Abstract
The red cells of patients with chronic hemolytic anemia due to cold agglutinins are agglutinated by antiglobulin serum in a nongamma reaction due to the coating of β-globulins, C′4 and C′3. The red cells of such patients are abnormally resistant to C′ hemolysis by cold agglutinin.
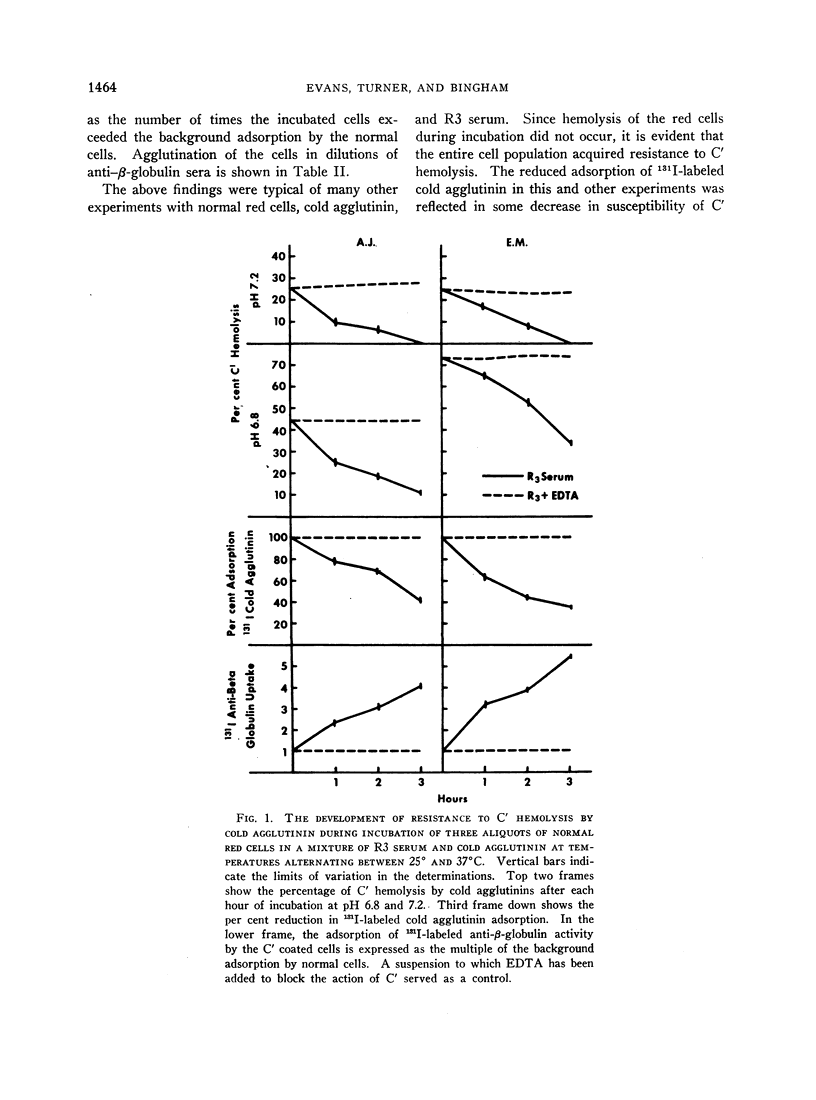
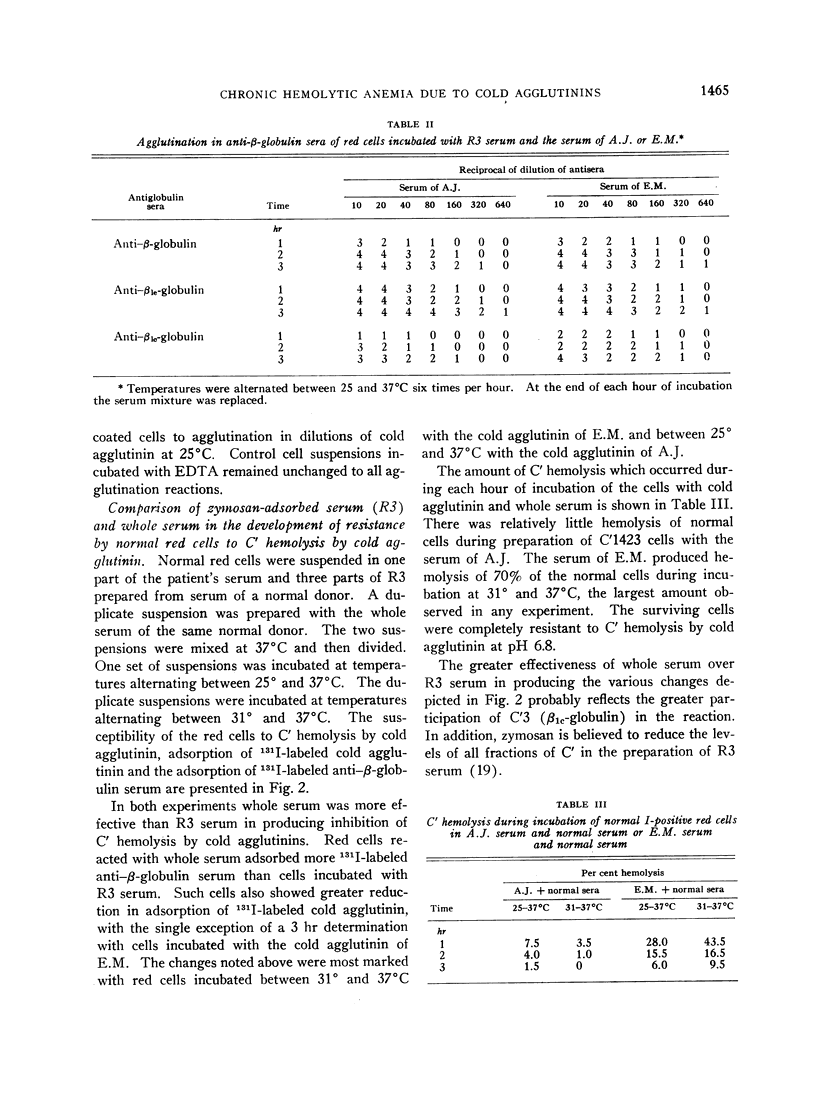
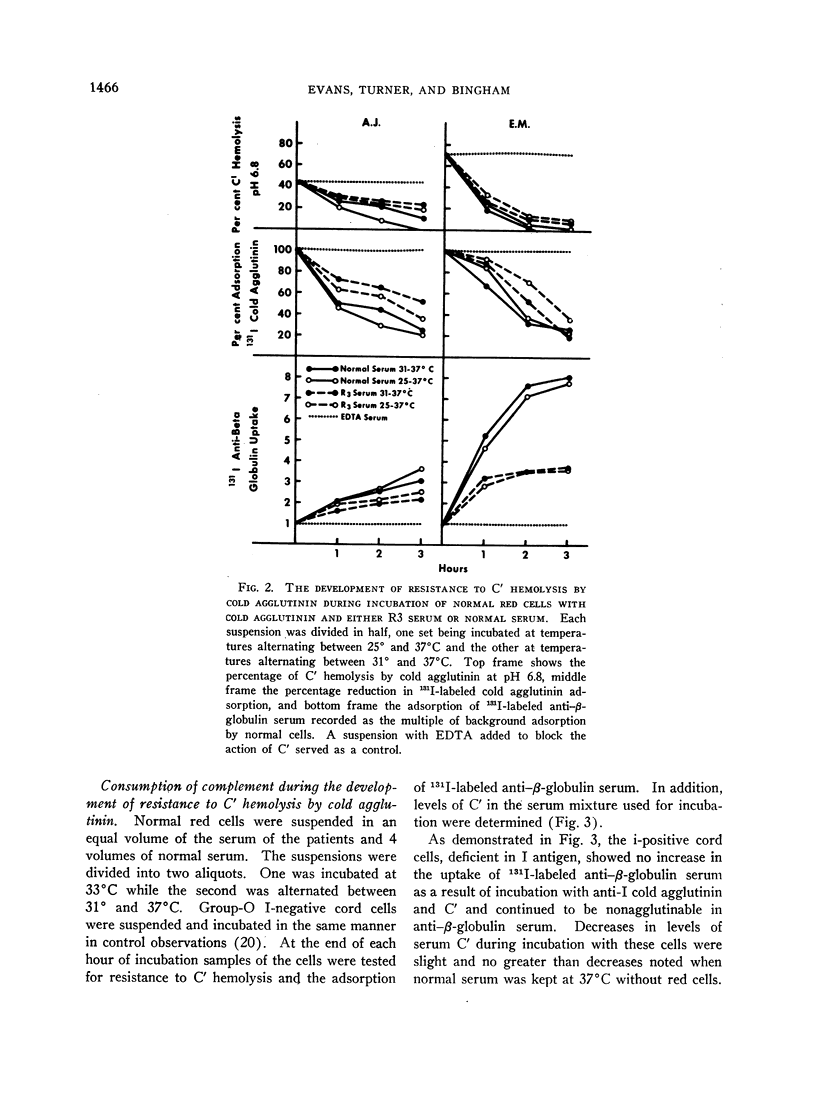
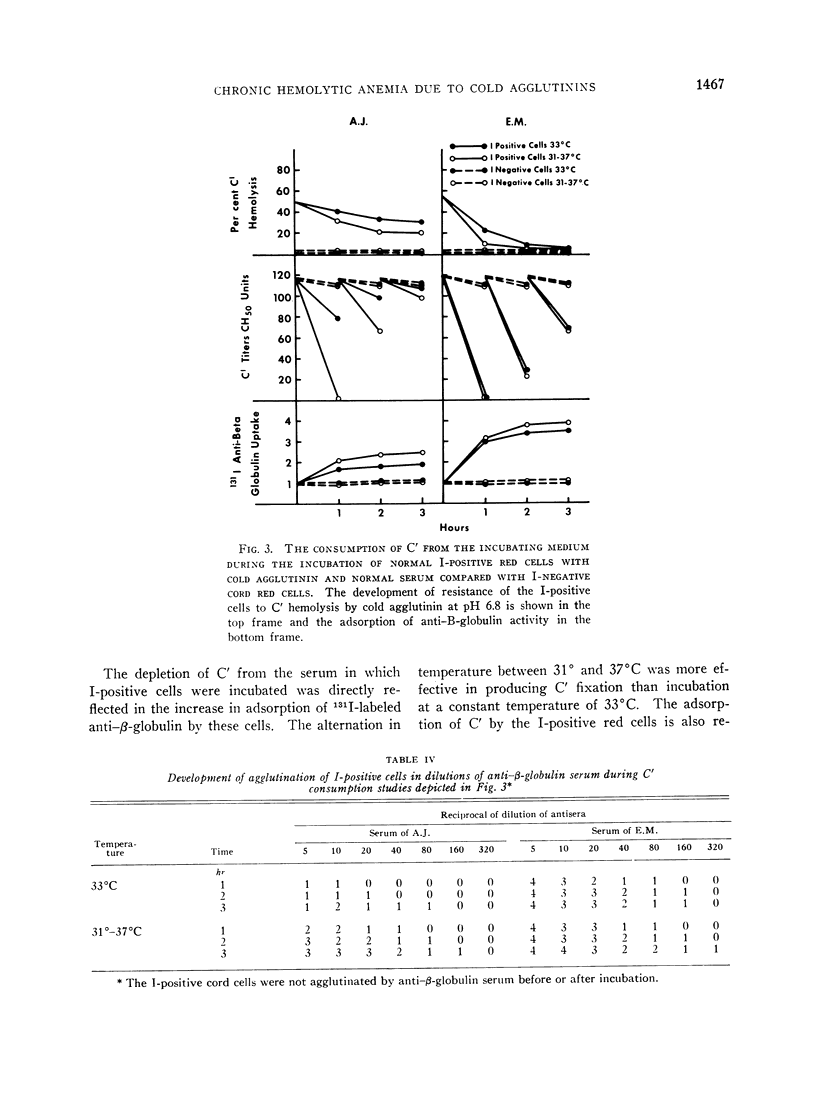
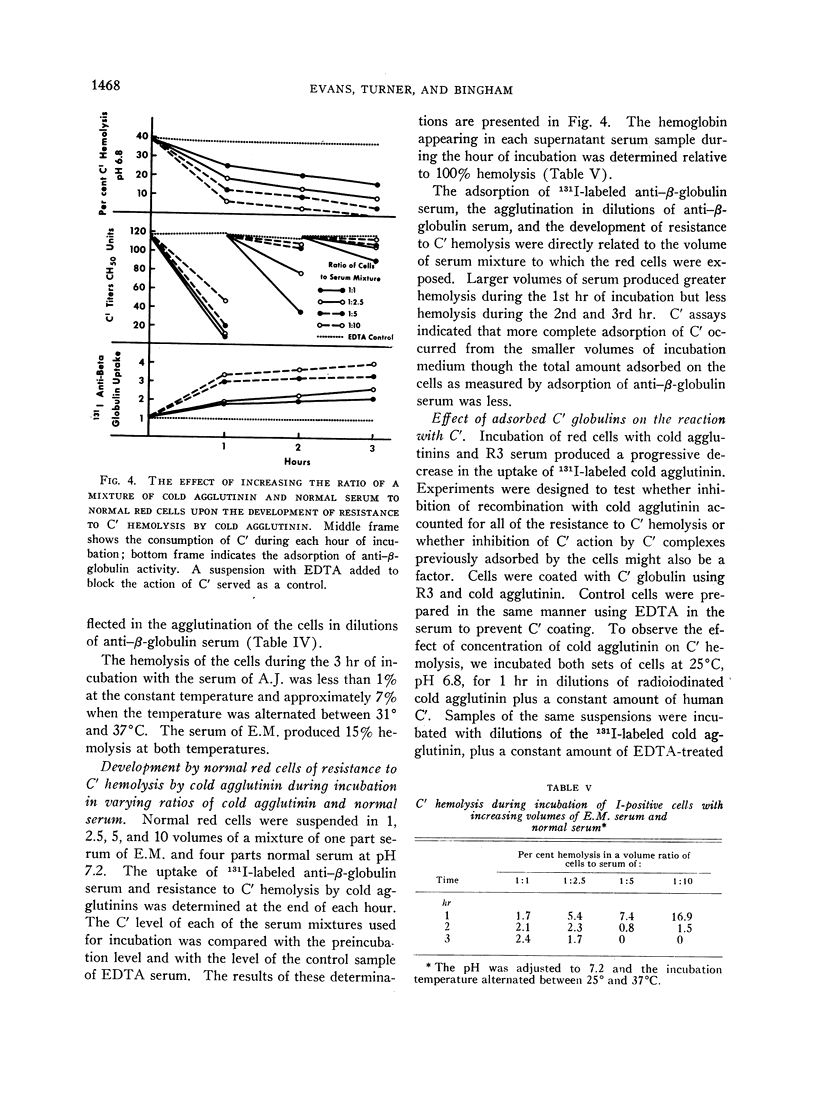
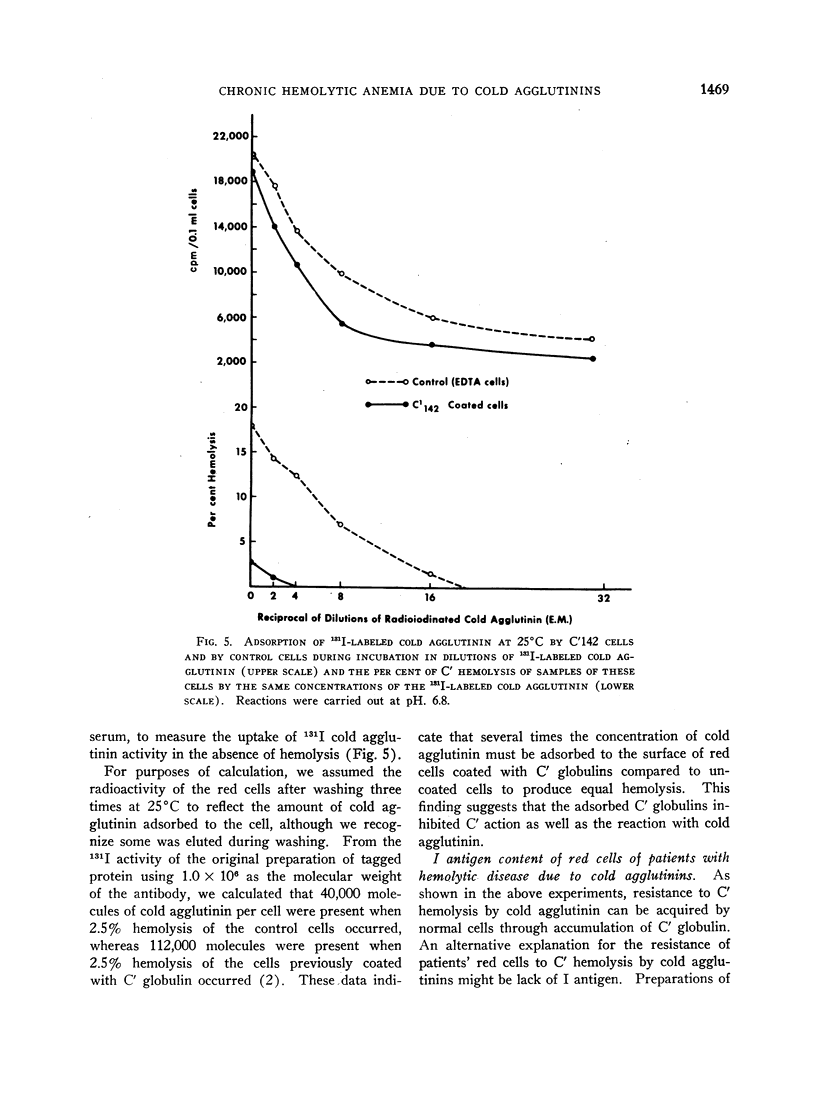
Normal red cells can be made equally resistant to C′ hemolysis by incubation with cold agglutinin and normal serum at temperatures which allow transient reactions between the red cells and cold agglutinins. The development of resistance to C′ hemolysis was related to increasing susceptibility to agglutination in anti-β1c- and anti-β1e-sera and by increasing uptake of 131I activity from labeled anti-β-globulin serum containing antibodies for both globulins. There was decrease in the adsorption of 131I-labeled cold agglutinin during the development of resistance to C′ hemolysis and reduced susceptibility to agglutination by cold agglutinins.
Since cold agglutinins have been demonstrated to dissociate from the red cell, leaving fractions of C′ globulin attached, it is postulated that repeated transient reactions produce the accumulation of incomplete C′ complexes. Steric hindrance by the adsorbed C′ complexes is probably responsible for the inhibition of the reaction with cold agglutinin. There is evidence that the adsorbed C′ complexes also interfere with the hemolytic action of C′ even when cold agglutinin has become reattached to the red cells.
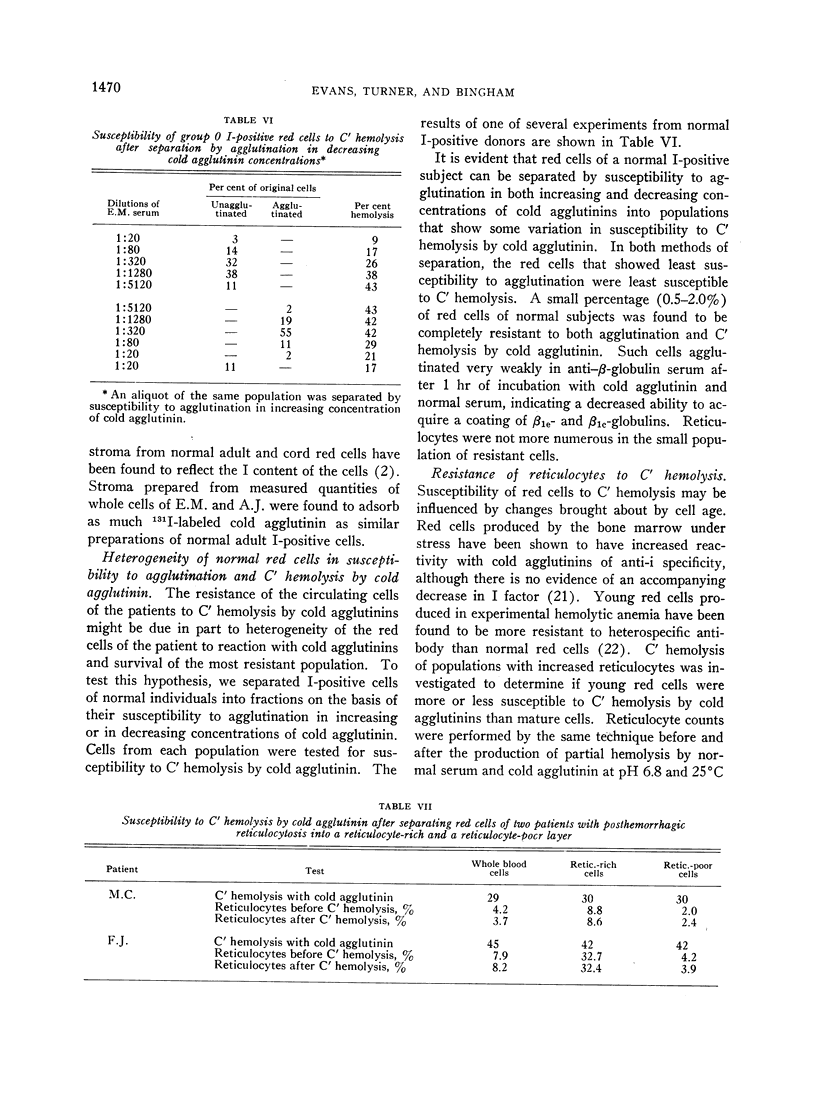
The accumulation of C′ complexes by cold agglutinins appears to be the most important factor in the abnormal resistance to C′ hemolysis exhibited by the patient's red cells. Other factors, such as the heterogeneity within a population of normal cells, appear to be of minor significance.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTEN K. F., BEER F. THE MEASUREMENT OF SECOND COMPONENT OF HUMAN COMPLEMENT (C'2HU) BY ITS INTERACTION WITH EAC'1AGP,4GP CELLS. J Immunol. 1964 Jun;92:946–957. [PubMed] [Google Scholar]
- BECKER E. L. Concerning the mechanism of complement action. V. The early steps in immune hemolysis. J Immunol. 1960 Mar;84:299–308. [PubMed] [Google Scholar]
- CONSTANTOULAKIS M., COSTEA N., SCHWARTZ R. S., DAMESHEK W. QUANTITATIVE STUDIES OF THE EFFECT OF RED-BLOOD-CELL SENSITIZATION ON IN VIVO HEMOLYSIS. J Clin Invest. 1963 Nov;42:1790–1801. doi: 10.1172/JCI104864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTEA N., SCHWARTZ R., CONSTANTOULAKIS M., DAMESHEK W. The use of radioactive antiglobulin for the detection of erythrocyte sensitization. Blood. 1962 Aug;20:214–232. [PubMed] [Google Scholar]
- CROOKSTON J. H., DACIE J. V., ROSSI V. Differences in the agglutinability of human red cells by the high-titre cold antibodies of acquired haemolytic anaemia. Br J Haematol. 1956 Jul;2(3):321–331. doi: 10.1111/j.1365-2141.1956.tb06705.x. [DOI] [PubMed] [Google Scholar]
- DACIE J. V., CROOKSTON J. H., CHRISTENSON W. N. Incomplete cold antibodies role of complement in sensitization to antiglobulin serum by potentially haemolytic antibodies. Br J Haematol. 1957 Jan;3(1):77–87. doi: 10.1111/j.1365-2141.1957.tb05773.x. [DOI] [PubMed] [Google Scholar]
- EVANS R. S., TURNER E., BINGHAM M. STUDIES WITH RADIOIODINATED COLD AGGLUTININS OF TEN PATIENTS. Am J Med. 1965 Mar;38:378–395. doi: 10.1016/0002-9343(65)90146-4. [DOI] [PubMed] [Google Scholar]
- GOLD E. R., LOCKYER W. J., TOVEY G. H. Use of lyophilized formol-treated red cells in blood-group serology. Nature. 1958 Oct 4;182(4640):951–951. doi: 10.1038/182951a0. [DOI] [PubMed] [Google Scholar]
- HARBOE M. INTERACTIONS BETWEEN 131-I TRACE-LABELLED COLD AGGLUTININ, COMPLEMENT AND RED CELLS. Br J Haematol. 1964 Jul;10:339–346. doi: 10.1111/j.1365-2141.1964.tb00710.x. [DOI] [PubMed] [Google Scholar]
- HELMKAMP R. W., GOODLAND R. L., BALE W. F., SPAR I. L., MUTSCHLER L. E. High specific activity iodination of gamma-globulin with iodine-131 monochloride. Cancer Res. 1960 Nov;20:1495–1500. [PubMed] [Google Scholar]
- HOREJSI J., SMETANA R. The isolation of gamma globulin from blood-serum by rivanol. Acta Med Scand. 1956 Jun 30;155(1):65–70. doi: 10.1111/j.0954-6820.1956.tb14351.x. [DOI] [PubMed] [Google Scholar]
- Hillman R. S., Giblett E. R. Red cell membrane alteration associated with "marrow stress". J Clin Invest. 1965 Oct;44(10):1730–1736. doi: 10.1172/JCI105280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES N. C., GARDNER B. The exchange of 131-I-labelled lipid and 131-I-labelled protein between red cells and serum. Biochem J. 1962 May;83:404–413. doi: 10.1042/bj0830404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAN S. Y., GARDNER F. H. LIFE SPAN OF RETICULOCYTES IN PAROXYSMAL NOCTURNAL HEMOGLOBINURIA. Blood. 1965 May;25:759–766. [PubMed] [Google Scholar]
- LAURELL A. B. COMPARISON OF THE AGGLUTINABILITY BY RHEUMATOID ARTHRITIC SERA OF SENSITIZED SHEEP CELLS, EXPOSED TO HUMAN C'1, C'4 AND C'2. Acta Pathol Microbiol Scand. 1963;59:73–78. [PubMed] [Google Scholar]
- LEWIS S. M., DACIE J. V., TILLS D. Comparison of the sensitivity to agglutination and haemolysis by a high-titre cold antibody of the erythrocytes of normal subjects and of patients with a variety of blood diseases including paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1961 Jan;7:64–72. doi: 10.1111/j.1365-2141.1961.tb00320.x. [DOI] [PubMed] [Google Scholar]
- MARCUS D. M., KABAT E. A., ROSENFIELD R. E. THE ACTION OF ENZYMES FROM CLOSTRIDIUM TERTIUM ON THE I ANTIGENIC DETERMINANT OF HUMAN ERYTHROCYTES. J Exp Med. 1963 Aug 1;118:175–194. doi: 10.1084/jem.118.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSH W. L. Anti-i: a cold antibody defining the Ii relationship in human red cells. Br J Haematol. 1961 Apr;7:200–209. doi: 10.1111/j.1365-2141.1961.tb00329.x. [DOI] [PubMed] [Google Scholar]
- MOLLER G. ISOANTIBODY-INDUCED CELLULAR RESISTANCE TO IMMUNE HAEMOLYSIS IN VIVO AND IN VITRO. Nature. 1964 Apr 25;202:357–359. doi: 10.1038/202357a0. [DOI] [PubMed] [Google Scholar]
- MUELLER-EBERHARD H. J., BIRO C. E. ISOLATION AND DESCRIPTION OF THE FOURTH COMPONENT OF HUMAN COMPLEMENT. J Exp Med. 1963 Sep 1;118:447–466. doi: 10.1084/jem.118.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J., NILSSON U., ARONSSON T. Isolation and characterization of two beta1-glycoproteins of human serum. J Exp Med. 1960 Feb 1;111:201–215. doi: 10.1084/jem.111.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON R. A., Jr An alternative mechanism for the properdin system. J Exp Med. 1958 Oct 1;108(4):515–535. doi: 10.1084/jem.108.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILSSON U. R., MUELLER-EBERHARD H. J. ISOLATION OF BETA IF-GLOBULIN FROM HUMAN SERUM AND ITS CHARACTERIZATION AS THE FIFTH COMPONENT OF COMPLEMENT. J Exp Med. 1965 Aug 1;122:277–298. doi: 10.1084/jem.122.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PILLEMER L., BLUM L., LEPOW I. H., WURZ L., TODD E. W. The properdin system and immunity. III. The zymosan assay of properdin. J Exp Med. 1956 Jan 1;103(1):1–13. doi: 10.1084/jem.103.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIROFSKY B., CORDOVA M. S., IMEL T. L. The nonimmunologic reaction of globulin molecules with the erythrocyte surface. Vox Sang. 1962;7:334–347. doi: 10.1111/j.1423-0410.1962.tb03262.x. [DOI] [PubMed] [Google Scholar]
- PONDMAN K. W., PEETOOM F. THE SIGNIFICANCE OF THE ANTIGEN-ANTIBODY COMPLEMENT REACTION. IV. THE TRANSFORMATION OF BETA-1-C-GLOBULIN INTO BETA-1-A-GLOBULIN. Immunochemistry. 1964 Jun;1:65–90. doi: 10.1016/0019-2791(64)90073-4. [DOI] [PubMed] [Google Scholar]
- Rochna E., Hughes-Jones N. C. The use of purified 125-I-labelled anti-gamma globulin in the determination of the number of D antigen sites on red cells of different phenotypes. Vox Sang. 1965 Nov-Dec;10(6):675–686. doi: 10.1111/j.1423-0410.1965.tb05179.x. [DOI] [PubMed] [Google Scholar]


