Abstract
Context: Intracellular lipid partitioning toward storage and the incomplete oxidation of fatty acids (FA) have been linked to insulin resistance.
Objective: To gain insight into how intracellular lipid metabolism is related to insulin signal transduction, we examined the effects of severe obesity, excess FA, and overexpression of the FA transporter, FA translocase (FAT)/CD36, in primary human skeletal myocytes.
Design, Setting, and Patients: Insulin signal transduction, FA oxidation, and metabolism were measured in skeletal muscle cells harvested from lean and severely obese women. To emulate the obesity phenotype in our cell culture system, we incubated cells from lean individuals with excess FA or overexpressed FAT/CD36 using recombinant adenoviral technology.
Results: Complete oxidation of FA was significantly reduced, whereas total lipid accumulation, FA esterification into lipid intermediates, and incomplete oxidation were up-regulated in the muscle cells of severely obese subjects. Insulin signal transduction was reduced in the muscle cells from severely obese subjects compared to lean controls. Incubation of muscle cells from lean subjects with lipids reduced insulin signal transduction and increased lipid storage and incomplete FA oxidation. CD36 overexpression increased FA transport capacity, but did not impair complete FA oxidation and insulin signal transduction in muscle cells from lean subjects.
Conclusions: Cultured myocytes from severely obese women express perturbations in FA metabolism and insulin signaling reminiscent of those observed in vivo. The obesity phenotype can be recapitulated in muscle cells from lean subjects via exposure to excess lipid, but not by overexpressing the FAT/CD36 FA transporter.
FAT/CD36 overexpression elevates rates of fatty acid oxidation and enhances insulin signal transduction in human muscle cells.
Obesity and insulin-resistant disorders are associated with metabolic alterations, such as elevated plasma fatty acid (FA) concentrations (1,2) that may lead to the accumulation of im lipid content (3,4,5). Although the exact mechanism remains unclear, it has been well documented that this accumulation of lipid within muscle cells is linked to the development of skeletal muscle insulin resistance (6,7,8,9). Additionally, more recent studies have established a link between muscle insulin resistance and excessive or incomplete β-oxidation (10). Regardless of the mechanisms, compelling evidence shows that chronic exposure to a surplus of lipid fuel can lead to the development of insulin-resistant disorders.
The accumulation of lipids can also be achieved via increased delivery to and uptake of FA by skeletal muscle cells. The rate of FA uptake is up-regulated in obese Zucker rats (11). In obese and type 2 diabetic human skeletal muscle, Bonen et al. (12) have shown elevated rates of long chain FA transport into giant sarcolemmal vesicles. The same group also reported that the greater rate of palmitate transport was associated with increased im triglyceride accumulation and an increase in sarcolemmal FA translocase (FAT)/CD36, suggesting the presence of FAT/CD36 is a critical determinant of FA uptake (12).
FAT/CD36 may be a rate-limiting factor in the uptake of FA because FAT/CD36 overexpression resulted in elevated rates of FA uptake in skeletal muscle (13,14), whereas FAT/CD36 deletion impaired the transport of FA across the plasma membrane (15). It is well documented that CD36-deficient mice exhibit elevated plasma free FA and triglyceride levels (15,16,17). In addition, mice null for CD36, demonstrate improved skeletal muscle insulin sensitivity (17). Thus, FAT/CD36 may play a key role in lipid-induced insulin resistance.
Animal and cell culture experiments suggest that augmented lipid supply to the skeletal muscle not only leads to elevations in the rate of FA uptake and the enhanced capacity for im lipid accumulation, but can eventually result in impaired insulin signal transduction (18,19). In the current investigation, we examined im lipid metabolism and insulin sensitivity in the skeletal muscle cells of lean compared with severely obese women. We hypothesized that FA uptake and metabolism would be elevated in myocytes from obese compared with lean women. Additionally, in an effort to emulate the obese phenotype in our cell culture system, we incubated primary muscle cells from lean women with FA or overexpressed FAT/CD36 to determine the effect of altered FA metabolism on intracellular lipid partitioning and storage. We hypothesized that FA treatment or FAT/CD36 overexpression in muscle cells of lean women would lead to perturbations in lipid metabolism and insulin signaling, thereby mimicking the phenotype of obesity.
Subjects and Methods
Healthy lean [body mass index (BMI), 22 ± 2.6 kg/m2; n = 8] and severely obese (BMI, 48 ± 3.8 kg/m2; n = 8) Caucasian women with a mean age of 29 yr in both groups were studied. Fasting insulin (133.3 ± 18.8 vs. 47.2 ± 4.0 pmol/liter) and glucose (5.5 ± 0.4 vs. 4.8 ± 0.1 mmol/liter) were higher (P < 0.001) in severely obese subjects compared with lean subjects. Although we did not measure nonesterified FA in this study, we have previously reported elevated plasma FA concentrations in severely obese subjects compared with lean (2). Subjects were screened for any metabolic abnormalities, including hypertension, vascular disease, and type 2 diabetes, and were recruited if they were not taking any medications that could interfere with lipid metabolism at the time of study. The current study was approved by the institutional review board at East Carolina University, and informed, written consent was obtained by all subjects before participation.
Human muscle cell culture
Human muscle cell culture techniques are detailed elsewhere (20). Skeletal muscle (50–100 mg) from the vastus lateralis was obtained using the percutaneous needle biopsy technique (21). The experiments were performed using low glucose DMEM media with or without addition of FA. The DMEM contains 5.5 mm glucose, 1 mm pyruvate, as well as high levels of most amino acids. Myoblasts were suspended in media supplemented with 10% fetal bovine serum, 0.5 mg/ml BSA, 0.5 mg/ml fetuin, 20 ng/ml human epidermal growth factor, 0.39 μg/ml dexamethasone, and 50 μg/ml gentamicin/amphotericin B, and cultured at 37 C in a humidified atmosphere of 5% CO2 and 95% O2. After reaching 70–80% confluence, myoblasts were subcultured onto type I collagen-coated plates for subsequent cellular experiments. Myoblasts were subcultured onto six- and 24-well type I collagen-coated plates at densities of 80 and 20 × 103 cells per well, respectively. When the myoblasts reached 80% confluence, differentiation was induced by changing to low-serum media consisting of 2% horse serum, 0.5 mg/ml BSA, 0.5 mg/ml fetuin, and 50 μg/ml gentamicin/amphotericin B for differentiation of myoblasts into myotubes. Human myotubes were harvested on d 8 of differentiation for all cellular experiments.
Preparation of adenovirus and adenoviral transduction
The cDNA encoding His-tagged CD36, kindly provided by Dr. Arend Bonen, was subcloned into an adenoviral vector as described by Gomez-Foix et al. (22). β-Galactosidase was used as a control virus. Skeletal muscle cells were transduced with adenovirus on d 5 of differentiation, and staining of myotubes was conducted on d 8 of differentiation as described in detail previously (23,24). An anti-His polyclonal antibody (Abcam, Cambridge, MA) was used to detect the CD36 fusion protein at 88 kDa, and goat antirabbit IgG conjugated to Alexa Fluor (Invitrogen Molecular Probes, Carlsbad, CA) was used to visualize the proteins via fluorescence microscopy. Briefly, for all subsequent experiments, cells were transduced with recombinant adenovirus encoding His-tagged rat CD36 at a final concentration of 1.5 × 1010 particles/ml. Twenty-four hours after transfection, the medium was removed and replaced with fresh differentiation media. Myotubes were harvested for respective experiments on d 8. We confirmed high transduction efficiency by immunocytochemistry using an anti-his antibody and estimated protein overexpression by Western blot analysis.
Metabolic assays
Muscle cells from lean individuals (no virus control) or lean individuals overexpressing CD36 adenovirus or β-galactosidase (control virus) were pretreated with or without a variable concentration of FA (100 or 250 μm; 1:1 oleate:palmitate). Cellular metabolic assays were performed after a 3-h incubation at 37 C in differentiation media, 12.5 mm HEPES, 0.5% BSA, 1 mm carnitine, and either 100 or 250 μm sodium oleate and 1 μCi/ml [1-14C]oleate (Sigma-Aldrich, St. Louis, MO). After the incubation period, the medium was transferred to new plates and assayed for the collection of 14CO2 production (complete oleate oxidation), which was quantified via liquid scintillation counting using 4 ml of Uniscint BD (National Diagnostics, Atlanta, GA). We have previously determined that myocyte rates of [14C]FA oxidation and incorporation into glycerolipids are linear during the 3-h assay. The quantity of cellular lipid esterified and oxidized was calculated from the specific activity of label in the incubation medium. The quantity of cellular uptake was calculated to obtain an estimate of skeletal muscle FA uptake.
We also measured incomplete oxidative products (acid-soluble metabolites) as described elsewhere (25). The results are reported as a ratio between incomplete (acid-soluble metabolites) to complete (1-14CO2) radiolabeled products to provide an indication of β-oxidative efficiency. The remaining cell pellet was washed twice with ice-cold PBS and harvested in 600 μl 0.05% sodium dodecyl sulfate, and cell lysates were stored at −80 C for subsequent protein determination and cellular lipid extraction.
For determination of rates of FA incorporation into various lipid species, the extraction of total cellular lipids and the incorporation of FA into glycerolipids were performed as previously described (26). A volume 500 μl of cell lysate was placed in a 13 × 100 glass screw top tube with ice-cold 1:2 chloroform-methanol (vol/vol) and vortexed. An additional 0.5ml of 100% chloroform and deionized water were added, and samples were centrifuged to separate the phases. The chloroform phase was transferred to a clean glass tube, gently evaporated under a stream of 100% N2, and redissolved in 100 μl of 2:1 chloroform-methanol for determination of total cellular lipids via liquid scintillation counting, and another 50 μl of each sample, including standards for determination of individual lipid species, were spotted in respective lanes on an oven-dried silica gel plate (Silica Gel GF; Analtech, Newark, DE), which was placed in a sealed tank containing solvent (60:40:3, heptane-isopropyl ether-acetic acid). The plates were scanned for visualization of the bands depicting phospholipids, monoacylglycerols, diacylglycerols, free FA, and triacylglycerols.
Western blot analysis
Insulin signal transduction was determined in cultured muscle cells from lean and obese individuals and for cells after overexpression of FAT/CD36 and/or after a 48-h incubation of either 100 or 250 μm 1:1 oleate:palmitate FA treatment or BSA (control treatment). After the incubation, the cells were serum starved for 5 h, insulin stimulated (100 nm porcine insulin) (Sigma-Aldrich) for 15 min, and immediately harvested in 200 μl of lysis buffer containing 20 mm Tris-HCl, 4% sodium dodecyl sulfate, 10 mm NaF, 1 mm EDTA, and 20% glycerol supplemented with phosphatase and protease inhibitor cocktails (Sigma-Aldrich) for subsequent Western blot analysis. Individual protein concentrations from the cell lysates were determined using the bicinchoninic acid assay kit (Pierce, Rockford, IL). Cellular protein (30 μg) was separated by SDS-PAGE, transferred to nitrocellulose membranes, and then probed overnight with specific primary antibodies diluted in 5% milk using Tris-buffered saline with 0.1% Tween. Activation of insulin signaling molecules measured by Western blot analysis was determined using an anti-pAKT(ser473) (Cell Signaling, Beverly, MA) antibody specific for human skeletal muscle. Cellular protein of the insulin receptor (IR) β-subunit phosphorylated at residue tyrosine 972 of the IR required for the IR to recruit and phosphorylate endogenous substrates and IR substrate (IRS)-1 (pS312) was normalized against the specific protein from the cell lysates, normalized to IRS total protein, and measured via an ELISA (Biosource International, Camarillo, CA).
Real-time PCR
For RNA studies, human myotubes were grown on collagen coated six-well plates. Total cellular RNA was isolated using the RNeasy total RNA isolation kit (QIAGEN, Valencia, CA) and quantified using RiboGreen reagent (Molecular Probes, Eugene, OR). cDNA for real-time PCR was synthesized from 2 μg of RNA using the high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA) in a 40-μl reaction. Samples were diluted 5-fold, and each reaction was carried out in triplicate for subsequent RT-PCR. RT-PCR was performed using an ABI PRISM 7700 Sequence Detection System instrument and software (Applied Biosystems), and sequence-specific FAM-labeled predesigned and prevalidated gene expression primer/probe sets for human CD36 (Hs00169627_m1, Applied Biosystems) were used.
Statistical methods
Data are presented as means ± se, with significance set at a threshold of P < 0.05. Data were analyzed using the Student’s t test or by ANOVA. To evaluate differences between groups and after treatment with CD36 adenovirus, comparisons were performed using ANOVA with repeated measures. Post hoc testing was performed using the Student’s t test when appropriate.
Results
Insulin signal transduction in primary muscle cells
Obesity is associated with impaired insulin signal transduction in skeletal muscle. Here, for the first time, we report that the changes in insulin signal transduction that are found in muscle of obese individuals are preserved in cultured muscle cells. Insulin-stimulated tyrosine phosphorylation of the IR (Fig. 1A) and Akt activation (phosphorylation) (Fig. 1C) were significantly reduced (P < 0.05), whereas IRS 312 serine phosphorylation was significantly higher (P = 0.043) (Fig. 1B), in muscle cells from severely obese compared with lean donors.
Figure 1.
Insulin signal transduction in muscle cells from lean and obese subjects. Insulin stimulated levels of IR tyrosine phosphorylation (A), IRS 312 serine phosphorylation (B), and Akt activation (phosphorylation) (C) in muscle cells from lean and severely obese women (n = 7). Data are represented as mean ± se. *, Significantly different from lean control (P < 0.05).
FA metabolism in primary muscle cells
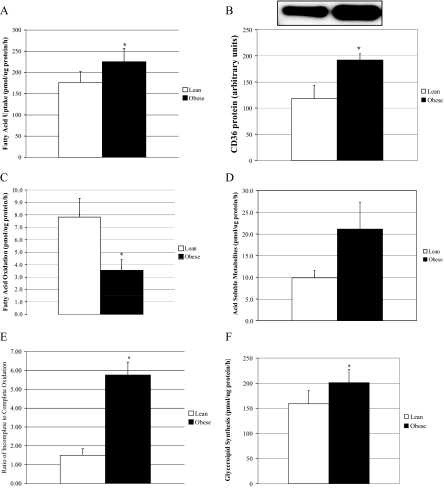
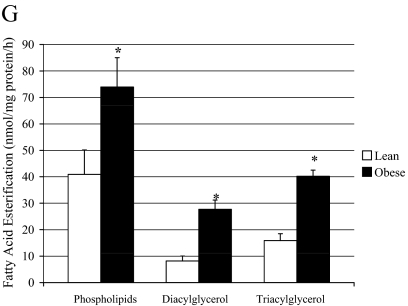
Previous studies have shown increased availability and uptake of FA in obese individuals (12), and our findings using our cell culture model agree. FA uptake in the muscle cells of obese subjects was significantly higher (P < 0.05) compared with lean controls (Fig. 2A). Because FA transport is mediated by a family of transport proteins, we measured FAT/CD36 protein and mRNA levels in cultured muscle cells of lean and obese individuals. FAT/CD36 protein content was significantly higher (P < 0.05) in the muscle cells from morbidly obese subjects compared with lean controls (Fig. 2B). We previously reported that skeletal muscle of obese individuals has a reduced capacity to fully oxidize FA, and this difference is maintained in our cell culture model (24). The findings in the current investigation are in agreement with previous observations. After incubation with 250 μm [1-14C]oleate, the complete oxidation of FA was significantly reduced in the obese muscle cells compared with lean controls (Fig. 2C). We also measured acid-soluble metabolites and the ratio between incomplete FA oxidation (measured as acid-soluble metabolites) and complete oxidation (14CO2) to provide a measure of oxidative efficiency (Fig. 2, D and E). The ratio of incomplete to complete oxidation products was significantly elevated 3.5-fold in the muscle cells from obese subjects compared with lean controls (Fig. 2E). As expected, glycerolipid synthesis was significantly increased (P < 0.05) with obesity (Fig. 2F). We further measured the incorporation of 1-14C-oleate into intracellular lipid intermediates in the primary muscle cells of lean and obese subjects. FA incorporation into phospholipids, diacylglycerol, and triacylglycerol was significantly higher in the muscle cells from obese subjects compared with lean controls (Fig. 2G).
Figure 2.
Lipid metabolism in muscle cells from lean and obese subjects. A, FA uptake; B, CD36 protein; C, complete oxidation of FA; D, acid-soluble metabolites; E, the ratio of incomplete oxidation of FA (acid-soluble metabolites) to the complete oxidation of FA; F, glycerolipid synthesis; and G, FA esterification into lipid intermediates in the muscle cells of lean and severely obese subjects (n = 8) incubated with 250 μm [1-14C]oleate. Data are represented as mean ± se. *, Significantly different from lean controls (P < 0.05).
Insulin signal transduction after a FA challenge
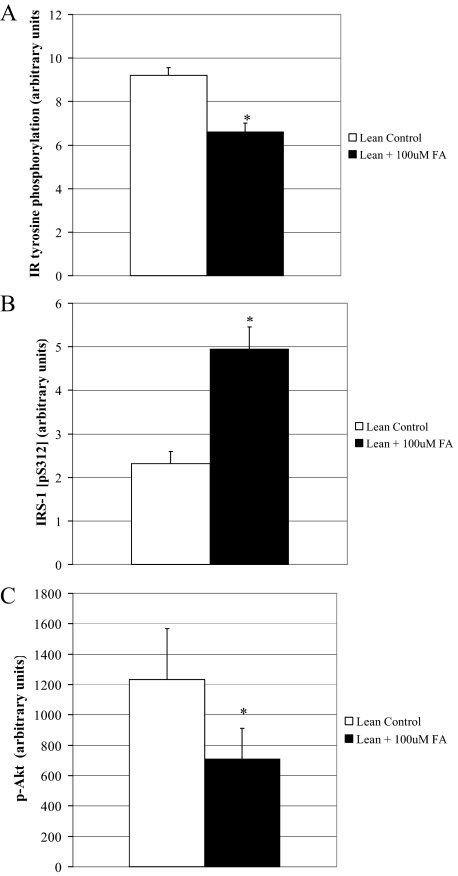
We challenged muscle cells from lean subjects with a FA incubation to determine whether we could induce insulin resistance in this cohort. After a 48-h incubation with 100 μm FA, insulin signal transduction was impaired (Fig. 3, A–C). Insulin-stimulated tyrosine phosphorylation of the IR and Akt activation (phosphorylation) were lower (P < 0.05) (Fig. 3, A and C), and IRS 312 serine phosphorylation was 2-fold higher (P < 0.05) after the FA challenge (Fig. 3B).
Figure 3.
Insulin signal transduction in muscle cells from lean subjects after a FA challenge. Insulin-stimulated level of IR tyrosine phosphorylation (A), IRS 312 serine phosphorylation (B), and Akt activation (phosphorylation) (C) in muscle cells from lean subjects after a 48-h FA challenge to induce insulin resistance (n = 7). Data are represented as mean ± se. *, Significantly different from lean control (P < 0.05).
FA metabolism in primary muscle cells from lean individuals after FA incubation
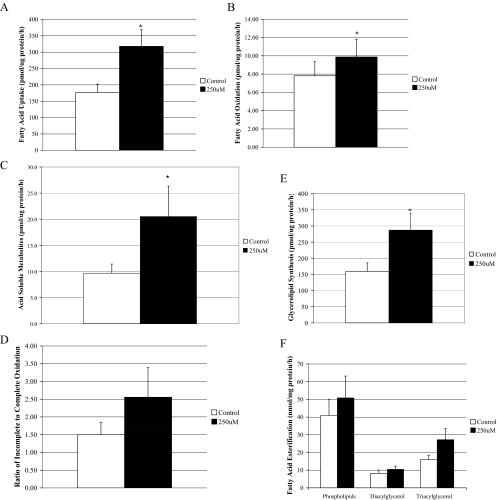
We also challenged the muscle cells from lean subjects with a 3-h FA incubation to determine how the presence of FA would impact intracellular lipid metabolism. FA uptake (Fig. 4A), the complete oxidation of FA (Fig. 4B), acid-soluble metabolites (Fig. 4C), and total accumulation of cellular lipids (Fig. 4E) significantly increased (P < 0.05) after treatment with 250 μm FA compared with 100 μm FA. Incorporation of FA into phospholipids (P = 0.06) (Fig. 4F) and the ratio of incomplete relative to complete oxidation (P = 0.07) (Fig. 4D) followed similar trends in response to the increase in FA dose. However, it is important to note that because insulin signaling and lipid metabolism experiments were conducted under different periods of FA incubation, the results are not directly comparable.
Figure 4.
Lipid metabolism in muscle cells from lean subjects after a FA challenge with 1:1 oleate:palmitate. A, FA uptake; B, complete oxidation of FA; C, acid-soluble metabolites; D, the ratio of incomplete oxidation of FA (acid-soluble metabolites) to the complete oxidation of FA; E, glycerolipid synthesis; and F, FA esterification into lipid intermediates in the muscle cells of lean and severely obese subjects (n = 8). Data are represented as mean ± se. *, Significantly different from the lower (100 μm) FA concentration (P < 0.05).
Overexpression of CD36 in primary cultured human muscle cells
The cDNA encoding His-tagged FAT/CD36 was subcloned into an adenoviral shuttle vector. We used an anti-His polyclonal primary antibody to determine CD36 overexpression transduction efficiency, which was then visualized via fluorescence microscopy and also used to detect the CD36 fusion protein at 88 kDa in lean human skeletal muscle cells (Fig. 5A). CD36 protein expression was induced in a dose-dependent manner (Fig. 5B), and CD36 protein content increased approximately 6-fold after CD36 overexpression (Fig. 5C). For the cell culture experiments, human muscle cells were transfected with CD36 adenovirus at a final concentration of 1.5 × 1010 particles.
Figure 5.
CD36 adenoviral transduction efficiency in muscle cells from lean subjects and insulin signal transduction in muscle cells from lean subjects after CD36 overexpression. A, CD36 overexpression transduction efficiency visualized via fluorescence microscopy in lean human multinucleated myotubes. The no virus control (β-gal) served as a negative control and did not present a fluorescent signal. B, CD36 expression was induced in a dose-dependent manner. C, CD36 protein content after CD36 overexpression in muscle cells from lean subjects. Insulin-stimulated level of IR tyrosine phosphorylation (D), IRS 312 serine phosphorylation (E), and Akt activation (phosphorylation) (F) in muscle cells from lean subjects after CD36 overexpression (n = 8). Data are represented as mean ± se. *, Significantly different from lean β-gal controls (P < 0.05).
Insulin signal transduction after CD36 overexpression
We overexpressed CD36 in the muscle cells from lean subjects to determine how the overexpression of CD36 would impact insulin signal transduction. Insulin-stimulated IR tyrosine phosphorylation and IRS 312 serine phosphorylation remained unchanged after CD36 overexpression in muscle cells from lean subjects compared with β-galactosidase controls (Fig. 5, D and E). However, to our surprise, downstream insulin-stimulated Akt phosphorylation was significantly increased (P = 0.004) after CD36 overexpression in lean muscle cells (Fig. 5F).
FA metabolism in lean muscle cells that overexpress FAT/CD36
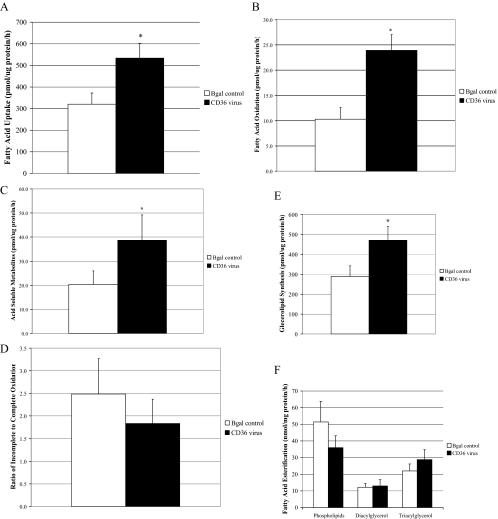
We next examined how overexpression of FAT/CD36 in muscle cells from lean subjects influenced metabolism of 14C oleate (100 μm) during an acute (3 h) incubation. Compared with the β-galactosidase control group, FAT/CD36 overexpression increased FA uptake (P < 0.05) (Fig. 6A), complete oxidation (Fig. 6B), acid-soluble metabolites (Fig. 6C), and total lipid accumulation (Fig. 6E). In contrast, FAT/CD36 overexpression did not affect the ratio of incomplete to complete oxidation (Fig. 6D).
Figure 6.
Lipid metabolism in muscle cells from lean subjects after CD36 overexpression. A, FA uptake; B, complete oxidation of FA; C, acid-soluble metabolites; D, the ratio of incomplete oxidation of FA (acid-soluble metabolites) to the complete oxidation of FA; E, glycerolipid synthesis; and F, FA esterification into lipid intermediates in the muscle cells of lean and severely obese subjects (n = 8) incubated with 250 μm [1-14C]oleate. Data are represented as mean ± se. *, Significantly different from Bgal controls (P < 0.05).
Discussion
This study examined the relationship between FA uptake, lipid metabolism, and insulin signaling in primary human myocytes harvested from lean and severely obese women. Shunting of FA toward storage and elevated rates of incomplete oxidation have previously been linked to insulin resistance (27). Herein we confirmed previous findings of elevated FA uptake, depressed fat oxidation, and increased storage with severe obesity (12,26). In addition, for the first time we demonstrated impaired insulin signal transduction in the primary muscle cells from obese women. The obesity phenotype was largely recapitulated when cells from lean women were exposed to FA. By contrast, FAT/CD36 overexpression in the skeletal muscle cells of lean subjects enhanced capacity for FA uptake, lipid synthesis, and β-oxidation but did not cause impairments in insulin signaling. Moreover, FAT/CD36 overexpression improved insulin-stimulated activation of Akt, contrary to our prediction.
Using several different experimental models (24,26,28,29), our laboratory has consistently shown reduced rates of FA oxidation in the skeletal muscle of severely obese individuals compared with lean controls. Findings from the current study are in line with the earlier reports. Research in the literature seems to suggest that obesity-induced insulin resistance can occur when the skeletal muscle is challenged with excess lipids. The end result is elevated im lipid storage and metabolism, which in turn has been implicated in the development of insulin resistance. For instance, excess skeletal muscle triglyceride and/or free FA could result in elevated long chain fatty acyl-CoA concentrations (30). Moreover, a number of investigations have shown that increased concentrations of fatty acyl-CoAs are associated with greater intracellular diacylglycerol and ceramide concentrations that have been linked to impaired transduction and subsequent glucose transport (31,32,33). In the current investigation, we observed elevations in the rate of FA uptake in the muscle cells from obese subjects, which is consistent with earlier reports in the literature (11,12). We also showed attenuated rates of FA oxidation, elevated rates of total lipid synthesis, along with elevations in individual lipid species in the muscle cells of severely obese subjects compared with lean controls. However, in the current study, it is important to note that we are using muscle cells from severely obese subjects (BMI range, 41.1 to 56.8 kg/m2), and thus our findings may not be representative of overweight/modestly obese individuals. Previous work by Hulver et al. (26) reported attenuated FA oxidation in the skeletal muscle with extreme obesity, but not overweight/obesity, suggesting that the accumulation of long chain FA is not completely dependent on reduced FA oxidation.
Depressed activity of insulin signaling is a hallmark characteristic of obesity. Previous investigations have reported reduced activity of Akt (34) along with impairments at the level of the IR and IRS-1 tyrosine phosphorylation (35). Insulin signal transduction was impaired with obesity, and we were able to induce impaired insulin signaling after a lipid challenge in the muscle cells from lean subjects. Lipid-induced abnormalities seem to be present in the insulin signaling cascade with obesity, and the accumulation of long chain FA via increased transport and/or decreased oxidation may lead to the development of insulin resistance. Moreover, we also observed increased rates of incomplete oxidation with severe obesity, and we were able to induce greater rates of incomplete oxidation after incubation with FA in lean subjects. Interestingly, emerging evidence in the literature has linked insulin resistance to impairments in skeletal muscle mitochondrial performance (36,37). For instance, when substrate flux through the β-oxidation pathway outpaces the activity of the Kreb’s cycle, rates of incomplete oxidation increase, resulting in a corresponding rise in acylcarnitine production and accumulation. Although the acylcarnitine metabolites are not thought to act as mediators of insulin resistance (38), they might reflect a state of mitochondrial stress that, in turn, disrupts glucose tolerance (27,39).
The expression of skeletal muscle FAT/CD36 has previously been shown to determine the extent of FA utilization both in animal models (14) and in cultured skeletal muscle cells of healthy individuals (13). In addition, previous groups have reported enhanced rates of skeletal muscle FA uptake after FAT/CD36 overexpression (13,14), findings that are in agreement with the current study. Increased rates of FA transport would be a likely mechanism for abnormal intracellular lipid accumulation. The accumulation of intracellular FA may also play a critical role in defects in the insulin signaling cascade, which may lead to the development of insulin resistance. Moreover, additional factors such as elevated plasma free FA levels and skeletal muscle mitochondrial dysregulation might contribute to perturbations in lipid balance and insulin action in obese individuals. In the current study, FAT/CD36 overexpression improved insulin-stimulated phosphorylation of Akt, without affecting upstream activators such as IRS-1. The mechanism underlying this effect is unknown.
Taken together, our findings indicate that complete FA oxidation is attenuated, whereas lipid deposition and incomplete oxidation are elevated in muscle cells of severely obese subjects. We can partially recapitulate the obese phenotype in muscle cells from lean subjects via exposure to excess lipid. By contrast, FAT/CD36 overexpression in cells from lean donors failed to impair insulin signal transduction. However, it is important to consider that the foregoing signaling assays were performed in the absence of exogenously added FA. Thus, the outcome might be different when cells expressing high levels of CD36 are exposed to a surfeit of fat, thereby mimicking the obese state.
Acknowledgments
The authors thank Dr. Arend Bonen for his contributions to the current research.
Footnotes
The current research project was supported by National Institutes of Health Grant DK 46212 (to G.L.D.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 28, 2010
Abbreviations: BMI, Body mass index; FA, fatty acid(s); FAT, FA translocase; IR, insulin receptor; IRS, IR substrate.
References
- Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD 2004 Splanchnic lipolysis in human obesity [see comment]. J Clin Invest 113:1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vice E, Privette JD, Hickner RC, Barakat HA 2005 Ketone body metabolism in lean and obese women. Metabolism 54:1542–1545 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Theriault R, Watkins SC, Kelley DE 2000 Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 49:467–472 [DOI] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster B, Wing RR, Simoneau JA 1999 Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 277:E1130–E1141 [DOI] [PubMed] [Google Scholar]
- Mandarino LJ, Consoli A, Jain A, Kelley DE 1996 Interaction of carbohydrate and fat fuels in human skeletal muscle: impact of obesity and NIDDM. Am J Physiol 270:E463–E470 [DOI] [PubMed] [Google Scholar]
- Boden G, Lebed B, Schatz M, Homko C, Lemieux S 2001 Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes 50:1612–1617 [DOI] [PubMed] [Google Scholar]
- Cooney GJ, Thompson AL, Furler SM, Ye J, Kraegen EW 2002 Muscle long-chain acyl CoA esters and insulin resistance. Ann NY Acad Sci 967:196–207 [DOI] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster BH 2001 Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 24:933–941 [DOI] [PubMed] [Google Scholar]
- Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH 1997 Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46:983–988 [DOI] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM 2008 Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7:45–56 [DOI] [PubMed] [Google Scholar]
- Luiken JJ, Arumugam Y, Dyck DJ, Bell RC, Pelsers MM, Turcotte LP, Tandon NN, Glatz JF, Bonen A 2001 Increased rates of fatty acid uptake and plasmalemmal fatty acid transporters in obese Zucker rats. J Biol Chem 276:40567–40573 [DOI] [PubMed] [Google Scholar]
- Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ 2004 Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J 18:1144–1146 [DOI] [PubMed] [Google Scholar]
- García-Martínez C, Marotta M, Moore-Carrasco R, Guitart M, Camps M, Busquets S, Montell E, Gómez-Foix AM 2005 Impact on fatty acid metabolism and differential localization of FATP1 and FAT/CD36 proteins delivered in cultured human muscle cells. Am J Physiol Cell Physiol 288:C1264–C1272 [DOI] [PubMed] [Google Scholar]
- Ibrahimi A, Bonen A, Blinn WD, Hajri T, Li X, Zhong K, Cameron R, Abumrad NA 1999 Muscle-specific over-expression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem 274:26761–26766 [DOI] [PubMed] [Google Scholar]
- Coburn CT, Knapp Jr FF, Febbraio M, Beets AL, Silverstein RL, Abumrad NA 2000 Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem 275:32523–32529 [DOI] [PubMed] [Google Scholar]
- Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL 1999 A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 274:19055–19062 [DOI] [PubMed] [Google Scholar]
- Goudriaan JR, Dahlmans VE, Teusink B, Ouwens DM, Febbraio M, Maassen JA, Romijn JA, Havekes LM, Voshol PJ 2003 CD36 deficiency increases insulin sensitivity in muscle, but induces insulin resistance in the liver in mice. J Lipid Res 44:2270–2277 [DOI] [PubMed] [Google Scholar]
- McGarry JD 2002 Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51:7–18 [DOI] [PubMed] [Google Scholar]
- Perseghin G, Petersen K, Shulman GI 2003 Cellular mechanism of insulin resistance: potential links with inflammation. Int J Obes Relat Metab Disord 27(Suppl 3):S6–S11 [DOI] [PubMed] [Google Scholar]
- Muoio DM, Way JM, Tanner CJ, Winegar DA, Kliewer SA, Houmard JA, Kraus WE, Dohm GL 2002 Peroxisome proliferator-activated receptor-α regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes 51:901–909 [DOI] [PubMed] [Google Scholar]
- Evans WJ, Phinney SD, Young VR 1982 Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14:101–102 [PubMed] [Google Scholar]
- Kim JY, Koves TR, Yu GS, Gulick T, Cortright RN, Dohm GL, Muoio DM 2002 Evidence of a malonyl-CoA-insensitive carnitine palmitoyltransferase I activity in red skeletal muscle. Am J Physiol Endocrinol Metab 282:E1014–E1022 [DOI] [PubMed] [Google Scholar]
- Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM 2005 Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab 2:251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA 2000 Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 279:E1039–E1044 [DOI] [PubMed] [Google Scholar]
- Hulver MW, Berggren JR, Cortright RN, Dudek RW, Thompson RP, Pories WJ, MacDonald KG, Cline GW, Shulman GI, Dohm GL, Houmard JA 2003 Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab 284:E741–E747 [DOI] [PubMed] [Google Scholar]
- Gomez-Foix AM, Coats WS, Baque S, Alam T, Gerard RD, Newgard CB 1992 Adenovirus-mediated transfer of the muscle glycogen phosphorylase gene into hepatocytes confers altered regulation of glycogen metabolism. J Biol Chem 267:25129–25134 [PubMed] [Google Scholar]
- Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM 2005 Peroxisome proliferator-activated receptor-γ co-activator 1α-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 280:33588–33598 [DOI] [PubMed] [Google Scholar]
- Cortright RN, Azevedo Jr JL, Zhou Q, Sinha M, Pories WJ, Itani SI, Dohm GL 2000 Protein kinase C modulates insulin action in human skeletal muscle. Am J Physiol Endocrinol Metab 278:E553–E562 [DOI] [PubMed] [Google Scholar]
- Thyfault JP, Kraus RM, Hickner RC, Howell AW, Wolfe RR, Dohm GL 2004 Impaired plasma fatty acid oxidation in extremely obese women. Am J Physiol Endocrinol Metab 287:E1076–E1081 [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Schmitz-Peiffer C, Saha AK, Ruderman NB, Biden TJ, Kraegen EW 1999 Muscle lipid accumulation and protein kinase C activation in the insulin-resistant chronically glucose-infused rat. Am J Physiol 277:E1070–E1076 [DOI] [PubMed] [Google Scholar]
- Ellis BA, Poynten A, Lowy AJ, Furler SM, Chisholm DJ, Kraegen EW, Cooney GJ 2000 Long-chain acyl-CoA esters as indicators of lipid metabolism and insulin sensitivity in rat and human muscle. Am J Physiol Endocrinol Metab 279:E554–E560 [DOI] [PubMed] [Google Scholar]
- Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI 1999 Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia [Errata (1999) 42:386 and 42:1269] 42:113–116 [DOI] [PubMed] [Google Scholar]
- Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL 1995 Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J Clin Invest 95:2195–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams 2nd JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ 2004 Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53:25–31 [DOI] [PubMed] [Google Scholar]
- Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ 2000 Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest 105:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI 2005 Mitochondrial dysfunction and type 2 diabetes. Science 307:384–387 [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI 2004 Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes [see comment]. N Engl J Med 350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM 2009 Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem 284:22840–22852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price III JW, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD 2009 Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]