Abstract
Recruitment of leukocytes to glomeruli is fundamental to the pathogenesis of many forms of glomerulonephritis. In a model of glomerulonephritis induced by in situ immune complex deposition, we previously observed that, in addition to leukocytes, platelets accumulate in glomerular capillaries, where they contribute to leukocyte recruitment. However, the mechanisms of platelet recruitment and the role of platelet-expressed P-selectin in leukocyte recruitment require further investigation. We used intravital microscopy to examine the mechanisms of platelet and leukocyte recruitment to glomeruli of mice following administration of an antibody against the glomerular basement membrane (anti-GBM antibody). Platelet recruitment was initiated within five minutes of administration of anti-GBM antibody. This was unaltered by inhibition of platelet GPIbα but was prevented by the absence of platelet GPVI. Fibrinogen was deposited in glomerular capillaries via a partially intercellular adhesion molecule 1 (ICAM-1)–dependent mechanism, and inhibition of αIIbβ3, fibrinogen and ICAM-1 inhibited platelet recruitment. Notably, neutrophil depletion also reduced platelet accumulation, indicating a cooperative interaction underlying recruitment of platelets and neutrophils. Finally, using bone marrow chimeras to restrict expression of P-selectin to platelets or endothelial cells, platelet but not endothelial P-selectin was required for glomerular leukocyte recruitment. Together these data indicate that platelet recruitment in this model is dependent on the combined actions of GPVI and the αIIbβ3/fibrinogen/ICAM-1 pathway and that platelet P-selectin is crucial for subsequent leukocyte recruitment.
In most tissues, leukocyte-endothelial cell interactions associated with inflammatory responses are restricted to postcapillary venules, where leukocytes undergo a well-characterized sequence of tethering, rolling, and arrest interactions before entering the tissue via transmigration.1 In general these interactions do not occur in capillaries, due to the minimal endothelial expression of specific adhesion molecules required, in particular, for the rolling step.2,3 However, in some specialized areas of the vasculature, particularly the glomerulus, capillaries can support leukocyte-endothelial cell interactions.4,5,6 Indeed, leukocyte recruitment to the glomerulus is a key contributor to the pathogenesis of many forms of glomerulonephritis (GN).4,7,8,9 To investigate the basis of this unusual form of leukocyte recruitment, we recently used intravital microscopy to visualize glomeruli undergoing an inflammatory response.4 We found that recruitment of leukocytes to glomerular capillaries occurs via a process of immediate arrest, bypassing the conventional requirement for an initial rolling interaction. Intriguingly, this form of recruitment retained a role for the P-selectin/PSGL-1 pathway normally associated with leukocyte rolling. Moreover, platelets accumulated in glomeruli during this response, where they played key roles in leukocyte recruitment and resultant glomerular injury. Together these findings suggested the existence of a unique mechanism for leukocyte recruitment in this vascular bed.
Clinical observations have reported platelet involvement in the pathogenesis of various forms of GN.10,11,12 In an immune complex model of GN, platelet depletion has been shown to reduce leukocyte infiltration and glomerular injury.13,14 However, little is known regarding the mechanisms of platelet recruitment to glomeruli. In addition to P-selectin, which is expressed upon platelet activation, platelets express a wide range of adhesive glycoprotein (GP) receptors with the potential to mediate platelet adhesion in the microcirculation. Platelet GPIbα can bind to von Willebrand factor (vWF) present on endothelial cells or the vascular wall and has also been shown to bind to P-selectin and Mac-1.15,16 Platelet GPVI is a receptor for collagen,17 and the αIIbβ3 integrin (GPIIb/IIIa) interacts with numerous ligands including fibrinogen, fibronectin, and thrombospondin.18,19,20,21,22 Examples of the contributions of these molecular pathways to platelet accumulation in the inflamed microvasculature include intestinal ischemia and reperfusion, in which platelet accumulation has been shown to be mediated via interaction with fibrinogen on the endothelial surface.20 Platelets have also been shown to interact with the inflamed endothelium via both endothelial and platelet P-selectin.23,24,25,26 However, in the glomerulus, capillary endothelial cells do not express P-selectin.2,27,28 Moreover, we observed that P-selectin blockade did not abolish platelet recruitment in the inflamed glomerulus, indicating that P-selectin was not involved in this process.4 This raises the possibility that one or more of the candidate platelet adhesion receptors described above mediates this response.
The molecular basis of the contribution of platelets to glomerular leukocyte recruitment also requires further investigation. Platelets have been shown to support leukocyte recruitment in numerous tissues under a range of inflammatory conditions.25,29,30,31 In many cases, platelet-expressed P-selectin has been found to be critical to this response,32,33 raising the possibility of a similar role in glomerular leukocyte recruitment. We investigated this issue previously by transferring isolated platelets into P-selectin–deficient mice undergoing glomerular inflammation.4 Leukocytes were recruited efficiently into glomeruli of mice that received wild-type platelets but not those that received P-selectin-deficient platelets, providing evidence that platelet-derived P-selectin was contributing to this response. However, under these experimental conditions, the majority of the circulating platelets remained of the recipient genotype. A more definitive approach would be to investigate bone marrow chimeras between wild-type and P-selectin–deficient mice, in which P-selectin expression can be restricted to either platelets or endothelial cells.
Therefore, the aims of the present study were to investigate the mechanisms of platelet recruitment to the inflamed glomerulus and to clarify the role of platelet-derived P-selectin in glomerular leukocyte recruitment. This was achieved using intravital microscopy to examine leukocyte and platelet recruitment in the intact glomerulus. These experiments supported a role for platelet-derived P-selectin in glomerular leukocyte recruitment and demonstrated roles for αIIbβ3/fibrinogen/intercellular adhesion molecule 1 (ICAM-1), GPVI, and neutrophils in platelet recruitment to the inflamed glomerulus.
Materials and Methods
Animals
Male C57BL/6 wild-type mice (supplied by Monash Animal Services, Clayton, Vic., Australia), weighing 27–35 g (aged 16 to 20 weeks), were used for intravital microscopy. Blood for platelet preparation was harvested from male wild-type C57BL/6 mice and FcRγ chain−/− mice (bred in-house).34 P-selectin−/− mice (bred in-house) were used for bone marrow chimera experiments. All experimental procedures were approved by the Monash University Animal Ethics Committee.
Antibodies and Reagents
Antibodies and reagents used for in vivo experiments were as follows: sheep anti-mouse-glomerular basement membrane (GBM) was prepared as described previously.4 To serve as a control antibody, normal sheep globulin (NSG) was prepared from nonimmune sheep serum using an identical procedure. To inhibit αIIbβ3, GPI562 (4 mg/kg), a small molecule αIIbβ3 antagonist, was used.35,36 Alboaggregin-B (280 μg/kg), a GPIbα-binding venom protein purified as a 25-kDa heterodimer from C.h. horridus (rattlesnake venom) as previously described (kindly provided by A/Prof. Rob Andrews, Australian Centre for Blood Diseases, Monash University, Australia), was used to inhibit GPIbα.37 Other inhibitory molecules used were as follows: goat polyclonal antibody against mouse fibrinogen (Nordic Immunology, Tiburg, Netherlands; 4 mg/kg)20; YN1/1.7.4, a rat monoclonal antibody against murine ICAM-1 (grown from hybridoma; 200 μg/mouse); RB6-8C5, a monoclonal antibody against Gr-1 (grown from hybridoma; 150 μg/mouse); and apyrase, an enzyme that metabolizes adenosine diphosphate (ADP), thereby limiting platelet activation (Sigma-Aldrich, Castle Hill, NSW, Australia; 0.2 U/g).38 For control experiments, isotype antibodies used included rat IgG2a and IgG2b (BD Biosciences, San Diego, CA).
Assessment of Platelet Aggregation in Vivo and in Vitro
The actions of various antithrombotic agents were assessed in an in vivo ferric chloride thrombosis model.39 In brief, in anesthetized C57BL/6 mice, FeCl3 was microinjected adjacent to a mesenteric arteriole, and platelet interactions were visualized with Differential Interference Contrast microscopy, using a Leica DMIRB inverted microscope (Leica Microsystems, Mt. Waverley), and a DAGE MTI charge-coupled device camera (SciTech, Preston, Vic., Australia). To assess the effect of alboaggregin-B, an in vitro flow chamber platelet adhesion assay was used.39 Mouse blood was anticoagulated with hirudin and flowed over a collagen-coated glass microcapillary at 1800 s−1 for 3 minutes. Differential Interference Contrast microscopy images were taken at 4.5 minutes after Tyrode’s buffer washout.
Unilateral Ureteric Ligation
To prepare kidneys for intravital microscopy, male mice (4 to 5 weeks old) underwent unilateral ureteric ligation as previously described.4,40 Twelve weeks were then allowed for the kidney to undergo renal hydronephrosis.
Renal Intravital Microscopy
Intravital microscopy of the hydronephrotic kidney was performed as previously described.4,40 Mice were anesthetized by i.p. injection of a cocktail of 150 mg/kg ketamine hydrochloride (Troy Lab, Smithfield, NSW, Australia) and 10 mg/kg xylazine (Pfizer, West Ryde, NSW, Australia). The jugular vein was then cannulated for administration of further anesthetic, platelets, and reagents. Mice were kept at a constant temperature of 37°C on a heating pad. The hydronephrotic kidney was exteriorized through a lateral skin incision, drained of urine using a 30G needle, and extended over a clear viewing platform using 4/0 silk tied to the kidney capsule. The kidney was superfused with bicarbonate-buffered saline at 37°C and covered with a coverslip. The renal microvasculature was observed with an intravital microscope (Axioplan 2 Imaging; Carl Zeiss, Australia) with a water immersion ×20 objective (×20/0.50 NA).
Platelet Isolation and Transfer
Platelets were isolated using a previously described method.35 Briefly, blood was withdrawn from the inferior vena cava of an anesthetized donor mouse and platelet-rich plasma was prepared via sequential centrifugation. Platelets were then pelleted from platelet-rich plasma and gently resuspended in Tyrode’s buffer (10 mmol/L HEPES, 12 mmol/L NaHCO3, pH 7.4, 137 mmol/L NaCl, 2.7 mmol/L KCl, 5 mmol/L glucose). Platelets were allowed to rest in Tyrode’s buffer for approximately 30 minutes at room temperature. Platelets isolated using this technique expressed minimal P-selectin, indicating that this protocol did not induce platelet activation. Subsequently, platelets (2 × 108) were labeled with 5, 6-carboxyfluorescein-succinimidyl-ester (CFSE) (5 mmol/L; Invitrogen, Mulgrave, Victoria, Australia) and slowly infused intravenously into hydronephrotic mice, which had been prepared for renal intravital microscopy. Most experiments used blood from C57BL/6 wild-type mice; however, in some experiments FcRγ chain−/− mice were used as platelet donors to provide platelets deficient in GPVI.
Analysis of Interactions within Glomeruli
To visualize leukocytes in experiments examining leukocyte recruitment, mice were injected with 50 μl of 0.05% rhodamine 6G (Sigma-Aldrich, Castle Hill, NSW, Australia) and the kidney was then examined via epifluorescence at 520–560 nm, using a 590-nm emission filter. Alternatively, CFSE-labeled platelets were observed by epifluorescence at 450–490 nm, using a 515-nm emission filter. Images were visualized using a low-light video camera (DAGE-MTI, IR1000; SciTech, Preston, Vic, Australia) and recorded for playback analysis using a digital video recorder.
Three to four randomly chosen glomeruli were recorded at different time intervals throughout the experiment and subsequently analyzed for different parameters. For the analysis of leukocyte recruitment, leukocyte adhesion was measured by the number of rhodamine 6G-labeled leukocytes that remained adherent for more than 30 seconds. To assess platelet-endothelial cell interactions, platelets were defined as adherent if they remained stationary within the glomerulus for at least 30 seconds. In addition, primary platelet adhesion (tethering) was assessed. Platelet interactions were defined as primary when rapidly-moving platelets were observed to tether on to the endothelial surface and return to the bloodstream in less than 10s. These data were expressed as number of platelet tethers per glomerulus per 30 s.
Generation of Bone Marrow Chimeras
Mice chimeric for P-selectin were generated via irradiation and bone marrow transfer as described previously.41 Briefly, mice were irradiated with two doses of 550 rad spaced three hours apart. Bone marrow (BM) cells were harvested from donor mice under sterile conditions, and recipient mice received 5 × 106 BM leukocytes within 24 hours of irradiation. Recipient mice were maintained under specific pathogen-free conditions for 8 weeks then used in intravital microscopy experiments. The following chimeric mice were generated: wild-type mice transplanted with P-selectin−/− BM (P-sel−/− into wild-type); P-selectin−/− mice transplanted with wild-type BM (wild-type into P-sel−/−). Additionally, wild-type into wild-type mice and P-sel−/− into P-sel−/− mice were generated as controls. To confirm BM reconstitution, platelets were isolated and assessed for expression of P-selectin under activating conditions via flow cytometry.42
Experimental Protocol
To examine platelet recruitment in the inflamed glomerulus, platelets were infused and five minutes allowed for equilibration, then either anti-GBM antibody (20 mg per mouse) or an equivalent amount of NSG was administered intravenously. Subsequently, 30-second recordings were made at various time intervals over a period of 1 hour.
In experiments investigating adhesion molecule function, Abs or various inhibitors were administered intravenously 5 minutes before infusion of CFSE-labeled platelets. To examine the effect of neutrophil depletion on platelet adhesion, mice were treated with the neutrophil-depleting antibody, RB6-8C5 (150 μg/mouse),4 24 hours before administration of fluorescently-labeled platelets. In a separate set of experiments, fibrinogen deposition in the inflamed glomerulus was investigated using Alexa Fluor 488-conjugated fibrinogen (Invitrogen; 17 mg/kg).20 This was administered intravenously 30 minutes before the administration of anti-GBM antibody or NSG.
To examine leukocyte recruitment in P-selectin chimeric mice, mice received 20 mg anti-GBM antibody intravenously and recordings of leukocyte recruitment in the glomeruli were performed 0, 60, and 120 minutes after anti-GBM antibody administration. To assess the role of ADP-dependent platelet activation, mice received apyrase (0.2 U/g, i.v.) 5 minutes before infusion of anti-GBM antibody.
Immunohistochemistry
Kidney samples were obtained 1 hour after administration of either NSG or anti-GBM antibody, or anti-GBM antibody and anti–ICAM-1 (YN1/1.7.4) and immediately snap-frozen in optimal cutting temperature (OCT) compound. Six-micrometer cryostat sections were prepared, fixed in ice-cold acetone for 20 minutes, and subsequently allowed to dry for at least 15 minutes. Fibrinogen staining was performed using a modification of a previously-published technique.43 Slides were incubated with 1:50 FITC-conjugated anti-mouse fibrinogen antibody (Emfret Analytics, Würzburg, Germany) in PBS with 2.5% BSA for 30 minutes and then rinsed gently three times in PBS. The samples were mounted and visualized using a Leica BMLB Laboratory microscope with ×40 objective (×40/0.70 NA). Quantitative assessment of glomerular deposition of fibrinogen was performed in 20–30 glomeruli per animal using image analysis. Images were captured with a Leica DC 300F digital camera (Leica Microsystems) using Photoshop CS software (Adobe Systems) at predefined exposure and gain levels. The mean fluorescence intensity within glomerular tuft minus background intensity in unstained tissue was determined using Scion Image (Scion Corp., Frederick, MD).4
Transmission Electron Microscopy
Kidneys were prepared for transmission electron microscopy as previously described.44 Briefly, mice were injected with either NSG or anti-GBM antibody (20 mg), and after 10 minutes tissues were flushed via intracardiac infusion of PBS then perfusion-fixed with glutaraldehyde (0.05% in phosphate buffer). Small segments of kidney were processed through to Epon Araldite, ultrathin (70 nm) sections prepared, and sections stained with uranyl acetate and lead citrate. Sections were examined using a Hitachi H7500 transmission electron microscope operating at 20 kV.
Statistical Analysis
All data presented are shown as the mean ± SEM. Student’s unpaired t-tests and one-way analysis of variance were performed to compare experimental groups and P < 0.05 was considered significant.
Results
We first performed experiments to confirm the specificity and effectiveness of reagents used to inhibit GPIbα and αIIbβ3. In an in vivo ferric chloride thrombosis model, a localized thrombus is formed by the microinjection of 6% ferric chloride. In this model, the GPIbα-binding venom protein, alboaggregin B, prevented primary platelet adhesion, whereas GPI562, which has been demonstrated to be a potent and selective inhibitor of αIIbβ3,36,45 prevented platelet aggregation (see Supplemental Figure S1 and Supplemental Video 1 at http://ajp.amjpathol.org). In addition, alboaggregin B prevented platelet attachment to collagen under high shear flow conditions in vitro (see Supplemental Figure S2 at http://ajp.amjpathol.org), interactions previously found to be GPIbα/vWF-dependent.46 Together these experiments confirm the in vivo effectiveness of these agents against their respective target molecules.
Anti-GBM Antibody Rapidly Induces Glomerular Platelet Recruitment
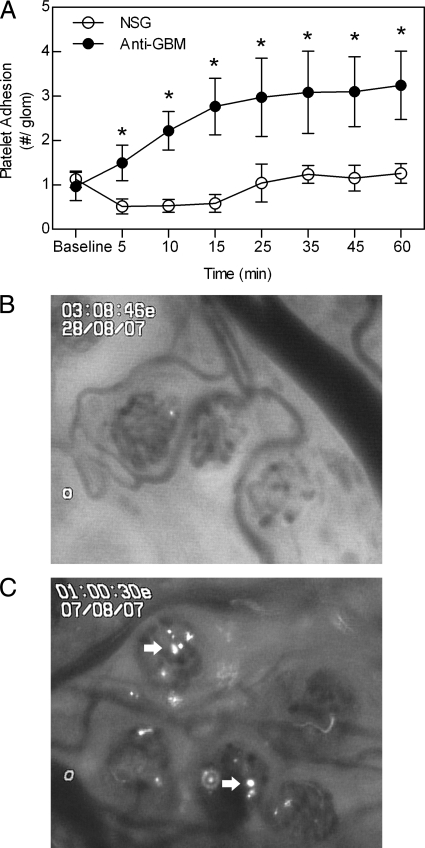
We next used intravital microscopy to determine the dynamics of glomerular platelet recruitment induced by anti-GBM antibody. This was achieved by transferring isolated fluorescently-labeled platelets into a mouse undergoing renal intravital microscopy. In mice treated with NSG as a control, minimal glomerular platelet accumulation was observed and levels remained stable throughout the 60 minute observation period (Figure 1A and B). In contrast, in anti-GBM antibody-treated mice, there was a significant increase in platelet adhesion within 5 minutes of inducing glomerular inflammation and platelet adhesion progressively increased for the next 20 minutes (Figure 1, A and B). Platelet accumulation in anti-GBM antibody-treated mice remained significantly elevated for the entire observation period. These observations demonstrate that anti-GBM antibody induces platelet adherence in glomerular capillaries within minutes of initiating an inflammatory response.
Figure 1.
Platelet accumulation in the glomerular microvasculature, as assessed using intravital microscopy. A: Glomerular accumulation of platelets was assessed via renal intravital microscopy, following i.v. infusion of 2 × 108 CFSE-labeled platelets. After an initial recording of platelet accumulation, mice were treated with anti-GBM antibody or NSG (20 mg, i.v., n = 6 per group), and platelet adhesion assessed at various time points over the ensuing 60 minutes. Data are shown as mean ± SEM. *P < 0.05 versus NSG-treated groups for each corresponding time point. B and C: Captured images of the renal microvasculature in an NSG-treated animal (B), and following anti-GBM antibody administration (C). Adherent platelets (arrows) are visible in the anti-GBM antibody-treated animal.
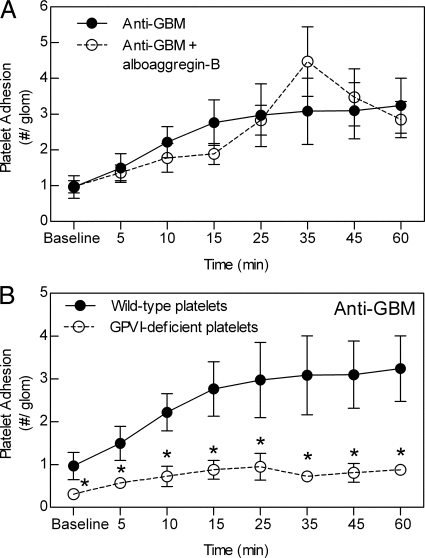
Platelet Collagen Receptor GPVI, but not GPIbα, Mediates Platelet Recruitment to the Inflamed Glomerular Microvasculature
To evaluate the role of platelet adhesion receptor GPIbα in platelet recruitment, mice were treated with alboaggregin-B before inducing glomerular inflammation. Alboaggregin-B–treated mice showed no reduction in the number of adherent platelets compared with mice treated with anti-GBM antibody alone (Figure 2A). We next assessed the role of platelet collagen receptor GPVI, using platelets isolated from FcRγ chain−/− mice. Platelets from these mice have been shown to lack expression of the GPVI receptor.47 GPVI-deficient platelets were significantly reduced in their ability to undergo adhesion in the inflamed glomerulus in comparison with wild-type platelets (Figure 2B).
Figure 2.
Roles of GPIbα and GPVI in anti-GBM antibody-induced glomerular platelet interactions. A: The role of GPIbα was assessed by comparing glomerular platelet adhesion in mice treated with anti-GBM antibody alone (n = 6) or with the GPIbα inhibitor, alboaggregin-B (280 μg/kg, i.v., n = 3). B: The role of GPVI was determined by comparison of recruitment of platelets from FcRγ chain−/− mice, which lack GPVI, with that of wild-type platelets. Platelets were isolated from either wild-type (n = 6) or FcRγ chain−/− mice (n = 6), and anti-GBM antibody-induced glomerular platelet adhesion assessed as for previous experiments. Data are shown as mean ± SEM. *P < 0.05 versus anti-GBM antibody-treated mice with wild-type platelets.
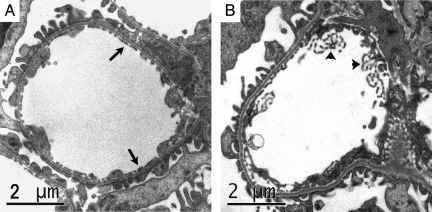
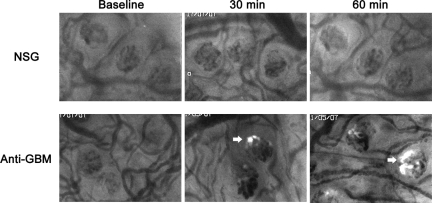
Evidence supporting a role for GPVI in platelet accumulation raised the possibility of a platelet/collagen interaction within the glomerulus. Therefore, we next used transmission electron microscopy to assess the effect of anti-GBM antibody on the glomerular endothelium in the initial stages of the response. Figure 3A shows a glomerular capillary from a mouse treated with NSG. The glomerular endothelium in these mice displayed typical thin discontinuous morphology. In contrast, in mice examined 10 minutes after anti-GBM antibody administration (Figure 3B), the endothelial morphology was markedly altered, showing membranous projections into the vascular lumen.
Figure 3.
Anti-GBM antibody alters the morphology of the glomerular endothelium within ten minutes. The structure of the glomerular endothelium was assessed using transmission electron microscopy. A: Glomerular capillary of a mouse shortly after receiving NSG, showing the typical discontinuous morphology of the glomerular endothelium (arrows) lining the capillary. B: Glomerular capillary of a mouse ten minutes after receiving anti-GBM antibody. The endothelial structure is markedly altered, with numerous protrusions into the vascular lumen (arrowheads). Scale bar = 2 μm.
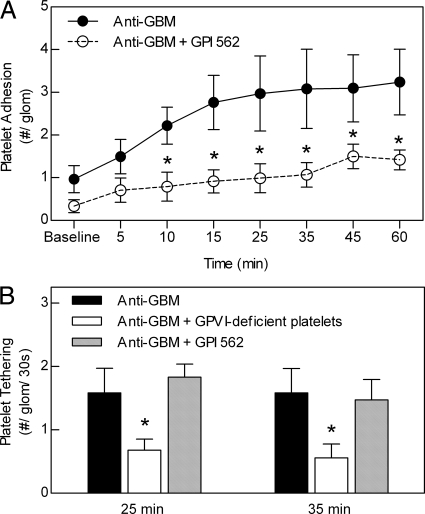
Anti-GBM Antibody-Induced Glomerular Platelet Adhesion Is Dependent on Platelet αIIbβ3 Integrin
The platelet αIIbβ3 integrin/fibrinogen/ICAM-1 pathway has been implicated in platelet adhesion in inflamed postcapillary venules.20 Therefore, we next examined the elements of this pathway in anti-GBM antibody-induced glomerular platelet recruitment. To investigate the role of αIIbβ3, mice were treated with GPI562.35 GPI562 treatment resulted in a significant reduction in the number of platelets adhering to the glomerulus, apparent within 10 minutes of initiation of the anti-GBM antibody response (Figure 4A). Previous studies indicate that αIIbβ3-mediated platelet interactions are responsible for stable platelet adhesion and aggregation, whereas GPVI mediates primary adhesion (or ‘tethering’) of platelets to the vascular wall.48,49 To further clarify the roles of these molecules in glomerular platelet accumulation, we assessed the effect of GPVI deficiency and αIIbβ3 inhibition on primary platelet adhesion (Figure 4B). Platelets lacking GPVI showed reduced primary adhesion within the glomerulus, whereas αIIbβ3 inhibition did not reduce the ability of platelets to undergo initial tethering to the glomerular surface, but did reduce subsequent stable platelet adhesion (Figure 4A).
Figure 4.
Inhibition of αIIbβ3 reduces anti-GBM antibody-induced glomerular platelet recruitment within glomeruli via an effect on stable adhesion. A: To assess the role of platelet αIIbβ3 in glomerular platelet recruitment, mice were pretreated with the αIIbβ3 inhibitor, GPI562 (4 mg/kg, i.v., n = 6) before assessment of anti-GBM antibody-induced platelet recruitment. Data were compared with that of mice treated with anti-GBM antibody alone (n = 6). Data are shown as mean ± SEM. *P < 0.05 versus anti-GBM antibody-treated group for each time point. B: Assessment of the contribution of platelet GPVI and αIIbβ3 to platelet tethering (primary adhesion) within the inflamed glomerulus. Transient (>10s) interactions of CFSE-labeled platelets with the glomerulus (tethering) were quantified in mice treated with anti-GBM antibody alone, or in mice that received anti-GBM antibody and GPVI-deficient platelets, or anti-GBM antibody plus GPI562, 25 and 35 minutes after anti-GBM antibody administration (n = 6 for all groups). Data are shown as mean ± SEM. *P < 0.05 versus anti-GBM antibody alone group.
Fibrinogen Is Deposited in the Inflamed Glomerulus and Contributes to Platelet Adhesion
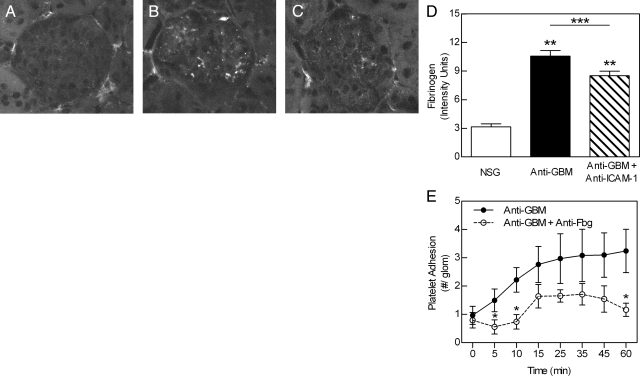
As fibrinogen has been observed to accumulate in the inflamed microvasculature in some inflammatory conditions,20 we next investigated deposition of fibrinogen in the inflamed glomerulus. We first assessed the glomerular accumulation of intravenously administered Alexa Fluor 488-conjugated fibrinogen during the anti-GBM antibody response. Mice treated with NSG displayed little or no fibrinogen deposition within the glomerulus throughout the 60-minute experiment (Figure 5). In contrast, in mice treated with anti-GBM antibody, fluorescent fibrinogen was observed accumulating in the glomerulus within 5 minutes of inducing inflammation. This progressively increased throughout the experiment such that by 30 minutes, substantial fibrinogen deposition had occurred (Figure 5). To address the possibility that infusion of exogenous fibrinogen resulted in an overestimation of fibrinogen deposition, we also assessed deposition of endogenous fibrinogen using immunohistochemistry. Anti-GBM antibody treatment resulted in a significant increase in the amount of fibrinogen detectable within glomeruli relative to that in NSG-treated mice (Figure 6, A, B, and D). To assess potential mechanisms of fibrinogen deposition, we examined the effect of inhibition of ICAM-1, a molecule constitutively expressed by endothelial cells, via which fibrinogen can attach to the endothelial surface.20,50 ICAM-1 inhibition induced a moderate but significant reduction in fibrinogen deposition (Figure 6, C and D). Finally, to examine the functional role of fibrinogen, we examined platelet adhesion in mice treated with a function-blocking anti-fibrinogen antibody.20 Inhibition of fibrinogen significantly reduced platelet adhesion 5, 10, and 60 minutes after anti-GBM antibody administration (Figure 6E).
Figure 5.
In vivo assessment of fibrinogen accumulation in inflamed glomeruli. To determine whether anti-GBM antibody induces fibrinogen accumulation in glomerular capillaries, mice were examined via intravital microscopy following administration of Alexa Fluor 488-conjugated fibrinogen (17 mg/kg, i.v.) 30 minutes before treatment with either NSG or anti-GBM antibody. In NSG-treated mice, no glomerular fibrinogen deposition is observed. In contrast, in anti-GBM antibody-treated mice, fibrinogen deposition (arrows) becomes apparent after 30 minutes and is further increased by 60 minutes. Images are representative examples of four (NSG) or five (anti-GBM antibody) experiments.
Figure 6.
Endogenous fibrinogen accumulates within the glomerulus in response to anti-GBM antibody and promotes platelet adhesion. A–D: Role of ICAM-1 in fibrinogen accumulation. Mice were treated with either NSG, anti-GBM antibody (20 mg, i.v.), or anti-GBM antibody following pretreatment with a mAb against ICAM-1, and the kidneys were removed after 1 hour. Kidney sections were stained for fibrinogen using FITC-conjugated anti-mouse fibrinogen antibody. Minimal glomerular fibrinogen deposition was observed in NSG-treated mice (A), whereas extensive deposits of fibrinogen were detectable in glomeruli of anti-GBM antibody-treated mice (B). In mice treated with anti-ICAM-1 (C), the intensity of fibrinogen staining was reduced relative to mice treated with anti-GBM antibody alone. D: Mean intensity of glomerular fibrinogen staining in mice treated with NSG (n = 6), anti-GBM antibody alone (n = 9), and anti-GBM antibody plus anti-ICAM-1 (n = 9), as assessed using image analysis. Data were assessed in >25 glomeruli per animal and are shown as mean ± SEM. **P < 0.0001 versus NSG-treated mice. ***P < 0.01 versus mice treated with anti-GBM antibody alone. E: To assess the role of fibrinogen in glomerular platelet recruitment, mice were pretreated with a function-blocking antibody against mouse fibrinogen (n = 6) before assessment of anti-GBM antibody-induced platelet recruitment. Data were compared with that of mice treated with anti-GBM antibody alone (n = 6). Data are shown as mean ± SEM. *P < 0.05 versus anti-GBM antibody-treated group for the time points indicated.
As ICAM-1 inhibition reduced fibrinogen deposition, it would also be expected to affect platelet adhesion. Antibody blockade of ICAM-1 eliminated the anti-GBM antibody-induced increase in glomerular platelet adhesion (Figure 7A). This finding is consistent with our previous observation of a key role for ICAM-1 in leukocyte adhesion, as reducing platelet adhesion would be expected to reduce leukocyte recruitment.4 However, an alternative interpretation is that ICAM-1 is acting as an adhesion molecule to directly mediate adhesion of neutrophils to the endothelium and that a reduction in neutrophil adhesion resulting from ICAM-1 inhibition also reduced the number of adherent platelets. Indeed, leukocytes have been shown to promote platelet adhesion in other inflammatory models.23,24 In mice treated with anti-GBM antibody, which typically have elevated neutrophil counts, treatment with neutrophil-depleting antibody RB6-8C5 caused a 75% reduction in circulating neutrophils (anti-GBM antibody alone – 50.3 ± 5.6% neutrophils, versus anti-GBM antibody + RB6-8C5 – 13.0 ± 2.0% neutrophils). In neutrophil-depleted mice treated with anti-GBM antibody, platelet adhesion was significantly reduced relative to mice treated with anti-GBM antibody alone (Figure 7B), indicating that neutrophils contributed to glomerular platelet accumulation.
Figure 7.
Inhibition of ICAM-1 and neutrophil depletion reduce anti-GBM antibody-induced glomerular platelet recruitment within glomeruli. A: To assess the role of ICAM-1 in glomerular platelet adhesion, mice were pretreated with a mAb against ICAM-1 (200 μg, i.v., n = 7) or isotype control mAb (n = 7) and anti-GBM antibody-induced platelet recruitment assessed as performed previously. Data are shown as mean ± SEM. *P < 0.05 versus isotype control group for each time point. B: To determine the effect of neutrophil depletion on glomerular platelet recruitment, mice were pretreated with RB6-8C5 (150 μg, i.p., n = 6) 24 hours before assessment of anti-GBM antibody-induced platelet recruitment. Data were compared with that of mice treated with anti-GBM antibody alone (n = 6). Data are shown as mean ± SEM. *P < 0.05 versus anti-GBM antibody-treated group for each time point.
Platelet-Derived P-Selectin Is Critical to Leukocyte Recruitment
We next turned to examine the molecular basis of leukocyte recruitment in this model. Acute platelet transfer studies in P-selectin−/− mice have implicated platelet P-selectin as contributing to leukocyte recruitment, although in these assays, endogenous platelets may still have been recruited to the glomerulus.4 Therefore, to examine this issue more definitively, we generated bone marrow chimeras between wild-type and P-selectin−/− mice to restrict expression of P-selectin to either platelets or endothelial/parenchymal cells. Control chimeras in which P-selectin was intact (wild-type into wild-type) or absent (P-sel−/− into P-sel−/−) confirmed our previous observation of a key role for P-selectin, in that glomerular leukocyte adhesion 2 hours after anti-GBM antibody was significantly reduced in P-sel−/− into P-sel−/− mice (Figure 8). In both wild-type into P-sel−/− and P-sel−/− into wild-type chimeras, analysis of platelet P-selectin expression confirmed bone marrow reconstitution with P-selectin–intact or –deficient platelets, respectively (data not shown). In wild-type into P-sel−/− mice, in which P-selectin expression was restricted to platelets, anti-GBM antibody-induced adhesion was not altered from the level observed in wild-type into wild-type mice (Figure 8). In contrast, in mice in which P-selectin was retained in the endothelium but absent in platelets (P-sel−/− into wild-type), leukocyte adhesion was significantly reduced and not different from that in mice in which P-selectin was completely absent. These data provide direct evidence of a key role for platelet-derived P-selectin in glomerular leukocyte adhesion.
Figure 8.
Platelet-derived P-selectin contributes to leukocyte recruitment in the inflamed glomerulus. Intravital microscopy was used to assess anti-GBM antibody-induced glomerular leukocyte recruitment in P-selectin chimeric mice. The following mice were examined: wild-type mice reconstituted with wild-type bone marrow (wild-type into wild-type, n = 6), P-selectin−/− mice reconstituted with wild-type bone marrow (wild-type into P-sel−/−, n = 7), wild-type mice reconstituted with P-selectin−/− bone marrow (P-sel−/− into wild-type, n = 7), and P-selectin−/− mice reconstituted with P-selectin−/− bone marrow (P-sel−/− into P-sel−/−, n = 6). Data show glomerular leukocyte adhesion assessed two hours after administration of anti-GBM antibody (20 mg, i.v.). Data are shown as mean ± SEM. *P < 0.05 versus wild-type into wild-type and wild-type into P-sel−/−.
Platelet Activation Is Required for Anti-GBM Antibody-Induced Leukocyte Adhesion
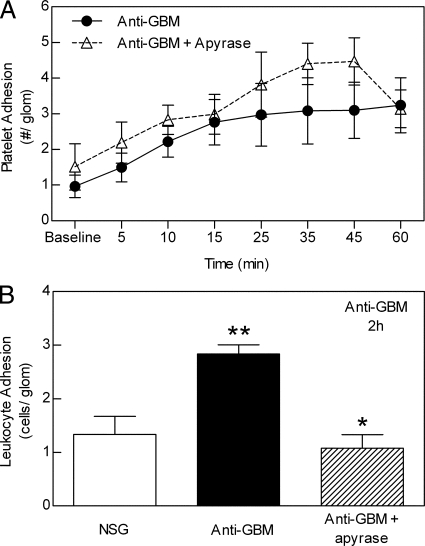
Finally, we assessed the requirement for ADP-dependent platelet activation in glomerular recruitment of platelets and leukocytes. To address this question, the effect of apyrase treatment was examined. Apyrase is an ecto-enzyme that rapidly metabolizes ADP and adenosine triphosphate (ATP) released from activated platelets and endothelial cells, thereby minimizing ADP-dependent platelet activation and recruitment.38 Apyrase treatment had no effect on platelet accumulation in anti-GBM antibody-treated mice (Figure 9A). In contrast, apyrase treatment significantly decreased the number of adherent leukocytes in response to anti-GBM antibody (Figure 9B). Taken together, these data indicate that ADP-dependent platelet activation is not required for anti-GBM antibody-induced platelet accumulation but does contribute to the associated leukocyte recruitment.
Figure 9.
ADP inhibition via apyrase reduces glomerular accumulation of leukocytes but not platelets. A: Effect of apyrase treatment on anti-GBM antibody-induced glomerular platelet accumulation. Mice were treated with the ADP-metabolizing enzyme apyrase (0.2 U/g, i.v., n = 6) before administration of anti-GBM antibody, and glomerular platelet adhesion was assessed using intravital microscopy as for previous experiments. Data were compared with mice treated with anti-GBM antibody alone (n = 6). Data are shown as mean ± SEM. B: Effect of apyrase treatment on anti-GBM antibody-induced glomerular leukocyte accumulation. Mice were treated with NSG, anti-GBM antibody alone, or anti-GBM antibody and apyrase (as above), and glomerular leukocyte adhesion was assessed using intravital microscopy. Data show leukocyte adhesion two hours after administration of either NSG, anti-GBM antibody alone, or anti-GBM antibody in apyrase-pretreated mice (n = 6 per group). Data are shown as mean ± SEM. **P < 0.05 versus NSG-treated mice, and *P < 0.05 versus anti-GBM antibody-treated mice.
Discussion
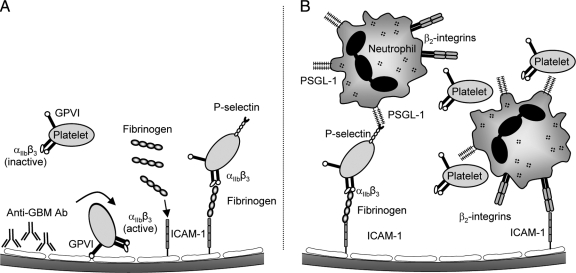
It is well established that in models of in situ immune complex formation in the glomerulus, glomerular leukocyte recruitment is central to the subsequent glomerular injury.4,51,52,53 Our recent work has shown that glomerular leukocyte recruitment and injury in this model are dependent on the recruitment of platelets.4 Previously we had used immunohistochemistry to observe extensive platelet accumulation 2 hours after anti-GBM antibody administration. However, the time course and mechanisms responsible for platelet adhesion within the glomerulus in this response were not investigated. In the present study, we used intravital microscopy to examine the interactions of platelets in the glomerulus throughout the inflammatory response. These experiments demonstrated that platelet accumulation in glomeruli commences rapidly after anti-GBM antibody administration, first being apparent within 5 minutes, and progressively increasing over the subsequent hour. This rapid time course of recruitment is consistent with a role for platelets in promoting leukocyte recruitment in response to anti-GBM antibody. This work also revealed roles for αIIbβ3, fibrinogen, ICAM-1, and GPVI, but not GPIbα, in mediating platelet accumulation. Interestingly, neutrophil depletion also reduced platelet accumulation, indicating that accumulation of platelets and neutrophils were interdependent. This work also confirmed a key role for platelet-derived P-selectin in mediating leukocyte recruitment and suggested that ADP-dependent platelet activation is required for recruitment of leukocytes, but not platelets. A schema illustrating the hypothesized mechanism whereby these pathways interact is depicted in Figure 10. These experiments are the first to describe these initial events in the development of the response to anti-GBM antibody, and suggest that one of the early events in the glomerular microcirculation following anti-GBM antibody administration is generation of a substrate amenable to platelet adhesion.
Figure 10.
Model of anti-GBM antibody-induced recruitment of platelets and neutrophils. Diagram of hypothesized mechanism by which platelets and neutrophils are recruited to the glomerulus in response to anti-GBM antibody. A: Following deposition of anti-GBM antibody, platelets adhere to the glomerular capillary in a GPVI-dependent fashion. This leads to activation of platelet αIIbβ3 integrin. Fibrinogen is also deposited in the glomerular microvasculature in a partially ICAM-1-dependent fashion. Platelet adhesion is then stabilized via an αIIbβ3-dependent interaction, using fibrinogen as one potential ligand. Platelets are further activated by an apyrase sensitive mechanism, presumably ADP, resulting in platelet P-selectin up-regulation. B: Neutrophils subsequently adhere in glomerular capillaries via interaction with platelet P-selectin, and additionally via β2 integrin-dependent adhesion, potentially with ICAM-1. Additional platelets accumulate in the glomerular microvasculature via interaction with adherent neutrophils.
The αIIbβ3 integrin, in addition to being a critical molecule in platelet-platelet interactions,54,55 has been implicated in inflammation-associated platelet adhesion within the vasculature.20,21,56,57 The soluble αIIbβ3 ligand fibrinogen has been shown to facilitate these interactions via ICAM-1-dependent deposition within the vasculature and subsequent interaction with platelet αIIbβ3.20 In the present study, the αIIbβ3 inhibitor GPI562 caused a significant reduction in platelet adhesion, as did inhibition of both fibrinogen and ICAM-1. In addition, fibrinogen deposition was observed in the inflamed glomerulus, occurring via a partially ICAM-1-dependent mechanism. Taken together, these data indicate that the αIIbβ3/fibrinogen/ICAM-1 pathway is also active in the inflamed glomerulus. However, these observations do not preclude interaction of αIIbβ3 with other ligands such as fibronectin, vitronectin, vWF, and thrombospondin, potentially present within the inflamed glomerular microvasculature.18,49,58
ICAM-1 plays a central role in leukocyte adhesion within the microvasculature, and inhibition of ICAM-1 has been shown to reduce leukocyte recruitment in multiple models of GN.59,60,61,62 Moreover, our previous findings showed that inhibition of the CD18/ICAM-1 pathway resulted in comparable inhibitory effects on leukocyte recruitment, suggesting that the major role of ICAM-1 in this model was to act as a β2-integrin ligand required for leukocyte adhesion in glomerular capillaries.4 The data in the present study suggest that ICAM-1 is also of central importance in mediating glomerular platelet adhesion. However, as we also observed that depletion of circulating neutrophils markedly inhibits platelet recruitment, the ability of ICAM-1 inhibition to reduce platelet accumulation may also reflect a reduction in ICAM-1–dependent neutrophil adhesion and neutrophil-associated platelet recruitment. Taken together, these findings indicate that ICAM-1 plays dual roles in this response—acting as a ligand for fibrinogen binding to the endothelial surface and thereby promoting platelet adhesion, and potentially acting as a ligand for β2-integrin-dependent neutrophil adhesion (Figure 10, A and B).
To examine the role of GPVI, we used platelets from FcRγ chain−/− mice. While deletion of the FcRγ chain results in loss of high and low affinity Fcγ receptors, this is not likely to alter the response of the platelets from these mice, as murine platelets do not express Fcγ receptors.63,64 However, absence of the FcRγ chain also results in loss of expression of GPVI in platelets. Use of these platelets demonstrated a role for GPVI in platelet accumulation within the inflamed glomerulus. A growing body of evidence now indicates that GPVI is the major collagen receptor on platelets.22,47,65 The present findings raise the possibility that collagen also contributes to platelet recruitment. Type IV collagen is the major constituent of basement membranes, including that of the glomerulus.58 Given the unique fenestrated nature of the glomerular endothelium, it is conceivable that subendothelial extracellular matrix molecules may be more accessible to circulating platelets than in other microvascular beds. Furthermore, loss of glomerular endothelium and exposure of the glomerular basement membrane has been reported in this model previously, 4 hours after anti-GBM antibody administration.44 However, whether this response underlies the immediate increase in platelet adhesion observed in the present experiments was unclear. To address this, we examined the glomerulus via transmission electron microscopy 10 minutes after initiation of the anti-GBM antibody response. These experiments revealed that endothelial morphology was perturbed very rapidly after administration of anti-GBM antibody. This altered morphology may allow increased exposure of subendothelial collagen shortly after deposition of anti-GBM antibody in the glomerulus, thereby facilitating interactions with circulating platelets mediated via GPVI.
In the present study, targeting either GPVI or αIIbβ3 was equally effective at inhibiting platelet recruitment, a finding consistent with a mechanism in which these molecules work in sequence, rather than independently. Our hypothesis is that the initial interaction of platelets within the glomerulus is mediated by a GPVI-mediated interaction with glomerular collagen and that this interaction facilitates subsequent αIIbβ3-mediated stable adhesion. This is consistent with existing evidence showing that the αIIbβ3 integrin must undergo a transition to a high affinity state to be capable of ligand binding.54,66,67 This activation process can occur via several pathways, although of relevance in this study is the fact that GPVI binding to collagen stimulates signaling through tyrosine kinase-based pathways that lead to αIIbβ3 activation.47,65,68 Moreover, this concept is supported by our observation that GPVI-deficient platelets showed reduced ability to undergo primary adhesion/tethering within the glomerulus, whereas this process was unaffected in animals undergoing αIIbβ3 inhibition. These findings are consistent with a mechanism whereby platelets initially interact with collagen within the glomerular capillaries via GPVI, and that this leads to αIIbβ3 activation (Figure 10A). This would render αIIbβ3 capable of ligand binding and therefore able to contribute to platelet adhesion in glomeruli.
Participation of platelets in leukocyte recruitment has been observed in numerous studies. In some cases, platelets have been found to promote leukocyte recruitment.25,29 Conversely, in other models, platelet accumulation within the inflamed microvasculature is facilitated via interaction with adherent leukocytes.23,24 In the model of glomerulonephritis used in the present study, our findings indicate that both mechanisms are active, and that recruitment of one cell type facilitates the other. Previously we have found that platelet depletion inhibits leukocyte recruitment and glomerular injury.4 In the present study, neutrophil depletion was found to reduce platelet accumulation. One possible scenario that encompasses these findings is that anti-GBM antibody-induced platelet accumulation is required to initiate the neutrophil recruitment response, but that once neutrophils are accumulating within the glomerular microvasculature, their presence facilitates additional platelet recruitment.
The mechanisms by which platelets interact with leukocytes and endothelial cells vary. In some cases, platelets are recruited to the inflamed site via interactions with endothelial P-selectin.26 Alternatively, platelets first bind to leukocytes via interaction of platelet P-selectin and leukocyte PSGL-1, with leukocyte-platelet complexes subsequently undergoing recruitment to the inflamed site.32,33 Here we used P-selectin chimeric mice to clarify the role of platelet-derived leukocyte recruitment in the glomerulus. This enabled us to restrict P-selectin expression to either platelets or endothelial cells. Under these conditions, normal leukocyte adhesion was only observed in mice with wild-type bone marrow, in which the platelets were capable of P-selectin expression. This finding further strengthens the hypothesis that in this model, platelet-derived P-selectin is critical to the leukocyte adhesion response.
An additional aspect of the response examined in this study was the role of ADP-dependent platelet activation in recruitment of platelets and leukocytes. ADP is a potent platelet-activating agent released from injured cells and activated platelets. In the present study, we inhibited ADP using the ADP-metabolizing enzyme, apyrase. In apyrase-treated mice, platelet accumulation was unaltered, whereas the same treatment resulted in a significant reduction in leukocyte recruitment. These data could be interpreted to indicate that ADP-dependent platelet activation is not required for platelet accumulation in the glomerulus but contributes to the platelet P-selectin up-regulation following platelet recruitment. Given that our previous work shows a key role for platelet P-selectin in leukocyte recruitment,4 these data are consistent with a process whereby quiescent platelets are recruited to the altered glomerular microvasculature where they subsequently become activated and express P-selectin, which subsequently mediates glomerular leukocyte recruitment. However, these data are arguably not consistent with observations of a functional role for αIIbβ3 in platelet accumulation, as this adhesion molecule must be activated to a high affinity state to be capable of ligand binding. Notably, we have recently observed that under certain conditions, αIIbβ3 can contribute to platelet aggregation in the absence of a role for soluble agonists, including ADP.39 This indicates the existence of subtleties in the functions of platelets in the intact circulation. In light of these findings, it is possible that αIIbβ3 is activated by alternative unidentified soluble agonists or even intracellular signaling, neither of which would be affected by ADP inhibition.
In conclusion, this study is the first to use intravital microscopy to characterize the molecular mechanisms of glomerular platelet recruitment induced by in situ immune complex deposition. This work has demonstrated that the timing of platelet recruitment is consistent with them having a central role in initiating leukocyte recruitment. Moreover, the αIIbβ3/fibrinogen/ICAM-1 and GPVI pathways were identified as contributing for platelet recruitment to the inflamed glomerular microvasculature. These findings raise the possibility that platelets may contribute to glomerular leukocyte recruitment in other forms of glomerulonephritis.
Acknowledgments
We thank Dr. Simone Schoenwaelder and A/Prof. Robert Andrews (Australian Centre for Blood Diseases, Monash University) for advice and provision of reagents, Julie Hickey and Sarah Walker (Monash Micro Imaging) for assistance with electron microscopy, and Cecilia Lo for technical assistance.
Footnotes
Address reprint requests to Michael J. Hickey, Ph.D., Centre for Inflammatory Diseases, Monash University, Department of Medicine, Monash Medical Centre, 246 Clayton Road, Clayton, Victoria, 3168, Australia. E-mail: michael.hickey@monash.edu.
Supported by a National Health and Medical Research Council (Australia) Program grant (#334067).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current address of M.P.K.: Centenary Institute, Sydney, NSW, Australia; of R.Y.Q.K.: Centenary Institute, Sydney, NSW, Australia.
References
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Jung U, Ley K. Regulation of E-selectin. P-selectin, and intercellular adhesion molecule 1 expression in mouse cremaster muscle vasculature. Microcirculation. 1997;4:311–319. doi: 10.3109/10739689709146794. [DOI] [PubMed] [Google Scholar]
- Ley K, Gaehtgens P. Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ Res. 1991;69:1034–1041. doi: 10.1161/01.res.69.4.1034. [DOI] [PubMed] [Google Scholar]
- Kuligowski MP, Kitching AR, Hickey MJ. Leukocyte recruitment to the inflamed glomerulus: a critical role for platelet-derived P-selectin in the absence of rolling. J Immunol. 2006;176:6991–6999. doi: 10.4049/jimmunol.176.11.6991. [DOI] [PubMed] [Google Scholar]
- Mizgerd JP, Meek BB, Kutkoski GJ, Bullard DC, Beaudet AL, Doerschuk CM. Selectins and neutrophil traffic: margination and Streptococcus pneumoniae-induced emigration in murine lungs. J Exp Med. 1996;184:639–645. doi: 10.1084/jem.184.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Johnston B, Lee SS, Bullard DC, Smith CW, Beaudet AL, Kubes P. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest. 1997;99:2782–2790. doi: 10.1172/JCI119468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth SR, Tipping PG. Leukocytes in glomerular injury. Semin Immunopathol. 2007;29:355–374. doi: 10.1007/s00281-007-0097-9. [DOI] [PubMed] [Google Scholar]
- Ikezumi Y, Kanno K, Karasawa T, Han GD, Ito Y, Koike H, Toyabe S, Uchiyama M, Shimizu F, Kawachi H. The role of lymphocytes in the experimental progressive glomerulonephritis. Kidney Int. 2004;66:1036–1048. doi: 10.1111/j.1523-1755.2004.00852.x. [DOI] [PubMed] [Google Scholar]
- Zachem CR, Alpers CE, Way W, Shankland SJ, Couser WG, Johnson RJ. A role for P-selectin in neutrophil and platelet infiltration in immune complex glomerulonephritis. J Am Soc Nephrol. 1997;8:1838–1844. doi: 10.1681/ASN.V8121838. [DOI] [PubMed] [Google Scholar]
- Barnes JL. Platelets in glomerular disease. Nephron. 1997;77:378–393. doi: 10.1159/000190313. [DOI] [PubMed] [Google Scholar]
- Zoja C, Remuzzi G. Role of platelets in progressive glomerular diseases. Pediatr Nephrol. 1995;9:495–502. doi: 10.1007/BF00866739. [DOI] [PubMed] [Google Scholar]
- Cameron JS. Platelets in glomerular disease. Annu Rev Med. 1984;35:175–180. doi: 10.1146/annurev.me.35.020184.001135. [DOI] [PubMed] [Google Scholar]
- Ideura T, Ogasawara M, Tomura S, Ida T, Chida Y, Kuriyama R, Takeuchi J, Motomiya T, Yamazaki H. Effect of thrombocytopenia on the onset of immune complex glomerulonephritis. Nephron. 1992;60:49–55. doi: 10.1159/000186704. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Alpers CE, Pritzl P, Schulze M, Baker P, Pruchno C, Couser WG. Platelets mediate neutrophil-dependent immune complex nephritis in the rat. J Clin Invest. 1988;82:1225–1235. doi: 10.1172/JCI113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DI, Chen Z, Xu H, Li CQ, Dong J, McIntire LV, Ballantye CM, Zhang Li, Furman MI, Berndt MC, Lopez JA. Platelet Glycoprotein Ib-alpha is a Counterreceptor for the Leukocyte Integrin Mac-1 (CD11b/CD18). J Exp Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo GM, Dong JF, Schade AJ, Gardiner EE, Kansas GS, Li CQ, McIntire LV, Berndt MC, Lopez JA. The glycoprotein Ib-IX-V complex is a platelet counterreceptor for P-selectin. J Exp Med. 1999;190:803–814. doi: 10.1084/jem.190.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieswandt B, Brakebusch C, Bergmeier W, Schulte V, Bouvard D, Mokhtari-Nejad R, Lindhout T, Heemskerk JW, Zirngibl H, Fassler R. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO J. 2001;20:2120–2130. doi: 10.1093/emboj/20.9.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 2007;21:99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Andrews RK, Shen Y, Gardiner EE, Berndt MC. Platelet adhesion receptors and (patho)physiological thrombus formation. Histol Histopathol. 2001;16:969–980. doi: 10.14670/HH-16.969. [DOI] [PubMed] [Google Scholar]
- Massberg S, Enders G, Matos FC, Tomic LI, Leiderer R, Eisenmenger S, Messmer K, Krombach F. Fibrinogen deposition at the postischemic vessel wall promotes platelet adhesion during ischemia-reperfusion in vivo. Blood. 1999;94:3829–3838. [PubMed] [Google Scholar]
- Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Massberg S, Gawaz M, Gruner S, Schulte V, Konrad I, Zohlnhofer D, Heinzmann U, Nieswandt B. A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J Exp Med. 2003;197:41–49. doi: 10.1084/jem.20020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Cooper D, Arumugam TV, Zhang JH, Nanda A, Granger DN. Platelet-leukocyte-endothelial cell interactions after middle cerebral artery occlusion and reperfusion. J Cereb Blood Flow Metab. 2004;24:907–915. doi: 10.1097/01.WCB.0000132690.96836.7F. [DOI] [PubMed] [Google Scholar]
- Cooper D, Chitman KD, Williams MC, Granger DN. Time-depenedent platelet-vessel wall interactions induced by intestinal ischaemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1027–G1033. doi: 10.1152/ajpgi.00457.2002. [DOI] [PubMed] [Google Scholar]
- Massberg S, Enders G, Leiderer R, Eisenmenger S, Vestweber D, Krombach F, Messmer K. Platelet-endothelial cell interactions during ischemia/reperfusion: the role of P-selectin. Blood. 1998;92:507–515. [PubMed] [Google Scholar]
- Frenette PS, Johnson RC, Hynes RO, Wagner DD. Platelets roll on stimulated endothelium in vivo: An interaction mediated by endothelial P-selectin. Proc Natl Acad Sci USA. 1995;92:7450–7454. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz AR, Mendrick DL, Cotran RS, Mayadas TN. P-selectin deficiency exacerbates experimental glomerulonephritis: a protective role for endothelial P-selectin in inflammation. J Clin Invest. 1999;103:649–659. doi: 10.1172/JCI5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Tavares J, Hickey MJ, Hutchison J, Michaud J, Sutcliffe IT, Kubes P. A role for platelets and endothelial selectins in tumor necrosis factor-alpha-induced leukocyte recruitment in the brain microvasculature. Circ Res. 2000;87:1141–1148. doi: 10.1161/01.res.87.12.1141. [DOI] [PubMed] [Google Scholar]
- Asaduzzaman M, Lavasani S, Rahman M, Zhang S, Braun OO, Jeppsson B, Thorlacius H. Platelets support pulmonary recruitment of neutrophils in abdominal sepsis. Crit Care Med. 2009;37:1389–1396. doi: 10.1097/CCM.0b013e31819ceb71. [DOI] [PubMed] [Google Scholar]
- Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- Fernandes LS, Conde ID, Wayne Smith C, Kansas GS, Snapp KR, Bennet N, Ballantyne C, McIntire LV, O'Brian Smith E, Klem JA, Mathew S, Frangogiannis N, Turner NA, Maresh KJ, Kleiman NS. Platelet-monocyte complex formation: effect of blocking PSGL-1 alone, and in combination with alphaIIbbeta3 and alphaMbeta2, in coronary stenting. Thromb Res. 2003;111:171–177. doi: 10.1016/j.thromres.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieswandt B, Bergmeier W, Schulte V, Rackebrandt K, Gessner JE, Zirngibl H. Expression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcRgamma chain. J Biol Chem. 2000;275:23998–24002. doi: 10.1074/jbc.M003803200. [DOI] [PubMed] [Google Scholar]
- Maxwell MJ, Yuan Y, Anderson KE, Hibbs ML, Salem HH, Jackson SP. SHIP1 and Lyn Kinase Negatively Regulate Integrin alpha IIb beta 3 signaling in platelets. J Biol Chem. 2004;279:32196–32204. doi: 10.1074/jbc.M400746200. [DOI] [PubMed] [Google Scholar]
- Choudhri TF, Hoh BL, Zerwes HG, Prestigiacomo CJ, Kim SC, Connolly ES, Jr, Kottirsch G, Pinsky DJ. Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting GP IIb/IIIa receptor-mediated platelet aggregation. J Clin Invest. 1998;102:1301–1310. doi: 10.1172/JCI3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews RK, Kroll MH, Ward CM, Rose JW, Scarborough RM, Smith AI, Lopez JA, Berndt MC. Binding of a novel 50-kilodalton alboaggregin from Trimeresurus albolabris and related viper venom proteins to the platelet membrane glycoprotein Ib-IX-V complex. Effect on platelet aggregation and glycoprotein Ib-mediated platelet activation, Biochemistry. 1996;35:12629–12639. doi: 10.1021/bi960704e. [DOI] [PubMed] [Google Scholar]
- Sun D, McNicol A, James AA, Peng Z. Expression of functional recombinant mosquito salivary apyrase: a potential therapeutic platelet aggregation inhibitor. Platelets. 2006;17:178–184. doi: 10.1080/09537100500460234. [DOI] [PubMed] [Google Scholar]
- Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, Carberry J, Fouras A, Jackson SP. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- Kuligowski MP, Kwan RY, Lo C, Wong C, James WG, Bourges D, Ooi JD, Abeynaike LD, Hall P, Kitching AR, Hickey MJ. Antimyeloperoxidase antibodies rapidly induce alpha-4-integrin-dependent glomerular neutrophil adhesion. Blood. 2009;113:6485–6494. doi: 10.1182/blood-2008-12-192617. [DOI] [PubMed] [Google Scholar]
- Ruth AJ, Kitching AR, Semple TJ, Tipping PG, Holdsworth SR. Intrinsic renal cell expression of CD40 directs Th1 effectors inducing experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2003;14:2813–2822. doi: 10.1097/01.asn.0000091381.60059.fb. [DOI] [PubMed] [Google Scholar]
- Tailor A, Granger DN. Hypercholesterolemia promotes P-selectin-dependent platelet-endothelial cell adhesion in postcapillary venules. Arterioscler Thromb Vasc Biol. 2003;23:675–680. doi: 10.1161/01.ATV.0000056742.97580.79. [DOI] [PubMed] [Google Scholar]
- Larson MK, Watson SP. Regulation of proplatelet formation and platelet release by integrin alpha IIb beta3. Blood. 2006;108:1509–1514. doi: 10.1182/blood-2005-11-011957. [DOI] [PubMed] [Google Scholar]
- Kuhn K, Ryan GB, Hein SJ, Galaske RG, Karnovsky MJ. An ultrastructural study of the mechanisms of proteinuria in rat nephrotoxic nephritis. Lab Invest. 1977;36:375–387. [PubMed] [Google Scholar]
- Kottirsch G, Zerwes HG, Cook NS, Tapparelli C. Beta- amino acid derivatives as orally active non-peptide fibrinogen receptor antagonists. Bioorg Med Chem Lett. 1997:727–732. [Google Scholar]
- Alevriadou BR, Moake JL, Turner NA, Ruggeri ZM, Folie BJ, Phillips MD, Schreiber AB, Hrinda ME, McIntire LV. Real-time analysis of shear-dependent thrombus formation and its blockade by inhibitors of von Willebrand factor binding to platelets. Blood. 1993;81:1263–1276. [PubMed] [Google Scholar]
- Chen H, Locke D, Liu Y, Liu C, Kahn ML. The platelet receptor GPVI mediates both adhesion and signaling responses to collagen in a receptor density-dependent fashion. J Biol Chem. 2002;277:3011–3019. doi: 10.1074/jbc.M109714200. [DOI] [PubMed] [Google Scholar]
- Moroi M, Jung SM, Shinmyozu K, Tomiyama Y, Ordinas A, Diaz-Ricart M. Analysis of platelet adhesion to a collagen-coated surface under flow conditions: the involvement of glycoprotein VI in the platelet adhesion. Blood. 1996;88:2081–2092. [PubMed] [Google Scholar]
- Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- Hill PA, Lan HY, Nikolic-Paterson DJ, Atkins RC. The ICAM-1/LFA-1 interaction in glomerular leukocytic accumulation in anti-GBM glomerulonephritis. Kidney Int. 1994;45:700–708. doi: 10.1038/ki.1994.94. [DOI] [PubMed] [Google Scholar]
- Coxon A, Cullere X, Knight S, Sethi S, Wakelin MW, Stavrakis G, Luscinskas FW, Mayadas TN. Fc gamma RIII mediates neutrophil recruitment to immune complexes. a mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity. 2001;14:693–704. doi: 10.1016/s1074-7613(01)00150-9. [DOI] [PubMed] [Google Scholar]
- Tang T, Rosenkranz A, Assmann KJ, Goodman MJ, Gutierrez-Ramos JC, Carroll MC, Cotran RS, Mayadas TN. A role for Mac-1 (CDIIb/CD18) in immune complex-stimulated neutrophil function in vivo: mac-1 deficiency abrogates sustained Fcgamma receptor-dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J Exp Med. 1997;186:1853–1863. doi: 10.1084/jem.186.11.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XR, Tipping PG, Apostolopoulos J, Oettinger C, D'Souza M, Milton G, Holdsworth SR. Mechanisms of T cell-induced glomerular injury in anti-glomerular basement membrane (GBM) glomerulonephritis in rats. Clin Exp Immunol. 1997;109:134–142. doi: 10.1046/j.1365-2249.1997.4091307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109:5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23:2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- Khandoga A, Biberthaler P, Enders G, Axmann S, Hutter J, Messmer K, Krombach F. Platelet adhesion mediated by fibrinogen-intercelllular adhesion molecule-1 binding induces tissue injury in the postischemic liver in vivo. Transplantation. 2002;74:681–688. doi: 10.1097/00007890-200209150-00016. [DOI] [PubMed] [Google Scholar]
- Bombeli T, Schwartz BR, Harlan JM. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPIbalpha. J Exp Med. 1998;187:329–339. doi: 10.1084/jem.187.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutaud A, Borza DB, Bondar O, Gunwar S, Netzer KO, Singh N, Ninomiya Y, Sado Y, Noelken ME, Hudson BG. Type IV collagen of the glomerular basement membrane. Evidence that the chain specificity of network assembly is encoded by the noncollagenous NC1 domains. J Biol Chem. 2000;275:30716–30724. doi: 10.1074/jbc.M004569200. [DOI] [PubMed] [Google Scholar]
- Miyatake N, Shikata K, Sugimoto H, Kushiro M, Shikata Y, Ogawa S, Hayashi Y, Miyasaka M, Makino H. Intercellular adhesion molecule 1 mediates mononuclear cell infiltration into rat glomeruli after renal ablation. Nephron. 1998;79:91–98. doi: 10.1159/000044997. [DOI] [PubMed] [Google Scholar]
- Wada J, Shikata K, Makino H, Morioka S, Hirata K, Ota K, Tamatani T, Miyasaka M, Horiuchi T, Noji S, Nishikawa K, Myokai F, Taniguchi S, Kanwar Y, Ota Z. The critical role of intercellular adhesion molecule-1 in Masugi nephritis in rats. Nephron. 1996;73:264–272. doi: 10.1159/000189050. [DOI] [PubMed] [Google Scholar]
- Xu H, Gonzalo JA, St Pierre Y, Williams IR, Kupper TS, Cotran RS, Springer TA, Gutierrez-Ramos JC. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J Exp Med. 1994;180:95–109. doi: 10.1084/jem.180.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sligh JJ, Ballantyne C, Rich S, Hawkins HK, Smith CW, Bradley A, Beaudet AL. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Kato Y, Hori S, Fujita N, Tsuruo T. A novel anti-platelet monoclonal antibody induces mouse platelet aggregation through an Fc receptor-independent mechanism. Biochem Biophys Res Commun. 1998;242:250–255. doi: 10.1006/bbrc.1997.7917. [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- Ma YQ, Qin J, Plow EF. Platelet integrin alpha(IIb)beta(3): activation mechanisms. J Thromb Haemost. 2007;5:1345–1352. doi: 10.1111/j.1538-7836.2007.02537.x. [DOI] [PubMed] [Google Scholar]
- Andrews RK, Gardiner EE, Shen Y, Berndt MC. Platelet interactions in thrombosis. IUBMB Life. 2004;56:13–18. doi: 10.1080/15216540310001649831. [DOI] [PubMed] [Google Scholar]
- Suzuki-Inoue K, Inoue O, Frampton J, Watson SP. Murine GPVI stimulates weak integrin activation in PLCgamma2-/- platelets: involvement of PLCgamma1 and PI3-kinase. Blood. 2003;102:1367–1373. doi: 10.1182/blood-2003-01-0029. [DOI] [PubMed] [Google Scholar]