Abstract
Objective
Age-related cognitive decline trajectories were compared in apolipoprotein E (APOE) e4 homozygotes (HMZ), heterozygotes (HTZ), and noncarriers (NC) in the absence of mild cognitive impairment (MCI) and Alzheimer’s dementia (AD).
Background
At how young an age memory decline diverges from that of noncarriers in healthy people with elevated genetic risk for late-onset AD due to APOE e4 is unknown.
Methods
Cognitively normal participants age 21-97 years were recruited with local ads, grouped using an APOE e4 enrichment paradigm, and had longitudinal neuropsychological testing. Anyone who developed MCI or dementia during followup was excluded. Acceleration of the rates of decline for predetermined cognitive measures were compared between APOE e4/4 HMZ, e3/4 HTZ, and e4 NC using a mixed model for longitudinal change with age.
Results
79 e4 HMZ, 238 HTZ and 498 NC were included. APOE e4 carriers were younger (mean 58.0 vs 61.4 years, p<0.001) and had more years of followup (5.3 v 4.7 years, p=0.01), with equivalent education (15.4 years) and gender (69% women). With accelerating declines beginning prior to age 60 in e4 carriers, longitudinal decline in memory in e4 carriers accelerated more than in NC (p=0.0253) with a possible e4 gene-dose effect (p=0.0231) in which longitudinal decline in e4 HMZ accelerated more than in NC (p=0.0087). Weaker similar effects were also found on a visuospatial and general mental status measure.
Conclusions
Age-related memory decline in APOE e4 carriers diverges from NC prior to age 60 and appears most severe in HMZ despite ongoing normal clinical status.
Cognitive profiles of normal aging emphasize declining frontally mediated skills including learning efficiency, working memory, and psychomotor speed (1-3), while memory loss has been shown repeatedly to be the earliest cognitive change in Alzheimer’s disease (AD) (4-9). Overlap in these cognitive profiles exists, however, and distinguishing normal aging from early AD can be difficult (10,11). The apolipoprotein E (APOE) e4 allele is the most prevalent genetic risk factor for AD and may account for up to half of all sporadic and familial late onset cases (12,13). APOE e4 has been correlated with earlier and more rapidly progressive memory decline in presymptomatic individuals. APOE e4 carriers in their 50s and 60s have more rapid memory loss and reduced learning efficiency than matched APOE e4 noncarriers (14-16), and such decline correlates with reduced cerebral metabolism as much as 5-10 years before the onset of cognitive symptoms (17). The transition from normal aging to AD has been sought in population based studies, but cross-sectional designs are limited by demographic differences between participants that influence neuropsychological test results while longitudinal studies often suffer from inadequate test scope, entry criteria that are loose enough to accommodate all community members, and attrition of participants (18). Additionally, many studies focus on the elderly (19-21), and so do not encompass the entire adult lifespan. Consequently, at how young an age memory decline in clinically healthy APOE e4 carriers diverges from that of noncarriers is not yet known.
To address this question we have performed longitudinal growth modeling on a unique, genetically enriched cohort using a mixed model approach for cross-sectional and longitudinal data to compare the age-related memory trajectories of APOE e4 homozygotes (HMZ), e3/4 heterozygotes (HTZ), and e4 noncarriers (NC) in the absence of mild cognitive impairment (MCI) and AD.
Methods
Study Participants and Enrollment
From January 1, 1994, through August 6, 2007, cognitively normal residents of Maricopa County age 21 years and older were recruited through local media ads into the Arizona APOE cohort, a longitudinal study of cognitive aging (15); and from January 1, 2000, through August 6, 2007, cognitively normal residents of Maricopa and Pima Counties over age 65 years were enrolled in either the Arizona APOE cohort or the Arizona Alzheimer’s Disease Center cohort. Demographic, family, and medical history data were obtained on each individual undergoing APOE genotyping, and identity was coded by a study assistant. All individuals gave their written, informed consent, approved by the Institutional Review Boards of all participating institutions, and agreed to have the results of the APOE test withheld from them as a precondition to their participation in this study. Genetic determination of APOE allelic status was performed using a polymerase chain reaction (PCR) based assay (22).
The recruitment strategy for the Arizona APOE cohort involved matching by age, gender, and education two e4 carriers and two e4 noncarriers. Within each set of four individuals, from the APOE test results, we evaluated all APOE e4 homozygotes (HMZ) who were individually matched to one APOE e4 heterozygote (HTZ) all with the e3/4 genotype, and two APOE e4 non-carriers (NC), but because far greater numbers of e3/4 HTZ and e4 NC were identified, not all matched sets of four included an e4/4 HMZ (especially in those over age 70 years). (APOE e2/4 heterozygotes were excluded as their overall level of genetic risk is uncertain due to the concomitant protective effects of the e2 allele and deleterious effects of the e4 allele). Each was then invited to return for screening tests that included a medical history, neurologic examination, the Folstein Mini-Mental Status Exam (MMSE; 23), the Hamilton Depression Rating Scale (Ham-D; 24), the Functional Activities Questionnaire (FAQ), Instrumental Activities of Daily Living (IADL), and Structured Psychiatric Interview for DSM-IIIR (25). There were no potentially confounding medical, neurological, or psychiatric problems (such as prior stroke, traumatic brain injury, memory or other form of cognitive impairment, parkinsonism, major depression, or substance abuse). None met the published criteria for MCI (6), AD (26), or any other form of dementia (27), or major depressive disorder (27). On the MMSE, participants had to score at least 27 based on published age and education-based norms (and must have scored at least 1 out of 3 on the recall subtest) (23). On the Ham-D, participants had to score 10 or less (24) at the time of their first visit. All FAQ and IADL questions had to indicate no loss of function. The entry criteria for the Arizona Alzheimer’s Disease Center cohort was identical save that there was no match paradigm.
Those fulfilling these requirements were administered an extensive standardized battery of neuropsychological tests that was repeated every one to two years. To insure that ours was a true normal aging sample, anyone who subsequently met published criteria for MCI (6), AD (26), or any other form of dementia (27) during followup were excluded from this analysis, and this included 16 participants (NC n=4, HTZ n=4, HMZ n=8). Diagnostic status at entry and followup was determined by a consensus panel of behavioral neurologists (RJC, MNS, GLA, SZR, JS, and BKW).
The study was designed by Drs. Caselli and Reiman, data gathered by Drs. Caselli, Osborne, Sabbagh, Connor, Ahern, Baxter, Rapcsak, Shi, Woodruff, Locke, Rademakers, and Ms. Hoffman Snyder. Drs. Caselli and Dueck vouch for the data and analysis. Data from each clinical site was stored and managed in a central database by Dr. Alexander stripped of patient identifiers and security encoded. Dr. Caselli wrote the first draft that all coauthors helped to revise with the statistical analyses and revisions provided by Dr. Dueck. Dr. Caselli decided to publish the paper with the consent of all coauthors.
Neuropsychological Testing
Because the goal of this study was to distinguish the aging trajectories of normal and pathological aging, a long term memory measure known to be impaired early in patients with AD was selected as the primary endpoint. Only a single measure was selected to avoid the issue of multiple comparisons. Based upon prior experience (18-20,24,28) the primary endpoint we selected a priori for this analysis was the Long Term Memory (LTM; Trial 7) score of the Auditory Verbal Learning Test (AVLT) (score range from worst to best is 0-15), (29). The AVLT was administered as part of a comprehensive neuropsychological test battery as previously described (15). Single measures were also selected a priori for general cognition and non-memory domains and included the Folstein MMSE (score range from worst to best is 0-30), the controlled oral word association test (COWAT; executive and language skills; higher scores are better [24]), and the Judgment of Line Orientation test (JLO; visuospatial function; score range from worst to best is 0-30) [24]).
Longitudinal Growth Modeling
The acceleration of the rate of decline for each of the predetermined measures for carriers (collectively and separately for HMZ and HTZ subgroups) was compared to NC by using a mixed model approach for modeling cross-sectional and longitudinal data (30,31). The model for Yij (the jth response for the ith individual) is as follows:
| [1] |
where Carrieri is the carrier status for the ith individual (1=Carrier; 0=NC); Agecij is the age minus 60 (i.e., centered age) of the ith individual at the time of the jth response; and b1i is an individual specific random effect allowing each subject to have a different intercept. From this model, the longitudinal growth model for NC is given by:
and the longitudinal growth model for carriers is given by:
A quadratic model was selected to allow for comparison of the acceleration in the rate of decline between groups. Age is centered to reduce the correlation between the age and age-squared terms as well as to aid in the interpretation of coefficients (e.g., β1 represents the mean response for a 60-year-old NC). From these models, a test of significance of β10 was used to assess the difference between carriers and NC in the quadratic longitudinal effect of aging on the outcome measure being modeled. Modeling was carried out using SAS PROC MIXED (SAS Version 9, SAS Institute, Cary, NC). In a subsequent analysis, the model was modified to replace the Carrieri variable with two indicator variables to assess differences between NC and HTZ (Heteroi: 1=HTZ; 0=Other) and between NC and HMZ (Homoi: 1=HMZ; 0=Other). This analysis was preplanned (RJC) though considered exploratory given the small number of HMZ. Baseline characteristics and followup were compared among groups by using the two-sample t-test / analysis of variance (ANOVA) F-test or Pearson chi-square test.
Results
317 APOE e4 carriers, including 79 e4 HMZ and 238 HTZ, and 498 NC were included. There were uneven numbers of carriers and noncarriers due to fewer healthy APOE e4 carriers over age 75 years identified (generally in the Arizona Alzheimer’s Disease Center cohort). Demographic data are summarized in table 1. Overall, carriers were younger than NC (mean 58.0 vs 61.4 years, p<0.001), and had a higher reported rate of having a first-degree relative with dementia (73.5% vs 52.8%, p<0.001). Adjusting for the presence of a first-degree relative with dementia did not significantly alter the results for any of the measures. Gender (68.8% vs 69.1% women, p=0.93), mean years of education (15.4 vs 15.4 years, p=0.83) and number of participants with more than one epoch of testing (76% vs 73.1%, p=0.35) did not differ between the carrier groups, but among those with more than one epoch of testing, e4 carriers had slightly more years of followup (5.3 v 4.7, p=0.01). The age and APOE genotype distribution of our cohort was uneven between age deciles with more participants overall, and a higher proportion of e4 carriers (especially HMZ) in the 50-59 and 60-69 year old deciles than in those younger and older.
Table 1.
Entry Demographics
| NC | e3/4 HTZ | e4/4 HMZ | p* | |
|---|---|---|---|---|
| Total N | 498 | 238 | 79 | |
| Age at entry by age deciles |
||||
| 21-29 (%) | 9 (1.8%) | 6 (2.5%) | 0 (0%) | |
| 30-39 (%) | 22 (4.4%) | 19 (8%) | 5 (6.3%) | |
| 40-49 (%) | 27 (5.4%) | 32 (13.4%) | 4 (5.1%) | |
| 50-59 (%) | 169 (33.9%) | 73 (30.7%) | 43 (54.4%) | |
| 60-69 (%) | 153 (30.7%) | 58 (24.4%) | 20 (25.3%) | |
| 70-79 (%) | 85 (17.1%) | 35 (14.7%) | 7 (8.9%) | |
| 80-89 (%) | 29 (5.8%) | 14 (5.9%) | 0 (0%) | |
| 90-99 (%) | 4 (0.8%) | 1 (0.4%) | 0 (0%) | |
| Mean age at entry (SD) |
61.4 (12.6) | 58.4 (13.9) | 56.8 (9.1) | 0.0007 |
| Years of education (SD) |
15.4 (2.6) | 15.4 (2.7) | 15.4 (2.6) | 0.98 |
| Gender (% women) |
69.1% | 68.9% | 68.4% | 0.99 |
| FDR w dementia (% w) |
52.8% | 68.8% | 87.2% | <0.0001 |
| >1 epoch testing | 73.1% | 73.1% | 84.8% | 0.08 |
| Years of followup** |
4.7 (2.8) | 5.1 (2.8) | 5.7 (3.2) | 0.01 |
SD=standard deviation; FDR=first-degree relative;
HMZ=homozygote; HTZ=heterozygote; NC=noncarrier; w=with.
analysis of variance F-test for age at entry, years of education, and years of followup; chi-square test for gender, FDR w dementia, and >1 epoch testing.
duration for those with more than 1 epoch of testing.
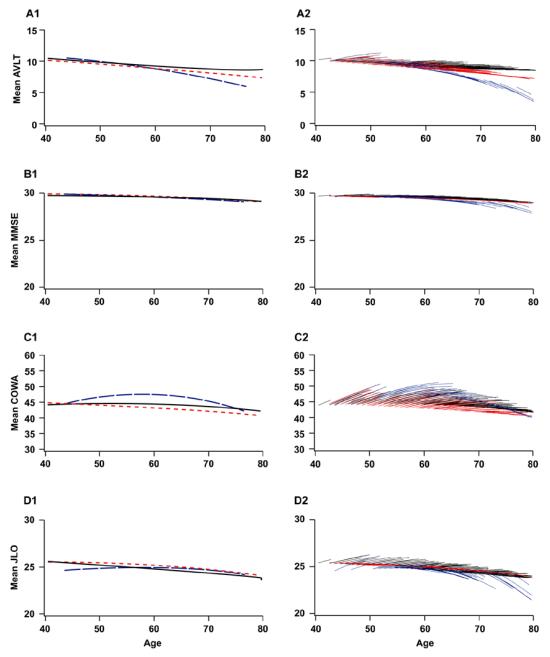
Table 2 summarizes and Figure 1 depicts the mixed model age trajectories in APOE e4 carriers and NC for AVLT-LTM, MMSE, COWAT, and JLO scores. On the AVLT-LTM, there is a significantly greater quadratic longitudinal effect of aging in APOE e4 carriers as compared to noncarriers (p=0.0253). Table 4a presents observed and fitted mean cross-sectional AVLT-LTM scores and annual changes in AVLT-LTM scores by age deciles based on the mixed model. In Table 4a, the mixed model for AVLT-LTM predicts decline in AVLT-LTM for APOE e4 carriers beginning in their 50s whereas the model does not predict such a decline in NCs until their 70s. There were no significant differences in the quadratic longitudinal effects of aging between carriers and NC on the MMSE (p=0.7508), COWAT (p=0.5709), or JLO (p=0.7775). However, there were significant differences in the linear longitudinal effects of aging between carriers and NC on the MMSE (p=0.0336) and the JLO (p=0.0088).
Table 2.
Mixed Models of APOE e4 Carriers and Noncarriers
| AVLT | MMSE | COWAT | JLO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | Estimate | SE | P | Estimate | SE | P | |
| Fixed Effects | ||||||||||||
| Intercept | 9.19 | 0.16 | <.0001 | 29.52 | 0.05 | <.0001 | 44.57 | 0.60 | <.0001 | 24.79 | 0.20 | <.0001 |
| Carrieri | −0.34 | 0.26 | 0.1973 | 0.02 | 0.09 | 0.8577 | −0.26 | 0.97 | 0.7927 | 0.29 | 0.33 | 0.3719 |
| Agei1-60 | −0.0853 | 0.0254 | 0.0008 | −0.0234 | 0.0102 | 0.0221 | −0.3979 | 0.0809 | <.0001 | −0.1118 | 0.0342 | 0.0011 |
| Carrieri × (Agei1-60) | 0.0381 | 0.0379 | 0.3145 | 0.0277 | 0.0150 | 0.0657 | −0.0189 | 0.1213 | 0.876 | 0.1360 | 0.0510 | 0.0078 |
| (Agei1-60)2 | 0.0021 | 0.0013 | 0.1076 | 0.0006 | 0.0005 | 0.2744 | 0.0078 | 0.0041 | 0.0586 | 0.0017 | 0.0016 | 0.2842 |
| Carrieri × (Agei1-60)2 | 0.0031 | 0.0020 | 0.1311 | 0.0000 | 0.0008 | 0.9636 | 0.0035 | 0.0065 | 0.5897 | 0.0000 | 0.0025 | 0.9964 |
| Ageij-60 | 0.0408 | 0.0244 | 0.0953 | 0.0060 | 0.0101 | 0.5511 | 0.3406 | 0.0743 | <.0001 | 0.0692 | 0.0331 | 0.0371 |
| Carrieri × (Ageij-60) | −0.0685 | 0.0351 | 0.0511 | −0.0309 | 0.0145 | 0.0336 | −0.0233 | 0.1065 | 0.8268 | −0.1257 | 0.0479 | 0.0088 |
| (Ageij-60)2 | −0.0013 | 0.0012 | 0.2804 | −0.0008 | 0.0005 | 0.0862 | −0.0116 | 0.0035 | 0.0011 | −0.0020 | 0.0015 | 0.1763 |
| Carrieri × (Ageij-60)2 | −0.0041 | 0.0018 | 0.0253 | −0.0002 | 0.0007 | 0.7508 | −0.0031 | 0.0055 | 0.5709 | −0.0006 | 0.0022 | 0.7775 |
| Random Effects | ||||||||||||
| Intercept | 6.55 | 0.41 | <.0001 | 0.60 | 0.06 | <.0001 | 102.40 | 6.01 | <.0001 | 9.42 | 0.62 | <.0001 |
| Residual | 3.61 | 0.13 | <.0001 | 0.64 | 0.03 | <.0001 | 32.42 | 1.19 | <.0001 | 4.85 | 0.20 | <.0001 |
AVLT = Auditory Verbal Learning Test (Long Term Memory Score); MMSE = Mini Mental Status Examination; COWAT = Controlled Oral Word Association Test; JLO = Judgement of Line Orientation; SE = Standard Error
Figure 1.
Mean cross-sectional (1) and longitudinal trajectories (2) of the AVLT-LTM (A), MMSE (B), COWAT (C), and JLO (D) by ApoE e4 carrier status (noncarrier vs carrier) based on the mixed model (Table 2). Cross-sectional means represent population mean values at the first examination. APOE e4 noncarriers (solid black line) and carriers (dotted red line). Longitudinal mean trajectories are population mean trajectories for the ages at the first and followup examinations observed in the sample. Predicted values at the first examination and predicted trajectories for each subject in the sample (not shown) are vertically shifted from the displayed means via the random intercept term. APOE e4 noncarriers (black lines) and carriers (red lines).
Table 4a.
Observed and Fitted Mean Auditory Verbal Learning Test Long Term Memory Score at First Examination and Annual Change by Age Deciles: APOE e4 Carriers and Noncarriers
| AVLT-LTM at First Examination | Annual Change in AVLT-LTM | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noncarriers | Carriers | Noncarriers | Carriers | |||||||||||||
| Observed(1) | Model(2) | Observed(1) | Model(2) | Observed(3) | Model(4) | Observed(3) | Model(4) | |||||||||
| Age Decile | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | ||||
| 20-29 | 9 | 12.44 | 2.07 | 12.26 | 6 | 11.00 | 2.97 | 11.03 | 1 | 0.25 | . | 0.14 | 2 | 0.50 | 0.71 | 0.40 |
| 30-39 | 22 | 10.64 | 2.63 | 10.27 | 24 | 10.13 | 2.85 | 10.24 | 12 | −0.50 | 1.06 | 0.12 | 12 | 0.31 | 0.53 | 0.29 |
| 40-49 | 27 | 10.48 | 2.42 | 10.44 | 36 | 10.00 | 3.57 | 9.83 | 18 | 0.12 | 0.82 | 0.09 | 16 | 0.14 | 1.15 | 0.18 |
| 50-59 | 169 | 9.63 | 3.10 | 9.50 | 114 | 9.92 | 3.10 | 9.55 | 92 | 0.10 | 0.81 | 0.07 | 76 | −0.03 | 0.96 | 0.08 |
| 60-69 | 148 | 8.89 | 2.91 | 8.84 | 78 | 8.10 | 3.51 | 8.21 | 134 | −0.05 | 1.18 | 0.04 | 88 | 0.02 | 1.18 | −0.03 |
| 70-79 | 83 | 8.70 | 3.53 | 8.73 | 40 | 8.18 | 2.77 | 7.94 | 78 | 0.12 | 0.99 | 0.02 | 39 | −0.61 | 1.65 | −0.13 |
| 80-89 | 29 | 9.38 | 2.97 | 8.81 | 14 | 6.43 | 3.52 | 6.48 | 39 | −0.33 | 1.66 | −0.01 | 14 | −0.46 | 1.49 | −0.24 |
| 90-99 | 4 | 10.50 | 4.43 | 9.18 | 1 | 11.00 | . | 10.36 | 8 | 0.21 | 1.39 | −0.03 | 1 | 0.48 | . | −0.35 |
AVLT = Auditory Verbal Learning Test (Long Term Memory score).
Observed AVLT score at first examination per subject summarized for each age decile.
Predicted (empirical best linear unbiased predictor) AVLT score at first examination per subject based on the AVLT mixed model (Table 2) averaged for each age decile.
Slope of simple linear regression line computed per subject (using all observations for that subject within the age decile) summarized for each age decile.
Predicted annual change in AVLT at the beginning of the age decile based on the AVLT mixed model (Table 2).
Table 3 and Figure 2 present the mixed model of the e4/4 HMZ and e3/4 HTZ subgroups in comparison to the NC. There was significant e4 gene-dose interaction with quadratic age in the AVLT-LTM mixed model (p=0.0231) supporting an e4 gene-dose effect; however, statistical significance was only reached for the HMZ subgroup (HMZ: p=0.0087; HTZ: p=0.1754). There was significant e4 gene-dose interaction with quadratic age in the MMSE mixed model (p=0.0117) and a trend towards significance in the JLO mixed model (p=0.0737) also suggesting an e4 gene-dose effect for these measures; however, similar to the AVLT-LTM model, statistical significance was only reached for the HMZ subgroup (MMSE: HMZ: p=0.01, HTZ: p=0.3641; JLO: HMZ: p=0.0431, HTZ: p=0.5658). The e4 gene-dose interaction with quadratic age in the COWAT mixed model was not statistically significant (p=0.4269).
Table 3.
Mixed Models of APOE e4 Homozygotes, Heterozygotes, and Noncarriers
| AVLT | MMSE | COWAT | JLO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | Estimate | SE | P | Estimate | SE | P | |
| Fixed Effects | ||||||||||||
| Intercept | 9.19 | 0.16 | <.0001 | 29.52 | 0.05 | <.0001 | 44.57 | 0.60 | <.0001 | 24.79 | 0.20 | <.0001 |
| Heteroi | −0.32 | 0.29 | 0.2732 | 0.05 | 0.10 | 0.6066 | −1.34 | 1.09 | 0.2183 | 0.37 | 0.36 | 0.3057 |
| Homoi | −0.42 | 0.42 | 0.3178 | −0.08 | 0.14 | 0.5936 | 2.74 | 1.57 | 0.0812 | 0.12 | 0.54 | 0.8284 |
| Agei1-60 | −0.0853 | 0.0254 | 0.0008 | −0.0234 | 0.0102 | 0.0222 | −0.3979 | 0.0809 | <.0001 | −0.1117 | 0.0342 | 0.0011 |
| Heteroi × (Agei1-60) | 0.0020 | 0.0420 | 0.9622 | 0.0274 | 0.0167 | 0.1022 | −0.0611 | 0.1337 | 0.6476 | 0.1248 | 0.0564 | 0.0271 |
| Homoi × (Agei1-60) | 0.0575 | 0.0658 | 0.3823 | 0.0241 | 0.0247 | 0.329 | 0.0879 | 0.2243 | 0.6953 | 0.1548 | 0.0870 | 0.0754 |
| (Agei1-60)2 | 0.0021 | 0.0013 | 0.1069 | 0.0006 | 0.0005 | 0.2705 | 0.0078 | 0.0041 | 0.0587 | 0.0017 | 0.0016 | 0.2843 |
| Heteroi × (Agei1-60)2 | 0.0017 | 0.0022 | 0.4376 | −0.0010 | 0.0009 | 0.2576 | 0.0033 | 0.0070 | 0.639 | −0.0021 | 0.0027 | 0.4405 |
| Homoi × (Agei1-60)2 | 0.0052 | 0.0042 | 0.2158 | 0.0032 | 0.0016 | 0.0497 | 0.0007 | 0.0141 | 0.9582 | 0.0065 | 0.0052 | 0.2091 |
| Ageij-60 | 0.0408 | 0.0244 | 0.0946 | 0.0060 | 0.0101 | 0.5546 | 0.3407 | 0.0743 | <.0001 | 0.0691 | 0.0331 | 0.0371 |
| Heteroi × (Ageij-60) | −0.0279 | 0.0394 | 0.479 | −0.0315 | 0.0163 | 0.0525 | 0.0138 | 0.1196 | 0.9082 | −0.1200 | 0.0532 | 0.0243 |
| Homoi × (Ageij-60) | −0.1457 | 0.0497 | 0.0034 | −0.0273 | 0.0206 | 0.184 | −0.0930 | 0.1509 | 0.5381 | −0.1257 | 0.0702 | 0.0734 |
| (Ageij-60)2 | −0.0013 | 0.0012 | 0.2793 | −0.0008 | 0.0005 | 0.0841 | −0.0116 | 0.0035 | 0.0011 | −0.0020 | 0.0015 | 0.1762 |
| Heteroi × (Ageij-60)2 | −0.0027 | 0.0020 | 0.1754 | 0.0007 | 0.0008 | 0.3641 | −0.0006 | 0.0060 | 0.9253 | 0.0014 | 0.0024 | 0.5658 |
| Homoi × (Ageij-60)2 | −0.0083 | 0.0032 | 0.0087 | −0.0034 | 0.0013 | 0.01 | −0.0123 | 0.0096 | 0.197 | −0.0080 | 0.0039 | 0.0431 |
| Random Effects | ||||||||||||
| Intercept | 6.53 | 0.41 | <.0001 | 0.60 | 0.06 | <.0001 | 101.97 | 6.00 | <.0001 | 9.46 | 0.62 | <.0001 |
| Residual | 3.59 | 0.13 | <.0001 | 0.64 | 0.03 | <.0001 | 32.43 | 1.19 | <.0001 | 4.84 | 0.20 | <.0001 |
AVLT = Auditory Verbal Learning Test (Long Term Memory score); MMSE = Mini Mental Status Examination; COWAT = Controlled Oral Word Association Test; JLO = Judgement of Line Orientation; SE = Standard Error
Figure 2.
Mean cross-sectional (1) and longitudinal trajectories (2) of the AVLT-LTM (A), MMSE (B), COWAT (C), and JLO (D) by ApoE e4 genotype (noncarrier vs heterozygotes vs homozygotes) based on the mixed model (Table 3). Cross-sectional means represent population mean values at the first examination. ApoE e4 noncarriers (solid black line), heterozygotes (dotted red line), and homozygotes (dashed blue line). Longitudinal mean trajectories are population mean trajectories for the ages at the first and followup examinations observed in the sample. Predicted values at the first examination and predicted trajectories for each subject in the sample (not shown) are vertically shifted from the displayed means via the random intercept term. APOE e4 noncarriers (black lines), heterozygotes (red lines), and homozygotes (blue lines).
Table 4b presents observed and fitted mean cross-sectional AVLT-LTM scores and annual changes in AVLT-LTM scores by age deciles for the e4 gene-dose subgroups. The mixed model for AVLT-LTM predicts decline in AVLT-LTM for APOE e4 HMZ beginning in their 50s whereas the model does not predict such a decline in APOE e4 HTZ until their 60s and for NC until their 70s.
Table 4b.
Observed and Fitted Mean Auditory Verbal Learning Test Long Term Memory Score at First Examination and Annual Change in Auditory Verbal Learning Test by Age Deciles: APOE e4 Homozygotes, Heterozygotes and Noncarriers
| AVLT-LTM at First Examination | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noncarriers | Heterozygotes | Homozygotes | ||||||||||
| Observed(1) | Model(2) | Observed(1) | Model(2) | Observed(1) | Model(2) | |||||||
| Age Decile | N | Mean | SD | N | Mean | SD | N | Mean | SD | |||
| 20-29 | 9 | 12.44 | 2.07 | 12.26 | 6 | 11.00 | 2.97 | 11.03 | 0 | . | . | . |
| 30-39 | 22 | 10.64 | 2.63 | 10.27 | 19 | 9.84 | 2.59 | 10.07 | 5 | 11.20 | 3.83 | 10.77 |
| 40-49 | 27 | 10.48 | 2.42 | 10.44 | 32 | 10.25 | 3.47 | 9.93 | 4 | 8.00 | 4.24 | 8.80 |
| 50-59 | 169 | 9.63 | 3.10 | 9.50 | 71 | 9.77 | 2.99 | 9.53 | 43 | 10.16 | 3.30 | 9.62 |
| 60-69 | 148 | 8.89 | 2.91 | 8.84 | 58 | 8.31 | 3.52 | 8.32 | 20 | 7.50 | 3.50 | 7.72 |
| 70-79 | 83 | 8.70 | 3.53 | 8.73 | 33 | 8.36 | 2.68 | 8.00 | 7 | 7.29 | 3.25 | 7.55 |
| 80-89 | 29 | 9.38 | 2.97 | 8.81 | 14 | 6.43 | 3.52 | 6.44 | 0 | . | . | . |
| 90-99 | 4 | 10.50 | 4.43 | 9.18 | 1 | 11.00 | . | 10.25 | 0 | . | . | . |
| Annual Change in AVLT-LTM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noncarriers | Heterozygotes | Homozygotes | ||||||||||
| Observed(3) | Model(4) | Observed(3) | Model(4) | Observed(3) | Model(4) | |||||||
| Age Decile | N | Mean | SD | N | Mean | SD | N | Mean | SD | |||
| 20-29 | 1 | 0.25 | . | 0.14 | 2 | 0.50 | 0.71 | 0.33 | 2 | 0.50 | 0.71 | 0.66 |
| 30-39 | 12 | −0.50 | 1.06 | 0.12 | 9 | 0.33 | 0.59 | 0.25 | 3 | 0.26 | 0.41 | 0.47 |
| 40-49 | 18 | 0.12 | 0.82 | 0.09 | 13 | 0.14 | 1.28 | 0.17 | 3 | 0.11 | 0.34 | 0.28 |
| 50-59 | 92 | 0.10 | 0.81 | 0.07 | 49 | −0.03 | 1.05 | 0.09 | 27 | −0.03 | 0.77 | 0.09 |
| 60-69 | 134 | −0.05 | 1.18 | 0.04 | 57 | −0.04 | 1.23 | 0.01 | 31 | 0.13 | 1.08 | −0.10 |
| 70-79 | 78 | 0.12 | 0.99 | 0.02 | 29 | −0.80 | 1.83 | −0.07 | 10 | −0.05 | 0.82 | −0.30 |
| 80-89 | 39 | −0.33 | 1.66 | −0.01 | 13 | −0.25 | 1.33 | −0.14 | 1 | −3.15 | . | −0.49 |
| 90-99 | 8 | 0.21 | 1.39 | −0.03 | 1 | 0.48 | . | −0.22 | 1 | 0.48 | . | −0.68 |
AVLT = Auditory Verbal Learning Test (Long Term Memory score).
Observed AVLT score at first examination per subject summarized for each age decile.
Predicted (empirical best linear unbiased predictor) AVLT score at first examination per subject based on the AVLT mixed model (Table 3) averaged for each age decile.
Slope of simple linear regression line computed per subject (using all observations for that subject within the age decile) summarized for each age decile.
Predicted annual change in AVLT at the beginning of the age decile based on the AVLT mixed model (Table 3).
Discussion
The main finding of this study is that APOE e4 affects age-related memory performance even in the absence of MCI and dementia, and does so in a gene-dose pattern that mirrors AD risk. Further, APOE e4 HMZ, whose symptomatic onset of AD is typically during their 60s (15,16), also experience declining MMSE scores and visuospatial skills during the same age-range despite their normal clinical status. By using a genetically enriched cohort and mixed model for cross-sectional and longitudinal data we were able to analyze adequate numbers of individuals in each of three APOE subgroups to demonstrate, for the first time, the profile and trajectory of cognitive aging in the absence of clinical impairment across the adult lifespan.
We expect AD to be more prevalent in APOE e4 carriers than noncarriers. The preferential effect of e4 carrier status on age-related memory decline, the subsequent decline in visuospatial function in e4/4 HMZ, and the similarity of our model-predicted memory decline with the original predictions of Corder et al for the age of onset of AD in each of these three genetic subgroups (12) raises the possibility that accelerated memory decline in e4 carriers is caused by subclinical AD. Other lines of evidence support this explanation. We have previously found that e4/4 HMZ in their early 60s develop accelerated neuropsychological decline in a cognitive-domain specific pattern (pre-MCI) that anticipates the onset of clinically symptomatic memory loss by several years (19). FDG-PET studies of individuals manifesting this pre-MCI pattern of memory decline have reduced cerebral metabolic rate for glucose (CMRgl) patterns in cortical regions that overlap those known to be affected by AD (20). More recently, we have shown that PIB-PET studies of asymptomatic APOE e4/4 HMZ at this age reveal early amyloid deposition in AD salient regions (32). Finally, AD-like neuropathology in thirty and forty year olds (33,34) and in nondemented elderly (35-37) is more prevalent and severe in APOE e4 carriers than NC.
Not all previous studies have supported this interpretation, however (38,39). Driscoll and colleagues compared the cognitive trajectories of 27 clinically normal elders lacking AD pathology after death to 21 clinically normal elders with mild AD pathology and found that not one of 12 neuropsychological measures (including three specific for memory) showed any steeper decline in those with AD pathology (39). Our data nonetheless show that healthy appearing APOE e4 carriers cognitively decline at a faster rate than NC, and that the decline has a cognitive profile that resembles AD. If we also accept that e4 carriers are likely to have a higher AD pathology burden at autopsy (40,41), the discrepancy appears difficult to reconcile. Possibly the memory decline we observed is mediated by a non-amyloid mechanism (42), analogous to the increased rate of ischemic heart disease (43), cerebral infarction (44) and mortality (45,46), observed in e4 carriers. We suspect, however, that the resolution lies in the different methodologies including the longer duration of prospective observation of larger numbers of genetically defined participants in our study that facilitated the elucidation of this relatively subtle difference in cognitive trajectories between the different APOE groups.
APOE e4 may be a genetic cause of dementia prior to age 60 (47), but the literature is divided on the potential effect of APOE e4 on cognitive functioning in healthy younger adults. Snowdon and colleagues reported psycholinguistic differences in twenty year old women that correlated with AD and neuropathological disease burden 60 years later (48). Whalley et al. found that school age performances on psychometric tests were predictive of dementia in old age (49), although subsequently it was shown this was explained by increased vascular dementia rather than AD (50). In contrast, we found no correlation between APOE genotype and intellectual achievement as measured by educational and occupational outcomes (51), although we have found middle age e4 HMZ to be more sensitive to the effects of fatigue (52) and anxiety (53) than noncarriers. Our current data show that age-related cognitive decline prior to age 50 is essentially identical in e4 carriers and noncarriers as a group, and any childhood or young adult differences are likely to be lifelong and superimposed on this APOE-age interaction.
A potential limitation of our study is that it is not population based, but instead genetically enriched for APOE e4. APOE e4 HMZ represent the single largest source of individuals whose risk for AD is nearly that of autosomal dominant mutation carriers. This small but important subgroup provides an opportunity to study the changes that may occur before the clinical onset of MCI and AD, and compiling such a cohort is not practical in a community based sample as current U.S. epidemiologic cohorts have demonstrated. In the absence of random community based sampling, however, we risk recruiting individuals concerned about their own cognitive health perhaps due in some to early stage AD. To address this we eliminated anyone who developed clinically symptomatic MCI or dementia at any point. While this might theoretically raise the possibility of survivor bias in our study, the number of clinical converters was small, and if anything would have reduced our sensitivity to the differential APOE e4 effect that we found. Also, the mean age of our cohort is quite young and 74% had at least two epochs of testing (85% for e4 HMZ) with mean followup duration of 5 years further reducing the likelihood that individuals with incipient symptoms were enrolled. Therefore, selection bias is unlikely to explain our findings.
Another potential limitation is the unbalanced distribution of age and APOE e4 carriers (especially HMZ) in this study. There were a greater number of participants over than under age 50 years, and a slightly older NC group (probably reflecting the predicted higher rates of symptomatic conversion in aging e4 carriers). Possibly APOE e4 effects at an even earlier age might be detected in a larger cohort of younger individuals and employing a memory measure with potentially greater sensitivity than the AVLT. The more participants, the greater the power to detect a change so the pattern of age imbalance (more older NC and fewer younger participants), if anything reduced the strength of our study making our findings all the more remarkable. Finally, the HMZ had slightly longer mean followup than NC. We elected not to limit the duration of followup data to achieve greater balance because the difference, though statistically significant, was small. We felt it was better strategically to err on the side of more than less data inclusion, and do not believe this had any impact on our results.
In summary, APOE e4 affects age-related memory trajectories with accelerating declines beginning prior to age 60 even in the absence of MCI and dementia, and does so in a gene-dose pattern that mirrors AD risk.
References
- 1.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 2.Verhaeghen P, Cerella J. Aging, executive control, and attention: a review of meta-analyses. Neurosci Behav Rev. 2002;26:849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- 3.Treitz FH, Heyder K, Daum I. Differential course of executive control changes during normal aging. Aging, Neuropsychology, and Cognition. 2007;14:370–393. doi: 10.1080/13825580600678442. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 5.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Bell L. Mild cognitive impairment represents early stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 7.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer’s disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 8.Albert M, Blacker D, Moss MB, Tanzi R, McArdle JJ. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21:158–169. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- 9.Howieson DB, Carlson NE, Moore MM, Wasserman D, Abendroth CD, Payne-Murphy J, Kaye JA. Trajectory of mild cognitive impairment onset. J Int Neuropsychol Soc. 2008;14:192–198. doi: 10.1017/S1355617708080375. [DOI] [PubMed] [Google Scholar]
- 10.Salthouse TA. Memory aging 18 to 80. Alzeimer Dis Assoc Disord. 2003;17:162–167. doi: 10.1097/00002093-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Burke D, Mackay DG. Memory, language, and ageing. Phil Trans R Soc Lond B. 1997;352:1845–1856. doi: 10.1098/rstb.1997.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of AD in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 13.Saunders AM, Strittmatter WJ, Schmechel D, St. George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-McLachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD. Association of apolipoprotein E allele ε4 with late onset familial and sporadic Alzheimer’s disease. Neurol. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 14.Baxter LC, Caselli RJ, Johnson SC, Reiman E, Osborne D. Apolipoprotein E epsilon 4 affects new learning in cognitively normal individuals at risk for Alzheimer’s disease. Neurobiol Aging. 2003 Nov;24(7):947–52. doi: 10.1016/s0197-4580(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 15.Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, Alexander GG. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004 Jun 8;62(11):1990–5. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- 16.Caselli RJ, Reiman EM, Locke DE, Hutton ML, Hentz JG, Hoffman-Snyder C, Woodruff BK, Alexander GE, Osborne D. Cognitive domain decline in healthy apolipoprotein E epsilon4 homozygotes before the diagnosis of mild cognitive impairment. Arch Neurol. 2007;64(9):1306–11. doi: 10.1001/archneur.64.9.1306. [DOI] [PubMed] [Google Scholar]
- 17.Caselli RJ, Chen K, Lee W, Alexander GE, Reiman EM. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Archives of Neurology. 2008;65:1231–1236. doi: 10.1001/archneurol.2008.1. [DOI] [PubMed] [Google Scholar]
- 18.Small SA. Age-related memory decline. Arch Neurol. 2001;58:360–364. doi: 10.1001/archneur.58.3.360. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC, Smith G, Kokmen E, Ivnik RJ, Tangalos EG. Memory function in normal aging. Neurol. 1992;42:396–401. doi: 10.1212/wnl.42.2.396. [DOI] [PubMed] [Google Scholar]
- 20.Small SA, Stern Y, Tang M, Mayeux R. Selective decline in memory function among healthy elderly. Neurol. 1999;52:1392–1396. doi: 10.1212/wnl.52.7.1392. [DOI] [PubMed] [Google Scholar]
- 21.Ferris SH, Aisen PS, Cummings J, et al. ADCS Prevention Instrument Project: overview and initial results. Alzheimer Dis Assoc Disord. 2006;20(suppl 3):S109–S123. doi: 10.1097/01.wad.0000213870.40300.21. [DOI] [PubMed] [Google Scholar]
- 22.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with Hha I. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 23.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental satae examination by age and education level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 24.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th edition Oxford University Press; New York: 2004. [Google Scholar]
- 25.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition, Revised American Psychiatric Association; Washington DC: 1987. [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurol. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition American Psychiatric Association; Washington D.C.: 1994. [Google Scholar]
- 28.Steinberg BA, Bieliauskas LA, Smith GE, Ivnik RJ, Malec JF. Mayo’s Older Americans Normative Studies: age- and IQ- adjusted norms for the Auditory Verbal Learning Test and the Visual Spatial Learning Test. The Clinical Neuropsychologist. 2005;19:464–523. doi: 10.1080/13854040590945193. [DOI] [PubMed] [Google Scholar]
- 29.Rey A. L’examen clinique en psychologie. Presses Universitaires; Paris: 1964. [Google Scholar]
- 30.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. John Wiley & Sons, Inc.; Hoboken: 2004. (Section 15.4) [Google Scholar]
- 31.Ware JH, Dockery DW, Louis TA, XU X, Ferris BG, Speizer FE. Longitudinal and cross-sectional estimates of pulmonary function decline in never-smoking adults. Am J Epidemiology. 1990;132:685–700. doi: 10.1093/oxfordjournals.aje.a115710. [DOI] [PubMed] [Google Scholar]
- 32.Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, et al. Fibrillar amyloid-β burden in cognitively normal people homozygous for the apolipoprotein E ε4 allele. (manuscript under review)
- 33.Ghebremedhin E, Schultz C, Braak E, Braak H. High frequency of apolipoprotein E epsilon4 allele in young individuals with very mild Alzheimer’s disease-related neurofibrillary changes. Exp Neurol. 1998;153:152–155. doi: 10.1006/exnr.1998.6860. [DOI] [PubMed] [Google Scholar]
- 34.Morishima-Kawashima M, Oshima N, Ogata H, Yamaguchi H, Yoshimura M, Sugihara S, Ihara Y. Effect of apolipoprotein E allele epsilon4 on the initial phase of amyloid beta-protein accumulation in the human brain. Am J Pathol. 2000;157:2093–2099. doi: 10.1016/s0002-9440(10)64847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crystal HA, Dickson D, Sliwinski M, Masur D, Blau A, Lipton RB. Associations of status and change measures of neuropsychological function with pathologic changes in elderly, originally nondemented subjects. Arch Neurol. 1996;53:82–87. doi: 10.1001/archneur.1996.00550010102023. [DOI] [PubMed] [Google Scholar]
- 36.Alafuzoff I, Helisalmi S, Mannermaa A, Soininen H. Severity of cardiovascular disease, apolipoprotein E genotype, and brain pathology in aging and dementia. Ann NY Acad Sci. 2000;903:244–251. doi: 10.1111/j.1749-6632.2000.tb06374.x. [DOI] [PubMed] [Google Scholar]
- 37.Snowden DA. Healthy aging and dementia: findings from the nun study. Ann Intern Med. 2003;139:450–454. doi: 10.7326/0003-4819-139-5_part_2-200309021-00014. [DOI] [PubMed] [Google Scholar]
- 38.Knopman D, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 39.Driscoll I, Resnick SM, Troncoso JC, An Y, O’Brien R, Zonderman AB. Impact of Alzheimer’s pathology on cognitive trajectories in nondemented elderly. Ann Neurol. 2006;60:688–695. doi: 10.1002/ana.21031. [DOI] [PubMed] [Google Scholar]
- 40.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Berry-Kravis E, Arnold SE. Amyloid mediates the association of apolipoprotein E e4 allele to cognitive function in older people. J Neurol Neurosurg Psychiatry. 2005;76:1194–1199. doi: 10.1136/jnnp.2004.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lambert JC, Mann D, Richard F, et al. IsAlzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76:928–933. doi: 10.1136/jnnp.2004.048983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horsburgh K, McCarron MO, White F, et al. The role of apolipoprotein E in Alzheimer’s disease, acute brain injury, and cerebrovascular disease: Evidence of common mechanisms and utility of animal models. Neurobiol Aging. 2000;21:245–255. doi: 10.1016/s0197-4580(00)00097-x. [DOI] [PubMed] [Google Scholar]
- 43.Olichney JM, Sabbagh MN, Hofstetter CR, et al. The impact of apolipoprotein E4 on cause of death in Alzheimer’s disease. Neurology. 1997;49:76–81. doi: 10.1212/wnl.49.1.76. [DOI] [PubMed] [Google Scholar]
- 44.Schneider JA, Bienias JL, Wilson RS, Berry-Kravis E, Evans DA, Bennett DA. The apolipoprotein E epsilon4 allele increases the odds of chronic cerebral infarction detected at autopsy. Stroke. 2005;36:954–959. doi: 10.1161/01.STR.0000160747.27470.2a. [DOI] [PubMed] [Google Scholar]
- 45.Hayden KM, Zandi PP, Lyketsos CG, et al. Apolipoprotein E genotype and mortality: findings from the Cache County Study. J Am Geriatr Soc. 2005;53:935–942. doi: 10.1111/j.1532-5415.2005.53301.x. [DOI] [PubMed] [Google Scholar]
- 46.Lane KA, Gao S, Hui SL, Murrell JR, Hall KS, Hendrie HC. Apolipoprotein E and mortality in African-Americans and Yoruba. J Alzheimers Dis. 2003;5:383–390. doi: 10.3233/jad-2003-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brickell KL, Steinbart EJ, Rumbaugh M, Payami H, Schellenberg GD, Van Deerlin V, Yuan W, Bird TD. Early-onset Alzheimer disease families with late-onset Alzheimer disease: a potential important subtype of familial Alzheimer disease. Arch Neurol. 2006;63:1307–1311. doi: 10.1001/archneur.63.9.1307. [DOI] [PubMed] [Google Scholar]
- 48.Snowden DA. Healthy aging and dementia: findings from the nun study. Ann Intern Med. 2003;139:450–454. doi: 10.7326/0003-4819-139-5_part_2-200309021-00014. [DOI] [PubMed] [Google Scholar]
- 49.Whalley LJ, Starr JM, Athawes R, Hunter D, Pattie A, Deary IJ. Childhood mental ability and dementia. Neurology. 2000;55:1455–1459. doi: 10.1212/wnl.55.10.1455. [DOI] [PubMed] [Google Scholar]
- 50.McGurn B, Deary IJ, Starr JM. Childhood cognitive ability and risk of late-onset Alzheimer and vascular dementia. Neurology. 2008;71:1051–1056. doi: 10.1212/01.wnl.0000319692.20283.10. [DOI] [PubMed] [Google Scholar]
- 51.Caselli RJ, Hentz JG, Osborne D, et al. Apolipoprotein E and intellectual achievement. J Am Ger Soc. 2002;50:49–54. doi: 10.1046/j.1532-5415.2002.50007.x. [DOI] [PubMed] [Google Scholar]
- 52.Caselli RJ, Reiman EM, Hentz JG, Osborne D, Alexander GE, Boeve BF. A distinctive interaction between memory and chronic daytime somnolence in asymptomatic APOE e4 homozygotes. Sleep. 2002;25:447–53. [PubMed] [Google Scholar]
- 53.Caselli RJ, Reiman EM, Hentz JG, Osborne D, Alexander GE. A distinctive interaction between chronic anxiety and problem solving in asymptomatic APOE e4 homozygotes. J Neuropsychiatry Clin Neurosci. 2004;16:320–9. doi: 10.1176/jnp.16.3.320. [DOI] [PubMed] [Google Scholar]