Abstract
Transforming growth factor-β1 (TGF-β1) promotes tissue fibrosis through the Smad3 signaling pathway. While phosphorylation is known to regulate Smad3 function, recent in vitro studies have suggested that acetylation may also regulate Smad3 function. This study investigated Smad3 acetylation in renal fibrosis. TGF-β1 stimulation of renal fibroblasts and tubular epithelial cells induced Smad3 acetylation and phosphorylation. Resveratrol, an activator of the Nicotinamide adenine dinucleotide (NAD) dependent protein deacetylase SIRT1, reversed acetylation but not phosphorylation of Smad3 and inhibited TGF-β1–induced up-regulation of collagen IV and fibronectin mRNA levels. Knockdown of SIRT1 expression abolished the inhibitory effect of resveratrol, and co-immunoprecipitation studies provide direct evidence of an interaction between acetylated Smad3 and SIRT1. The role of Smad3 acetylation in renal fibrosis was then examined in the unilateral ureteric obstruction (UUO) model. Immunoprecipitation studies showed acetylation and phosphorylation of Smad3 by day 2 UUO, which was sustained to day 7 in association with development of interstitial fibrosis. Resveratrol inhibited acetylation but not phosphorylation of Smad3 at day 2 UUO, and resveratrol treatment inhibited interstitial fibrosis at day 7 UUO. In conclusion, these studies support a pathological role for Smad3 acetylation in renal fibrosis and suggest that deacetylation of Smad3 may be a novel therapeutic target for fibrotic disease.
The transforming growth factor-β1 (TGF-β1)/Smad3 signaling pathway plays a central role in the pathogenesis of tissue fibrosis.1,2 Smad3 null mice have a profound resistance to interstitial fibrosis induced by unilateral ureter obstruction (UUO)3 and to renal fibrosis in streptozotocin-induced diabetic glomerulopathy.4 Smad3 is directly phosphorylated by the TGF-β1 receptor I serine kinase, and this posttranslational modification is critical for TGF-β1/Smad3 signaling leading to a profibrotic response.1,5,6
Recent cell culture studies have identified that Smad2 and Smad3 can be acetylated in response to TGF-β1 stimulation.7,8 Acetylation involves the transfer of the acetyl moiety from acetyl coenzyme-A to the ε-amino groups of lysine residues.9 This posttranslational modification can regulate diverse biological processes, with the balance between the acetylation and deacetylation of histone proteins making a significant impact on chromatin structure and transcription.10 Smad3 can be acetylated at the Lys-378 in the MH2 domain by p300/CBP, and this regulates Smad3 DNA binding activity and Smad3 transcriptional activity.8 Furthermore, the p300 acetyltransferase, which acts as a transcriptional coactivator via acetylation of transcription factors, is required for maximal TGF-β1–induced collagen synthesis in cultured fibroblasts,11 suggesting that acetylation of Smad3 may play an important role in TGF-β1-driven tissue fibrosis.12
A number of deacetylase enzymes that remove acetyl groups from protein lysine residues have been described, although the particular enzyme that deacetylates Smad3 has not been identified. One of the best characterized deacetylase enzymes is SIRT1, a Nicotinamide adenine dinucleotide (NAD)+-dependent class III protein deacetylase, which can protect cultured cells against metabolic, geneotoxic, hypoxic, and heat stress by deacetylating a number of key transcription factors.13 Resveratrol (3,4′,5-trihydroxy-trans-stilbene) is a natural plant polyphenol. There is increasing evidence that Resveratrol has protective effects in rodent models of stress and disease.14,15,16 Resveratrol can stimulate SIRT1 activity, which can then mediate antiinflammatory effects by deacetylation of transcriptional regulators such as nuclear transcription factor-kappa B (NF-κB).17 However, the ability of Resveratrol to inhibit Smad3 acetylation has not been established, nor whether Resveratrol can inhibit Smad3 acetylation through augmentation of SIRT1 activity, nor whether Resveratrol can suppress renal fibrosis.
In the present study, we tested the hypothesis that acetylation of Smad3 plays a role in TGF-β1–driven renal fibrosis. This was addressed by examining Smad3 acetylation and phosphorylation in interstitial fibrosis induced by UUO in the mouse. The effects of Resveratrol on Smad3 acetylation and interstitial fibrosis were examined in vivo, while the ability of SIRT1 to interact with and deacetylate Smad3 was examined in vitro.
Materials and Methods
Experimental Animals
Male C57BL/6J mice (20 to 25 g) were obtained from Monash Animal Services, Monash University, Australia. All animal experiments were performed with approval from the local Animal Ethics Committee and adhered to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. UUO surgery was performed under isofluorane anesthesia. The left ureter was visualized following a flank incision and ligated with a vascular clamp. Sham mice underwent the same procedure, except that the ureter was not ligated. Mice were sacrificed 6 hours, 2 days, or 7 days after surgery. The initial time course study used three mice per group. The main study used groups of six mice that underwent UUO surgery and were then given Resveratrol (Sigma-Aldrich, Castle Hill, Australia) at 5 mg/kg/day in DMSO or vehicle alone (same volume of DMSO) by once daily i.p. injection from the time of surgery until being sacrificed on day 7. Finally, groups of three mice underwent UUO and on day 2 were given two i.p. injections of 5 mg/kg Resveratrol or DMSO with a 5-hour interval and then sacrificed 1 hour after the second injection to examine Smad3 acetylation and phosphorylation.
Cell Culture
NRK49F rat fibroblasts and rat NRK52E proximal tubular epithelial cells were grown in a 5% CO2 atmosphere at 37°C in Dulbecco’s modified Eagle’s medium (Invitrogen, Mount Waverly, Vic, Australia) containing 10% fetal bovine serum. Cells were seeded into 100-mm culture dishes or 6-well culture plates (Nunc, Naperville, CT), allowed to adhere overnight, and then changed into medium containing 0.1% fetal bovine serum. Cells were stimulated with recombinant human TGF-β1 (R&D Systems, Minneapolis, MN) for different periods before harvesting. To examine Smad3 phosphorylation and acetylation, cells were pretreated with vehicle (0.025% DMSO) and Resveratrol (5 to 10 μmol/L) for 30 minutes before TGF-β1 stimulation. Each experiment was repeated at least three times.
Immunoprecipitation and Western Blotting
Kidney and cell culture samples were sonicated and resuspended in 0.4 ml of RIPA lysis buffer. Protein concentration estimations were performed with a detergent-compatible protein assay kit (Bio-Rad, Hercules, CA). Samples containing 500 μg total protein were immunoprecipitated with the rabbit anti-Smad3 antibody (Cell Signaling Technology, Danvers, MA) followed by Western blotting with mouse anti-phosphoserine (Calbiochem, Kilsyth, Victoria, Australia) or mouse anti-Smad3 (Santa Cruz Biotechnology, Santa Cruz, CA). Rabbit anti-phospho-Smad2 (Cell Signaling Technology) and mouse anti-Smad2 (Abcam, Cambridge, UK) were used in Western blotting. Detection of acetylated Smad3 or acetylated Smad2 was performed by immunoprecipitation with mouse anti-Smad3 antibody (Santa Cruz Biotechnology) or mouse anti-Smad2 (Abcam) antibody followed by Western blotting with rabbit anti-acetylated lysine antibody, rabbit anti-Smad3 (both Cell signaling Technology), or rabbit anti-Smad2 (Abcam). Rabbit anti-Smad7 (Invitrogen, Carlsbad, CA), rabbit anti-SIRT1 (Abcam), rabbit anti-phospho-p38 MAPK, and mouse anti-p38 MAPK (both Cell Signaling Technology) were used in Western blotting. Blots were then incubated with peroxidase-conjugated goat anti-rabbit, goat anti-mouse IgG, or goat anti-mouse IgM (Sigma-Aldrich) and bound antibody was detected by ECL Plus (Amersham, Little Chalfont, UK) and captured by Fujifilm model LAS-3000 (Fujifilm Corporation, Australia). Densitometry analysis was performed with Gel Pro analyzer (Media Cybernetics, Silver Spring, MD).
RNA Extraction and Real-Time RT-PCR
Total RNA from cultured cells and kidney samples were isolated and one-step real-time RT-PCR and real-time PCR performed using SYBR Green PCR Reagents (Sigma), the Thermoscript RT-PCR system (Invitrogen), and the Opticon DNA Engine (MJ Research Inc., South San Francisco, CA), according to manufacturer’s instructions. In each reaction, 0.5 μg of total RNA was reverse transcribed before the following PCR conditions: 94°C for 2 minutes followed by 40 cycles at 94°C for 15 seconds, 58°C for 30 seconds, 72°C for 30 seconds, with final extension at 72°C for 10 minutes. Primers used in this study were: α-SMA, fwd 5′-ACTGGGACGACATGGAAAAG-3′, rev 5′-CATCTCCAGAGTCCAGCACA-3′; collagen IV, fwd 5′-TGACCCTGGTGATGTTCTCA-3′, rev 5′-GCCACACCTTGTATGCCTTT; fibronectin, fwd 5′-ATGATGAGGTGCACGTGTGT-3′, rev 5′-TCTCCCAGGAGTCACCAATC-3′; GAPDH, fwd 5′-ACCCCCAATGTATCCGTTGT-3′, rev 5′-TACTCCTTGGAGGCCATGTA-3′. Amplicon sizes were 240 bp (α-SMA), 285 bp (collagen IV), 256 bp (fibronectin), and 299 bp (GAPDH). The relative amount of mRNA was calculated using the comparative Ct (ΔCt) method compared with GAPDH and expressed as the mean ± SD.
Confocal Microscopy Analysis
Cryostat sections of tissues fixed in 4% paraformaldehyde (Sigma-Aldrich) were blocked with 2% bovine serum albumin in PBS and incubated with the following antibodies: Cy3-conjugated mouse anti–α-SMA antibody; goat anti-collagen type IV followed by chicken anti-goat Alexa Fluor 647 (Molecular Probes, Eugene, OR); rabbit anti-fibronectin followed by goat anti-rabbit Alexa Fluor 555; or rat anti-mouse F4/80 antibody (Serotec, Oxford, UK) followed by goat anti-rat Alexa Fluor 594. Sections were counterstained with 4,6-diamidino-2-phenylindole to visualize nuclei. The degree of tubulointerstitial fibrosis was measured in 40 randomly selected high power fields (×600) in each animal using Image J software (rsbweb. nih.gov/ij) by analyzing the percentage of the total cortical area accounted for by immunostaining for α-SMA, collagen IV, or fibronectin. The number of F4/80+ macrophages was counted directly. All scoring was performed blind on coded slides.
Measurement of TGF-β1 in Renal Tissues
Total and active TGF-β1 levels in kidney lysates containing 100 μg total protein in RIPA lysis buffer were quantified by ELISA (R&D System Inc.) according to the manufacturer’s instructions.
Co-Immunoprecipitation of Smad3 and SIRT1
NRK49F cells were stimulated with 4 ng/ml TGF-β1 for 1, 3, and 6 hours. Smad3 was immunoprecipitated overnight from cell lysates using mouse anti-Smad3 antibody and Protein A/G agarose beads (Santa Cruz Biotechnology). Precipitates were then analyzed by Western blotting with rabbit anti-p300 (Santa Cruz Biotechnology) and rabbit anti-SIRT1 (LifeSpan BioSciences, Seattle, WA). To confirm interaction, NRK49F cells were cotransfected with pCMV-SPORT-Smad3 and pECE-Flag-SIRT1 plasmids for 18 hours, then cultured in serum-free DMEM for 6 hours and then stimulated with or without TGF-β1 for 1, 3, and 6 hours. Rabbit anti-Smad3 (Cell Signaling Technology) and mouse anti-Flag (Sigma-Aldrich) were used for immunoprecipitation/Western blotting.
Luciferase Reporter Assay, Expression Plasmids, and siRNA Transfection
The SBE4-Luc plasmid (29), pECE-Flag-SIRT1(30); pECE-Flag-SIRT1-H363Y (SIRT1 dominant negative mutant) (30) were purchased from Addgene Inc (Cambridge, MA); pCMV-SPORT6-Smad3 was purchased from Source BioScience (Nottingham, UK). PRL-TK and Dual-Luciferase assay kit were purchased from Promega (Madison, WI). NRK49F cells were transfected with different plasmids using lipofectamine 2000 (Invitrogen) with maximum of 1.0 μg total DNA per well. For Resveratrol (10 μmol/L) or SIRT1 activator 3 (10 μmol/L, Cayman Chemical Co., Ann Arbor, Michigan) pharmacological treatments, cells were transfected with SBE4-Luc and PRL-TK plasmids and media was replaced 18 hours later with drug containing media. Cells were harvested for luciferase assay 18 hours after Resveratrol or SIRT1 activator 3 treatments were initiated. To investigate the effects of Smad3 and SIRT1 interaction on SBE4-Luc activities, NRK49F cells were transfected with SBE4-Luc, PRL-TK, pCMV-SPORT6-Smad3 and pECE-Flag-SIRT1 plasmids and media was replaced 18 hours after transfection with TGF-β1-containing media. At 36 hours posttransfection, cells were harvested for luciferase assays. Control siRNA, SIRT1 siRNA, Smad2 siRNA and Smad3 siRNA were purchase from Invitrogen. For cell culture studies, NRK49F cells were transfected in triplicate with SIRT1 siRNA or control siRNA by Lipofectamine 2000. Forty-eight hours after transfection, the transfected cells were subjected to TGF-β1 stimulation for 3 and 24 hours. Cells were harvested for immunoblotting or real-time PCR analysis.
Statistical Analyses
Data are shown as mean ± SD with statistical analyses performed using one-way analysis of variance with post hoc analysis using Tukey’s multiple comparison test with GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA).
Results
Reversal of Smad3 Acetylation via SIRT1 Inhibits the TGF-β1-Induced Profibrotic Response in Vitro
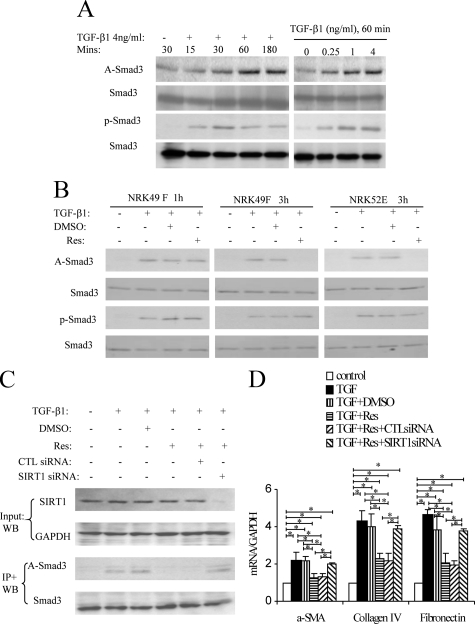
Immunoprecipitation/Western blot studies showed that TGF-β1 stimulation of NRK49F fibroblasts rapidly induced Smad3 acetylation and phosphorylation, peaking at 60 minutes, and this response was dose-dependent (Figure 1A). To examine the role of Smad3 acetylation in the TGF-β1 response, we used Resveratrol, which is known to activate the deacetylase activity of SIRT1.17 Resveratrol did not prevent TGF-β1–induced Smad3 acetylation at 1 hour in NRK49F fibroblasts, but Smad3 acetylation was reversed at 3 hours (Figure 1B; see Supplemental Figure 1 at http://ajp.amjpathol.org), demonstrating that Resveratrol can induce deacetylation of Smad3. Resveratrol also reduced Smad3 acetylation 3 hours after TGF-β1 stimulation in NRK52E tubular epithelial cells (Figure 1B). However, Resveratrol did not prevent Smad3 phosphorylation at 1 hour or 3 hours in either cell type (Figure 1B), enabling the study of Smad3 acetylation independent of Smad3 phosphorylation. We also showed that Resveratrol is not toxic to either NRK52E or NRK49F cells on the basis apoptosis rates after a 24 hours culture period (see Supplemental Figure 2 at http://ajp.amjpathol.org).
Figure 1.
TGF-β1-induced Smad3 acetylation (A-Smad3) and phosphorylation (p-Smad3) in rat renal NRK49F fibroblasts. A: Immunoprecipitation (IP)/Western blotting (WB) shows a time course of TGF-β1 (4 ng/ml)-induced Smad3 acetylation and phosphorylation, and a TGF-β1 dose-response at the 60-minute time point. B: IP/WB analysis of the effect of Resveratrol (Res) on TGF-β1-induced Smad3 acetylation and phosphorylation. The addition of 10 μmol/L Res to NRK49F cells suppressed Smad3 acetylation at three hours but not one hour, while the addition of 5 μmol/L Res suppressed Smad3 acetylation at 3 hours in NRK52E cells. C: WB in top panel shows that SIRT1 siRNA but not control siRNA knocks down SIRT1 protein levels in NRK49F cells. IP/WB analysis in bottom panel shows that TGF-β1-induced Smad3 acetylation at three hours is suppressed by Res, but this suppression is lost in the presence of SIRT1 siRNA. D: Real-time RT-PCR analysis of mRNA levels in NRK49F cells 24 hours after TGF-β1 stimulation. The addition of 10 μmol/L Res suppressed TGF-β1-induced up-regulation of α-SMA, collagen IV, and fibronectin, and this effect of Res was prevented by SIRT1 siRNA but not control siRNA. Data are mean ± SD. *P < 0.05.
Next we examined whether the ability of Resveratrol to reverse Smad3 acetylation is dependent on SIRT1. siRNA efficiently knocked-down SIRT1 protein levels, and this abolished the ability of Resveratrol to deacetylate Smad3 in TGF-β1–stimulated NRK49F fibroblasts (Figure 1C). SIRT1 knockdown also abolished Resveratrol inhibition of TGF-β1–induced up-regulation of α-SMA, collagen IV, and fibronectin mRNA levels (Figure 1D), demonstrating a functional role for Smad3 acetylation in the fibrotic response.
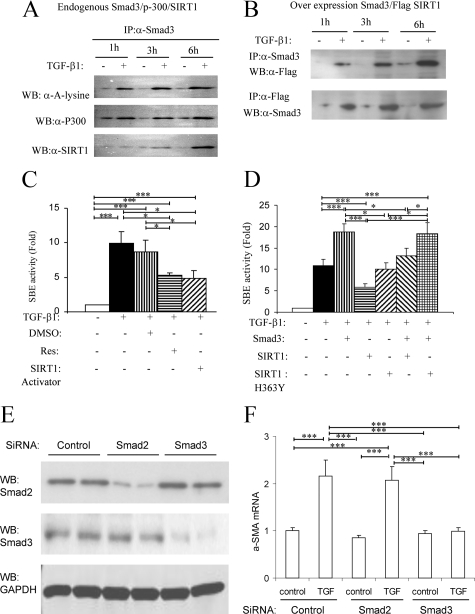
Immunoprecipitation of endogenous Smad3 from TGF-β1–stimulated NRK49F cells identified a direct interaction between Smad3 and SIRT1 (120 kDa) and between Smad3 and p300 (300 kDa) (Figure 2A). The bands detected by the p300 and SIRT1 antibodies are consistent with the known molecular weights of these proteins. Interestingly, the Smad3-SIRT1 interaction was most prominent 3 hours after TGF-β1 stimulation, consistent with the lack of effect of Resveratrol on Smad3 acetylation at 1 hour and the reversal of acetylation at 3 hours (Figure 1B). Overexpression of Smad3 and Flag-tagged SIRT1 in NRK49F cells also demonstrated co-immunoprecipitation of Smad3 and SIRT1 at 3 hours and 6 hours after TGF-β1 stimulation (Figure 2B).
Figure 2.
Evidence of direct interaction of Smad3 and SIRT1 in TGF-β1–stimulated NRK49F cells. A: Smad3 immunoprecipitation (IP) from TGF-β1 (4 ng/ml for 1, 3 or 6 hours) stimulated cells was examined by Western blotting (WB) showing Smad3 acetylation (α-A-lysine), co-immunoprecipitation of Smad3 and p300, and of Smad3 with SIRT1. B: NRK49F cells were cotransfected with plasmids expressing Smad3 and Flag-SIRT1. IP/WB showed co-immunoprecipitation of Smad3 and SIRT1 at three hours and six hours after TGF-β1 stimulation. C: NRK49F cells were transfected with a Smad-binding element (SBE)-luciferase reporter construct and a control reporter (PRL-TK) to measure Smad transcriptional activity. After 18 hours, cells were stimulated with TGF-β1 for a further 18 hours in the presence of 10 μmol/L Resveratrol (Res), DMSO vehicle, or SIRT1 activator three and then cells harvested and dual-luciferase activity assayed. Data are mean ± SD. *P < 0.05; ***P < 0.001. D: NRK49F cells were transfected with the SBE and control reporters and with plasmids expressing Smad3, SIRT1, or SIRT1 H363Y (dominant negative form). After 18 hours, cells were stimulated with TGF-β1 for a further 18 hours and then cells harvested and dual-luciferase activity assayed. Data are mean ± SD. *P < 0.05; ***P < 0.001. E and F: NRK49F cells were transfected with control siRNA, Smad2 siRNA, and Smad3 siRNA. After 48 hours, cells were harvested for western blotting (E) or stimulated with TGF-β1 for a further 24 hours and then cells harvested and real-time PCR was performed to detect α-SMA. Data are mean ± SD. ***P < 0.001.
TGF-β1/Smad3 transcriptional activity was examined in NRK49F cells using a luciferase reporter containing four copies of the Smad-binding element (SBE). TGF-β1 induced a 10-fold increase in Smad transcriptional activity, and this was reduced by approximately 50% by two different SIRT1 activators, Resveratrol and SIRT1 activator 3 (Figure 2C). In further studies, transfection of NRK49F cells with a Smad3-expressing plasmid augmented TGF-β1–induced transcriptional activity, whereas overexpression of SIRT1 had an inhibitory effect on TGF-β1–induced transcriptional activity (Figure 2D). Co-expression of both Smad3 and SIRT1 counteracted each other, having no net effect (Figure 2D). Overexpression of a dominant negative form of SIRT1 (SIRT1 H363Y) failed to modify Smad transcriptional activity (Figure 2D). Finally, siRNA was used to knock down Smad3 and Smad2 expression in NRK49F cells. Cells in which Smad3 was knocked down were unresponsive to TGF-β1–induced up-regulation of α-SMA mRNA. In contrast, knockdown of Smad2 did not affect this response, suggesting that the profibrotic actions of TGF-β1 in renal fibroblasts operate primarily via Smad3 (Figure 2, E and F).
Resveratrol Inhibits Smad3 Acetylation and Interstitial Fibrosis in the Obstructed Kidney
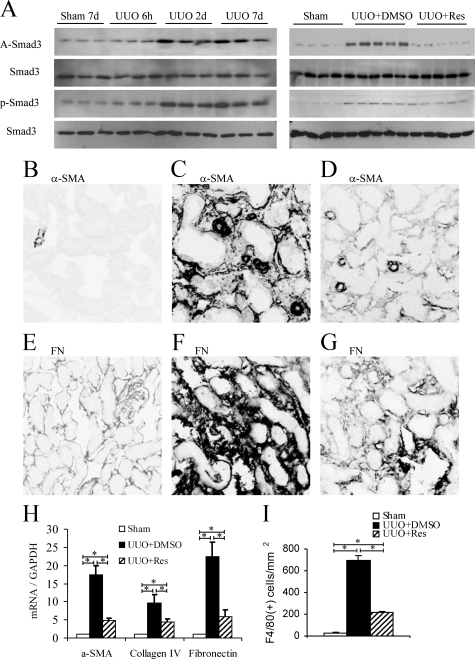
Smad3 acetylation was examined in whole kidney samples by immunoprecipitation/Western blotting. Little Smad3 acetylation was detectable in normal mouse kidney or at 6 hours after UUO; however, significant Smad3 acetylation was evident on day 2 UUO, which was maintained to day 7 UUO (Figure 3A). In a similar pattern, significant Smad3 phosphorylation was seen on days 2 and 7 UUO (Figure 3A). Mild fibrotic changes were seen on day 2 UUO in terms of interstitial accumulation of α-smooth muscle actin (SMA)+ myofibroblasts and increased matrix deposition (not shown), which became pronounced on day 7 (Figure 3 and see Supplemental Figure 3 at http://ajp.amjpathol.org).
Figure 3.
Smad3 acetylation following unilateral ureter obstruction (UUO). A: Left panel shows immunoprecipitation/Western blotting of Smad3 acetylation (A-Smad3) and phosphorylation (p-Smad3) in a time course of UUO or sham-operated mice. Right panel shows that Resveratrol (Res) treatment prevented Smad3 acetylation, but not Smad3 phosphorylation, on day 2 UUO. Immunofluorescence staining at day 7 UUO showing α-smooth muscle actin (α-SMA) in sham-operated (B), UUO+Vehicle (C), and UUO+Res (D); and immunofluorescence staining for fibronectin in sham operated (E), UUO+Vehicle (F), and UUO+Res (G). H: Real-time RT-PCR analysis of kidney α-SMA, collagen IV, and fibronectin mRNA levels at day seven UUO. I: Quantification of immunofluorescence staining for F4/80+ macrophages at day seven UUO. Data are mean ± SD. *P < 0.05.
Resveratrol treatment markedly attenuated Smad3 acetylation at day 2 UUO, but had no effect on Smad3 phosphorylation (Figure 3A). In addition, Resveratrol treatment did not affect Smad2 phosphorylation or acetylation, up-regulation of Smad7 expression or phosphorylation of p-38 MAPK at day 2 UUO (see Supplemental Figure 3A and 3B at http://ajp.amjpathol.org), demonstrating a degree of selectivity of Resveratrol action in vivo. Resveratrol substantially inhibited the fibrotic response at day 7 UUO as shown by a reduction in α-SMA+ myofibroblast accumulation, deposition of fibronectin and collagen IV (Figure 3, B–G, and see Supplemental Figure 3C at http://ajp.amjpathol.org), and reduced kidney mRNA levels for α-SMA, collagen IV, and fibronectin (Figure 3H). In addition, Resveratrol treatment reduced the interstitial macrophage infiltrate that accompanies fibrosis in this model (Figure 3I).
Discussion
This study provides the first demonstration that Smad3 undergoes acetylation in the development of tissue fibrosis and provides evidence that the deacetylase, SIRT1, may be an important regulator of this process.
In vitro studies found that TGF-β1 stimulation of renal fibroblasts or tubular epithelial cells induced Smad3 acetylation. This Smad3 acetylation was reversed by Resveratrol, resulting in suppression of TGF-β1 induced Smad transcriptional activity including transcription of specific profibrotic genes. Resveratrol is known to activate the deacetylase SIRT1, but this compound can also inhibit a number of other signaling pathways.18,19,20,21,22 Therefore, we used several approaches to examine whether the ability of Resveratrol to reverse Smad3 acetylation operated via SIRT1. First, knockdown of SIRT1 protein levels negated the effect of Resveratrol, arguing that the compound was not operating via other pathways. Second, overexpression of SIRT1 provided a protective effect against TGF-β1–induced Smad transcriptional activity. Third, overexpression of a dominant negative form of SIRT1 (H363Y) had no impact on TGF-β1–induced Smad transcriptional activity. Fourth, a second and independent SIRT1 activator also inhibited TGF-β1–induced Smad transcriptional activity. Fifth, immunoprecipitation/Western blotting studies identified a physical interaction between Smad3 and SIRT1, arguing that SIRT1 directly deacetylates Smad3. This was evident in studies of both endogenous and overexpressed Smad3 and SIRT1. Importantly, this interaction was prominent at 3 hours after TGF-β1 stimulation, the time at which Smad3 deacetylation occurred.
This is the first description of a Smad3-SIRT1 interaction or of SIRT1 deacetylating Smad3. This may be a common mechanism for the regulation of members of the Smad family because one previous study has reported that SIRT1 can deacetylate Smad7, which prevents TGF-β1–induced apoptosis in cultured glomerular mesangial cells.23 The finding that Smad3 acetylation plays an important role in TGF-β1–induced transcriptional activity in renal fibroblasts and tubular epithelial cells is consistent with the original study identifying Smad3 acetylation in 293 and HepG2 cell lines.8 We also identified an interaction between Smad3 and p300 in NRK49F fibroblasts. This was evident at 1 hour after TGF-β1 stimulation when Smad3 acetylation reached peak levels and is consistent with a known role of p300 in promoting Smad3 acetylation in 293 and HepG2 cells,8 and that p300 governs the intensity of the profibrotic response to TGF-β1 in dermal fibroblasts.11
In vivo studies showed that Smad3 acetylation is an early response in the induction of interstitial fibrosis in the obstructed kidney, and this was sustained during the development of severe fibrosis. This acetylation occurred in conjunction with phosphorylation of Smad3, suggesting that these posttranslational modifications may cooperate in the development of tissue fibrosis. Resveratrol treatment suppressed Smad3 acetylation, but not phosphorylation, in the early phase of interstitial fibrosis in the obstructed kidney enabling dissection of the role of Smad3 acetylation and phosphorylation. While Resveratrol can potentially affect several pathways when delivered systemically in the UUO model, the action of Resveratrol was at least partially selective for Smad3 acetylation because the treatment did not affect Smad3 phosphorylation or the acetylation or phosphorylation of Smad2. Furthermore, Resveratrol did not affect other mechanisms known to modify fibrosis in this model (p38 MAPK activation and up-regulation of Smad7 expression). However, the in vivo studies are limited in that we cannot prove beyond doubt that the effect of Resveratrol in the UUO model is entirely due to inhibition of Smad3 acetylation. Definitive evidence would require specific mutation of the Smad3 gene to selectively prevent Smad3 acetylation but not phosphorylation. Despite these limitations, these in vivo findings are supported by the specific effect of Resveratrol on SIRT1 activity as established in the cell culture studies. Resveratrol was effective in suppressing key markers of interstitial fibrosis including myofibroblast accumulation, extracellular matrix deposition, and macrophage accumulation.
In summary, this study has identified Smad3 acetylation in the development of tissue fibrosis. The ability of Resveratrol to inhibit renal fibrosis in the obstructed kidney was attributed to activation of SIRT1, which deacetylated Smad3 and suppressed the TGF-β1–induced fibrotic response. Thus, prevention or reversal of Smad3 acetylation represents a new therapeutic strategy for suppression of tissue fibrosis.
Acknowledgments
Confocal imaging was performed at the Monash MicroImaging Facility at Monash University.
Footnotes
Address reprint requests to Jinhua Li, MBBS, Ph.D., Department of Anatomy and Developmental Biology, Monash University, Clayton, Victoria 3800, Australia. E-mail: jinhua.li@med.monash.edu.au.
Supported by Kidney Health Australia, Monash University, Faculty of Medicine, Nursing and Health Sciences Strategic Grant Scheme and the National Health and Medical Research Council (NHMRC) of Australia. J.L. is the recipient of a NHMRC Peter Doherty Postdoctoral Fellowship and a NHMRC Career Development Award.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Koka V, Lan HY. Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology. 2005;10:48–56. doi: 10.1111/j.1440-1797.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Maezawa Y, Yokote K, Joh K, Kobayashi K, Kawamura H, Nishimura M, Roberts AB, Saito Y, Mori S. Mice lacking Smad3 are protected against streptozotocin-induced diabetic glomerulopathy. Biochem Biophys Res Commun. 2003;305:1002–1007. doi: 10.1016/s0006-291x(03)00885-4. [DOI] [PubMed] [Google Scholar]
- Li JH, Huang XR, Zhu HJ, Oldfield M, Cooper M, Truong LD, Johnson RJ, Lan HY. Advanced glycation end products activate Smad signaling via TGF-beta-dependent and independent mechanisms: implications for diabetic renal and vascular disease. FASEB J. 2004;18:176–178. doi: 10.1096/fj.02-1117fje. [DOI] [PubMed] [Google Scholar]
- Li J, Campanale NV, Liang RJ, Deane JA, Bertram JF, Ricardo SD. Inhibition of p38 mitogen-activated protein kinase and transforming growth factor-beta1/Smad signaling pathways modulates the development of fibrosis in adriamycin-induced nephropathy. Am J Pathol. 2006;169:1527–1540. doi: 10.2353/ajpath.2006.060169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsson M, Kanduri M, Grönroos E, Heldin CH, Ericsson J. The DNA binding activities of Smad2 and Smad3 are regulated by coactivator-mediated acetylation. J Biol Chem. 2006;281:39870–33980. doi: 10.1074/jbc.M607868200. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Itoh Y, Abe K, Okamoto T, Daitoku H, Fukamizu A, Onozaki K, Hayashi H. Smad3 is acetylated by p300/CBP to regulate its transactivation activity. Oncogene. 2007;26:500–508. doi: 10.1038/sj.onc.1209826. [DOI] [PubMed] [Google Scholar]
- Yang X, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Ghosh AK, Pannu J, Mori Y, Takagawa S, Chen G, Trojanowska M, Gilliam AC, Varga J. Fibroblast expression of the coactivator p300 governs the intensity of profibrotic response to transforming growth factor beta. Arthritis Rheum. 2005;52:1248–1258. doi: 10.1002/art.20996. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Varga J. The transcriptional coactivator and acetyltransferase p300 in fibroblast biology and fibrosis. J Cell Physiol. 2007;213:663–671. doi: 10.1002/jcp.21162. [DOI] [PubMed] [Google Scholar]
- Guarente L. Cell biology. Hypoxic hookup. Science. 2009;324:1281–1282. doi: 10.1126/science.1175679. [DOI] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Cadenas S, Barja G. Resveratrol, melatonin, vitamin E, and PBN protect against renal oxidative DNA damage induced by the kidney carcinogen KBrO3. Free Radic Biol Med. 1999;26:1531–1537. doi: 10.1016/s0891-5849(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Giovannini L, Migliori M, Longoni BM, Das DK, Bertelli AA, Panichi V, Filippi C, Bertelli A. Resveratrol, a polyphenol found in wine, reduces ischemia reperfusion injury in rat kidneys. J Cardiovasc Pharmacol. 2001;37:262–270. doi: 10.1097/00005344-200103000-00004. [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JR, O'Brian CA. Resveratrol antagonizes EGFR-dependent Erk1/2 activation in human androgen-independent prostate cancer cells with associated isozyme-selective PKC alpha inhibition. Invest New Drugs. 2004;22:107–117. doi: 10.1023/B:DRUG.0000011787.75522.ec. [DOI] [PubMed] [Google Scholar]
- Huang Z, Wang C, Wei L, Wang J, Fan Y, Wang L, Wang Y, Chen T. Resveratrol inhibits EMMPRIN expression via P38 and ERK1/2 pathways in PMA-induced THP-1 cells. Biochem Biophys Res Commun. 2008;374:517–521. doi: 10.1016/j.bbrc.2008.07.058. [DOI] [PubMed] [Google Scholar]
- Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis. 2006;27:1465–1474. doi: 10.1093/carcin/bgi349. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, Nair AS, Shishodia S, Aggarwal BB. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- Venkatesan B, Ghosh-Choudhury N, Das F, Mahimainathan L, Kamat A, Kasinath BS, Abboud HE, Choudhury GG. Resveratrol inhibits PDGF receptor mitogenic signaling in mesangial cells. role of PTP1B. FASEB J. 2008;22:3469–3482. doi: 10.1096/fj.08-109488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume S, Haneda M, Kanasaki K, Sugimoto T, Araki S, Isshiki K, Isono M, Uzu T, Guarente L, Kashiwagi A, Koya D. SIRT1 inhibits transforming growth factor beta-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. J Biol Chem. 2007;282:151–158. doi: 10.1074/jbc.M605904200. [DOI] [PubMed] [Google Scholar]