vomiting and dehydration and had subsequently been treated with hydrocortisone and fludrocortisone, but no hormonal data are available. She reported menarche at 12 yr of age and regular menstruation, but progesterone at d 22 of the cycle was low at 0.2 nmol/liter, indicating anovulatory cycles. Her ACTH was 2070 pg/ml, and she had unmeasurable cortisol (<1 μg/dl), aldosterone (<2.5 ng/dl), testosterone (<10 ng/dl), 17OHP (<60 ng/dl), androstenedione (<30 ng/dl), and DHEA sulfate (<15 μg/dl) (Table 1). Her plasma and urine steroid profiles were compatible with complete adrenal insufficiency. A magnetic resonance imaging scan showed normal-sized adrenals.
Mutation analysis, mutagenesis, and transfection
With informed consent, leukocyte genomic DNA was extracted, and the seven exons of the StAR gene were amplified by PCR and sequenced using previously described oligonucleotides and amplification conditions (12). Mutant full-length StAR cDNA expression vectors (13) were generated by PCR-based, site-directed mutagenesis (primers are shown in Table 2) and verified by direct sequencing. The PCR conditions were: 95 C for 30 sec, 16 cycles of 95 C for 30 sec, 55 C for 1 min, and 68 C for 18 min. Nonsteroidogenic monkey kidney COS-1 cells were grown in DMEM supplemented with 10% fetal calf serum and antibiotics at 37 C in a humidified 5% CO2 incubator. Cells were divided into 12-well plates (Falcon; BD Biosciences, Lincoln Park, NJ) and cotransfected using Effectene (QIAGEN, Valencia, CA) at approximately 50% confluence. Cotransfections were done with a pCMV-StAR expression vector and the F2 plasmid expressing a fusion protein of the cholesterol side-chain cleavage system (H2N-P450scc-adrenodoxin reductase-adrenodoxin-COOH) (14). To monitor transfection efficiency, cells were also cotransfected with 5 ng of Renilla luciferase reporter plasmid (pRL-CMV) (Promega, Madison, WI) per well. Culture media were collected 48 h later, and pregnenolone production was measured by enzyme immunoassay (ALPCO Diagnostics, Salem, NH). The sensitivity of this assay is 5.4 ng/dl. Data are presented as the mean ± sem for at least three independent experiments, each performed in triplicate.
Table 2.
Oligonucleotide sequences for site-directed mutagenesis
| Name | Sequence | NCBI accession no.; location |
|---|---|---|
| R188C | TTTGTGAGCGTGTGCTGTGCCAAGC | NM_000349; 826 |
| R192C | GCGCTGTGCCAAGTGCCGAGGCTCCAC | NM_000349; 838 |
| G221D | AGGGCGGAGCACGATCCCACTTGCATG | NM_000349; 926 |
| L260P | ATCAACCAGGTCCCGTCCCAGACCCAG | NM_000349; 1043 |
| F267S | ACCCAGGTGGATTCTGCCAACCACCTG | NM_000349; 1064 |
Boldface and underlined bases indicate a nucleotide change. The numbering of nucleotides is based on the NCBI human StAR cDNA sequence NM_000349. The numbering of the amino acid residues is based on the NCBI human StAR protein reference sequence NP_000340.2.
Preparation of vectors and proteins and in vitro biochemical assays
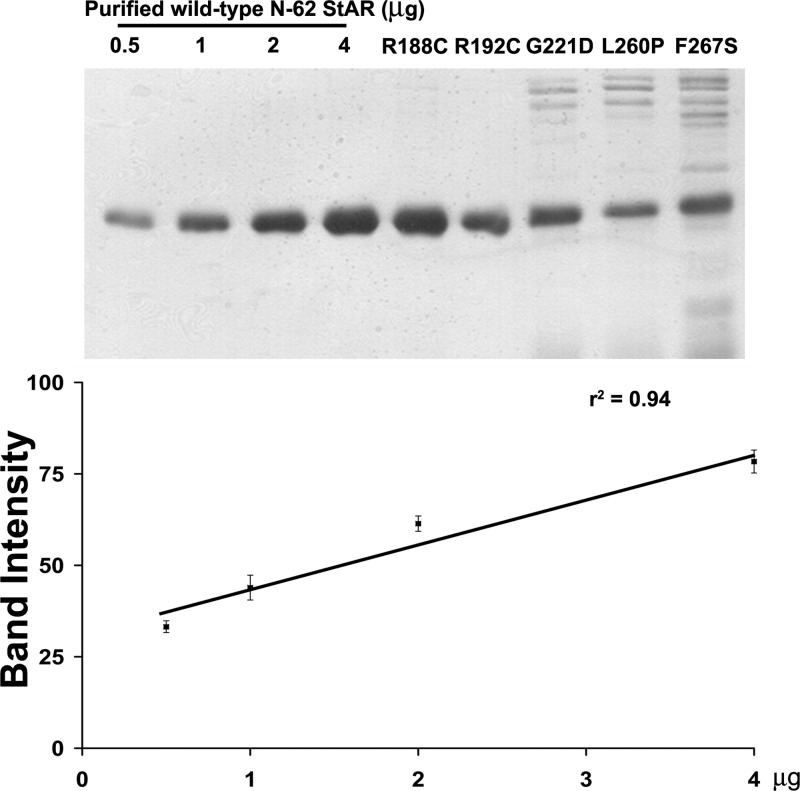
Each StAR mutation was inserted into the sequence of N-62 StAR in the modified pTWIN1 intein expression plasmid (15). The plasmids were individually transformed into Escherichia coli BL21DE3. StAR expression was induced with 0.5 mm isopropyl-β-d-thiogalactopyranoside at 28 C for 6–8 h. Bacteria were lysed by sonication and cleared of debris; the supernatant was loaded onto chitin-binding columns (New England Biolabs, Ipswich, MA), washed, and eluted according to the manufacturer’s recommendations. The N-62 StAR was collected and dialyzed against 300 mm NaCl, 20 mm Tris (pH 7.5) (15,16). Wild-type N-62 StAR was quantitated by the Bradford method, and mutant proteins were quantitated with a standard curve of wild-type N-62 StAR (Fig. 1). Mitochondria were prepared from mouse MA-10 Leydig cells as described (13). Purified N-62 StAR proteins (5 μm) were added to mitochondria (5 μg of Bradford protein) in 50 μl of 125 mm KCl, 5 mm MgCl2, 10 mm KH2PO4, 25 mm HEPES (pH 7.4), 250 ng/ml trilostane, 100 μm GTP, and 10 mm isocitrate, and incubated for 1 h; conversion of mitochondrial cholesterol to pregnenolone was measured by enzyme immunoassay, as above.
Figure 1.
Quantification of StAR protein. Top panel, The indicated amounts of purified wild-type N-62 StAR protein and mutant purified N-62 StAR proteins were separated by 12% SDS-PAGE. The band intensity was measured by Scion Image software. Bottom panel, The standard curve of StAR protein. The amount of mutant N-62 StAR proteins was plotted against its band intensity.
Cholesterol binding assay
Cholesterol binding capacity was measured by mixing purified N-62 StAR proteins (1 μm) in PBS with various amounts of fluorescent 22-(n-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino-23,24- bisnor-5-cholen-3β-ol) (NBD-cholesterol) (Invitrogen, Carlsbad, CA) in 96-well plates (final volume, 100 μl). The samples were incubated 1 h at 37 C and excited at 480 nm, and steady-state fluorescence emission at 540 nm (cutoff, 515 nm) was monitored with a SpectraMax M2 microplate reader (Molecular Devices Inc., Sunnyvale, CA) (16).
Results
StAR mutations
Patient 1 was homozygous for the mutation 5033C>T, changing arginine 188 to cysteine (R188C) (NCBI genomic DNA reference sequence NG_011827.1, and StAR protein reference sequence NP_000340.2) (17,18). Homozygous R188C was found in one of the initial patients with nonclassic lipoid CAH (9) and in four families reported in Ref. 10. Patient 2 was a compound heterozygote for 6732T>C, changing leucine 260 to proline (L260P); and 6753T>C, changing phenylalanine 267 to serine (F267S). L260P was described in Swiss patients with classic lipoid CAH (19); F267S is a novel mutation. Patient 3 was compound heterozygous for R188C and tryptophan 250 stop mutation (W250X). Homozygous W250X was previously found in a Serbian 46,XY phenotypic female with classic lipoid CAH (20). Patient 4 was homozygous for the novel mutation 5780G>A, changing glycine to aspartic acid (G221D).
Functional studies of the StAR mutants in transfected cells
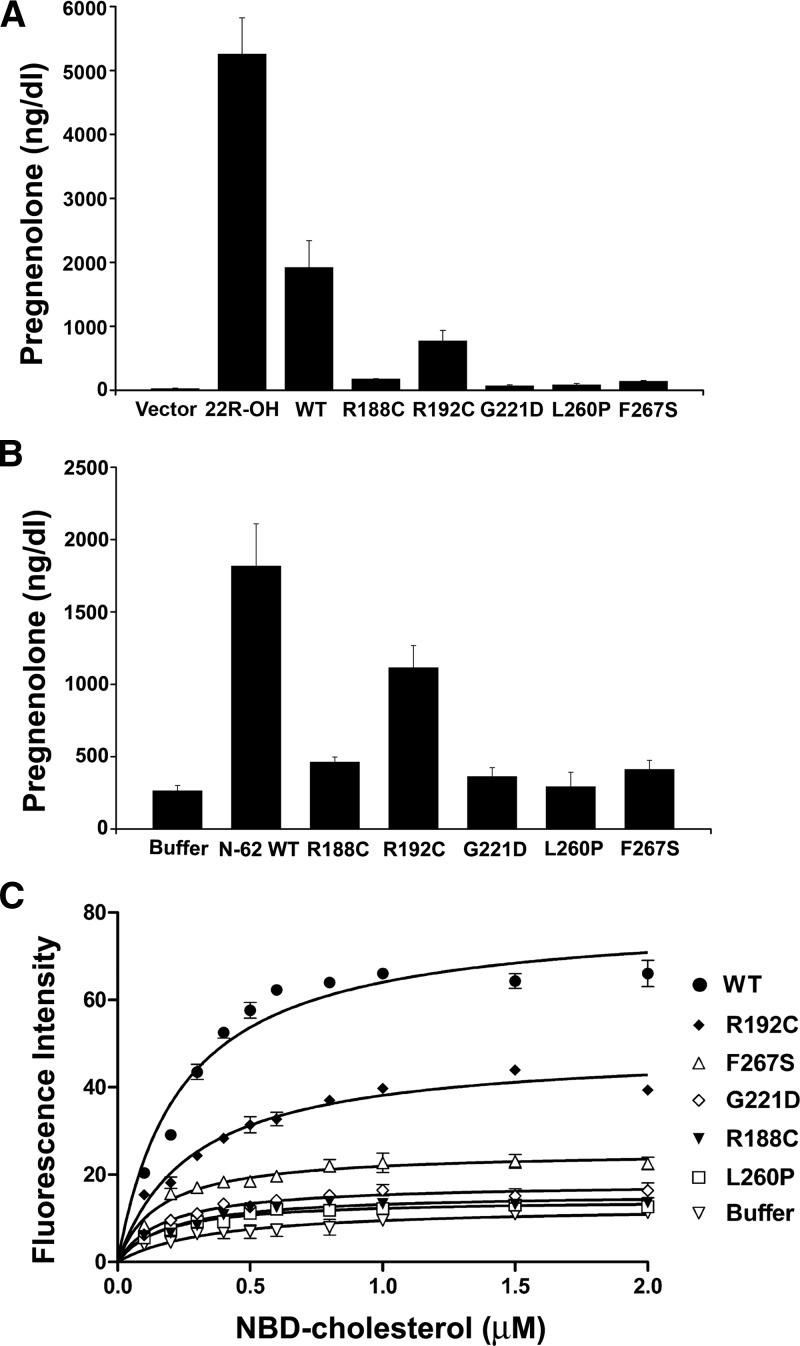
We studied the functional consequences of the four missense StAR mutants identified here and the mutation R192C recently found in a family with nonclassic lipoid CAH, but which had not been characterized functionally (10). We cotransfected nonsteroidogenic COS-1 cells with either wild-type or mutant StAR and a vector expressing the F2 fusion of the cholesterol side chain cleavage system (14) and compared the amount of pregnenolone produced. An empty vector was used as a negative control, and 22R-hydroxycholesterol, which bypasses the action of StAR and thus indicates the maximal enzymatic capacity of the P450scc system (4,7,13) was added as a positive control. Using endogenous cellular cholesterol and cholesterol in the serum in the culture media as substrate, COS-1 cells expressing F2 and wild-type StAR made 1917 ± 422 ng/dl of pregnenolone, whereas cells transfected with the empty vector produced a low level of pregnenolone (23 ± 11 ng/dl), indicating the presence of StAR-independent steroidogenesis. The R188C mutant produced 175 ± 8 ng/dl of pregnenolone, whereas the G221D, L260P, and F267S mutants generated 68 ± 17, 82 ± 23, and 138 ± 16 ng/dl, respectively. By contrast, R192C produced substantially more pregnenolone at 769 ± 169 ng/dl. When the background of StAR-independent steroidogenesis is subtracted, the R188C, R192C, G221D, L260P, and F267S mutants had 8.0, 39.4, 2.4, 3.1, and 6.1% of wild-type activity, respectively (Fig. 2A). In this assay, pregnenolone production in all five StAR mutations was significantly higher than vector control (P < 0.05).
Figure 2.
Activities of StAR mutants. A, Activity of full-length StAR in whole cells. COS-1 cells were cotransfected with expression vectors for the cholesterol side-chain cleavage system (F2) and either wild-type (WT) or mutant StAR, and pregnenolone was measured 48 h later by immunoassay. The StAR-independent substrate 22(R)-hydroxycholesterol (22R-OH) was added to the cell culture media to determine the maximum steroidogenic capacity of the cells. Data are expressed as the mean ± sem from at least three independent experiments, each performed in triplicate. B, Activity of isolated N-62 StAR on mitochondria in vitro. Purified proteins were added to mitochondria from steroidogenic mouse MA-10 Leydig cells, and pregnenolone production from endogenous mitochondrial cholesterol was measured. Data are expressed as mean ± sem from four experiments, each performed in duplicate. C, Cholesterol binding assay. Binding of various concentrations of NBD-cholesterol by wild-type and mutant StAR was measured by fluorescence; control is buffer without protein. Data are expressed as mean ± sem for three experiments, each performed in triplicate.
Activities of purified StAR proteins with steroidogenic mitochondria
To assess the activities of StAR mutants in a cell-free system, we expressed N-62 StAR protein using a self-splicing bacterial intein system. Purified N-62 StAR protein (Fig. 1) was added to mitochondria isolated from mouse Leydig MA-10 cells, and StAR activity was measured as pregnenolone produced from endogenous mitochondrial cholesterol. In this assay, wild-type N-62 StAR elicited 1816 ± 293 ng/dl pregnenolone, and the buffer control lacking StAR produced 263.3 ± 37.7 ng/dl pregnenolone, indicating a level of StAR-independent steroidogenesis approximately 14% of the StAR-induced level. After subtracting the buffer control, the R188C, R192C, G221D, L260P, and F267S mutants elicited 12.8, 54.8, 6.3, 1.8, and 9.5% of wild-type activity, respectively (Fig. 2B). In comparison to the buffer control, R188C (P < 0.0001), R192C (P < 0.0001), G221D (P = 0.02), and F267S (P = 0.002) elicited significantly more pregnenolone, whereas the activity of L260P was not different from the buffer paired (two-tailed Student’s t test; P = 0.57).
Cholesterol-binding capacity of the mutant StAR proteins
Purified recombinant proteins were combined with various concentrations of NBD-cholesterol, and cholesterol binding was measured fluorometrically. The binding of fluorescent NBD-cholesterol to StAR is very similar to that of [14C]cholesterol; hence, this readily measured form of cholesterol is appropriate for measuring the cholesterol binding capacity of StAR (16). After subtracting the buffer control, the maximal cholesterol binding capacity of the R188C, R192C, G221D, L260P, and F267S mutants was 6.7, 55.3, 10.2, 4.6, and 20.9% of the wild-type value (Fig. 2C). Thus, the impaired activity of the mutants generally correlated with their impaired capacities to bind cholesterol.
Discussion
StAR moves cholesterol from the outer mitochondrial membrane (OMM) to the inner mitochondrial membrane, thus providing the cholesterol substrate for steroidogenesis (4,21). StAR exerts this action on the OMM (13,22), where it must undergo a conformational change that results from its interaction with protonated OMM phospholipids (15,23,24,25). The essential role of StAR in adrenal and gonadal steroidogenesis was established by finding recessive, loss-of-function mutations of StAR in lipoid CAH (4,5,7,8). Until recently, lipoid CAH was known exclusively as a very severe disorder of steroidogenesis characterized by greatly diminished or absent synthesis of all adrenal and gonadal steroids (26). Affected individuals with classic lipoid CAH are phenotypically female and have severe salt loss in early infancy. Nonetheless, affected 46,XX females spontaneously develop secondary sex characteristics and experience cyclical vaginal bleeding, but the cycles are anovulatory, and the patients have progressive hypergonadotropic hypogonadism later in life (12,27). These unusual findings were explained by the two-hit model (7). In early lipoid CAH, StAR-independent mechanisms can still move some cholesterol into mitochondria, resulting in a low level of steroidogenesis, and increased corticotropin secretion (first hit). Over time, lipid droplets accumulate, damage the cell, and eventually destroy steroidogenic capacity (second hit). In the ovary, follicular cells remain unstimulated and thus undamaged until they are sequentially recruited in monthly cycles beginning at puberty. By contrast, the fetal testicular Leydig cells are affected early in gestation due to stimulation by chorionic gonadotropin.
In 2006, Baker et al. described three children from two families who presented with adrenal insufficiency at 2–4 yr of age; the males had normal genitalia. These patients were homozygous for StAR mutations V187M and R188C; functional studies of these mutants showed that they retained approximately 20% of wild-type activity (9). These data delineated a new disorder, nonclassic lipoid CAH. Later, Metherell et al. (10) reported five families with nonclassic lipoid CAH who had initially been misdiagnosed as having familial glucocorticoid deficiency. Four families carried R188C, and one carried R192C. All affected probands presented with Addisonian phenotypes at 2–7 yr of age, but one affected male remained undiagnosed and untreated until 58 yr of age (10). Several patients with nonclassic StAR mutations have progressive hypergonadotropic hypogonadism and azoospermia, potentially compromising fertility (10). Thus, identifying patients with nonclassic lipoid CAH is important because, unlike patients with familial glucocorticoid deficiency, they may have compromised fertility and mild salt loss.
Although massive adrenal enlargement is a classic hallmark of lipoid CAH (26), this sign is not pathognomonic. Small adrenals have been reported in patients with classic lipoid CAH (11), and such enlargement has not been reported in patients with nonclassic phenotypes (9,10), although hypoplastic adrenals with calcifications, suggesting cirrhotic, end stage fat deposition, have been reported (10). None of the patients in our study had evidence of adrenal enlargement by abdominal imaging. Similarly, patients with CYP11A1 mutations causing P450scc deficiency, who have clinical manifestations that are indistinguishable from StAR mutations, have not been reported with enlarged adrenals (28). Thus, in the setting of adrenal insufficiency, massively enlarged adrenals indicate classic lipoid CAH; normal-sized adrenals cannot exclude it but instead suggest P450scc deficiency or nonclassic lipoid CAH.
StAR mutations have been described in many ethnic groups but are common in Japan, Korea, and some isolated populations (1,7,8,19). The R188C mutation found in our Thai patient has also been reported in patients from Canada, Jordan, India, and Pakistan (9,10), suggesting a recurrent mutation. StAR mutations in intronic regions can also cause lipoid CAH (5,29). Most StAR missense mutations are found in the carboxy-terminal 40% of the 285-amino acid StAR protein (1) and totally eliminate StAR activity. Only five previously described mutations were associated with residual StAR activity in transfected COS-1 cells: V187M (22%) (9), R188C (14%) (9), A218V (6%) (7), M225T (29%) (8), and L275P (10%) (7). A gain-of-function StAR mutation, Q128R, has been reported recently in a 46,XX phenotypic female infant who had salt loss at 4 months, but the coexistence of homozygous frameshift null mutation ablated all StAR activities, so the potential effect of the Q128R mutation was not evidenced in the patient (30). The mechanism by which this mutant gains function is unknown.
The manifestations and severity of disease differed substantially in our patients. Patient 1, who had the mildest phenotype, was homozygous for R188C. In our assays of intracellular activity, mitochondrial activity, and cholesterol-binding capacity, R188C retained 8, 12.8, and 6.7% of wild-type activity, respectively; when we studied this mutant previously, the values for these assays were 13.6, 17.7, and 21.0% (9). Hence, whereas there is some variability in these biochemical assays, it seems that 10–20% of activity will dramatically alter the classic phenotype. The report of the patient carrying R192C, which retained about 50% of wild-type activity, indicated even milder disease (10). By contrast, patient 2, carrying both F267S (∼10% activity) and L260P (∼3% activity) had fairly severe disease, as did patient 4, who was homozygous for G221D (∼6% activity). Among the three assays we used, the direct assays of StAR activity, rather than the cholesterol-binding assay, appear to provide the best correlation with the clinical phenotype. A disparity between the activity assays and the binding assay is not surprising, because StAR mutation R182L was homozygous in patients with classic lipoid CAH and lacked activity in functional assays (7,11) but retained normal capacity to bind cholesterol, indicating that cholesterol binding is necessary but not sufficient for StAR activity (16). Thus, there is a fairly good correlation between StAR activity in vitro and the clinical phenotypes.
The crystal structures of three proteins closely related to StAR have been determined (31,32,33), permitting the construction of reliable computational models of human StAR (25,34). StAR’s structure features a hydrophobic pocket of the size and shape needed to bind cholesterol; the amphipathic C-terminal α-helix (C-helix) forms the “floor” of the pocket, with hydrophobic residues pointing inward and hydrophilic residues pointing outward. The structure shows no direct access to this pocket. Liposome protection experiments show that only the C-terminal α-helix (C-helix) interacts with the OMM (25), and the position of the C-helix is stabilized by a network of hydrogen bonds with the adjacent structures (25,34). Molecular dynamics simulations indicate that interaction of StAR with protonated phospholipids on the OMM disrupts these hydrogen bonds, inducing a conformational change (molten globule transition), permitting the C-helix to swing open to allow the binding of cholesterol into the sterol binding pocket of StAR; consistent with this model, disulfide mutants that immobilize the C-helix but that do not alter StAR’s conformation interfere with StAR activity and cholesterol binding (15,35).
Many mutations that cause lipoid CAH, including those identified in this study, are located in the C-terminal helix or elsewhere in the sterol-binding pocket. Leu260 and Phe267 are conserved hydrophobic residues in the C-helix, forming part of the cholesterol-binding site. Arg188 and Arg192 lie in sheet β6, and G221 is in the loop joining sheets β8 and β9, which contribute to the sterol-binding pocket. Consistent with this, the reduced activity of these mutants was associated with reduced cholesterol-binding capacity. The mechanism by which StAR facilitates the movement of cholesterol into the mitochondrion is incompletely understood, but it appears to involve several other proteins on the OMM. Functional data indicate that the 18-kDa peripheral benzodiazepine receptor (PBR; also called the mitochondrial translocator protein) must be present for StAR to act (36), and homo-bifunctional protein cross-linking data show that StAR comes into close contact with the voltage-dependent anion channel-1 and with a phosphate-carrier protein on the OMM (37). Additional data suggest that StAR participates in a large macromolecular complex, which, in addition to StAR, PBR, and voltage-dependent anion channel, includes PBR-associated protein 7, protein kinase A regulatory subunit Iα, and adenine nucleotide transporter (38,39). Substantial additional work is needed to delineate the role of each of these proteins in mitochondrial cholesterol import; the potential discovery of patients carrying mutations in any of these could be most informative.
Supplementary Material
Acknowledgments
We thank Dr. Lin Lin for sequencing DNA from patients 3 and 4, Drs. Carrie Burns and Anna Biason-Lauber for help with patients 2 and 3, and the multiple specialists who participated in the care of these patients.
Footnotes
This work was supported by National Institutes of Health Grant DK37922 (to W.L.M.). T.S. was supported by funds from Chulalongkorn University and by the University of California, San Francisco, Division of Pediatric Endocrinology. J.C.A. was supported by a Wellcome Trust Senior Fellowship in Clinical Science (079666).
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 5, 2010
Abbreviations: DHEA, Dehydroepiandrosterone; lipoid CAH, congenital lipoid adrenal hyperplasia; 17OHP, 17-hydroxyprogesterone; OMM, outer mitochondrial membrane; PBR, peripheral benzodiazepine receptor; PRA, plasma renin activity; StAR, steroidogenic acute regulatory protein.
References
- Miller WL 1997 Congenital lipoid adrenal hyperplasia: the human gene knockout for the steroidogenic acute regulatory protein. J Mol Endocrinol 19:227–240 [DOI] [PubMed] [Google Scholar]
- Matteson KJ, Chung BC, Urdea MS, Miller WL 1986 Study of cholesterol side-chain cleavage (20,22 desmolase) deficiency causing congenital lipoid adrenal hyperplasia using bovine-sequence P450scc oligodeoxyribonucleotide probes. Endocrinology 118:1296–1305 [DOI] [PubMed] [Google Scholar]
- Lin D, Gitelman SE, Saenger P, Miller WL 1991 Normal genes for the cholesterol side chain cleavage enzyme, P450scc, in congenital lipoid adrenal hyperplasia. J Clin Invest 88:1955–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Sugawara T, Strauss 3rd JF, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL 1995 Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 267:1828–1831 [DOI] [PubMed] [Google Scholar]
- Tee MK, Lin D, Sugawara T, Holt JA, Guiguen Y, Buckingham B, Strauss 3rd JF, Miller WL 1995 T→A transversion 11 bp from a splice acceptor site in the human gene for steroidogenic acute regulatory protein causes congenital lipoid adrenal hyperplasia. Hum Mol Genet 4:2299–2305 [DOI] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ 1996 Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev 17:221–244 [DOI] [PubMed] [Google Scholar]
- Bose HS, Sugawara T, Strauss 3rd JF, Miller WL 1996 The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N Engl J Med 335:1870–1878 [DOI] [PubMed] [Google Scholar]
- Nakae J, Tajima T, Sugawara T, Arakane F, Hanaki K, Hotsubo T, Igarashi N, Igarashi Y, Ishii T, Koda N, Kondo T, Kohno H, Nakagawa Y, Tachibana K, Takeshima Y, Tsubouchi K, Strauss 3rd JF, Fujieda K 1997 Analysis of the steroidogenic acute regulatory protein (StAR) gene in Japanese patients with congenital lipoid adrenal hyperplasia. Hum Mol Genet 6:571–576 [DOI] [PubMed] [Google Scholar]
- Baker BY, Lin L, Kim CJ, Raza J, Smith CP, Miller WL, Achermann JC 2006 Nonclassic congenital lipoid adrenal hyperplasia: a new disorder of the steroidogenic acute regulatory protein with very late presentation and normal male genitalia. J Clin Endocrinol Metab 91:4781–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherell LA, Naville D, Halaby G, Begeot M, Huebner A, Nürnberg G, Nürnberg P, Green J, Tomlinson JW, Krone NP, Lin L, Racine M, Berney DM, Achermann JC, Arlt W, Clark AJ 2009 Nonclassic lipoid congenital adrenal hyperplasia masquerading as familial glucocorticoid deficiency. J Clin Endocrinol Metab 94: 3865–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose HS, Sato S, Aisenberg J, Shalev SA, Matsuo N, Miller WL 2000 Mutations in the steroidogenic acute regulatory protein (StAR) in six patients with congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab 85:3636–3639 [DOI] [PubMed] [Google Scholar]
- Bose HS, Pescovitz OH, Miller WL 1997 Spontaneous feminization in a 46,XX female patient with congenital lipoid adrenal hyperplasia due to a homozygous frameshift mutation in the steroidogenic acute regulatory protein. J Clin Endocrinol Metab 82:1511–1515 [DOI] [PubMed] [Google Scholar]
- Bose HS, Lingappa VR, Miller WL 2002 Rapid regulation of steroidogenesis by mitochondrial protein import. Nature 417:87–91 [DOI] [PubMed] [Google Scholar]
- Harikrishna JA, Black SM, Szklarz GD, Miller WL 1993 Construction and function of fusion enzymes of the human cytochrome P450scc system. DNA Cell Biol 12:371–379 [DOI] [PubMed] [Google Scholar]
- Baker BY, Yaworsky DC, Miller WL 2005 A pH-dependent molten globule transition is required for activity of the steroidogenic acute regulatory protein, StAR. J Biol Chem 280:41753–41760 [DOI] [PubMed] [Google Scholar]
- Baker BY, Epand RF, Epand RM, Miller WL 2007 Cholesterol binding does not predict activity of the steroidogenic acute regulatory protein, StAR. J Biol Chem 282:10223–10232 [DOI] [PubMed] [Google Scholar]
- Sugawara T, Holt JA, Driscoll D, Strauss 3rd JF, Lin D, Miller WL, Patterson D, Clancy KP, Hart IM, Clark BJ, Stocco DM 1995 Human steroidogenic acute regulatory protein: functional activity in COS-1 cells, tissue-specific expression, and mapping of the structural gene to 8p11.2 and a pseudogene to chromosome 13. Proc Natl Acad Sci USA 92:4778–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Lin D, Holt JA, Martin KO, Javitt NB, Miller WL, Strauss 3rd JF 1995 Structure of the human steroidogenic acute regulatory protein (StAR) gene: StAR stimulates mitochondrial cholesterol 27-hydroxylase activity. Biochemistry 34:12506–12512 [DOI] [PubMed] [Google Scholar]
- Flück CE, Maret A, Mallet D, Portrat-Doyen S, Achermann JC, Leheup B, Theintz GE, Mullis PE, Morel Y 2005 A novel mutation L260P of the steroidogenic acute regulatory protein gene in three unrelated patients of Swiss ancestry with congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab 90:5304–5308 [DOI] [PubMed] [Google Scholar]
- Korsch E, Peter M, Hiort O, Sippell WG, Ure BM, Hauffa BP, Bergmann M 1999 Gonadal histology with testicular carcinoma in situ in a 15-year-old 46,XY female patient with a premature termination in the steroidogenic acute regulatory protein causing congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab 84:1628–1632 [DOI] [PubMed] [Google Scholar]
- Miller WL, Strauss 3rd JF 1999 Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. J Steroid Biochem Mol Biol 69:131–141 [DOI] [PubMed] [Google Scholar]
- Arakane F, Sugawara T, Nishino H, Liu Z, Holt JA, Pain D, Stocco DM, Miller WL, Strauss 3rd JF 1996 Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial import sequence: implications for the mechanism of StAR action. Proc Natl Acad Sci USA 93:13731–13736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose HS, Whittal RM, Baldwin MA, Miller WL 1999 The active form of the steroidogenic acute regulatory protein, StAR, appears to be a molten globule. Proc Natl Acad Sci USA 96:7250–7255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Bose HS, Harris FM, Miller WL, Bell JD 2001 Binding of steroidogenic acute regulatory protein to synthetic membranes suggests an active molten globule. J Biol Chem 276:17044–17051 [DOI] [PubMed] [Google Scholar]
- Yaworsky DC, Baker BY, Bose HS, Best KB, Jensen LB, Bell JD, Baldwin MA, Miller WL 2005 pH-Dependent interactions of the carboxyl-terminal helix of steroidogenic acute regulatory protein with synthetic membranes. J Biol Chem 280:2045–2054 [DOI] [PubMed] [Google Scholar]
- Hauffa BP, Miller WL, Grumbach MM, Conte FA, Kaplan SL 1985 Congenital adrenal hyperplasia due to deficient cholesterol side-chain cleavage activity (20, 22-desmolase) in a patient treated for 18 years. Clin Endocrinol (Oxf) 23:481–493 [DOI] [PubMed] [Google Scholar]
- Fujieda K, Tajima T, Nakae J, Sageshima S, Tachibana K, Suwa S, Sugawara T, Strauss 3rd JF 1997 Spontaneous puberty in 46,XX subjects with congenital lipoid adrenal hyperplasia. Ovarian steroidogenesis is spared to some extent despite inactivating mutations in the steroidogenic acute regulatory protein (StAR) gene. J Clin Invest 99:1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CJ, Lin L, Huang N, Quigley CA, AvRuskin TW, Achermann JC, Miller WL 2008 Severe combined adrenal and gonadal deficiency caused by novel mutations in the cholesterol side chain cleavage enzyme, P450scc. J Clin Endocrinol Metab 93:696–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama E, Nishi N, Onishi S, Itoh S, Ishii Y, Miyanaka H, Fujita K, Ichikawa Y 1997 A novel splicing junction mutation in the gene for the steroidogenic acute regulatory protein causes congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab 82:2337–2342 [DOI] [PubMed] [Google Scholar]
- Bens S, Mohn A, Yüksel B, Kulle AE, Michalek M, Chiarelli F, Nuri Ozbek M, Leuschner I, Grötzinger J, Holterhus PM, Riepe FG 2010 Congenital lipoid adrenal hyperplasia: functional characterization of three novel mutations in the StAR gene. J Clin Endocrinol Metab 95:1301–1308 [DOI] [PubMed] [Google Scholar]
- Tsujishita Y, Hurley JH 2000 Structure and lipid transport mechanism of a StAR-related domain. Nat Struct Biol 7:408–414 [DOI] [PubMed] [Google Scholar]
- Romanowski MJ, Soccio RE, Breslow JL, Burley SK 2002 Crystal structure of the Mus musculus cholesterol-regulated START protein 4 (StarD4) containing a StAR-related lipid transfer domain. Proc Natl Acad Sci USA 99:6949–6954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick SL, Chan WW, Agate DS, Olsen LR, Vetting MW, Rajashankar KR, Cohen DE 2002 Structure of human phosphatidylcholine transfer protein in complex with its ligand. Nat Struct Biol 9:507–511 [DOI] [PubMed] [Google Scholar]
- Mathieu AP, Fleury A, Ducharme L, Lavigne P, LeHoux JG 2002 Insights into steroidogenic acute regulatory protein (StAR)-dependent cholesterol transfer in mitochondria: evidence from molecular modeling and structure-based thermodynamics supporting the existence of partially unfolded states of StAR. J Mol Endocrinol 29:327–345 [DOI] [PubMed] [Google Scholar]
- Miller WL 2007 StAR search—what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol Endocrinol 21:589–601 [DOI] [PubMed] [Google Scholar]
- Hauet T, Yao ZX, Bose HS, Wall CT, Han Z, Li W, Hales DB, Miller WL, Culty M, Papadopoulos V 2005 Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into leydig cell mitochondria. Mol Endocrinol 19:540–554 [DOI] [PubMed] [Google Scholar]
- Bose M, Whittal RM, Miller WL, Bose HS 2008 Steroidogenic activity of StAR requires contact with mitochondrial VDAC1 and phosphate carrier protein. J Biol Chem 283:8837–8845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Rone MB, Papadopoulos V 2006 Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem 281:38879–38893 [DOI] [PubMed] [Google Scholar]
- Rone MB, Fan J, Papadopoulos V 2009 Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim Biophys Acta 1791:646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.