Abstract
Objective
Inhibition of angiotensin II receptor type 1 (AT1) reduces chronic inflammation associated with hypertension. We asked whether AT1 receptor inhibition would reduce the innate inflammatory response induced by bacterial lipopolysaccharide (LPS).
Methods
We used unstimulated human circulating monocytes obtained from healthy donors by counterflow centrifugal elutriation. Monocytes were studied in vitro after incubation with LPS (50 ng/ml) with and without 1 μmol/l candesartan, an AT1 receptor blocker. Angiotensin II receptor mRNA expression was determined by reverse transcriptase-PCR and receptor binding by autoradiography; inflammatory factor mRNA expression was studied by reverse transcriptase-PCR and cytokine release by ELISA.
Results
Human monocytes did not express detectable AT1 receptors, and angiotensin II did not induce inflammatory factor mRNA expression or cytokine release. However, candesartan substantially reduced the LPS-induced expression of the mRNAs for the LPS recognition protein cluster of differentiation 14, the proinflammatory cytokines tumor necrosis factor alpha, interleukin-1 beta and interleukin-6 and the lectin-like oxidized low-density lipoprotein receptor. In addition, candesartan reduced the activation of the nuclear factor kappa B pathway, the tumor necrosis factor alpha and interleukin-6 secretion, and the ROS formation induced by LPS, without affecting the secretion of interleukin-10.
Conclusion
We hypothesize that the anti-inflammatory effects of candesartan in these cells are likely mediated by mechanisms unrelated to AT1 receptor blockade. Our results demonstrate that candesartan significantly reduces the innate immune response to LPS in human circulating monocytes. The anti-inflammatory effects of candesartan may be of importance not only in hypertension but also in other inflammatory disorders.
Keywords: angiotensin receptor blockers, cytokines, inflammation, inhibitor of kappa B alpha, interleukin 6, interleukin-1 beta, renin–angiotensin system, transcription factors, tumor necrosis factor alpha
Introduction
In addition to its role in the regulation of blood pressure (BP), angiotensin II (Ang II), the active principle of the renin–angiotensin system (RAS), is a key proinflammatory factor in blood vessels through stimulation of its angiotensin II receptor type 1 (AT1) [1–3]. Enhanced RAS activity, frequently associated with hypertension and diabetes, is an important determinant of endothelial dysfunction contributing to the development of atherosclerosis and peripheral vascular complications [3–7]. Ang II AT1 receptor blockers (ARBs) reverse the chronic peripheral and brain vascular inflammatory reaction associated with hypertension [8–10]. The anti-inflammatory effects of ARBs include decrease in oxidative stress and reversal of endothelial dysfunction by mechanisms unrelated to BP decrease [11–14]. This indicates that anti-inflammatory effects of ARBs are therapeutically important but not dependent on their effect on BP.
The role of Ang II in the regulation of inflammatory responses was established early during evolution and precedes its participation in cardiovascular control [15]. In invertebrates, Ang II regulates the innate immune response through mechanisms controlled by the nervous system [15]. The innate immune response is the initial inflammatory response to infection. This requires, as a first step, the recognition of specific microbial antigens including the endotoxin lipopolysaccharide (LPS), a major component of the outer membranes of Gram-negative bacteria. Recognition of LPS by the innate immune system leads to immediate cell activation and the release of proinflammatory cytokines. LPS recognition is facilitated by a cluster of differentiation 14 (CD14), which exists in both soluble and membrane-anchored forms [16]. The bound LPS then associates with Toll-like receptor 4 (TLR4), a signaling receptor of the Toll receptor family [17]. As a consequence, the transcription factor nuclear factor kappa B (NFκB) is activated, leading to transcriptional stimulation of proinflammatory genes and promotion of monocyte and macrophage attachment to the vascular endothelium, thus initiating the inflammatory chain of events in the vasculature that is a characteristic of hypertension, arteriosclerosis and diabetes [18,19].
Circulating monocytes are prime targets for LPS [20]. These cells play a major role in the development of both atherosclerosis and inflammation [18,21]. Monocytes differentiated into macrophages express a complete RAS, including AT1 receptors [22]. These observations indicate that the inhibition of AT1 receptor stimulation in the crucial macrophage/endothelial cell link of the innate immune response may contribute to explain the anti-inflammatory effects of ARBs.
We asked whether AT1 receptors participate in the innate immune response produced by bacterial endotoxin, and we attempted to clarify the mechanisms underlying such effect. To answer this question, we studied the effect of the ARB candesartan in circulating human monocytes incubated with LPS. We examined the effects of LPS and candesartan on LPS receptors, gene expression and secretion of proinflammatory cytokines and the NFκB pathway.
Materials and methods
Human monocytes
Human peripheral blood mononuclear cells were obtained by density gradient centrifugation of heparinized blood, collected by leukaphoresis of healthy volunteers [Department of Transfusion Medicine, National Institutes of Health (NIH)].
Purified populations of nonactivated monocytes were prepared by counterflow centrifugal elutriation on a Beckman elutriation system [23]. Monocyte preparations were enriched to above 90% as determined by flow cytometry. Monocytes were subsequently allowed to attach to the cell culture plates for 2 h at 37°C in humidified 5% CO2–95% air, and nonadherent cells were removed.
Cell culture conditions
Human monocytes were seeded at 2 × l06 cells/ml in serum-free high-glucose Dulbecco's-modified Eagle's medium (DMEM), (Invitrogen, Carlsbad, California, USA) containing 50 μg/ml gentamicin, and cultured in 100-mm culture dishes or six-well plates at 37°C in humidified 5% CO2–95% air. Monocytes were preincubated with vehicle; the ARBs, candesartan (1 μmol/l), losartan (10 μmol/l) (Sigma, St. Louis, Missouri, USA) or telmisartan (10 μmol/l) (Sigma); the antioxidant, N-acetyl–cysteine (NAC) (10 mmol/l) (Sigma); or peroxisome proliferator-activated receptor gamma (PPARγ) agonist, troglitazone (10 μmol/l) (Sigma) for 2 h. After preincubation, the monocytes were incubated in the presence of LPS (50 ng/ml) (Escherichia coli serotype 055:B5; Sigma). LPS was added for 2 h for PCR analysis, for 4 h for tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6) determinations and for 24 h for interleukin-10 (IL-10) determination in cell culture supernatants. Separated monocyte preparations preincubated with vehicle were incubated with Ang II (100 nmol/l; Bachem, Torrance, California, USA), 50 ng/ml LPS or physiological saline for 2 or 24 h to determine the effects of Ang II or LPS stimulation on Ang IIAT1 and angiotensin II receptor type 2 (AT2) receptor and inflammatory marker mRNA expression. The data presented were collected from blood samples of 21 different donors studied individually. All the experiments were approved by the NIH's Office of Human Subjects Research (OHSR).
Angiotensin II receptor binding
Monocyte pellets were prepared by centrifuging monocytes in Dulbecco's PBS in 1.5 ml polypropylene tubes at 1000g for 5 min at 4°C followed by freezing in isopentane on dry ice [23]. Frozen monocyte pellets from four donors were cut individually into 16-μm thick consecutive sections in a cryostat at −20°C. The sections were thaw-mounted onto gelatin-coated glass slides and dried under vacuum at 4°C overnight.
Quantification of Ang II bound to AT1 and AT2 receptor types was performed as previously described [23], by incubating the sections with 0.5 nmol/l [125I]Sarcosine1–Ang II ([125I]Sar1–Ang II) (Peninsula Laboratories, Belmont, California, USA), which was iodinated by the Peptide Radioiodination Service Center (School of Pharmacy, University of Mississippi, Mississippi, USA). The labeled ligand was selectively displaced in consecutive sections, with 10 μmol/l losartan, a selective AT1 receptor antagonist, or 10 μmol/l PD123319 (Sigma), a selective AT2 receptor antagonist. The nonspecific binding was determined by displacement with 5 μmol/l Ang II. Optical densities of autoradiograms generated by incubation with the 125I ligand were normalized after comparison with 14C standards as described [24] and were quantified by computerized densitometry using the Scion Image 4.0.2 for Windows (Scion Corporation, Frederick, Maryland, USA) based on the NIH Image Program of the National Institutes of Health. The binding to AT1 and AT2 receptors was defined as the binding displaced by losartan and PD123319, respectively [24].
Measurement of mRNA expression levels by real-time PCR
Total RNA was isolated individually from lysed human monocytes using 1 ml TRIzol (Invitrogen), followed by purification using an RNeasy Mini kit (Qiagen, Valencia, California, USA) in accordance with our previous studies [25]. cDNA was prepared by reverse transcription of 2 μg of total RNA using Super-Script III first-Strand Synthesis kit (Invitrogen). Quantitative real-time PCR was performed on DNA Engine Opticon (MJ Research, Waltham, Massachusetts, USA). PCR reaction was performed in a 20 μl volume containing 10 μl SYBR Green PCR Master Mix (Applied Biosystems, Foster City, California, USA), 2 μl of diluted cDNA and 0.3 μmol/l of each primer for a specific target. The primers are listed in Table 1. The amplification conditions consisted of one denaturation/activation cycle at 95°C for 10 min, followed by 40–45 cycles at 95°C for 15 s and 56 or 60°C for 60 s followed by final extension step for 10 min at 72°C. Serial dilutions of cDNA from the same source as samples were used to obtain a calibration curve. The individual targets for each sample were quantified by determining the cycle threshold (Ct) and by using calibration curves. The relative amount of the target was normalized with the housekeeping gene 18S rRNA.
Table 1. List of PCR primers used in this study.
| Product | Accession # | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|---|
| 18S rRNA | X03205 | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
| AT1 | S77410 | ACCGCCCCTCAGATAATGTAAG | TGAAGTGCTGCAGAGGAATGTT |
| AT2 | NM_000686 | CATTGACCTGGCACTTCCTT | AAACACACTGCGGAGCTTCT |
| CD14 | AB446505 | TGCGCAACACAGGAATGG | CTCGGAGCGCTAGGGTTTAC |
| IκBα | NM_020529 | CGGACTGCCCTTCACCTC | ACATCAGCCCCACACTTCAA |
| IL-1β | NM_000576 | GTCATTCGCTCCCACATTCT | CTACTTCTTGCCCCCTTTGA |
| IL-6 | NM_000600 | CCAGTACCCCCAGGAGAAGAT | GAGGATGTACCGAATTTGTTTGTC |
| LOX-1 | NM_002543 | CTTTGATGCCCCACTTATTTAG | GGTTTGCCTTCTTCTGACAT |
| TLR4 | AB445638 | TTTCAGCTCTGCCTTCACTACA | ATCACCTTTCGGCTTTTATGG |
| TNFα | NM_000594 | CCAGCTGGAGAAGGGTGAC | AGGCGTTTGGGAAGGTTG |
AT1, angiotensin II receptor type 1; AT2, angiotensin II receptor type 2; CD14, cluster of differentiation 14; IκBα, inhibitor of kappa B alpha; IL, interleukin; LOX-1, lectin-like oxidized low-density lipoprotein receptor 1; rRNA, ribosomal RNA; TLR4, Toll-like receptor 4; TNFα, tumor necrosis factor alpha.
For determination of AT1 and AT2 receptor expression, the products of PCR amplification were separated on 4% agarose gel and visualized by staining with ethidium bromide to verify the size of amplicon.
ELISA
Human TNFα, IL-6 and IL-10 concentrations in culture medium were measured by human TNFα and IL-6 ELISA kits (Biosource, Camarillo, California, USA) and human IL-10 Quantikine kits (R&D, Minneapolis, Minnesota, USA) according to the manufacturer's protocol.
Detection of intracellular reactive oxygen species
The level of intracellular ROS was determined by the change in fluorescence resulting from the oxidation of the fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Invitrogen) [26]. Monocytes (2 × 105 cells per well) were exposed to 1 μmol/l candesartan or 10 mmol/l NAC for 2 h. After incubation, the cells were exposed to 50 ng/ml LPS for 2 h. Cells were washed three times by PBS (140 mmol/l Na2HPO4, 1.8 mmol/l KH2PO4, pH 7.2, 138 mmol/l NaCl and 2.7 mmol/l KCl) and incubated with 10 μmol/l of H2DCFDA for 20 min at 37°C. After two washes in PBS, the cells were lysed in a mixture of 90% dimethyl sulfoxide (Sigma) and 10% PBS. The degree of fluorescence, corresponding to intracellular ROS, was determined using VICTOR3 plate reader (Perkin-Elmer, Torrance, California, USA) with 485-nm excitation and 535-nm emission filters.
Statistical analysis
Statistical significance was determined with the Wilcoxon matched paired test. Multiple group comparisons were performed by one-way analysis of variance followed by Newman–Keuls posttest. Differences were considered statistically significant at a P value of less than 0.05.
Results
Expression of angiotensin II receptor types in human circulating monocytes
Analysis of human circulating monocytes did not reveal significant presence of AT1 or AT2 receptors. Reverse transcriptase-PCR analysis showed very low, barely detectable, AT1 or AT2 mRNA levels in only about half of the samples, thus making quantification unreliable (Fig. 1). Receptor binding analysis, as determined by incubating sections from monocyte pellets with radiolabeled Sar1–Ang II and selective AT1 and AT2 ligands, did not produce signals significantly higher than background (Fig. 2).
Fig. 1.
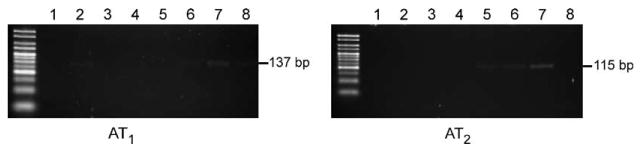
Expression of angiotensin II receptor type mRNAs in human monocytes. Total RNA from human monocytes was reversely transcribed into cDNA and expression of AT1 and AT2 receptors was analyzed by reverse transcriptase-PCR. Products of PCR amplification were resolved by electrophoresis on 4% agarose gel and stained with ethidium bromide as described in Materials and Methods. Each lane, 1 through 8, was generated from a monocyte sample from a different individual donor, and AT1 and AT2 receptors were studied in sample aliquots from each of the individual donors. AT1 mRNA, of expected size 137 bp, was detected only in lanes 2, 6, 7 and 8. AT2 mRNA, expected size 115 bp, was detected only in lanes 5, 6 and 7.
Fig. 2.
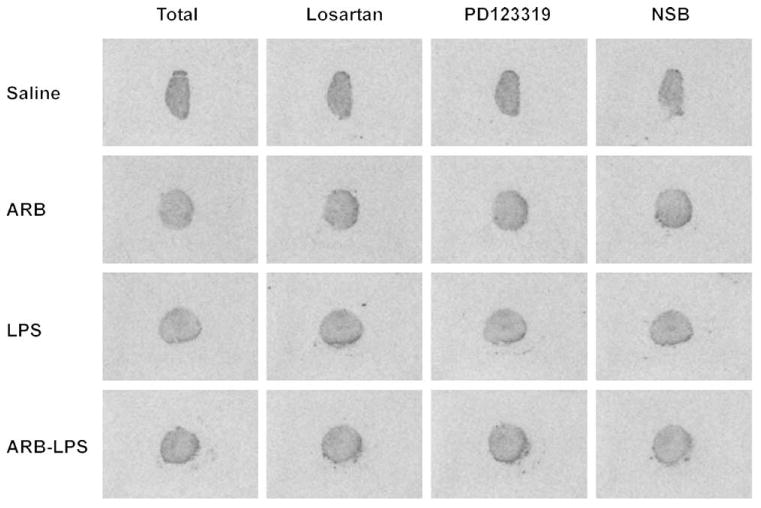
Expression of angiotensin II receptor type binding in human monocytes. Human monocytes were incubated for 24 h with physiological saline, 1 μmol/l candesartan (ARB), 50 ng/ml LPS or both LPS and candesartan (ARB–LPS). At the end of incubation, monocytes were pelleted and frozen pellets were cut to 16-μm consecutive sections for receptor binding analysis as described in Materials and Methods. Total pictures show binding obtained after incubation with [125I]Sar1–Ang II only and represent binding to both AT1 and AT2 receptors. Losartan pictures were obtained from consecutive sections incubated as in total with the addition of losartan, a selective AT1 receptor antagonist, to displace binding to AT1 receptors. Figures represent binding to AT2 receptors. PD123319 pictures were obtained from consecutive sections incubated as in total with the addition of PD123319, a selective AT2 receptor antagonist, to displace binding to AT2 receptors. Figures represent binding to AT1 receptors. NSB was obtained from consecutive sections incubated as in total with addition of unlabeled Ang II as described in Materials and Methods. Note that there is no difference in density in any of the sections studied, indicating absence of AT1 and AT2 receptors, and that incubation in the presence of LPS or candesartan did not induce expression of AT1 or AT2 receptor binding. Figures are representative of three determinations in section pellets obtained from three different donors and run in three different experiments. Ang II, angiotensin II; ARB, angiotensin II receptor blocker; LPS, lipopolysaccharide; NSB, nonspecific binding.
Incubation of human circulating monocytes for 24 h in the presence of 100 nmol/l Ang II or 50 ng/ml LPS did not induce the expression of Ang II AT1 or AT2 receptor mRNA, as determined by reverse transcriptase-PCR (results not shown).
Effects of incubation with lipopolysaccharide on human monocytes
Incubation of monocytes with LPS alone did not change the expression of mRNA for the TLR4 and CD14 receptors (Fig. 3a), but induced the mRNA expression of TNFα, interleukin-1 beta (IL-1β), IL-6, inhibitor of kappa B alpha (IκBα) and the lectin-like oxidized low-density lipoprotein receptor 1 (LOX-1) (Fig. 3b), and the secretion of TNFα, IL-6 and IL-10 (Fig. 3c). In addition, LPS strongly enhanced ROS production (Fig. 4).
Fig. 3.
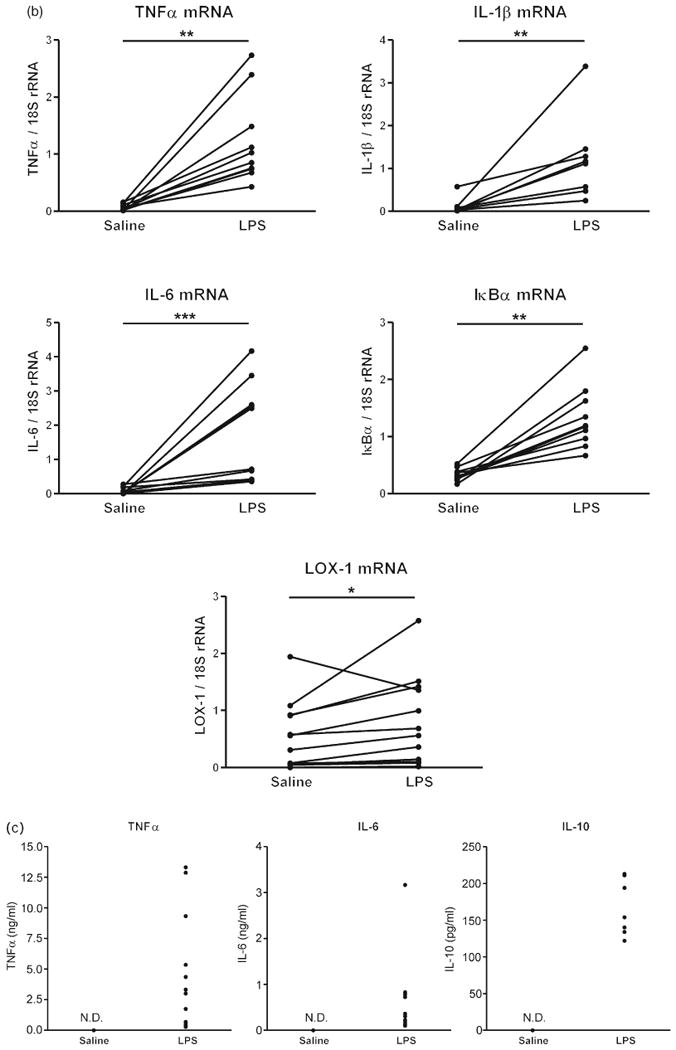
(a) Effects of lipopolysaccharide on lipopolysaccharide receptor mRNAs in human monocytes. Human monocytes were incubated with physiological saline or 50 ng/ml LPS and CD14, and TLR4 mRNAs were quantified by reverse transcriptase-PCR as described in Materials and Methods (n = 8–13, assayed individually). Statistical significance was determined with the Wilcoxon matched paired test. (b) Effects of LPS on inflammatory marker mRNAs in human monocytes. Human monocytes were incubated with physiological saline or 50 ng/ml LPS, and mRNAs for inflammatory markers were quantified by reverse transcriptase-PCR as described in Materials and Methods. Statistical significance was determined with the Wilcoxon matched paired test. *P < 0.05, **P < 0.01, ***P < 0.001, LPS vs. Saline (n = 8–13, assayed individually). (c) Effects of LPS on cytokine secretion in human monocytes. Human monocytes were incubated with physiological saline or 50 ng/ml LPS and cytokine release was determined in culture medium by specific ELISAs as described in Materials and Methods (n = 8–13, assayed individually). IκBα, inhibitor of kappa B alpha; IL, interleukin; LOX-1, lectin-like oxidized low-density lipoprotein receptor 1; LPS, lipopolysaccharide; ND, nondetectable; rRNA, ribosomal RNA; TLR4, Toll-like receptor 4; TNF, tumor necrosis factor.
Fig. 4.
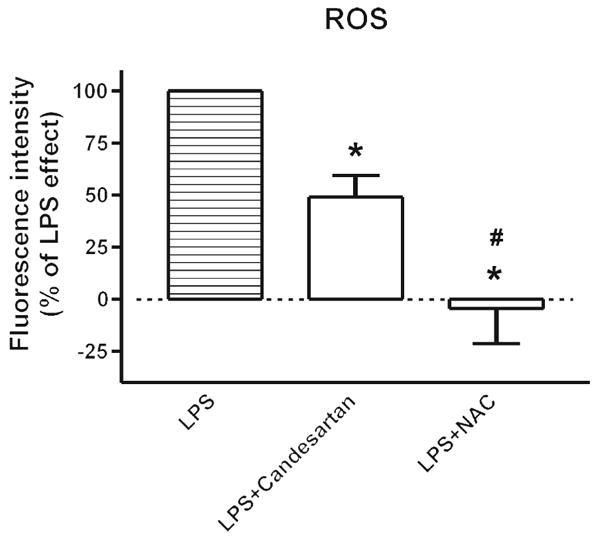
Candesartan inhibits lipopolysaccharide-induced reactive oxygen species generation in human monocytes. Human monocytes were incubated with 50 ng/ml LPS alone or in the presence of 1 μmol/l candesartan or 10 mmol/l NAC for 2 h, and then incubated with 10 μmol/l H2DCFDA for ROS detection as described in Material and Methods. Data are expressed as a percentage of the response to LPS (basal subtracted) and are presented as means ± SEM of three monocyte preparations assayed individually. Individual groups were compared by one-way ANOVA followed by Newman–Keuls posttest. *P < 0.05 compared with LPS, #P < 0.05 compared with LPS + candesartan. ANOVA, analysis of variance; H2DCFDA, 2′,7′-dichlorodihydrofluorescein diacetate; LPS, lipopolysaccharide; NAC, N-acetyl–cysteine; ROS, reactive oxygen species.
Effects of angiotensin II on human monocytes
Incubation of monocytes with Ang II did not influence either TNFα secretion (not shown) or the expression of TNFα, IκBα or LOX-1 mRNA, whether the monocytes were incubated with Ang II for 2 or for 24 h (Fig. 5).
Fig. 5.
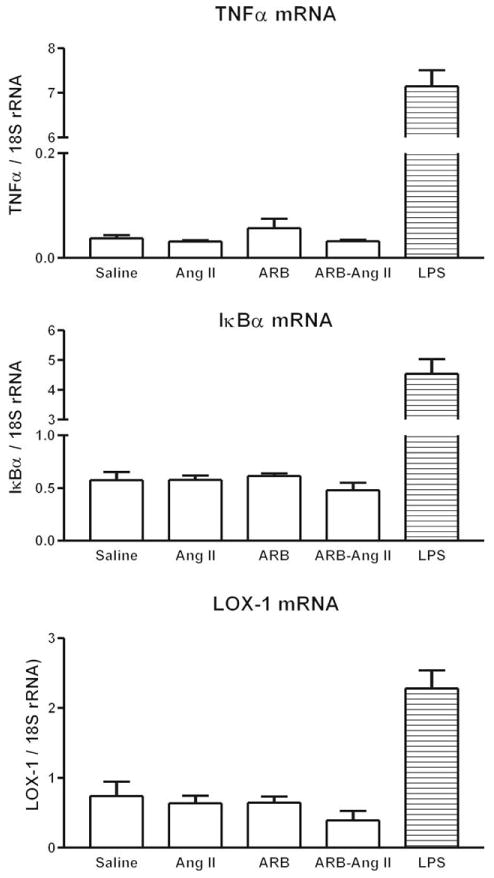
Effect of angiotensin II on inflammatory marker mRNAs in human monocytes. Human monocytes were incubated with physiological saline, 100 nmol/l Ang II, 1 μmol/l candesartan (ARB), Ang II and candesartan (ARB–Ang II) or 50 ng/ml LPS, and mRNAs were quantified by reverse transcriptase-PCR as described in Materials and Methods. Results are means ± SEM of three different monocyte preparations assayed individually. Ang II, angiotensin II; ARB, angiotensin II receptor blocker; IκBα, inhibitor of kappa B alpha; LOX-1, lectin-like oxidized low-density lipoprotein receptor 1; LPS, lipopolysaccharide; rRNA, ribosomal RNA; TNF, tumor necrosis factor.
Effects of candesartan on the lipopolysaccharide-induced innate immune response in human monocytes
Preliminary results indicated that a maximal decrease in LPS-induced effects was observed when monocytes were preincubated with candesartan for at least 1 h. We next determined, by analysis of the effect of candesartan on LPS-induced IL-6 release, the most effective inhibitory concentration. We found that a concentration of 1 μmol/l candesartan had maximum inhibitory effects on LPS-induced IL-6 release (Fig. 6). For this reason, 1 μmol/l concentration of the ARB and an incubation time of 2 h were used for subsequent experiments.
Fig. 6.
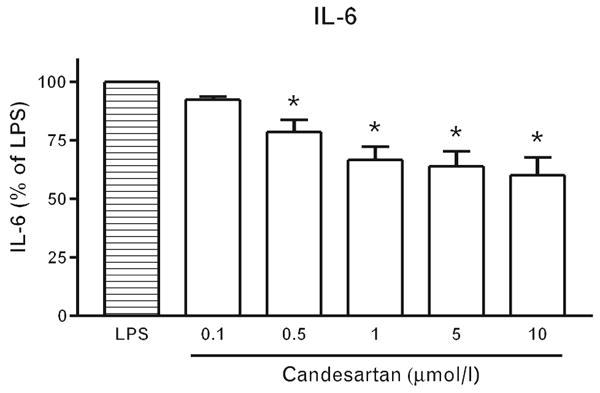
Dose-dependent inhibition of lipopolysaccharide-induced interleukin 6 release by candesartan. Human monocytes were incubated with LPS alone or in the presence of various candesartan concentrations (0.1–10 μmol/l). After 4 h of incubation, IL-6 release was quantified in culture medium by specific ELISA as described in Material and Methods. Results are means ± SEM of three different monocyte preparations assayed individually. Individual groups were compared by one-way ANOVA followed by Newman–Keuls posttest. *P < 0.05 compared with LPS. ANOVA, analysis of variance; IL, interleukin; LPS, lipopolysaccharide.
Preincubation of monocytes with candesartan alone did not produce any significant changes to the observed parameters. In monocytes exposed to LPS, candesartan significantly reduced the expression of CD14 mRNA but did not alter the expression of TLR4 (Fig. 7a). LPS-induced expression of TNFα, IL-1β, IL-6, LOX-1 and IκBα mRNA was significantly attenuated by preincubation with candesartan (Fig. 7b). Incubation with candesartan also significantly reduced the LPS-induced secretion of the proinflammatory cytokines TNFα and IL-6 without affecting the LPS-induced secretion of the anti-inflammatory cytokine IL-10 (Fig. 7c).
Fig. 7.
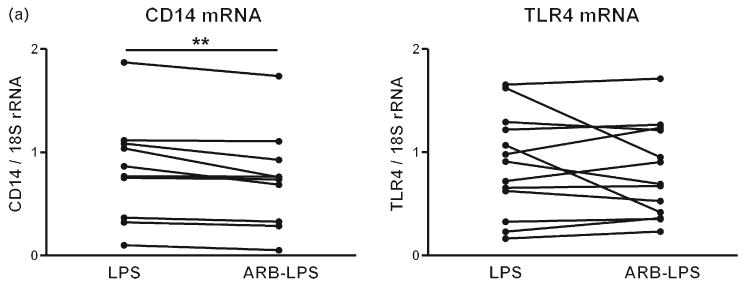
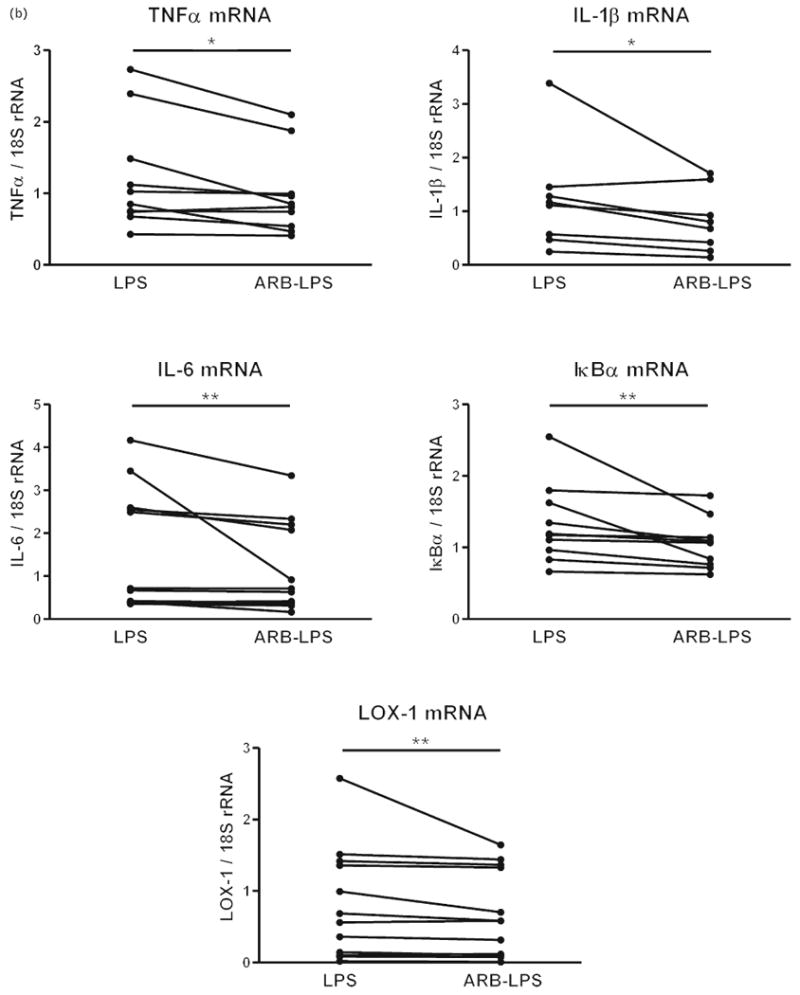
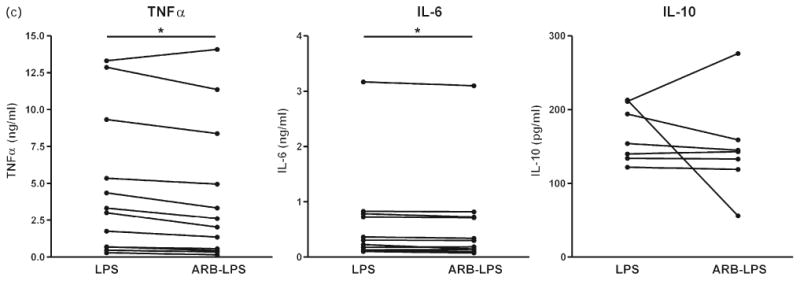
(a) Effect of candesartan and lipopolysaccharide on lipopolysaccharide receptor mRNA expression in human monocytes. Human monocytes were incubated with LPS alone or in the presence of candesartan (ARB–LPS), and the expression of CD14 and TLR4 mRNAs was quantified by reverse transcriptase-PCR as described in Materials and Methods. Monocytes from eight to 12 individual donors were studied individually. Statistical significance was determined with the Wilcoxon matched paired test. **P < 0.01 vs. LPS-treated group. (b). Effect of candesartan and LPS on inflammatory marker mRNA expression in human monocytes. Human monocytes were incubated with LPS alone or in the presence of candesartan (ARB–LPS), and mRNAs for inflammatory markers were quantified by reverse transcriptase-PCR as described in Materials and Methods. Monocytes from eight to 12 individual donors were studied individually. Statistical significance was determined with the Wilcoxon matched paired test. *P < 0.05 vs. LPS-treated group. **P < 0.01 vs. LPS-treated group. (c) Effect of candesartan on LPS-induced secretion of cytokines from human monocytes. Human monocytes were incubated with LPS alone or in the presence of candesartan (ARB–LPS) and cytokine release was quantified in culture medium by specific ELISAs as described in Material and Methods. Monocytes from six to 12 individual donors were studied individually. Statistical significance was determined with the Wilcoxon matched paired test. *P < 0.05. vs. LPS-treated group. ARB, angiotensin II receptor blocker; IκBα, inhibitor of kappa B alpha; IL, interleukin; LOX-1, lectin-like oxidized low-density lipoprotein receptor 1; LPS, lipopolysaccharide; rRNA, ribosomal RNA; TLR4 Toll-like receptor 4; TNF tumor necrosis factor.
In addition, preincubation with candesartan partially reduced the LPS-induced ROS formation in human monocytes (Fig. 4). Although pronounced, the effect of the ARB did not achieve complete inhibition of the LPS-induced ROS formation as produced by preincubation with the antioxidant NAC (Fig. 4).
We studied the effect of comparable concentrations of the ARBs, candesartan, telmisartan and losartan, on LPS-induced IL-6 release, and compared these effects with those of the PPARγ agonist, troglitazone, and the antioxidant, NAC. We found pronounced decreases in LPS-induced IL-6 release after incubation with telmisartan and candesartan, a smaller effect after incubation with losartan (Fig. 8). The effects of telmisartan and candesartan were comparable to those obtained after troglitazone incubation, but did not reach the complete inhibition of the LPS effect produced by NAC (Fig. 8).
Fig. 8.
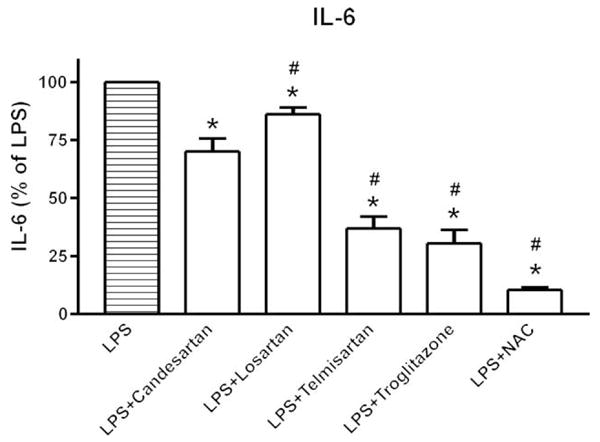
Comparison of angiotensin II receptor blockers, troglitazone and N-acetyl–cysteine on lipopolysaccharide-induced interleukin-6 release. Human monocytes were incubated with LPS alone or in the presence of 1 μmol/l candesartan, 10 μmol/l losartan, 10 μmol/l telmisartan, 10 μmol/l troglitazone or 10 mmol/l NAC. IL-6 release was quantified in culture medium by specific ELISA as described in Material and Methods. Results are presented as means ± SEM of three different monocyte preparations assayed individually. Individual groups were compared by one-way ANOVA followed by Newman–Keuls posttest. *P < 0.05 compared with LPS, #P < 0.05 compared with LPS + candesartan. ANOVA, analysis of variance; IL, interleukin; LPS, lipopolysaccharide; NAC, N-acetyl–cysteine.
Discussion
The principal observation of our study is that in circulating human monocytes, devoid of significant Ang II AT1 receptor expression and unresponsive to Ang II, incubation in the presence of the ARB candesartan effectively attenuates the innate immune response produced by LPS. These results suggest that candesartan decreases LPS effects by mechanisms independent of AT1 receptor blockade.
We found that LPS induced a strong inflammatory response in circulating human monocytes, in agreement with previous studies [18,20,21,27]. The purpose of our study was to determine the responses to LPS and the ARB in circulating monocytes obtained from a randomly selected human population. We found large differences in individual responses to LPS in our volunteer sample population, but very consistent responses to candesartan. It is possible that some of the observed differences in the individual responses to LPS may be attributable to sex, age, inflammatory status, medications or to genetic differences, factors not considered in our study.
LPS enhanced transcription and release of proinflammatory cytokines such as TNFα, IL-1β and IL-6, substantially increased IκBα mRNA expression and enhanced ROS formation. We interpret the increase in IκBα mRNA as a consequence of LPS-induced NF-κB activation, resulting in enhanced transcription of proinflammatory genes and subsequent stimulation of IκBα transcription to balance NF-κB effects [8,28]. This indicates, in agreement with previous observations [18,20,29–31], that the effects of LPS in human monocytes are, at least partially, induced by activation of NF-κB.
Human circulating monocytes from normal individuals express LOX-1 [32], a protein responsible for binding and uptake of the oxidized form of low-density lipoprotein in endothelial cells and macrophages and an important factor in the early stages of atherosclerosis [33]. LOX-1 has been reported as a target for LPS, and endotoxin administration upregulates LOX-1 in rats [34]. Our finding that LPS upregulates LOX-1 mRNA in human monocytes further supports the hypothesis that inflammatory mechanisms participate in the initiation and development of atherosclerosis [33].
Activation of signal transduction pathways requires LPS recognition by CD14 and association with TLR4. Earlier studies [35–37] demonstrated that LPS upregulated transcription of its recognition molecule CD14 and its receptor TLR4 in a number of target cells, including human monocytes. Under the conditions of our experiments and at the time selected for analysis, however, LPS did not alter CD14 or TLR4 transcription in circulating human monocytes. This indicated that LPS regulates its recognition molecules and receptors in a time-dependent, complex and cell-specific manner. When analyzing cultured cells, the results may be dependent on culture conditions. It has been reported that high glucose concentrations such as those present in the incubation medium used here enhance TLR4 expression in human monocytes [38]. However, expression of TLR4 mRNA is significantly decreased in diabetic rats [39], and the influence of variable glucose concentrations in the response to LPS was not studied here.
Ang II promotes the innate immune response, inflammation and oxidative stress by mechanisms similar to those involved in the LPS effects [1,2,18,40]. A complete RAS system is activated during monocyte differentiation into macrophages, and in differentiated macrophages Ang II, through AT1 receptor stimulation, promotes inflammation and oxidative stress [41]. In addition, expression of AT1 receptor mRNA has been described in circulating human monocytes [42] and there is substantial evidence for proinflammatory effects of Ang II in preactivated circulating monocytes in hypertensive patients [43]. However, we failed to demonstrate significant numbers of AT1 receptors in our preparations of human circulating monocytes, in agreement with previous observations [23,44]. Nondifferentiated human monocytes do not produce Ang II [22], and we have found that incubation of circulating monocytes with Ang II does not increase TNFα release or the expression of TNFα, IκBα or LOX-1 mRNA. We conclude that in circulating human monocytes, prior to differentiation, Ang II AT1 receptor-mediated effects are of no functional significance.
Nonetheless, we found that the ARB candesartan substantially reduced the LPS-induced proinflammatory response to LPS in our monocyte preparations. We incubated the monocytes with 1 μmol/l candesartan, a concentration producing maximum effects and similar to plasma concentrations in candesartan-treated human patients [45,46] and to those necessary to reduce Ang II effects in cell cultures [47,48].
The ARB significantly decreased LPS-induced IκBα, TNFα, IL-1β, IL-6 and LOX-1 transcription, and reduced TNFα and IL-6 secretion without affecting secretion of the anti-inflammatory cytokine IL-10.
Taken together, our findings reveal that the ARB candesartan significantly limits the monocyte response to LPS even in the absence of significant Ang II AT1 receptor expression. Thus, the anti-inflammatory effects of candesartan appear to be independent of its AT1 receptor blocking properties.
The mechanisms by which the ARB candesartan decreases LPS inflammatory effects are not clear. Several possible mechanisms are worth mentioning. LPS increases ROS formation, an effect mediated through a TLR4-dependent pathway and leading to NFκB activation [49,50]. The expression of CD14 mRNA was reduced when monocytes were incubated with LPS in the presence of candesartan, when compared with LPS alone. This indicates that the ARB may interfere with the initial steps for the recognition of LPS and its association with its TLR4 receptor. Interference with LPS recognition may explain the decrease in LPS-induced ROS formation produced by candesartan in our experiments [49].
Some ARBs are agonists of PPARγ, a negative regulator of inflammation [51–54], and the ARB telmisartan was reported as a more effective PPARγ agonist than candesartan [54]. In addition, administration of a PPARγ antagonist partially inhibits the anti-inflammatory effects of the ARB telmisartan [55]. Although candesartan was reported as a poor PPARγ agonist in vitro, when administered to rats in vivo, candesartan upregulates PPARγ transcription [56]. In our experiments, we demonstrate that the inhibitory effect on LPS-induced IL-6 release produced by telmisartan is comparable to that produced by PPARγ agonist, troglitazone, but less effective than that produced by the antioxidant NAC. In turn, candesartan is more effective than an equivalent concentration of losartan, but less effective than telmisartan or troglitazone. Our results compare well with the reported effects of ARBs on adipogenesis promotion, a PPARγ-dependent process [54]. Although our experiments do not clarify the mechanism of action of candesartan, the possibility remains that at least some of its effects may be the result of PPARγ agonist activity. The non-Ang II effects of ARBs may also be the result of agonist activity on 1,25-dihydroxyvitamin-D and C–C motif chemokine receptor type 2b, as recently suggested on the basis of theoretical modeling [53], and perhaps as a consequence of reduced LPS recognition by TLR4 [57]. Additionally, candesartan has been recently reported to exert powerful direct antioxidant actions, independent of its AT1 receptor blocking capacity [58].
ARBs have been considered selective, high-affinity ligands for Ang II AT1 receptors. Clear indications that ARBs exert pharmacological actions unrelated to AT1 receptor blockade raise the possibility that these compounds may bind to additional non-Ang II sites. Indeed, it has been demonstrated that the ARB losartan binds to a still uncharacterized site, expressed in large numbers in the kidney, liver and adrenal gland that is not recognized by Ang II [59,60]. The possible expression of such a site in human monocytes has not been explored.
Perspectives and significance
Our results demonstrate that the ARB candesartan, a well tolerated compound widely used for the treatment of hypertension, has significant anti-inflammatory effects unrelated to BP control and to the RAS. Our results support the hypothesis that some metabolic and anti-inflammatory effects of ARBs are independent of the effects of these compounds on Ang II AT1 receptors [51,52,61].
Candesartan is able to substantially reduce, but not eliminate, the LPS-induced innate immune response in human monocytes, indicative of general anti-inflammatory effects. Although the innate immune response is essential for eliminating pathogens, preparing the transfer to the more specific, acquired immune response and regaining homeostasis, an overstimulated innate immune response can have deleterious effects for the organism leading to chronic inflammation and degenerative disorders. For this reason, limiting and controlling the innate immune response may be therapeutically beneficial, and we interpret our present data as a demonstration that the magnitude of the inflammatory response to LPS can be controlled by candesartan in humans.
Candesartan and other ARBs have been reported to exert actions unrelated to AT1 receptor blockade. Because of the lack of significant AT1 receptor expression in human circulating monocytes, the anti-inflammatory effects of candesartan can be best explained by alternative mechanisms, such as influence on PPARγ, direct antioxidant effects or stimulation of still uncharacterized additional receptors. We suggest that candesartan and other ARBs may have a role beyond hypertension, in the treatment of a number of inflammatory disorders.
Acknowledgments
This study was supported by the Division of Intramural Research Programs, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services, USA. Candesartan was a gift from Astra Zeneca, R&D, Mölndal, Sweden. J.P. is currently affiliated with the Institute of Neurobiology, Slovak Academy of Sciences.
Abbreviations
- Ang II
angiotensin II
- ARB
angiotensin II receptor blocker
- AT1
angiotensin II receptor type 1
- AT2
angiotensin II receptor type 2
- CD14
cluster of differentiation 14
- H2DCFDA
2′,7′-dichlorodihydrofluorescein diacetate
- IκBα
inhibitor of kappa B alpha
- IL-1β
interleukin-1 beta
- IL-10
interleukin-10
- IL-6
interleukin-6
- LOX-1
lectin-like oxidized low-density lipoprotein receptor 1
- LPS
lipopolysaccharide
- NAC
N-acetyl–cysteine
- NFκB
nuclear factor kappa B
- PPARγ
peroxisome proliferator-activated receptor gamma
- RAS
renin–angiotensin system
- ROS
reactive oxygen species
- TLR4
toll-like receptor 4
- TNFα
tumor-necrosis factor alpha
References
- 1.Pastore L, Tessitore A, Martinotti S, Toniato E, Alesse E, Bravi MC, et al. Angiotensin II stimulates intercellular adhesion molecule-1 (ICAM-1) expression by human vascular endothelial cells and increases soluble ICAM-1 release in vivo. Circulation. 1999;100:1646–1652. doi: 10.1161/01.cir.100.15.1646. [DOI] [PubMed] [Google Scholar]
- 2.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–651. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 3.Cheng ZJ, Vapaatalo H, Mervaala E. Angiotensin II and vascular inflammation. Med Sci Monit. 2005;11:RA194–RA205. [PubMed] [Google Scholar]
- 4.Savoia C, Schiffrin EL. Inhibition of the renin angiotensin system: implications for the endothelium. Curr Diab Rep. 2006;6:274–278. doi: 10.1007/s11892-006-0060-5. [DOI] [PubMed] [Google Scholar]
- 5.Dzau VJ. Theodore Cooper Lecture. Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–1052. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- 6.Nickenig G. Central role of the AT(1)-receptor in atherosclerosis. J Hum Hypertens. 2002;16:S26–S33. doi: 10.1038/sj.jhh.1001436. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 8.Dandona P, Kumar V, Aljada A, Ghanim H, Syed T, Hofmayer D, et al. Angiotensin II receptor blocker valsartan suppresses reactive oxygen species generation in leukocytes, nuclear factor-kappa B, in mononuclear cells of normal subjects: evidence of an antiinflammatory action. J Clin Endocrinol Metab. 2003;88:4496–4501. doi: 10.1210/jc.2002-021836. [DOI] [PubMed] [Google Scholar]
- 9.Ando H, Zhou J, Macova M, Imboden H, Saavedra JM. Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke. 2004;35:1726–1731. doi: 10.1161/01.STR.0000129788.26346.18. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Ando H, Macova M, Dou J, Saavedra JM. Angiotensin II AT1 receptor blockade abolishes brain microvascular inflammation and heat shock protein responses in hypertensive rats. J Cereb Blood Flow Metab. 2005;25:878–886. doi: 10.1038/sj.jcbfm.9600082. [DOI] [PubMed] [Google Scholar]
- 11.Ghiadoni L, Virdis A, Magagna A, Taddei S, Salvetti A. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension. 2000;35:501–506. doi: 10.1161/01.hyp.35.1.501. [DOI] [PubMed] [Google Scholar]
- 12.Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, et al. Angiotensin II type 1 receptor antagonist decreases plasma levels of tumor necrosis factor alpha, interleukin-6 and soluble adhesion molecules in patients with chronic heart failure. J Am Coll Cardiol. 2000;35:714–721. doi: 10.1016/s0735-1097(99)00594-x. [DOI] [PubMed] [Google Scholar]
- 13.Dohi Y, Ohashi M, Sugiyama M, Takase H, Sato K, Ueda R. Candesartan reduces oxidative stress and inflammation in patients with essential hypertension. Hypertens Res. 2003;26:691–697. doi: 10.1291/hypres.26.691. [DOI] [PubMed] [Google Scholar]
- 14.Koh KK, Quon MJ, Han SH, Chung WJ, Lee Y, Shin EK. Anti-inflammatory and metabolic effects of candesartan in hypertensive patients. Int J Cardiol. 2006;108:96–100. doi: 10.1016/j.ijcard.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 15.Salzet M, Deloffre L, Breton C, Vieau D, Schoofs L. The angiotensin system elements in invertebrates. Brain Res Brain Res Rev. 2001;36:35–45. doi: 10.1016/s0165-0173(01)00063-7. [DOI] [PubMed] [Google Scholar]
- 16.Jerala R. Structural biology of the LPS recognition. Int J Med Microbiol. 2007;297:353–363. doi: 10.1016/j.ijmm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 18.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 19.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto SJ, Morohoshi K, Suzuki T, Matsushima K. Lipopolysaccharide-inducible gene expression profile in human monocytes. Scand J Infect Dis. 2003;35:619–627. [PubMed] [Google Scholar]
- 21.Hawiger J. Innate immunity and inflammation: a transcriptional paradigm. Immunol Res. 2001;23:99–109. doi: 10.1385/IR:23:2-3:099. [DOI] [PubMed] [Google Scholar]
- 22.Kim MP, Zhou M, Wahl LM. Angiotensin II increases human monocyte matrix metalloproteinase-1 through the AT2 receptor and prostaglandin E2: implications for atherosclerotic plaque rupture. J Leukoc Biol. 2005;78:195–201. doi: 10.1189/jlb.1204715. [DOI] [PubMed] [Google Scholar]
- 23.Egidy G, Friedman J, Viswanathan M, Wahl LM, Saavedra JM. CGP-42112 partially activates human monocytes and reduces their stimulation by lipopolysaccharides. Am J Physiol. 1997;273:C826–C833. doi: 10.1152/ajpcell.1997.273.3.C826. [DOI] [PubMed] [Google Scholar]
- 24.Tsutsumi K, Saavedra JM. Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am J Physiol. 1991;261:R209–R216. doi: 10.1152/ajpregu.1991.261.1.R209. [DOI] [PubMed] [Google Scholar]
- 25.Yamakawa H, Jezova M, Ando H, Saavedra JM. Normalization of endothelial and inducible nitric oxide synthase expression in brain microvessels of spontaneously hypertensive rats by angiotensin II AT1 receptor inhibition. J Cereb Blood Flow Metab. 2003;23:371–380. doi: 10.1097/01.WCB.0000047369.05600.03. [DOI] [PubMed] [Google Scholar]
- 26.Yang CS, Lee HM, Lee JY, Kim JA, Lee SJ, Shin DM, et al. Reactive oxygen species and p47phox activation are essential for the Mycobacterium tuberculosis-induced proinflammatory response in murine microglia. J Neuroinflammation. 2007;4:27–45. doi: 10.1186/1742-2094-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki T, Hashimoto S, Toyoda N, Nagai S, Yamazaki N, Dong HY, et al. Comprehensive gene expression profile of LPS-stimulated human monocytes by SAGE. Blood. 2000;96:2584–2591. [PubMed] [Google Scholar]
- 28.Velasco M, Diaz-Guerra MJ, Martin-Sanz P, Alvarez A, Bosca L. Rapid up-regulation of IkappaBbeta and abrogation of NF-kappaB activity in peritoneal macrophages stimulated with lipopolysaccharide. J Biol Chem. 1997;272:23025–23030. doi: 10.1074/jbc.272.37.23025. [DOI] [PubMed] [Google Scholar]
- 29.Zhang FX, Kirschning CJ, Mancinelli R, Xu XP, Jin Y, Faure E, et al. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–7614. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]
- 30.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y, Wahl LM. Oxidative stress augments the production of matrix metalloproteinase-1, cyclooxygenase-2, and prostaglandin E2 through enhancement of NF-kappa B activity in lipopolysaccharide-activated human primary monocytes. J Immunol. 2005;175:5423–5429. doi: 10.4049/jimmunol.175.8.5423. [DOI] [PubMed] [Google Scholar]
- 32.Draude G, Hrboticky N, Lorenz RL. The expression of the lectin-like oxidized low-density lipoprotein receptor (LOX-1) on human vascular smooth muscle cells and monocytes and its down-regulation by lovastatin. Biochem Pharmacol. 1999;57:383–386. doi: 10.1016/s0006-2952(98)00313-x. [DOI] [PubMed] [Google Scholar]
- 33.Morawietz H. LOX-1 and atherosclerosis: proof of concept in LOX-1-knockout mice. Circ Res. 2007;100:1534–1536. doi: 10.1161/CIRCRESAHA.107.101105. [DOI] [PubMed] [Google Scholar]
- 34.Nagase M, Abe J, Takahashi K, Ando J, Hirose S, Fujita T. Genomic organization and regulation of expression of the lectin-like oxidized low-density lipoprotein receptor (LOX-1) gene. J Biol Chem. 1998;273:33702–33707. doi: 10.1074/jbc.273.50.33702. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J Immunol. 2000;165:3541–3544. doi: 10.4049/jimmunol.165.7.3541. [DOI] [PubMed] [Google Scholar]
- 36.Singh AK, Jiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology. 2004;201:197–207. doi: 10.1016/j.tox.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Vakharia K, Hinson JP. Lipopolysaccharide directly stimulates cortisol secretion by human adrenal cells by a cyclooxygenase-dependent mechanism. Endocrinology. 2005;146:1398–1402. doi: 10.1210/en.2004-0882. [DOI] [PubMed] [Google Scholar]
- 38.Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces toll-like receptor expression in human monocytes. Mechanism of activation. Diabetes. 2008;57:3090–3098. doi: 10.2337/db08-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Froy O, Hananel A, Chapnik N, Madar Z. Differential effect of insulin treatment on decreased levels of beta-defensins and Toll-like receptors in diabetic rats. Mol Immunol. 2007;44:796–802. doi: 10.1016/j.molimm.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Sun GY. LPS induces permeability injury in lung microvascular endothelium via AT(1) receptor. Arch Biochem Biophys. 2005;441:75–83. doi: 10.1016/j.abb.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 41.Okamura A, Rakugi H, Ohishi M, Yanagitani Y, Takiuchi S, Moriguchi K, et al. Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages. J Hypertens. 1999;17:537–545. doi: 10.1097/00004872-199917040-00012. [DOI] [PubMed] [Google Scholar]
- 42.Miura R, Nakamura K, Miura D, Miura A, Hisamatsu K, Kajiya M, et al. Aldosterone synthesis and cytokine production in human peripheral blood mononuclear cells. J Pharmacol Sci. 2006;102:288–295. doi: 10.1254/jphs.fp0060801. [DOI] [PubMed] [Google Scholar]
- 43.Dörfell Y, Lätsch C, Stuhlműller B, Schreiber S, Scholze S, Bermester GR, Scholze J. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension. 1999;34:113–117. doi: 10.1161/01.hyp.34.1.113. [DOI] [PubMed] [Google Scholar]
- 44.Simon MR, Kamlay MT, Khan M, Melmon K. Angiotensin II binding to human mononuclear cells. Immunopharmacol Immunotoxicol. 1989;11:63–80. doi: 10.3109/08923978909082143. [DOI] [PubMed] [Google Scholar]
- 45.Weinberg AJ, Zappe DH, Ashton M, Weinberg MS. Safety and tolerability of high-dose angiotensin receptor blocker therapy in patients with chronic kidney disease: a pilot study. Am J Nephrol. 2004;24:340–345. doi: 10.1159/000078950. [DOI] [PubMed] [Google Scholar]
- 46.Lee JW, Naidong W, Johnson T, Dzerk A, Miyabayashi T, Motohashi M. Development and validation of column-switching high-performance liquid chromatographic methods for the determination of a potent AII receptor antagonist, TCV-116, and its metabolites in human serum and urine. J Chromatogr B Biomed Appl. 1995;670:287–298. doi: 10.1016/0378-4347(95)00146-8. [DOI] [PubMed] [Google Scholar]
- 47.Seeger H, Lippert C, Wallwiener D, Mueck AO. Valsartan and candesartan can inhibit deteriorating effects of angiotensin II on coronary endothelial function. J Renin Ang Aldost System. 2001;2:141–143. doi: 10.3317/jraas.2001.016. [DOI] [PubMed] [Google Scholar]
- 48.Yanagitani Y, Rakugi H, Okamura A, Moriguchi K, Takiuchi S, Ohishi M, et al. Angiotensin II type 1 receptor-mediated peroxide production in human macrophages. Hypertension. 1999;33(part II):335–339. doi: 10.1161/01.hyp.33.1.335. [DOI] [PubMed] [Google Scholar]
- 49.Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharm. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Nishi K, Oda T, Takabuchi S, Oda S, Fukuda K, Adachi T, et al. LPS induces hypoxia-inducible factor 1 activation in macrophage-differentiated cells in a reactive oxygen species-dependent manner. Antioxid Redox Signal. 2008;10:983–995. doi: 10.1089/ars.2007.1825. [DOI] [PubMed] [Google Scholar]
- 51.Kurtz TW, Pravenec M. Antidiabetic mechanisms of angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists: beyond the renin-angiotensin system. J Hypertens. 2004;22:2253–2261. doi: 10.1097/00004872-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe T, Suzuki J, Yamawaki H, Sharma VK, Sheu SS, Berk BC. Losartan metabolite EXP3179 activates Akt and endothelial nitric oxide synthase via vascular endothelial growth factor receptor-2 in endothelial cells: angiotensin II type 1 receptor-independent effects of EXP3179. Circulation. 2005;112:1798–1805. doi: 10.1161/CIRCULATIONAHA.104.509760. [DOI] [PubMed] [Google Scholar]
- 53.Marshall TG, Lee RE, Marshall FE. Common angiotensin receptor blockers may directly modulate the immune system via VDR, PPAR and CCR2b. Theor Biol Med Model. 2006;3:1–33. doi: 10.1186/1742-4682-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erbe DV, Gartrell K, Zhang YL, Suri V, Kirincich SJ, Will S, et al. Molecular activation of PPARgamma by angiotensin II type 1-receptor antagonists. Vascul Pharmacol. 2006;45:154–162. doi: 10.1016/j.vph.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Nagai N, Oike Y, Izumi-Nagai K, Urano T, Kubota Y, Noda K, et al. Angiotensin II type 1 receptor-mediated inflammation is required for choroidal neovascularization. Arterioscler Thromb Vasc Biol. 2006;26:2252–2259. doi: 10.1161/01.ATV.0000240050.15321.fe. [DOI] [PubMed] [Google Scholar]
- 56.Zorad S, Dou JT, Benicky J, Hutanu D, Tybitanclova K, Zhou J, Saavedra JM. Long-term angiotensin II AT1 receptor inhibition produces adipose tissue hypotrophy accompanied by increased expression of adiponectin and PPARgamma. Eur J Pharmacol. 2006;552:112–122. doi: 10.1016/j.ejphar.2006.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl Jl, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361–370. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 58.Chen S, Ge Y, Si J, Rifai A, Dworkin LD, Gong R. Candesartan suppresses chronic renal inflammation by a novel antioxidant action independent of AT1R blockade. Kidney Int. 2008;74:1128–1138. doi: 10.1038/ki.2008.380. [DOI] [PubMed] [Google Scholar]
- 59.Grove KL, Speth RC. Angiotensin II and nonangiotensin II displaceable binding sites for [3H]losartan in the rat liver. Biochem Pharmacol. 1993;46:1653–1660. doi: 10.1016/0006-2952(93)90335-t. [DOI] [PubMed] [Google Scholar]
- 60.Chansel D, Vandermeersch S, Pham P, Ardaillou R. Characterization of [3H]losartan in isolated rat glomeruli. Eur J Pharmacol. 1993;247:193–198. doi: 10.1016/0922-4106(93)90077-m. [DOI] [PubMed] [Google Scholar]
- 61.Grothusen C, Umbreen S, Konrad I, Stellos K, Schulz C, Schmidt B, et al. EXP3179 inhibits collagen-dependent platelet activation via glycoprotein receptor-VI independent of AT1-receptor antagonism. Arterioscler Thromb Vasc Biol. 2007;27:1184–1190. doi: 10.1161/ATVBAHA.106.138693. [DOI] [PubMed] [Google Scholar]


