Abstract
Rationale
Elastin is a ubiquitous extracellular matrix (ECM) protein that is highly organized in heart valves and arteries. Because elastic fiber abnormalities are a central feature of degenerative valve disease, we hypothesized that elastin deficient mice would manifest viable heart valve disease.
Objective
To analyze valve structure and function in elastin insufficient mice (Eln+/−) at neonatal, juvenile, adult and aged adult stages.
Methods and Results
At birth, histochemical analysis demonstrated normal ECM organization in contrast to the aorta. However, at juvenile and adult stages thin elongated valves with ECM disorganization, including elastin fragment infiltration of the annulus, were observed. The valve phenotype worsened by the aged adult stage with overgrowth and proteoglycan replacement of the valve annulus. The progressive nature of elastin insufficiency was also shown by aortic mechanical testing that demonstrated incrementally abnormal tensile stiffness from juvenile to adult stages. Eln+/− mice demonstrated increased valve interstitial cell (VIC) proliferation at the neonatal stage and varied VIC activation at early and late stages. Gene expression profile analysis identified decreased TGF-β mediated fibrogenesis signaling in Eln+/− valve tissue. Juvenile Eln+/− mice demonstrated normal valve function, but progressive valve disease (predominantly aortic regurgitation) was identified in 17% of adult and 70% of aged adult Eln+/− mice by echocardiography.
Conclusions
These results identify the Eln+/− mouse as a model of latent aortic valve disease and establish a role for elastin dysregulation in valve pathogenesis.
Keywords: valve disease, extracellular matrix, valvular interstitial cells
Valve disease remains an important public health problem. Therapy for valve disease is primarily surgical and nearly 100,000 valve replacement procedures are performed in the US annually.1,2 The need for re-intervention is common, especially among pediatric patients, and there is increasing interest in improving the longevity of bioprosthetic valves.3–5 The etiology of valve disease remains unclear; however, increasing evidence implicates aberrant developmental mechanisms underlying pathogenesis.6–8 Extracellular matrix (ECM) protein abnormalities have been associated with human valve disease,5,8,9 and elastic fiber abnormalities are a common if not universal finding.10,11 However, little is known of the regulatory role elastin plays in valve disease.
Elastin (MIM*130160), the primary component of elastic fibers, is an ECM protein involved in tissue integrity and mobility, as well as paracrine signaling.12 Elastin haploinsufficiency results in complex cardiovascular problems, and approximately 10–45% of patients have valve abnormalities.13,14 Based on previous studies identifying elastic fiber abnormalities in syndromic and nonsyndromic aortic valve disease,10,11,15 we hypothesized that the elastin insufficient mouse (Eln+/−) would manifest viable aortic valve disease. The null mouse (Eln−/−) dies perinatally secondary to severe arterial obstruction reminiscent of supravalvar aortic stenosis,12 while arteriopathy in the Eln+/− mouse manifests as systemic hypertension.16 The Eln+/− mouse has one functional allele, expresses 50% elastin, and has normal longevity. Interestingly, elastin haploinsufficiency has negligible effects on aging.16,17 In arterial tissue, elastin and collagen are organized as a two component system allowing elastic fibers to function in low stress and collagen fibers to function in high stress. In valves, as in all non-arterial tissue, these ECM proteins function together in all stress states as composite tissue.18,19,20 To date, the valve phenotype of the elastin mutant mice has not been reported.
The mature aortic valve is a complex anatomical structure composed of loose connective tissue cusps hinged to a crown-shaped collagen-rich annulus within the aortic root.21,22 Valve cusps are organized in three overlapping layers of ECM (fibrosa, spongiosa, ventricularis), and each of these layers has distinct working capacities.5,11 Elastin is expressed in the ventricularis during late valvulogenesis just prior to birth, and elastic fiber assembly and organization occurs postnatally in the valve.8,23,24 Valvular interstitial cells (VIC) are the predominant cell type and their dynamic functional properties have resulted in an emerging understanding of distinct VIC phenotypes.5,25 Diseased aortic valves demonstrate abnormalities in both ECM and VIC organization.8,10,26
In the current study, we found complex abnormalities in valve tissue and identified progressive pathology in both aortic valve and aorta in elastin insufficient mice. Specifically, we demonstrate progressive postnatal aortic valve malformation and latent aortic valve disease in the Eln+/− mouse. These findings suggest a potential mechanism whereby compensatory VIC activation results in increased proliferation, decreased TGF-β signaling, and increased ECM synthesis, which in turn results in maladaptive ECM remodeling and over time aortic valve disease. Maladaptive ECM remodeling and disorganization are hallmarks of human valve disease. Specific abnormalities in the valve annulus-aortic root complex were shown in vivo and in vitro suggesting a central role for this region of valve tissue in pathogenesis.
Methods
The elastin mutant mice were created using a typical gene targeting approach. Specifically, wild type (Eln+/+) and elastin insufficient (Eln+/−) mice were derived on a mixed genetic background and a pure C57BL6 line was developed.12 ECM expression was determined using pentachrome staining, immunostaining and quantitative real time RT-PCR as previously described.8,27 Comprehensive aortic valve morphometrics, ultrastructural analysis and mechanical studies were performed. Gene expression profiling was performed using GeneChip Mouse Genome 430 2.0 Array (Affymetrix), and data were analyzed using Ingenuity Pathway Analysis (Ingenuity® Systems, www.ingenuity.com). Valve structure and function was evaluated using transthoracic echocardiography.28 A detailed Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
Results
Aortic valves are normal at birth and demonstrate progressive postnatal structural changes
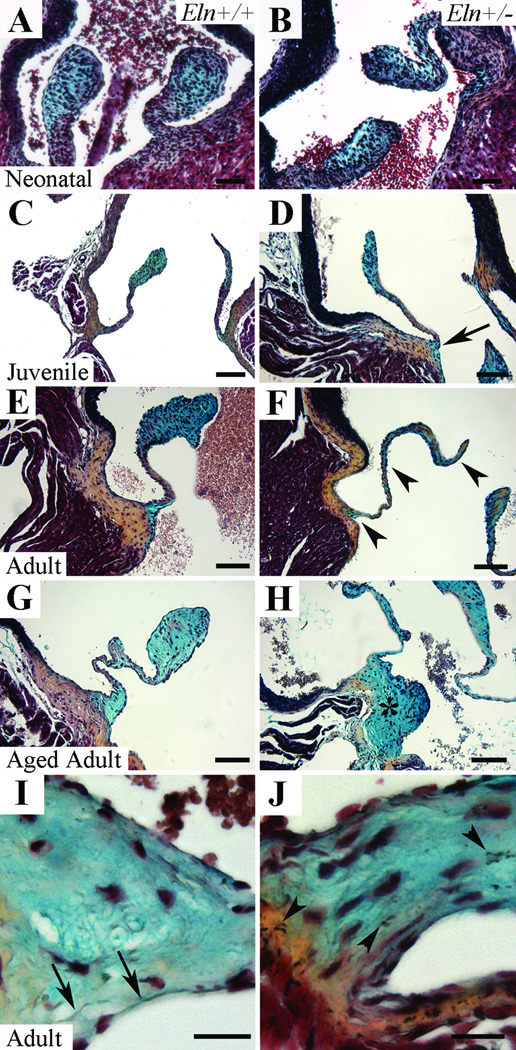
To evaluate aortic valve morphology and ECM organization in the Eln+/− mouse over time, we performed histochemical analysis. Bilaminar matrix stratification of the neonatal aortic valve was not qualitatively different when Eln+/+ mice were compared with Eln+/− (Figure 1A,B) and Eln−/− mice (Supplemental Figure I). Beginning at the juvenile stage when elastic fiber assembly and organization is occurring in the valve, the Eln+/− aortic valve was attenuated at the hinge point suggesting this anatomic area is the primary affected tissue (Figure 1C,D). In addition, elastin fragments were seen in both the cusp and annulus of the Eln+/− mouse beginning at this stage (Supplemental Figure II). No abnormalities were appreciated in the aorta at the neonatal or juvenile stages. Subsequently, at the adult stage, the Eln+/− aortic valve became disproportionately elongated and thinned compared to controls (Figure 1F); these abnormalities progressed with time ultimately resulting in a repeatedly kinked structure in the aged adult (Figure 1G). Importantly, there was overgrowth and proteoglycan replacement of the typically collagen-rich valve annulus in the aged adult (Figure 1H). Normal elastin filament organization localized to the ventricularis layer was seen in juvenile, adult and aged adult Eln+/+ mice (Figure 1I); notably, elastin was not present in the annulus at any stage. ECM disorganization was manifest in part as dispersed elastin fragments that infiltrated the annulus (Figure 1J). There was no collagen accumulation (Figure 1) or calcification (data not shown) in either aorta or aortic valve tissue at any stage in the Eln+/− or Eln+/+ mice. Taken together, these findings identify progressive valve histopathology due to elastin haploinsufficiency that begins postnatally.
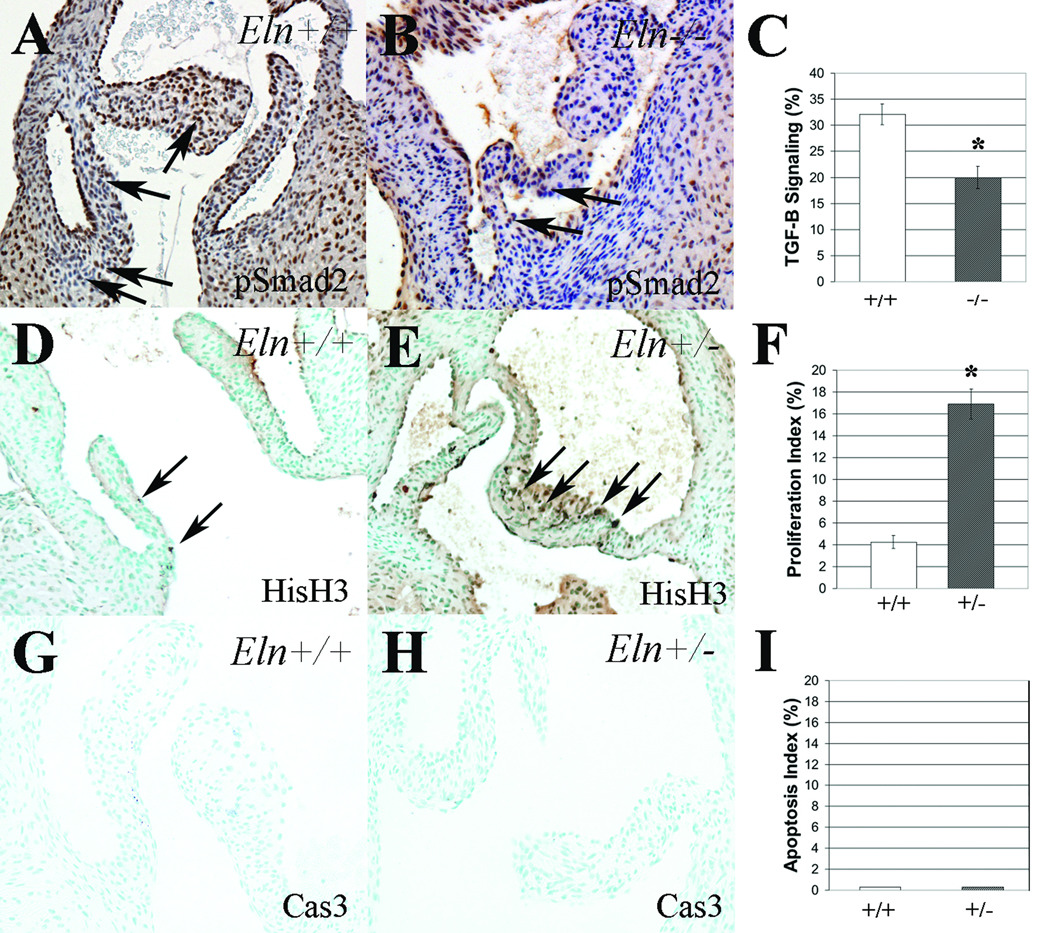
Figure 1. Progression of anatomic valve abnormalities in Eln+/− aortic valves by histochemistry.
Aortic valve cusps in Eln+/+ (A,C,E,G) and Eln+/− (B,D,F,H) mice at neonatal (A,B), juvenile (C,D), adult (E,F), and aged adult (G,H) stages. The Eln+/− mice demonstrate attenuation of the hinge (arrow, D), progressive valve cusp thinning and elongation (arrowheads, F), and ultimately overgrowth and proteoglycan replacement of the annulus (asterisk, H) when compared with age-matched wild type controls. At high magnification, normal elastic fiber organization is appreciated in the ventricularis of Eln+/+ mice (arrows, I), while elastic fiber fragments are seen throughout the cusp and infiltrating the annulus of Eln+/− mice (arrowheads, J). Scale bar 100µm.
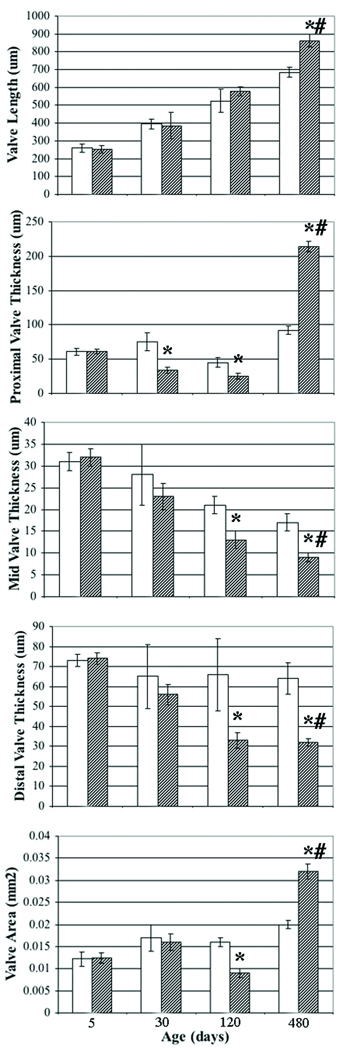
To quantify complex and progressive changes in valve structure, comprehensive morphometrics were used (Figure 2). All measures were normal at the neonatal stage. At the juvenile stage, all indices were normal except the thickness of the proximal aspect of the valve (hinge), which was abnormally thin. At the adult stage, the thickness of the middle and distal aspects of the valve decreased significantly as did the valve area. Interestingly, while the thickness of the proximal aspect of the valve was attenuated in the juvenile and adult, it became markedly thickened in the aged adult. The remainder of the cusp progressively thinned with age. Cusp length was significantly increased in the aged adult. The thickness of the aorta was decreased (data not shown), consistent with previous reports.16 In addition to differences by genotype, all indices measured in Eln+/− mice showed significant differences with age (p < 0.001). Taken together, these measurements confirm the progressive nature of valve malformation and demonstrate dynamic changes in regional valve anatomy.
Figure 2. Comprehensive aortic valve morphometrics in Eln+/− mice.
Progressive abnormalities are demonstrated in Eln+/− mice (hatched) when compared with Eln+/+ mice (white). Valve length, valve thickness (proximal, middle and distal), and valve area are shown at neonatal, juvenile, adult and aged adult stages (5, 30, 120 and 480 days, respectively). Mean ± standard deviation. * p < 0.05 Eln+/+ vs. Eln+/−. # p < 0.001 aged adult vs. adult, juvenile and neonatal.
Mechanical studies in aorta also show disease progression over time
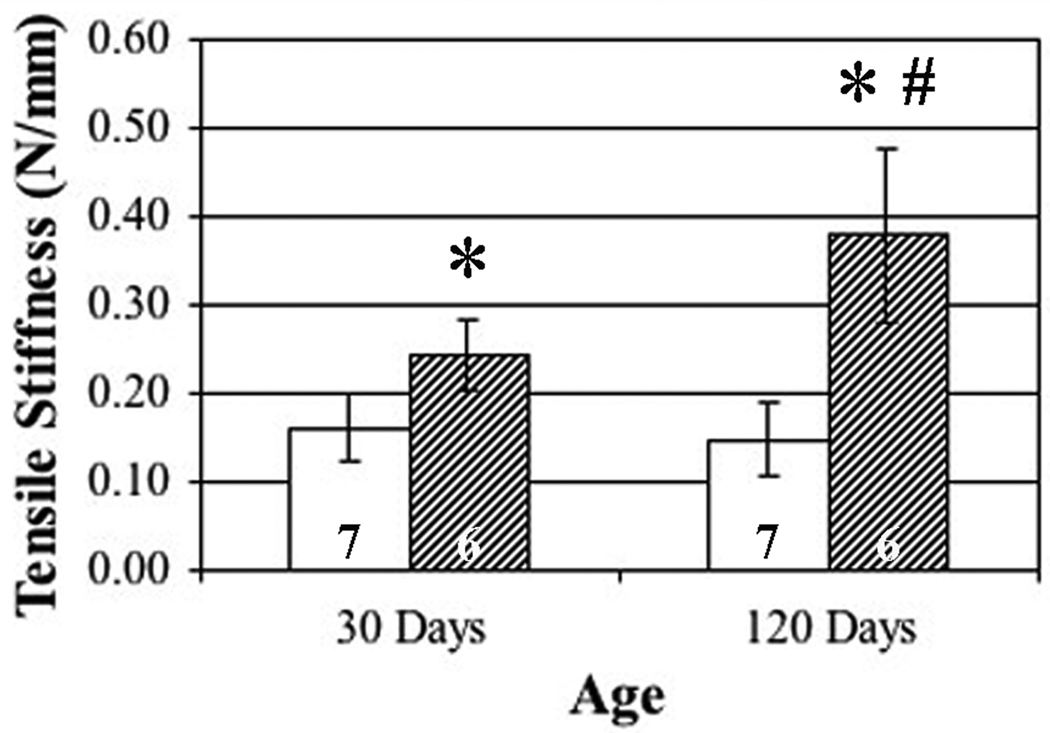
Mechanical studies show progressive aortopathy (Figure 3). Specifically, at the juvenile stage, Eln+/− mice demonstrate significantly increased aortic tensile stiffness when compared with age-matched wild type controls. In addition, adult stage Eln+/− mice demonstrate a significant increase in tensile stiffness when compared with both wild type controls and the juvenile transgenic. Since fibrillar collagen does not accumulate in the aorta, increased stiffness may be due to other factors or a shift in the elastin-collagen ratio.24 Importantly, aortic tensile stiffness in the adult wild type was not different from the juvenile wild type, suggesting the progressive aortopathy in the Eln+/− mice is due to maladaptation related to elastin haploinsufficiency rather than the effects of aging.16,18
Figure 3. Mechanical studies showing progressive aortic tensile stiffness abnormalities in Eln+/− aortas.
Aortas from Eln+/− mice (hatched) were significantly stiffer than Eln+/+ mice (white) at both juvenile (0.162 vs. 0.244 N/mm, 30 days) and adult (0.148 vs. 0.378 N/mm, 120 days) stages (asterisks, p < 0.05). Adult Eln+/− aortas were incrementally stiffer than juvenile Eln+/− aortas (0.244 vs. 0.378 N/mm, number sign, p < 0.05), demonstrating progressive aortopathy. Six or seven mice were studied per group.
Ultrastructural analysis reveals ECM disorganization and proteoglycan accumulation
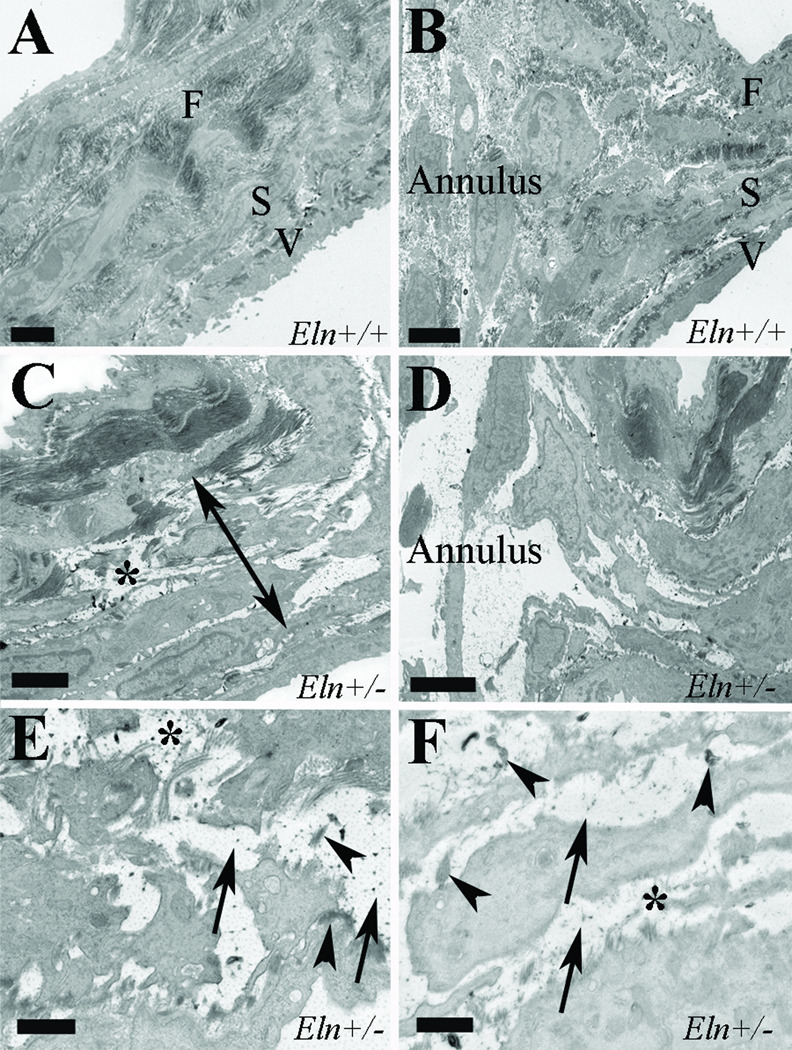
To further evaluate the nature of the morphological changes in aortic valves resulting from elastin haploinsufficiency, transmission electron microscopy was used in juvenile mice (Figure 4). Collagen bundles were disoriented and separated in the cusp, but appeared qualitatively unchanged in amount (Figure 4C). The central spongiosa was expanded with qualitatively markedly increased proteoglycans (Figure 4C), and these findings exended into the annulus region (Figure 4D). Elastic fibers were decreased and fragmented, and elastin globules were dispersed throughout the cusp no longer restricted to the ventricularis layer (Figure 4C,E), and elastin globules infiltrated the annulus (Figure 4D,F). In addition, there were increased synthetic organelles in the Eln+/− valve consistent with an activated VIC phenotype. Taken together, these findings confirm loss of trilaminar ECM stratification in Eln+/− valve as evidenced by abnormalities in each of the three cusp layers and the annulus at the juvenile stage.
Figure 4. Ultrastructural evidence of ECM abnormalities in Eln+/− aortic valves.
Aortic valves in juvenile Eln+/+ (A,B) and Eln+/− (C–F) mice in the cusp (A,C,E) and annulus (B,D,F) regions. Eln+/− mice demonstrate collagen fiber disarray (asterisks, C,E), expanded spongiosa (double headed arrow, C) with increased proteoglycans (arrows, E,F) and elastic fiber fragmentation and dispersion (arrowheads, E,F). In addition, elastin globules have infiltrated the annulus region in Eln+/− valves. F fibrosa, S spongiosa, V ventricularis. Scale bars are 2µm (A,B), 1µm (C,D) and 500nm (E,F).
Immunohistochemistry reveals decreased TGF signaling, increased interstitial cell proliferation and an activated VIC phenotype
Since gene expression studies identified subtle changes in TGF-β signaling, we sought to determine the effect of elastin loss of function on TGF-β signaling. Compared to Eln+/+ neonatal mice, there was decreased TGF-β signaling in neonatal Eln+/− mice (data not shown) and Eln−/− mice (Figure 5A–C). Specifically, the pSmad2 immunoreactivity index decreased from 32% to 20%, disproportionately affecting the annulus. pSmad2 immunoreactivity was modestly but not statistically significantly decreased in Eln+/− mice, consistent with the gene expression data. In neonatal Eln+/− mice, there was increased cell proliferation (Figure 5D–F). Specifically, the proliferation index in Eln+/− aortic valves increased from 4% to 17%, consistent with previous findings in aortic tissue.12 No proliferation was observed in juvenile Eln+/− or Eln+/+ mice in either valve or aortic tissue (data not shown), suggesting the increased proliferation appreciated in the neonatal heterozygote is transient. Not surprisingly, there was no evidence of apoptosis in either the Eln+/− or Eln+/+ mice at neonatal (Figure 5G–I) or juvenile (data not shown) stages.
Figure 5. Interstitial cell maladaptation in elastin mutant aortic valves.
TGF-β signaling, as indicated by phospho-Smad-2 immunostaining (pSmad2), was significantly reduced in Eln−/− aortic valves when compared with Eln+/+ mice (A–C). Proliferation was significantly increased in neonatal Eln+/− mice when compared with controls (D–F), using antibodies directed against phospho-Histone H3 (HisH3). No apoptosis was seen at this stage in either Eln+/− or Eln+/+ mice (G–I) using antibodies directed against cleaved caspase-3 (Cas3). Mean ± standard error. * p < 0.05.
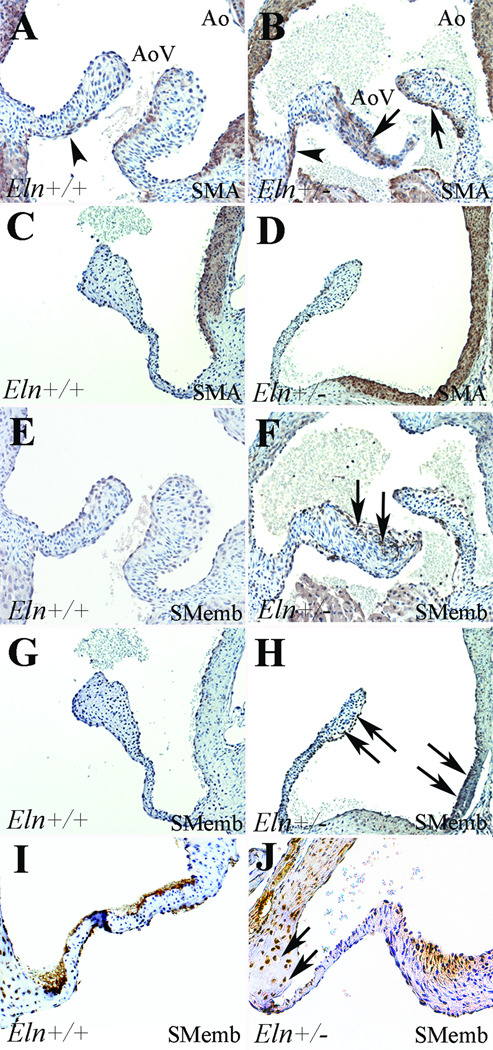
VIC phenotype was assessed by analysis of SMA and SMemb expression (Figure 6). SMA expression was shown in the proximal ventricularis of Eln+/+ mice at neonatal and juvenile stages (Figure 6A,C), consistent with previous findings and normal elastic fiber expression.8,23,29 However, in neonatal Eln+/− mice, SMA was increased in the ventricularis and present in all three cusp layers (Figure 6B). On the other hand, SMemb expression was absent at neonatal and juvenile stages in Eln+/+ mice. However, in Eln+/− mice at both stages, SMemb was expressed in the arterial aspect of the aortic valve cusps and the aortic root (Figure 6F,H), consistent with spatially restricted VIC activation involving different interstitial cells than those expressing SMA. Further, SMemb was present in the annulus of adult Eln+/− mice but not wild type controls (Figure 6I,J) and was absent from both genotypes in aged adult mice (data not shown). Taken together, there appears to be varied subsets of VICs activated in the Eln+/− mice at different stages resulting in increased cell proliferation at the neonatal stage and increased ECM synthesis at the juvenile stage. These findings suggest that VIC regulation plays multiple functional roles in postnatal valve malformation and ultimately disease manifestation.
Figure 6. VIC activation in Eln+/− aortic valves.
SMA expression (A–D) was shown in the ventricularis of neonatal Eln+/+ mice (arrowhead, A), but in neonatal Eln+/− mice, SMA expression was increased in this region (arrowhead, B) and was also present throughout the cusp (arrows, B). There was no valve SMA expression in either Eln+/+ or heterozygous juvenile mice (C,D). There was no expression of SMemb in Eln+/+ mice at neonatal or juvenile stages (E,G); however, at both stages in the Eln+/− mice, there was expression in the arterial aspect of the cusp as well as within the valve annulus – aortic root complex (arrows, F,H), consistent with VIC activation. In adult Eln+/− mice (I,J), SMemb expression was restricted to the valve annulus region (arrows, J).
Gene expression profile analysis identifies developmental ECM abnormalities and complex signaling pathway interactions
To further examine regulatory and ECM abnormalities in the Eln+/− mouse, microarray studies were performed. Comparing the Eln+/− versus Eln+/+ mouse, there were 1751 differentially expressed probe sets in aortic valve tissue, corresponding with 743 genes demonstrating increased expression and 604 genes demonstrating decreased expression. Likewise, there were 696 differentially expressed probe sets in aortic tissue, corresponding with 451 genes showing increased expression and 178 genes showing decreased expression. Differentially expressed genes with known biologic roles in valve or artery development are shown in Table 1. Interestingly, some genes (eg. hapln-1, mmp-15 and vegf-b) were up in one tissue group and down in the other. Importantly, elastin expression was reduced approximately one half, consistent with haploinsufficiency,12,16 validating the array methodology. In addition, microarray gene expression levels were confirmed in select genes by quantitative real time RT-PCR (Supplemental Table I).
Table 1.
Differential gene expression in Eln+/− mice
| Aortic Valve | Aorta | ||
|---|---|---|---|
| Gene | Fold Change | Gene | Fold Change |
| Col-2a1 | + 2.7 | Prg-4 | + 4.0 |
| Tbx-5 | + 2.4 | Wisp-1 | + 2.0 |
| Mmp-15 | + 2.4 | Axin-2 | + 1.9 |
| Fgf-12 | + 2.3 | Col-8a1 | + 1.9 |
| Col-11a1 | + 2.3 | Hapln-1 | + 1.9 |
| Acta-1 | + 2.2 | Ankrd-1 | + 1.7 |
| Vegf-b | + 1.9 | Bmp-6 | + 1.6 |
| Myh-6 | + 1.9 | Fgf-1 | + 1.6 |
| Fbn-2 | + 1.8 | Vcam-1 | + 1.6 |
| Gpc-1 | + 1.8 | Gata-6 | + 1.5 |
| Fln-a | − 1.6 | Vegf-b | − 1.5 |
| Hapln-1 | − 1.7 | Tnx-b | − 1.5 |
| Smad-3 | − 1.7 | Notch-3 | − 1.6 |
| Tgfb-r1 | − 1.7 | Hey-1 | − 1.7 |
| Myh-11 | − 1.7 | Corin | − 1.7 |
| Eln | − 2.4 | Mmp-15 | − 1.8 |
| Acta-2 | − 2.7 | Timp-4 | − 1.8 |
| Actg-2 | − 2.7 | Wnt-8b | − 1.8 |
| Bmp-r1b | − 2.9 | Itga-5 | − 2.2 |
| Jumonji | − 16.7 | Jumonji | − 12.5 |
Pathway analysis of differentially expressed genes from valve tissue identified several significant signaling pathways implicated in valve disease pathogenesis, including Fibrogenesis, Integrin, Actin cytoskeleton and VEGF pathways (Supplemental Table II). Among those pathways that were identified in both aortic valve and aorta tissue, differentially expressed genes were classified as contributing to “valve only”, “aorta only” or “both valve and aorta”. Subanalysis of the Fibrogenesis signaling pathway showed that 18 genes were differentially expressed in valve only, 5 in aorta only, and 7 in both valve and aorta (Supplemental Figure III). Interestingly, expression of both tgfbr1 and smad3 was decreased nearly 2-fold in Eln+/− valve only, suggesting TGF-β mediated fibrogenesis pathway signaling was significantly reduced in a spatially restricted manner. Further, fibrillar collagens (types I and III) were not misexpressed. Differentially expressed genes in the Fibrogenesis and Actin cytoskeleton pathways overlapped suggesting functional interaction, and several smooth muscle contractile genes are misexpressed (Supplemental Figure III, Supplemental Table II), consistent with the idea that these genes are critical in maintaining valve structure and function.
Echocardiography demonstrates in vivo development of latent progressive aortic valve disease
To assess valve structure and function in vivo, echocardiography was performed at serial stages in Eln+/− and age-matched Eln+/+ mice (Figure 7). Aortic dimensions were normal at all stages except the aged adult stage at which time the valve annulus (effective orifice diameter) and aortic root dimensions were dilated (Table 2). Importantly, the sinotubular junction dimension was normal at all stages (data not shown). Interestingly, valve annulus dimensions and function for the other three valves were normal at all stages (data not shown).
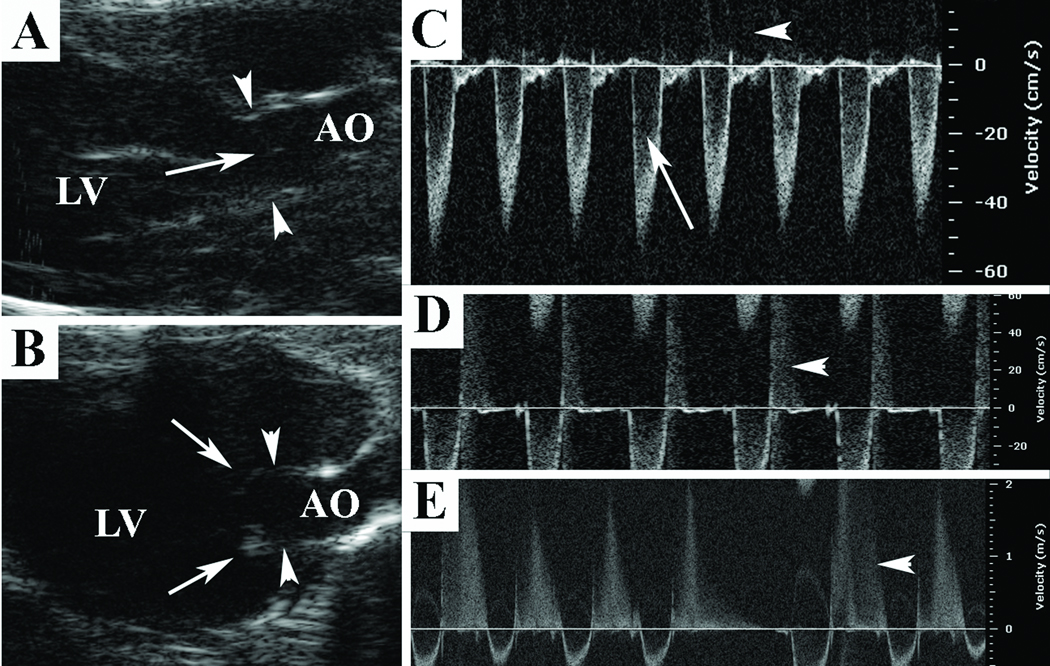
Figure 7. Echocardiography analysis of Eln+/− aortic valve structure and function.
At the aged adult stage, 2-D images (A,B) demonstrate normal central closure without redundancy in Eln+/+ mice (arrow, A) and cusp redundancy with frank valve prolapse at end-diastole in Eln+/− mice (arrows, B); aortic valve annulus hinge points are demonstrated by arrowheads (A,B). Doppler interrogation demonstrates a normal left ventricular outflow tract signal (arrow, C) without aortic regurgitation (arrowhead, C) in an aged adult Eln+/+ mouse; mild aortic regurgitation in an adult Eln+/− mouse (arrowhead, D) and moderate holodiastolic aortic regurgitation in an aged adult Eln+/− mouse (arrowhead, E).
Valve function was normal at the juvenile stage with disease manifesting in adulthood and worsening in senescence. At the juvenile stage, 6/6 Eln+/− mice demonstrated normal valve function; however, at the adult stage, 1/6 (17%) mice demonstrated mild aortic regurgitation (Figure 7D). At the aged adult stage, Eln+/− aortic valve cusps frankly prolapsed in diastole (Figure 7B) and 7/10 (70%) demonstrated aortic valve disease. There were 6 mice with aortic regurgitation (3 mild and 3 moderate, Figure 7E) and 3 mice with aortic stenosis (2 mice had both stenosis and regurgitation). The incidence of disease in the Eln+/− mice was significantly increased over time (p < 0.01). Ventricular dysfunction was present in 8/18 aged adult Eln+/− mice and these mice were excluded from analysis; 4/8 had aortic regurgitation. There was no clear histologic correlation between aortic valve disease types (stenosis vs. regurgitation). There was no valve disease in age-matched wild type controls at any stage. These findings complement both histologic and mechanical studies that demonstrate a progressive disease process.
Discussion
In this study, we have shown that elastin haploinsufficiency causes aortic valve malformation and disease in a mouse model, identifying elastin as a crucial factor in the progressive nature of aortic valve disease pathogenesis. These studies distinguish important regional differences in valve malformation and implicate destabilization of the valve annulus as a contributing factor to valve disease. Multiple transgenic mouse models have demonstrated severe cardiac malformation including valve defects;30–32 however, they are typically embryonic lethal and therefore present limited insight into postnatal valve disease pathogenesis. The postnatal valve malformation in the Eln+/− mouse can be characterized as a “late” valvulogenesis defect (post-cushion formation, post-endothelial mesenchymal transformation), and in this way bears a similarity to human valve disease. The progressive malformation and latent disease manifestation parallels the natural history of human valve disease. Finally, the valve cusp histopathology in elastin haploinsufficiency mimics the findings of human degenerative aortic valve disease, suggesting common mechanisms of elastin dysregulation underlie valve disease pathology. These studies are clinically relevant because they improve our understanding of valve disease pathogenesis and have the potential to contribute to the development of new therapeutics. Taken together, these findings demonstrate a central role for elastin in postnatal valve disease, supporting evidence that aberrant developmental programs cause disease phenotypes that manifest later in life.
The findings of the current study demonstrate that elastin haploinsufficiency results in VIC dysregulation and over time maladaptive ECM remodeling providing an underlying mechanism for disease manifestation. The important functional role of VICs has been emphasized, however VIC phenotyping remains controversial and incompletely understood in part due to the plasticity of these cells.23,25 In this study, we show that elastin haploinsufficiency results in different types of VIC activation that varies spatially and temporally. At the neonatal stage, there is increased expression of both SMA and SMemb concomitant with altered cell proliferation and increased ECM synthesis. At the juvenile stage, the increased expression of SMemb only is spatially restricted and involves the valve annulus-aortic root complex, without changes in either proliferation or apoptosis. Further, SMemb expression is localized to the annulus in adult mice but not aged adult mice, suggesting other factors contribute to valve maintenance and continued ECM remodeling after disease onset. VIC activation is temporally related to aberrant ECM remodeling that results in valve malformation and ultimately valve disease. Since SMA is normally present in the valve, increased expression may reflect maladaptive compensation or uncoordinated VIC differentiation. On the other hand, since SMemb is not typically expressed in the valve, ectopic expression may reflect a different explicitly pathologic response, eg. reactivated endothelial mesenchymal transformation leading to a maladaptive fibrogenesis response.33–35 Since a subset of VICs share a number of attributes with vascular smooth muscle cells, it may be that regulatory phenotypic modulation is also shared.36,37 Future studies exploring VIC activation after disease onset may provide valuable insight into VIC phenotyping, ECM remodeling and the progressive nature of aortic valve disease.
ECM proteins, signaling molecules and transcription factors have been associated with valve disease.6 Gene expression profiling in the current study identified specific genes and pathways involved in the development of aortic valve malformation and disease. Subanalysis of the Fibrogenesis pathway identified patterns of differential gene expression implicating VIC dysregulation and maladaptive ECM remodeling due to fibrogenesis abnormalities. Unexpectedly, we found that elastin insufficient mice demonstrate decreased TGF-β. Given the central role of elastin in elastic fiber formation, we expected TGF-β to be increased as has been found in the elastic fiber fibrillin1 and fibulin4 deficient mice.9,38 In the context of valve disease, fibrogenesis abnormalities may represent a spectrum of pathology with fibrosis (too much collagen) on one end and myxomatous change (too much proteoglycan) on the other end. Further, in the Eln+/− mice, jumonji was significantly reduced in aortic valve tissue, consistent with the finding of increased cell proliferation. Jumonji has been shown to negatively regulate normal proliferation and tissue growth,39 and in the context of elastin haploinsufficiency is downregulated with a concomitant maladaptive increase in cell proliferation that may contribute to early ECM abnormalities and ultimately disease. A limitation of the current study is that the “aortic valve” group contained valve cusp, valve annulus and aortic root. However, the aortic root is known to have different developmental origins than the aorta,40 and therefore from a mechanistic standpoint, the aortic root and valve annulus may represent a functional unit of transitional tissue. Importantly, the microarray was performed prior to the onset of valve disease and therefore is not the result of secondary changes due to hemodynamic perturbation or end stage disease. This type of subanalysis may direct the mechanistic evaluation of inter-related pathways and networks or specific disease-causing mutations, including crosses to other mice with connective tissue phenotypes and conditional transgenic mouse models controlling spatial and temporal gene expression.
These studies clearly demonstrate histopathology intrinsic to the valve cusp, but histochemistry, immunohistochemistry, gene expression and echocardiography findings suggest the region of the valve implicated in disease manifestation is the valve annulus within the aortic root. These observations add significant new implications to the analysis of valve structure and function in the Eln+/− mouse and may be clinically relevant in the broader study of human valve disease associated with aortopathy. In light of the clinical association between aortic valve disease and aortopathy,41,42 elastic fiber dysregulation in the valve annulus may be particularly relevant in elucidating the pathogenesis of these common and related disorders. These findings challenge the clinical taxonomy and suggest further investigation of the aortic valve annulus region is warranted. The complex anatomy of the aortic valve annulus as it relates to the aortic root has been well-described.21,22 In humans, disease causing gene mutations may be classified as those resulting in both aortic valve disease and aortopathy (ACTA2, MYH11, FBN1, ELN), valve disease but not aortopathy (FLNA, NOTCH1), or aortopathy without valve disease (TGFBR1, COL3). Interestingly, many of these genes were downregulated in elastin insufficient mouse valve tissue (but not aorta tissue), suggesting the primary pathologic tissue is the valve annulus-aortic root complex. These observations raise fundamental questions about both the origin and functional capacity of the valve annulus-aortic root complex and aortopathy. Taken together, these findings confirm that elastin dysregulation plays a central role in human valve disease ultimately leading to variations of aortopathy and aortic valve disease.
Combining functional, molecular and engineering approaches to the evaluation of regional valve tissue may significantly inform our understanding of valve disease natural history and pathogenesis. Synthesizing these approaches will provide important opportunities for early clinical recognition43,44 and potentially refine approaches to valve bioprosthesis development.4,45 For example, one explanation for “acquired” aortic valve disease in these mutant mice may be the combination of a predisposing genotype that results in subtle but maladaptive ECM remodeling and physiologic stresses, including diastolic shear stress at the valve hinge and lateral compressive forces on the valve annulus-aortic root complex. The findings of the current study have significant clinical implications. Longitudinal studies of patients with elastin haploinsufficiency and valve disease may reveal an increased incidence of latent aortic root dilation and aortic regurgitation providing a clinical population with a well defined genotype to study mechanisms of valve disease and aortopathy. Further, elucidating ECM and VIC characteristics will be particularly important in the context of bioprosthetic valve development. Because this area of research is increasingly interested in “living” valve tissue grafts composed of matrix scaffolds seeded with cells, these cell-matrix relationships may inform durability issues.45,46 Finally, different valve sparing root replacement surgery approaches demonstrate different long-term valve disease outcomes, suggesting the integrity of the valve annulus within the aortic root is paramount. Specifically, preservation of the aortic root (reimplantation approach) provides annular stabilization while disruption of the aortic root (remodeling approach) predisposes to annular dilation, significant aortic regurgitation and an increased incidence of subsequent aortic valve replacement.47 The long-standing association between a “dilated aortic root” and aortic regurgitation may actually represent primary valve annulus disease, which questions the premise that valve disease is a secondary complication of aortopathy. This is an important distinction when considering etiology and potential pharmacologic therapies.
Novelty and Significance.
What is known?
-
-
Valve disease is characterized by extracellular matrix (ECM) and valve interstitial cell (VIC) abnormalities.
-
-
Animal models of valve disease are limited.
What new information does this article contribute?
-
-
Provides a genetic mouse model of latent valve disease that has origins in development.
-
-
Identifies elastin as a crucial factor in aortic valve disease pathogenesis.
-
-
Implicates the valve annulus region in disease manifestation.
Valve disease is an important public health problem. Elastin, an ECM protein, is a primary component of valve cusp tissue. Despite a long-standing focus on collagen abnormalities in valve disease, elastin dysregulation is a universal if underappreciated finding in human valve disease. Accordingly, we examined the effects of elastin insufficiency on valve structure and function. We identified progressive aortic valve malformation and latent valve disease in elastin insufficient mice. Pathogenesis in this model is due in part to VIC activation resulting in hyperproliferation and maladaptive ECM remodeling in the valve cusp, but surprisingly moreso in the valve annulus. This study identifies important concepts, including the primary role of elastin in valve pathogenesis and the central role of the valve annulus in disease manifestation. These findings provide a new focus for translational research efforts to develop novel therapeutic targets for valve disease.
Supplementary Material
Acknowledgements
We thank Dr. Katherine Yutzey (Cincinnati Children’s) for scientific advice, and Amy Opoka, Shawn Smith, Betty Glascock, Matthew Gruber and Swathi Balaji for their assistance.
Sources of Funding
This work was supported by grants from Cincinnati Children’s Research Foundation (RBH), American Society of Echocardiography (SW), and NIH HL69712 (DWB), HL74728 (DWB), HD43005 (RBH) and HL85122 (RBH).
Non-standard abbreviations
- Ao
aorta
- AoV
aortic valve
- Cas3
cleaved caspase-3
- ECM
extracellular matrix
- HisH3
phospho-Histone H3
- pSmad2
phospho-Smad-2
- RT-PCR
reverse transcriptase polymerase chain reaction
- SMA
alpha smooth muscle actin
- SMemb
nonmuscle myosin heavy chain
- TGF-β
transforming growth factor beta
- VEGF
vascular endothelial growth factor
- VIC
valve interstitial cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J. 2004;147:425–439. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS. Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2008;118:e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748. 2008. [DOI] [PubMed] [Google Scholar]
- 3.Keane JF, Driscoll DJ, Gersony WM, Hayes CJ, Kidd L, O'Fallon WM, Pieroni DR, Wolfe RR, Weidman WH. Second natural history study of congenital heart defects. Results of treatment of patients with aortic valvar stenosis. Circulation. 1993;87:I16–I27. [PubMed] [Google Scholar]
- 4.Gallegos RP. Selection of Prosthetic Heart Valves. Curr Treat Options Cardiovasc Med. 2006;8:443–452. doi: 10.1007/s11936-006-0032-8. [DOI] [PubMed] [Google Scholar]
- 5.Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008;118:1864–1880. doi: 10.1161/CIRCULATIONAHA.108.805911. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 8.Hinton RB, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 9.Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoen FJ. Aortic valve structure-function correlations: role of elastic fibers no longer a stretch of the imagination. J Heart Valve Dis. 1997;6:1–6. [PubMed] [Google Scholar]
- 11.Vesely I. The role of elastin in aortic valve mechanics. J Biomech. 1998;31:115–123. doi: 10.1016/s0021-9290(97)00122-x. [DOI] [PubMed] [Google Scholar]
- 12.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 13.Keane JF, Fellows KE, LaFarge CG, Nadas AS, Bernhard WF. The surgical management of discrete and diffuse supravalvar aortic stenosis. Circulation. 1976;54:112–117. doi: 10.1161/01.cir.54.1.112. [DOI] [PubMed] [Google Scholar]
- 14.Eronen M, Peippo M, Hiippala A, Raatikka M, Arvio M, Johansson R, Kahkonen M. Cardiovascular manifestations in 75 patients with Williams syndrome. J Med Genet. 2002;39:554–558. doi: 10.1136/jmg.39.8.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinton RB, Deutsch GH, Pearl JM, Hobart HH, Morris CA, Benson DW. Bilateral semilunar valve disease in a child with partial deletion of the Williams-Beuren syndrome region is associated with elastin haploinsufficiency. J Heart Valve Dis. 2006;15:352–355. [PubMed] [Google Scholar]
- 16.Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest. 2003;112:1419–1428. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pezet M, Jacob MP, Escoubet B, Gheduzzi D, Tillet E, Perret P, Huber P, Quaglino D, Vranckx R, Li DY, Starcher B, Boyle WA, Mecham RP, Faury G. Elastin haploinsufficiency induces alternative aging processes in the aorta. Rejuvination Res. 2008;11:97–112. doi: 10.1089/rej.2007.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY, Mecham RP. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1209–H1217. doi: 10.1152/ajpheart.00046.2005. [DOI] [PubMed] [Google Scholar]
- 19.Hirano E, Knutsen RH, Sugitani H, Cilberto CH, Mecham RP. Functional rescue of elastin insufficiency in mice by the human elastin gene: implications for mouse models of human disease. Circ Res. 2007;101:523–531. doi: 10.1161/CIRCRESAHA.107.153510. [DOI] [PubMed] [Google Scholar]
- 20.Sacks MS, Merryman DW, Schmidt DE. On the biomechanics of heart valve function. J Biomech. 2009;42:1804–1824. doi: 10.1016/j.jbiomech.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson RH. Clinical anatomy of the aortic root. Heart. 2000;84:670–673. doi: 10.1136/heart.84.6.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yacoub MH, Kilner PJ, Birks EJ, Misfeld M. The aortic outflow and root: a tale of dynamism and crosstalk. Ann Thorac Surg. 1999;68:S37–S43. doi: 10.1016/s0003-4975(99)00745-6. [DOI] [PubMed] [Google Scholar]
- 23.Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 24.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171:1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 27.Zhao B, Etter L, Hinton RB, Benson DW. BMP and FGF regulatory pathways in semilunar valve precursor cells. Dev Dyn. 2007;236:971–980. doi: 10.1002/dvdy.21097. [DOI] [PubMed] [Google Scholar]
- 28.Hinton RB, Alfieri CM, Witt SA, Glascock BJ, Khoury PR, Benson DW, Yutzey KE. Mouse heart valve structure and function: echocardiographic and morphometric analyses from the fetus through the aged adult. Am J Physiol Heart Circ Physiol. 2008;294:H2480–H2488. doi: 10.1152/ajpheart.91431.2007. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima Y, Mironov V, Yamagishi T, Nakamura H, Markwald RR. Expression of smooth muscle alpha-actin in mesenchymal cells during formation of avian endocardial cushion tissue: a role for transforming growth factor beta3. Dev Dyn. 1997;209:296–309. doi: 10.1002/(SICI)1097-0177(199707)209:3<296::AID-AJA5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 30.Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- 31.Ranger AM, Grusby MJ, Hodge MR, Gravallese EM, de la Brousse FC, Hoey T, Mickanin C, Baldwin HS, Glimcher LH. The transcription factor NF-ATc is essential for cardiac valve formation. Nature. 1998;392:186–190. doi: 10.1038/32426. [DOI] [PubMed] [Google Scholar]
- 32.Dor Y, Camenisch TD, Itin A, Fishman GI, McDonald JA, Carmeliet P, Keshet E. A novel role for VEGF in endocardial cushion formation and its potential contribution to congenital heart defects. Development. 2001;128:1531–1538. doi: 10.1242/dev.128.9.1531. [DOI] [PubMed] [Google Scholar]
- 33.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mercado-Pimentel ME, Runyan RB. Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs. 2007;185:146–156. doi: 10.1159/000101315. [DOI] [PubMed] [Google Scholar]
- 35.Milewicz DM, Guo DC, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Kwartler CS, Pannu H. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet. 2008;9:283–302. doi: 10.1146/annurev.genom.8.080706.092303. [DOI] [PubMed] [Google Scholar]
- 36.Filip DA, Radu A, Simionescu M. Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circ Res. 1986;59:310–320. doi: 10.1161/01.res.59.3.310. [DOI] [PubMed] [Google Scholar]
- 37.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 38.Hanada K, Vermeij M, Garinis GA, de Waard MC, Kunen MG, Myers L, Maas A, Duncker DJ, Meijers C, Dietz HC, Kanaar R, Essers J. Perturbations of vascular homeostasis and aortic valve abnormalities in fibulin-4 deficient mice. Circ Res. 2007;100:738–746. doi: 10.1161/01.RES.0000260181.19449.95. [DOI] [PubMed] [Google Scholar]
- 39.Toyoda M, Kojima M, Takeuchi T. Jumonji is a nuclear protein that participates in the negative regulation of cell growth. Biochem Biophys Res Commun. 2000;274:332–336. doi: 10.1006/bbrc.2000.3138. [DOI] [PubMed] [Google Scholar]
- 40.Bruneau BG. The developmental genetics of congenital heart disease. Nature. 2008;451:943–948. doi: 10.1038/nature06801. [DOI] [PubMed] [Google Scholar]
- 41.Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119:880–890. doi: 10.1161/CIRCULATIONAHA.108.795401. [DOI] [PubMed] [Google Scholar]
- 42.Szabo Z, Crepeau MW, Mitchell AL, Stephan MJ, Puntel RA, Yin Loke K, Kirk RC, Urban Z. Aortic aneurismal disease and cutis laxa caused by defects in the elastin gene. J Med Genet. 2006;43:255–258. doi: 10.1136/jmg.2005.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of 'degenerative' valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 44.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 45.Sutherland FW, Perry TE, Yu Y, Sherwood MC, Rabkin E, Masuda Y, Garcia GA, McLellan DL, Engelmayr GC, Jr, Sacks MS, Schoen FJ, Mayer JE., Jr From stem cells to viable autologous semilunar heart valve. Circulation. 2005;111:2783–2791. doi: 10.1161/CIRCULATIONAHA.104.498378. [DOI] [PubMed] [Google Scholar]
- 46.Sales VL, Mettler BA, Engelmayr GC, Aikawa E, Bischoff J, Martin DP, Exarhopoulos A, Moses MA, Schoen FJ, Sacks MS, Mayer JE. Endothelial progenitor cells as a sole source for ex vivo seeding of tissue-engineered heart valves. Tissue Eng Part A. 2010;16:257–267. doi: 10.1089/ten.tea.2009.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bethea BT, Fitton TP, Alejo DE, Barreiro CJ, Cattaneo SM, Dietz HC, Spevak PJ, Lima JA, Gott VL, Cameron DE. Results of aortic valve-sparing operations: experience with remodeling and reimplantation procedures in 65 patients. Ann Thorac Surg. 2004;78:767–772. doi: 10.1016/j.athoracsur.2004.03.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.