Abstract
VCP (VCP/p97) is a ubiquitously expressed member of the AAA+-ATPase family of chaperone-like proteins that regulates numerous cellular processes including chromatin decondensation, homotypic membrane fusion, and ubiquitin-dependent protein degradation by the proteasome. Mutations in VCP cause a multisystem degenerative disease consisting of inclusion body myopathy, Paget’s disease of bone, and frontotemporal dementia (IBMPFD). Here we show that VCP is essential for autophagosome maturation. We generated cells stably expressing dual-tagged LC3 (mCherry-EGFP-LC3) which permit monitoring of autophagosome maturation. We determined that VCP deficiency by RNAi-mediated knockdown or over-expression of dominant-negative VCP results in significant accumulation of immature autophagic vesicles, some of which are abnormally large, acidified and exhibit cathepsin B activity. Furthermore, expression of disease-associated VCP mutants (R155H and A232E) also causes this autophagy defect. VCP was found to be essential to autophagosome maturation under basal conditions and in cells challenged by proteasome inhibition, but not in cells challenged by starvation, suggesting that VCP might be selectively required for autophagic degradation of ubiquitinated substrates. Indeed, a high percentage of the accumulated autophagic vesicles contain ubiquitin-positive contents, a feature that is not observed in autophagic vesicles that accumulate following starvation or treatment with Bafilomycin A. Finally, we show accumulation of numerous, large LAMP-1 and LAMP-2 –positive vacuoles and accumulation of LC3-II in myoblasts derived from patients with IBMPFD. We conclude that VCP is essential for maturation of ubiquitin-containing autophagosomes and that defect in this function may contribute to IBMPFD pathogenesis.
Keywords: autophagy, VCP, p97, ubiquitin, IBMPFD, ERAD
Introduction
Macroautophagy (hereafter autophagy) is a catabolic process in which sequestered cytosolic components are delivered to a specialized compartment for degradation by lysosomal hydrolases. Substrates, including proteins and organelles, are initially sequestered within double membraned, immature autophagic vesicles that are not acidified and do not contain lysosomal hydrolases. Early studies appreciated that delivery of lysosomal hydrolases occurs via fusion with lysosomes 1. As this process has been elucidated, it is become apparent that autophagosome maturation is a complex sequential, step-wise process that involves fusion with multiple components of the endo-lysosomal system to produce autolysosomes with degradative capacity 2. This maturation process is incompletely understood at present, but the molecular players are rapidly becoming elucidated. For example, it has become evident that components of the ESCRT complex machinery are essential to autophagosome maturation, either in the process of autophagosome closure, fusion with target vesicles, or both 2, 3. Several members of the Rab family of small GTPases, which regulate most if not all membrane trafficking pathways, play important roles at several steps of autophagosome maturation 4, 5. It is likely that autophagosome maturation also involves soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) proteins that drive membrane fusion through a process regulated by N-ethylmaleimide-sensitive factor (NSF) 6. As the process of autophagosome maturation is further illuminated, additional molecular players in the process will undoubtedly become apparent.
VCP (p97 in mouse, TER94 in Drosophila melanogaster, and CDC48 in S. cerevisiae) is a highly conserved AAA+-ATPase that regulates a wide array of cellular processes7. VCP is a 97 kD protein composed of an N-terminal domain followed by tandem ATPase domains. VCP functions as a homo-hexameric ring formed by the ATPase domains with the N-terminal domain oriented outward to permit interaction with adapter proteins. Hydrolysis of ATP drives a conformational change in VCP and this is required for VCP function 8. A catalytically dead mutant version of VCP, generated by mutations that impair both ATPase domains (E305Q/E578Q), functions as dominant negative when expressed exogenously and has been extensively used to interrogate VCP function 9. VCP coordinates a number of ubiquitin-regulated processes through its ability to segregate ubiquitinated substrates from unmodified partners 7. VCP is essential to some aspects of ubiquitin-dependent proteasomal degradation including endoplasmic reticulum-associated degradation (ERAD) 10, 11, degradation of some cytosolic proteins by the ubiquitin-fusion domain (UFD) pathway 12, and rapid degradation of nascent peptides during heat shock 13. VCP is also essential to some non-proteolytic aspects of ubiquitin signaling, including chromatin decondensation following mitosis 14, nuclear envelope formation 15 and homotypic membrane fusion during biogenesis of the ER and Golgi apparatus 16.
Mutations in VCP cause a dominantly inherited, multisystem degenerative disorder characterized by inclusion body myopathy, frontotemporal dementia and Paget’s disease of bone (also known as IBMPFD) (for a review see Ref 17). The pathology of this disorder is characterized by extensive accumulation of ubiquitin conjugates in affected tissues, suggesting impairment of protein degradation pathways. In addition, the muscle pathology in IBMPFD, which is the best characterized aspect of this disease, shows accumulation of numerous vacuoles and a defining feature of IBMPFD-related myopathy is the accumulation of large, ubiquitin-positive “rimmed vacuoles” of uncertain origin 18. The molecular basis for pathogenesis in IBMPFD is unknown. In this study, we show that VCP mutations that are causative for IBMPFD do not cause detectable impairment in ubiquitin-dependent degradation by the proteasome, but do impair maturation of ubiquitin-containing autophagosomes. Further, we show that myoblasts derived from IBMPFD patients show abnormal accumulation of large LAMP1- and LAMP2-positive vacuoles and LC3-II protein consistent with a defect in autophagy. This study strongly suggests that defective autophagy contributes to IBMPFD including the pathological accumulation of ubiquitin conjugates.
Results
Disease-associated mutations in VCP do not impair ubiquitin-dependent degradation by the proteasome
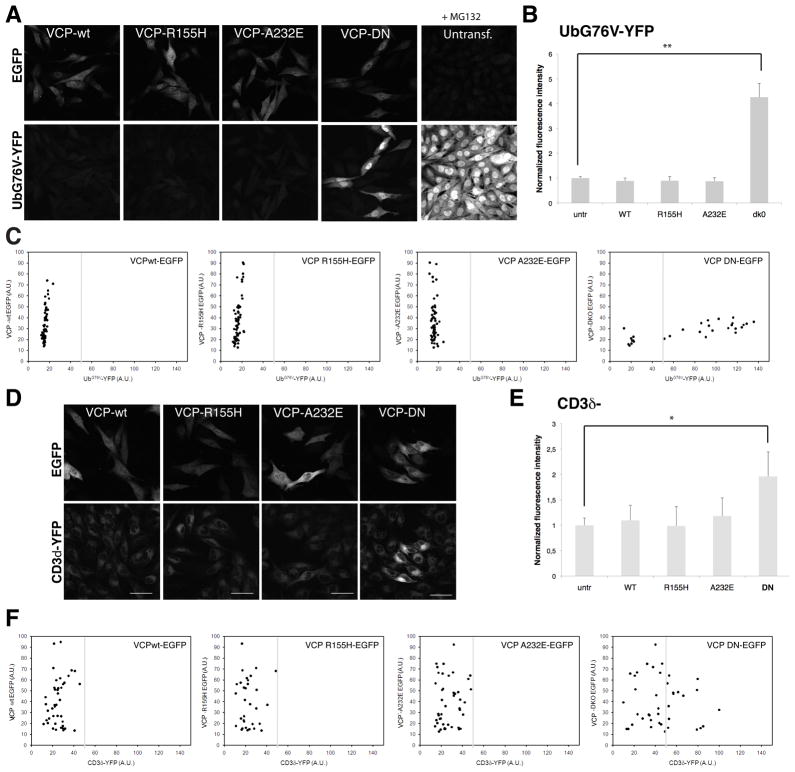
A prominent characteristic of the pathology in IBMPFD is the accumulation of ubiquitin conjugates in affected tissues. This implies a defect in turnover of ubiquitinated proteins and could result from impairment of either the ubiquitin-proteasome system (UPS) or of autophagy19. A well characterized function of VCP is its involvement in ubiquitin-dependent degradation by the ERAD and UFD pathways and, indeed, expression of dominant negative VCP (VCP-DN) strongly impairs these pathways. It has been suggested that mutations that cause IBMPFD impair ERAD, but this speculation is based on the observation that a mutant form of cystic fibrosis transmembrane conductance regulator (CFTR Δ508) is present at higher levels in cells co-transfected with mutant VCP20. This result is difficult to interpret, however, since the CFTRΔ508 mutant is also degraded by autophagy21, 22. To address the impact of disease-causing VCP mutations on proteasome-dependent degradation more specifically, we turned to another approach. We used reporter cells stably expressing UbG76V-YFP that provide a fluorescent signal that is highly sensitive to impairment of the UFD pathway of proteasomal degradation23. As expected, these cells show impairment of the UFD pathway in response to expression of VCP-DN (Fig. 1A–C and Suppl. Fig. 1). However, there was no impairment of the UFD pathway in cells expressing the disease-associated mutants VCP-R155H or VCP-A232E (Fig. 1A–C and Suppl. Fig. 1). We used a second reporter cell line that expresses CD3δ-YFP that provides a fluorescent signal that is highly sensitive to impairment of the ERAD pathway of proteasomal degradation 24. The ERAD pathway is impaired in cells expressing VCP-DN as expected but, again, there was no impairment of the ERAD pathway in cells expressing the disease-associated mutants VCP-R155H or VCP-A232E (Fig. 1D–F). Thus we find no evidence that disease-causing mutations in VCP impair its ability to support ubiquitin-dependent degradation by the proteasome.
Figure 1. Disease-associated VCP mutants do not cause a general impairment of ubiquitin-dependent proteasomal degradation.
(A) Mel Juso cells stably expressing the ubiquitin fusion degradation (UFD) substrate UbG76V-YFP were transiently transfected with expression vectors encoding GFP-tagged wild type VCP (VCP-wt), VCP-R155H, VCP-A232E and dominant negative VCP (VCP-DN). The VCP-wt and disease-associated mutants did not cause accumulation of the UbG76V-YFP substrate in contrast to the VCP-DN mutant. As a control, untransfected UbG76V-YFP Mel Juso cells were treated for 6 hrs with 10 μM of the proteasome inhibitor MG132. Scale bars equal 50 μm. (B) Quantification of the YFP fluorescence in UbG76V-YFP Mel Juso cells expressing wt and mutant VCPs. Values were normalized for the fluorescence intensity of untransfected cells. VCP-DN caused a significant increase in UbG76V-YFP levels (**unpaired t test, P < 0.001). Error bars represent standard deviation (n=30–150 cells). (C) Scatter plots of the YFP and GFP levels in UbG76V-YFP Mel Juso cells expressing GFP-tagged wt and mutant VCPs. (D) Mel Juso cells stably expressing the ERAD substrate CD3δ-YFP were transiently transfected with expression vectors encoding GFP-tagged VCP-wt, VCP-R155H, VCP-A232E and VCP-DN. The VCP-wt and disease-associated mutants did not cause accumulation of the CD3δ-YFP substrate whereas the VCP-DN mutant caused a general increase in CD3δ-YFP levels. Scale bars equal 50 μm. Error bars represent standard deviation. (E) Quantification of the YFP fluorescent values in CD3δ-YFP Mel Juso cells expressing wt and mutant VCPs. VCP-DN caused a significant increase in CD3δ-YFP levels (**unpaired t test, p<0.001). Error bars represent standard deviation (n=30–150 cells). (F) Scatter plots of the YFP and GFP levels in CD3δ-YFP Mel Juso cells expressing GFP-tagged wt and mutant VCP.
VCP is essential for autophagosome maturation
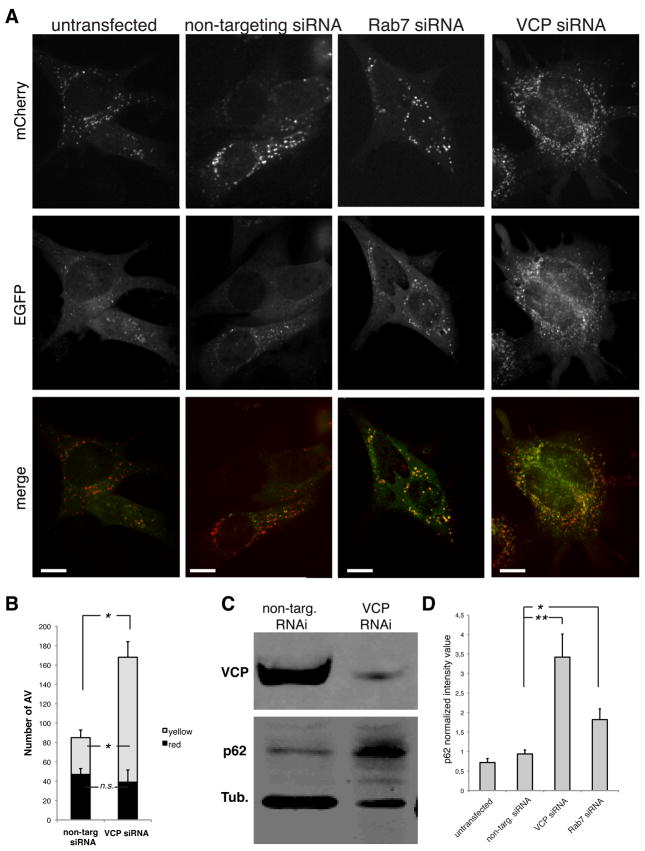
In order to monitor the process of autophagosome maturation, we generated mouse embryonic fibroblasts (MEFs) stably expressing the tandem tagged, fluorescent reporter mCherry-EGFP-LC3b 25. This reporter consists of a fusion protein of the fluorescent proteins mCherry, enhanced green fluorescent protein (EGFP) and microtubule-associated 1 light chain 3 isoform b (LC3b), a membrane-associated marker of autophagic vesicles. At neutral pH both mCherry and EGFP emit fluorescence and LC3-positive vesicles appear yellow in merged images. Since EGFP is pH-sensitive, at acidic pH only mCherry emits fluorescence and LC3-positive vesicles appear red. Thus, these reporter cells permit monitoring of autophagosome maturation since the fluorescence emission spectra changes during acidification. In untreated cells, or in cells transfected with non-targeting siRNA, the predominate type of LC3 vesicles present are small red foci indicative of mature autolysosomes, although some small yellow vesicles indicative of immature autophagosomes are also present (Fig. 2A–B). By contrast, RNAi-mediated knockdown of Rab7, a protein required for autophagosome maturation 4, results in significant accumulation of yellow foci, indicative of immature autophagosomes (Fig. 2A–B). We also observe a significant accumulation of immature autophagosomes following RNAi-mediated knockdown of VCP, indicating that this protein is also involved in autophagosome maturation (Fig. 2A–B). Here we use the term “maturation” to indicate the processes that occur after autophagosome formation, through acidification, including fusion-dependent processes. To corroborate this autophagy defect, we monitored the level of p62, a protein that is degraded by autophagy and accumulates when autophagy is impaired. VCP knockdown results in ~3.5-fold accumulation of p62 (Fig. 2C–D) thus further implicating VCP in autophagy.
Figure 2. RNAi knock down of VCP impairs autophagosome maturation.
(A) mCherry-EGFP-LC3b stable MEFs transfected with non-targeting, Rab7 or VCP siRNA. Scale bars equal 10 μm. (B) Quantification of autophagosomes and autophagolysosomes under basal conditions in cells transfected with non-targeting or VCP-targeting siRNA. * indicates p<0.01; n.s. indicates non-significant difference, error bars indicate standard errors. (C). Immunoblot against VCP, p62 and tubulin in non-targeting and VCP-targeting siRNA in MEFs. p62 and tubulin were observed simultaneously using a Li-Cor Odyssey system. (D) Quantification of p62 normalized against tubulin in cells transfected with non-targeting, VCP-targeting or Rab7-targeting siRNA. * indicates p<0.01; ** p<0.001; error bars indicate standard deviation from triplicates.
Disease-associated mutations in VCP impair autophagy maturation
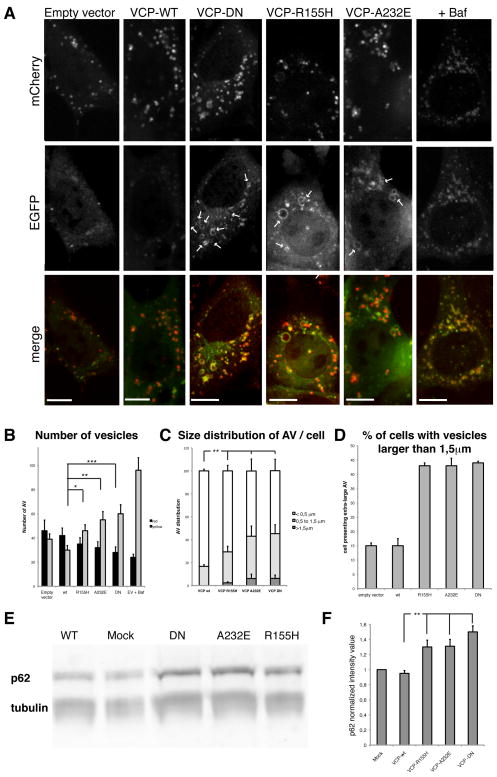
We next sought to determine whether disease-associated mutations of VCP impair autophagy. Expression of VCP-DN in mCherry-EGFP-LC3b reporter MEFs, but not expression of wild type VCP, resulted in accumulation of increased numbers of yellow foci consistent with an accumulation of immature autophagosomes (Fig. 3A–B). In addition, we observed accumulation of numerous large vacuoles over 1.5 μm in diameter consisting of a yellow ring surrounding a red lumen consistent with abnormal, partially acidified autophagic vesicles that was only rarely observed in cells transfected with empty vector or wild type VCP (Fig. 3A, C–D). Neither wild type nor mutant VCP was observed to associate with LC3-positive vesicles (Suppl. Fig. 2). Moreover, cells expressing mutant VCP accumulate p62 as shown by Western blot analyses consistent with a defect in clearance of this protein by autophagy (Fig. 3E–F). Thus, disease-associated VCP mutations also impair autophagosome maturation, suggesting that a defect autophagy may contribute to pathogenesis in IBMPFD.
Figure 3. Defective autophagosome maturation in cells expressing disease-associated VCP mutants.
(A) mCherry-EGFP-LC3b stable MEFs transfected with the empty vector or expressing VCP-wt, VCP-DN, VCP-R155H or VCP-A232E. Arrows indicate large LC3-positive vesicles. Scale bars equal 10 μm. (B) Quantification of autophagosomes (yellow) and autophagolysosomes (red) per cell under basal conditions. Cells were transfected with the empty vector, or with VCP-wt, VCP-DN, VCP-R155H or VCP-A232E. Empty vector transfected cells were also treated with bafilomycin (ev +Baf). * indicates p<0.05; ** p<0.01; *** p<0.001. Error bars indicate standard errors. (C) Size distribution of autophagic vesicles (AV) in cells transfected with wt or mutant forms of VCP. Size is estimated in μm. ** indicates p<0.01. (D) Quantification of the percent of cells containing “extra-large” vesicles (i.e. over 1.5 μm diameter) in cells transfected with empty vector, wt-VCP or mutant forms of VCP. Experiment was performed twice in triplicate. (E) Mutant forms of VCP lead to accumulation of p62 in HEK293T cells. Western blot against p62/rabbit and tubulin/mouse using a Li-Cor Odyssey system. (F) Quantification of p62 Western-Blot. p62 quantification was normalized to tubulin quantity. Error bars indicates standard deviation of three independent replicates. ** indicates p<0.01.
The experiments described above were performed in basal conditions. We next sought to evaluate whether VCP is required for autophagosome maturation under different conditions of autophagy induction. Interestingly, we observed that expression of VCP-DN or disease-associated versions of VCP did not impair autophagosome maturation under starvation conditions; although, as expected, autophagosome maturation during starvation was impaired by treatment with the drug bafilomycin (Suppl. Fig. 3). In contrast, similar to the results under basal conditions, expression of VCP-DN or disease associated versions of VCP did impair autophagosome maturation in cells challenged with the proteasome inhibitor epoxomicin (Suppl. Fig. 3). These results indicate a conditional requirement for VCP in autophagosome maturation.
Mutant forms of VCP impair autophagosome maturation at a late stage after acidification and delivery of lysosomal hydrolases
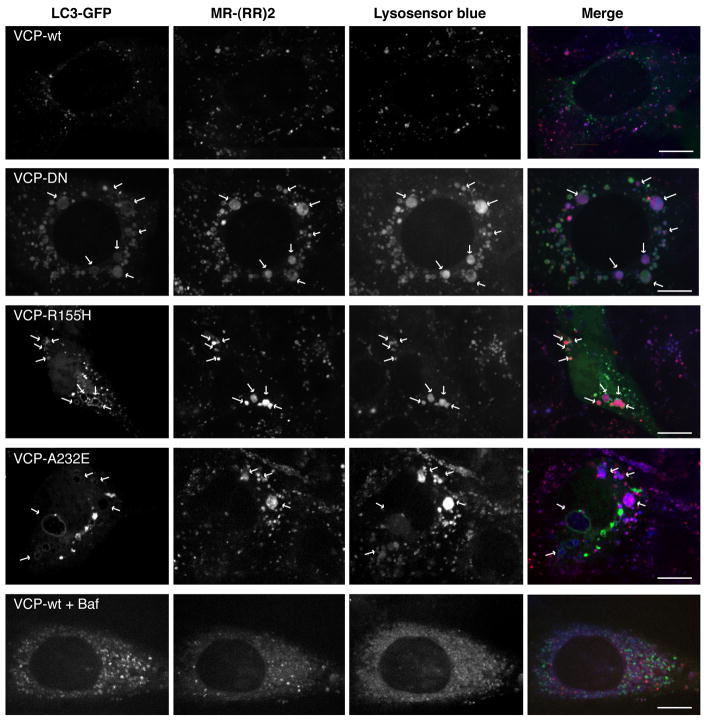
The appearance of abnormal, large autophagic vacuoles with a yellow rim surrounding a red lumen implies the accumulation of autophagosomes that are at least partially acidified. To confirm this suspicion, we co-transfected MEFs with GFP-LC3 and wild type or mutant forms of VCP, followed by labeling with the acidic dye LysoSensor Blue or the Cathepsin B substrate MR-(RR)2. Cells expressing wild type VCP did not accumulate LC3-positive vesicles and showed the normal distribution and size of lysosomes (Fig. 4). However as we suspected, many of the large vacuoles that accumulate in cells expressing mutant VCP were LysoSensor Blue-positive indicating that they were acidified (Fig. 4). These vesicles also exhibited Cathepsin B protease activity which confirmed acidification and indicated that these vesicles contain lysosomal protease (Fig. 4). In contrast, the vesicles that accumulate following Bafilomycin A treatment were smaller and showed no evidence of acidification or Cathepsin B activity, as expected (Fig. 4). We conclude that impairment of VCP function, including disease-causing mutations in VCP, results in defective autophagosome maturation, but is not simply a defect in autophagosome-lysosome fusion, since a significant number of large, acidified, Cathepsin B-containing vesicles are able to form.
Figure 4. The large LC3-positive vesicles that accumulate in cells expressing mutant VCP are acidified and exhibit cathepsin B activity.
Cells were co-transfected with GFP-LC3 and either wild type or mutant forms of VCP, and then labeled with acidotrophic dye LysoSensor Blue and Cathepsin B substrate MR-(RR)2. As a control, cells expressing VCP-wt were also treated with Bafilomycin A. Arrows indicate large GFP-LC3-positive vesicles that are labeled with LysoSensor Blue and MR(RR)2. Scale bars equal 10 μm. The control for efficiency of co-transfection is shown in Suppl. Fig. 4.
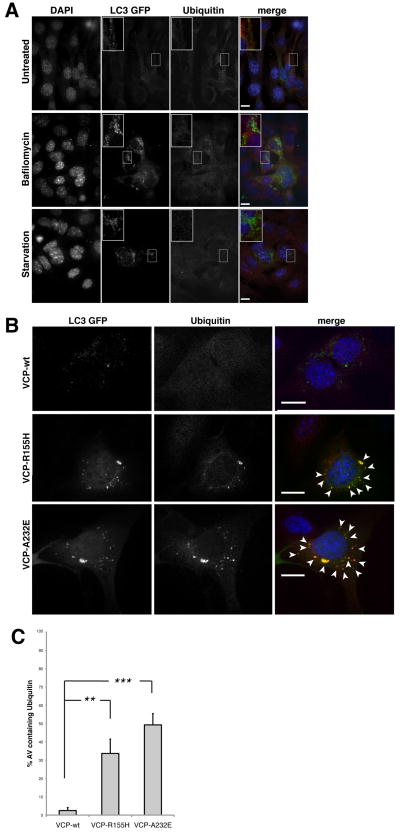
Disease-associated mutations in VCP lead to accumulation of autophagosomes that are laden with ubiquitin
The pathology of IBMPFD is characterized by accumulation of ubiquitin conjugates indicative of a defect in protein degradation. We found no evidence of a defect in the ERAD and UFD pathways of proteasomal degradation in cells expressing disease-associated mutant VCP (Fig. 1). However, we did find VCP to be essential for autophagosome maturation under basal conditions (Fig. 2–3) and following challenge with the proteasome inhibitor epoxomicin, but not under starvation conditions (Suppl. Fig. 3). Thus, we reasoned that VCP might be particularly important to clearance of ubiquitinated substrates by autophagy. We immunostained cells for ubiquitin and observed that wild type MEFs rarely show ubiquitin-positive, LC3-positive vesicles, even when autophagosomes accumulate in response to Bafilomycin A or starvation (Fig. 5A). By contrast, the majority of vesicles that accumulate in mutant VCP-expressing cells contain ubiquitin-positive material in their lumen (Fig. 5B–C and see Suppl. Fig. 5 for magnification). These results suggest that defective maturation of ubiquitin-containing autophagosomes may account for the pathology characteristic of IBMPFD.
Figure 5. The large LC3-positive vesicles that accumulate in mutant VCP-expressing cells contain ubiquitin-positive material.
(A) Cells transfected with LC3-GFP and immunostained with anti-ubiquitin (red) were observed under basal conditions (upper panel), or after Bafilomycin A treatment (middle panel) or starvation (lower panel). (B) Cells were co-transfected with indicated plasmid and GFP-LC3 and then immunostained with anti-ubiquitin (red). Scale bars equal 10 μm. Arrows indicate LC3-positive vesicles containing ubiquitin-positive material. (C) Graph representing the percentage ubiquitin-containing autophagosomes in cells expressing wild type or mutant VCP. Errors bars represent standard deviations of three independent replicates. ** means p<0,005; ***, p<0,001. See Suppl. Fig. 4 for analysis of co-transfection efficiency and Suppl. Fig. 5 for magnification indicating ubiquitin staining is lumenal.
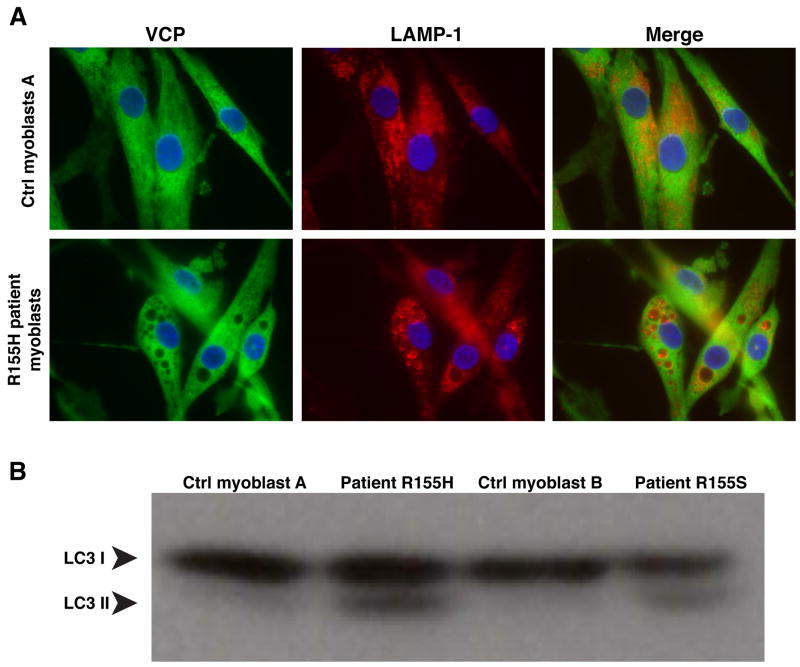
IBMPFD patient myoblasts show accumulation of large LAMP1- and LAMP2-positive vacuoles
To determine whether autophagy impairment was present in patients with IBMPFD, we studied myoblasts derived from IBMPFD patients carrying R155H or R155S mutations and compared these to myoblasts derived from normal individuals. In normal myoblasts, LAMP1 and LAMP2 staining associates with numerous small, vesicles as expected (Figure 6A and Suppl. Fig. 6). By contrast, in myoblasts derived from IBMPFD patients, nearly all cells showed accumulation of very large LAMP1- and LAMP2-positive vacuoles (Figure 6A and Suppl. Fig. 6). Furthermore, LC3-II accumulates in myoblasts derived from IBMPFD patients, providing further evidence of an autophagy defect (Fig. 6B). Combined with the preceding data, these findings strongly suggest that disease-causing mutations in VCP lead to autophagy impairment that contributes to the pathogenesis of IBMPFD. Endogenous VCP did not associate with the membranes of these LAMP-positive vacuoles (Fig. 6A) consistent with our observation that exogenous VCP/p97 expressed in MEFs did not associate with membranes (Suppl. Fig 2). However, we cannot exclude the possibility that VCP/p97 associates with autophagosome or lysosomal membranes transiently or at some low level in a way that is functionally significant as it does with membranes of the nuclear envelope, endoplasmic reticulum and the Golgi apparatus.
Figure 6. IBMPFD patient myoblasts show accumulation of large LAMP-positive vesicles and LC3-II protein.
(A) Immunostaining for VCP and LAMP-1 in myoblasts derived from normal control or an IBMPFD patient. Cells were observed with a 63× objective. Please see Suppl. Fig. 6 for cell lines from additional patients and additional markers. (B) Western blot analysis of LC3 in myoblast lysates from 2 control samples and samples from 2 IBMPFD patients.
Discussion
These data indicate that VCP is essential for proper maturation of autophagosomes under basal conditions and in the setting of proteasome impairment, but not in the setting of starvation. Furthermore, the autophagosomes that accumulate due to impaired VCP function contain ubiquitin-positive contents. Thus, it appears that VCP may be selectively required for autophagic degradation of ubiquitinated substrates. Importantly, disease-associated mutations in VCP also lead to defective autophagosome maturation and accumulation of vesicles that contain ubiquitin-positive contents. This is in contradistinction to a lack of any impairment of the UPS (including the ERAD and UFD pathways), suggesting that impaired autophagosome maturation is the basis for the accumulation of ubiquitin conjugates and ubiquitin-positive vacuoles in IBMPFD. The observed defect in maturation of autophagosomes caused by VCP mutations is not simply an impairment of autophagosome-lysosome fusion, since we clearly see acidification and protease activity in the large, accumulated vacuoles. Interestingly, a similar defect in autophagosome maturation, with accumulation of acidified and cathepsin D-positive vesicles, has been described in Alzheimer disease 26, 27. Finally, we show accumulation of numerous, large LAMP-1 and LAMP-2 –positive vacuoles and accumulation of LC3-II in myoblasts derived from patients with IBMPFD. We conclude that VCP is essential for maturation of ubiquitin-containing autophagosomes and that defect in this function may contribute to IBMPFD pathogenesis.
The role of VCP in autophagosome maturation shown here is particularly interesting in light of clinical overlap between IBMPFD and diseases caused by mutations in other genes related to autophagy; specifically, p62/SQSTM1 (p62) and Vps2/CHMP2B (CHMP2B). Mutations in p62, which is essential for the delivery of ubiquitinated proteins to the autophagy-lysosomal system 28, cause Paget’s disease of bone that is indistinguishable from that caused by mutations in VCP29, suggesting that these proteins may function in a common biological pathway. Mutations in CHMP2B cause frontotemporal dementia that shares the same type of ubiquitin pathology as that caused by mutations in VCP30, suggesting that these proteins may also function in a common biological pathway. CHMP2B is a component of the ESCRT-III complex that regulates the formation of early endosomes. Depletion of ESCRT subunits or over-expression of CHMP2B proteins inhibits autophagic degradation of ubiquitinated proteins31. Indeed, VCP may be added to list of proteins found at the interface of the endo-lysosomal system and autophagy that are associated with neurodegenerative disease 32.
It should also be noted that among VCP’s binding partners is HDAC6 33, which we previously showed was essential for degradation of ubiquitinated protein through autophagy 34, 35. Overexpression of HDAC6 in cells expressing disease mutants of VCP partially rescue degradation of ubiquitinated proteins 36 suggesting that these two proteins work cooperatively in a common pathway leading to the autophagic clearance of ubiquitinated protein.
In conclusion, this study shows that VCP is essential for clearance of ubiquitinated protein by autophagy. It will be of highly interest to determine if such a phenomenon in an in vivo model is reproducible in order to define its role in IBMPFD and develop adapted therapeutics.
Materials and Methods
Cell culture
MEFs were cultured with DMEM (Hyclone) supplemented with GlutaMax-1X (GIBCO) and 10% FBS (Hyclone). Starvation was realized washing cells with PBS and then adding a low glucose medium supplemented with inactivated dialyzed FBS (Hyclone). Epoxomicin was used at a final concentration of 10nM; bafilomycin was used at a final concentration of 10 μM. The human melanoma Mel JuSo cell lines stable expressing the UPS reporters UbG76V-YFP and CD3δ-YFP 24 were cultured in Iscove’s modified Dulbecco medium (Sigma-Aldrich) supplemented with 10% fetal calf serum (Sigma-Aldrich), 10 units/ml penicillin and 10 μg/ml streptomycin (Sigma-Aldrich). Primary myoblast cell lines from patients with the heterozygous R155H and R155S mutations as well as from two control subjects were obtained from The Muscle Tissue Culture Collection (MTCC)/EuroBioBank (Munich, Germany). All characteristics have been previously published by Vesa et colleagues 37. Mutant myoblasts were generated from muscle biopsies showing typical clinical phenotype and histological findings of IBMPFD 37. Control cell lines were generated from age-matched control subjects without any pathological findings in histology. Cells were maintained in Skeletal Muscle Cell Growth Medium (PromoCell, Heidelberg, Germany) supplemented with the supplement mix (PromoCell), 10% FBS (HyClone, Logan, UT), Gentamicin (Gibco, Carlsbad, CA) and GlutaMAX-1 (Gibco) in 5% CO2 at 37°C.
Plasmids and transfection
RFP-LC3 and mCherry-EGFP-LC3b pDEST vectors were obtained from Terje Johannsen. We isolated an insert containing the coding sequences for mCherry-EGFP-LC3b by digestion with NheI and SphI. This insert was subcloned into the retroviral vector LCP into BamHI and SphI, which was used to make stably-expressing MEFs using techniques described previously 38. GFP-LC3 vector was obtained from Craig Thompson. Wt and mutant VCP tagged with Flag at the N-terminus were subloned into a pcDNA3.1. Transfections with these plasmids were accomplished with Fugene reagent (Roche). To determine which cells were transfected with FLAG-VCP, we co-transfected with a vector expressing the fluorescent protein Cerulean. In our hands, control experiments using immunofluorescence determined that >93% of transfected cells were co-transfected with both constructs (supp. fig. 4). The VCP wt and mutant plasmids were constructed by PCR amplification of the VCP ORFs lacking the stop codons from pcDNA3.1+/VCP-WT, pcDNA3.1+/VCP-R155H, pcDNA3.1+/VCP-A232E, pcDNA3.1+/VCP-DKO, and insertion into the BamHI and HindIII sites of pEGFP-N1 (Clontech). These plasmids were transfected using Lipofectamine Plus (Invitrogen) according to the manufacturer’s protocol. RNAi knockdown were performed by transfection of ON-TARGET plus-smartpool siRNA (Dharmacon, ref 040859 for Rab7 and 057592 for VCP) with Lipofectamine RNAi Max (Invitrogen).
Microscopy
Confocal microscopy for autophagy observation was performed with Marianas and Marianas 2 confocal microscopes, with 63× objectives. All analyses were performed with Slidebook 5.0 software. To analyse only transfected cells, we cotransfected VCP plasmids with a cerulean containing plasmid. The efficiency of co-transfection has been determined < 93% (Suppl. Fig. 3). The cerulean plasmid has no impact on autophagy (Figure 1A, empty vector panel). LysoSensor Blue (Molecular Probes, DND-167) was used at a final concentration of 10 μM. Cathepsin B activity was determined using Magic Red Cathepsin B detection kit (Immunochemistry Technologies, LLC) following recommendations of the manufacturer. Anti-Ubiquitin immunofluorescence microscopy was performed with P4D1 antibody (Santa Cruz) on cells previously co-transfected with GFP-LC3 and VCP wt or mutants. Lamp1 PA1-654A antibody was purchased from Affinity BioReagents, Lamp2 sc-18822 antibody was purchased from Santa Cruz biotechnology, VCP MA-3004 was purchased from Affinity BioReagents. For microscopy related to monitoring ERAD and UFD substrates, after 24hrs, Mel JuSo cells were fixed with 4% paraformaldehyde (Sigma) and examined with a Zeiss LSM510 META confocal laser scanning microscope (Plan-Neofluar 40×/1.3 oil objective). In order to achieve clear separation of the EGFP and EYFP fluorescence, the META system was used with a spectrum range 497–508nm for EGFP, and 550–583nm for EYFP. Quantitative analyses of fluorescence intensities were performed using the Volocity software (Improvision PerkinElmer).
Western Blot
For p62 Western blots, HEK293T cells or MEFs were transfected as described above. Sixteen (Fugene) or 67 (Lipofectamine RNAi-Max) hours post-transfection, transfection media were replaced by rich medium for 5 hours. Cells were then lysed using RIPA and sonication. Entire lysates were loaded. Anti-p62 (rabbit) and anti-tubulin (mouse) were provided by Sigma. The Odyssey system (Li-Cor) was used for detection, and the ImageJ software was used for quantification. For LC3 western-blot, control and patient myoblasts have been lysed in RIPA and centrifuged. Anti-LC3 antibody (PM036) was provided by Upstate. Cells were then lysed using RIPA and sonication. Entire lysates were loaded. Anti-p62 (rabbit) and anti-tubulin (mouse) were provided by Sigma. The Odyssey system (Li-Cor) was used for detection, and the ImageJ software was used for quantification. For LC3 western-blot, control and patient myoblasts have been lysed in RIPA and centrifuged. Anti-LC3 antibody (PM036) was provided by Upstate.
Supplementary Material
Western blot analysis of lysates Mel Juso cells stably expressing the ubiquitin fusion degradation (UFD) substrate UbG76V-YFP that were untransfected (UT) or transiently transfected with expression vectors encoding GFP-tagged wild type VCP (wt), VCP-R155H (R155H), VCP-A232E (A232E) and dominant negative VCP (DN). As a control, untransfected UbG76V-YFP Mel Juso cells were treated for 6 hrs with 10 μM of the proteasome inhibitor MG132. The steady state levels of the GFP-tagged VCPs and UbG76V-YFP were determined using a GFP-specific antibody.
A. MEFs were co-transfected with RFP-LC3 and EGFP-VCP-wt (upper panel) or EGFP-VCP-DN (lower panel). WT and DN VCP are cytoplasmic molecules and do not co-localize with LC3 positive vesicles. B. Co-transfection efficiency control. Graph representing efficiency of co-transfection of EGFP-VCP and RFP-LC3.
Quantification of proportions of autophagosomes and autophagolysosomes under basal conditions (A) or in cells treated with epoxomycin (B) or starvation (C). Cells were transfected with the empty vector, or with VCP-wt, VCP-DN, VCP-R155H or VCP-A232E. Empty vector transfected cells were also treated with bafilomycin (ev +Baf). * indicates p<0.05; ** p<0.01; *** p<0.001.
Graph representing efficiency of co-transfection of EGFP-VCP and RFP-LC3.
Example of a very large LC3-positive vesicle from a R155H transfected cell immunostained with anti-ubiquitin (red). N indicates nucleus (with DAPI staining).
Control individual corresponds to control B from figure 6.
Acknowledgments
We thank the Cell and Tissue Imaging Core of St. Jude Children’s Research Hospital for assistance with confocal microscopy, Kelly McCastlain and Kristin Walters for help with retroviral transfection, Terje Johansen for the gift of a plasmid containing mCherry-EGFP-LC3b, and Craig Thompson for the gift of a plasmid containing GFP-LC3. The Muscle Tissue Culture Collection is part of the German network on muscular dystrophies (MD-NET, service structureS1, 01GM0601) funded by the German ministry of education and research (BMBF, Bonn, Germany). This work was supported by NIH grant AG031587 and a grant from the Dana Foundation to JPT, by the Swedish Research Council, the Swedish Cancer Society, the Nordic Center of Excellence Neurodegeneration and the Karolinska Institute to NPD.
Abbreviations
- AAA+
ATPase associated with diverse cellular activities
- AL
Autolysosomes
- ERAD
Endoplasmic reticulum-associated degradation
- IBMPFD
Inclusion body myopathy with Paget’s disease of bone and frontotemporal dementia
- UPS
Ubiquitin-proteasome system
- VCP
Valosin-containing protein
- LAMP
Lysosome-associated membrane protein
References
- 1.De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–92. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 2.Tooze SA, Razi M. The essential role of early endosomes in autophagy is revealed by loss of COPI function. Autophagy. 2009;5:874–5. doi: 10.4161/auto.9066. [DOI] [PubMed] [Google Scholar]
- 3.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–52. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez MG, Munafo DB, Beron W, Colombo MI. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci. 2004;117:2687–97. doi: 10.1242/jcs.01114. [DOI] [PubMed] [Google Scholar]
- 5.Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, et al. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117:4837–48. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 6.Atlashkin V, Kreykenbohm V, Eskelinen EL, Wenzel D, Fayyazi A, Fischer von Mollard G. Deletion of the SNARE vti1b in mice results in the loss of a single SNARE partner, syntaxin 8. Mol Cell Biol. 2003;23:5198–207. doi: 10.1128/MCB.23.15.5198-5207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jentsch S, Rumpf S. Cdc48 (p97): a “molecular gearbox” in the ubiquitin pathway? Trends Biochem Sci. 2007;32:6–11. doi: 10.1016/j.tibs.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Rouiller I, DeLaBarre B, May AP, Weis WI, Brunger AT, Milligan RA, et al. Conformational changes of the multifunction p97 AAA ATPase during its ATPase cycle. Nat Struct Biol. 2002;9:950–7. doi: 10.1038/nsb872. [DOI] [PubMed] [Google Scholar]
- 9.Dalal S, Rosser MF, Cyr DM, Hanson PI. Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway. Mol Biol Cell. 2004;15:637–48. doi: 10.1091/mbc.E03-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowis D, McConnell E, Wojcik C. Destabilization of the VCP-Ufd1-Npl4 complex is associated with decreased levels of ERAD substrates. Exp Cell Res. 2006;312:2921–32. doi: 10.1016/j.yexcr.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Ballar P, Fang S. Regulation of ER-associated degradation via p97/VCP-interacting motif. Biochem Soc Trans. 2008;36:818–22. doi: 10.1042/BST0360818. [DOI] [PubMed] [Google Scholar]
- 12.Wojcik C, Rowicka M, Kudlicki A, Nowis D, McConnell E, Kujawa M, et al. Valosin-containing protein (p97) is a regulator of endoplasmic reticulum stress and of the degradation of N-end rule and ubiquitin-fusion degradation pathway substrates in mammalian cells. Mol Biol Cell. 2006;17:4606–18. doi: 10.1091/mbc.E06-05-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medicherla B, Goldberg AL. Heat shock and oxygen radicals stimulate ubiquitin-dependent degradation mainly of newly synthesized proteins. J Cell Biol. 2008;182:663–73. doi: 10.1083/jcb.200803022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramadan K, Bruderer R, Spiga FM, Popp O, Baur T, Gotta M, et al. Cdc48/p97 promotes reformation of the nucleus by extracting the kinase Aurora B from chromatin. Nature. 2007;450:1258–62. doi: 10.1038/nature06388. [DOI] [PubMed] [Google Scholar]
- 15.Hetzer M, Meyer HH, Walther TC, Bilbao-Cortes D, Warren G, Mattaj IW. Distinct AAA-ATPase p97 complexes function in discrete steps of nuclear assembly. Nat Cell Biol. 2001;3:1086–91. doi: 10.1038/ncb1201-1086. [DOI] [PubMed] [Google Scholar]
- 16.Uchiyama K, Jokitalo E, Kano F, Murata M, Zhang X, Canas B, et al. VCIP135, a novel essential factor for p97/p47-mediated membrane fusion, is required for Golgi and ER assembly in vivo. J Cell Biol. 2002;159:855–66. doi: 10.1083/jcb.200208112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimonis VE, Mehta SG, Fulchiero EC, Thomasova D, Pasquali M, Boycott K, et al. Clinical studies in familial VCP myopathy associated with Paget disease of bone and frontotemporal dementia. Am J Med Genet A. 2008;146A:745–57. doi: 10.1002/ajmg.a.31862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimonis VE, Fulchiero E, Vesa J, Watts G. VCP disease associated with myopathy, Paget disease of bone and frontotemporal dementia: review of a unique disorder. Biochim Biophys Acta. 2008;1782:744–8. doi: 10.1016/j.bbadis.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Nedelsky NB, Todd PK, Taylor JP. Autophagy and the ubiquitin-proteasome system: collaborators in neuroprotection. Biochim Biophys Acta. 2008;1782:691–9. doi: 10.1016/j.bbadis.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weihl CC, Dalal S, Pestronk A, Hanson PI. Inclusion body myopathy-associated mutations in p97/VCP impair endoplasmic reticulum-associated degradation. Hum Mol Genet. 2006;15:189–99. doi: 10.1093/hmg/ddi426. [DOI] [PubMed] [Google Scholar]
- 21.Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem. 2005;280:40282–92. doi: 10.1074/jbc.M508786200. [DOI] [PubMed] [Google Scholar]
- 22.Fu L, Sztul E. ER-associated complexes (ERACs) containing aggregated cystic fibrosis transmembrane conductance regulator (CFTR) are degraded by autophagy. Eur J Cell Biol. 2009;88:215–26. doi: 10.1016/j.ejcb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dantuma NP, Lindsten K, Glas R, Jellne M, Masucci MG. Short-lived green fluorescent proteins for quantifying ubiquitin/proteasome-dependent proteolysis in living cells. Nat Biotechnol. 2000;18:538–43. doi: 10.1038/75406. [DOI] [PubMed] [Google Scholar]
- 24.Menendez-Benito V, Verhoef LG, Masucci MG, Dantuma NP. Endoplasmic reticulum stress compromises the ubiquitin-proteasome system. Hum Mol Genet. 2005;14:2787–99. doi: 10.1093/hmg/ddi312. [DOI] [PubMed] [Google Scholar]
- 25.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–45. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 26.Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci. 2008;28:6926–37. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling D, Song HJ, Garza D, Neufeld TP, Salvaterra PM. Abeta42-induced neurodegeneration via an age-dependent autophagic-lysosomal injury in Drosophila. PLoS One. 2009;4:e4201. doi: 10.1371/journal.pone.0004201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–4. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hocking LJ, Lucas GJ, Daroszewska A, Mangion J, Olavesen M, Cundy T, et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet. 2002;11:2735–9. doi: 10.1093/hmg/11.22.2735. [DOI] [PubMed] [Google Scholar]
- 30.Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–8. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 31.Lee JA, Beigneux A, Ahmad ST, Young SG, Gao FB. ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr Biol. 2007;17:1561–7. doi: 10.1016/j.cub.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 32.Nixon RA, Yang DS, Lee JH. Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy. 2008;4:590–9. doi: 10.4161/auto.6259. [DOI] [PubMed] [Google Scholar]
- 33.Seigneurin-Berny D, Verdel A, Curtet S, Lemercier C, Garin J, Rousseaux S, et al. Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol Cell Biol. 2001;21:8035–44. doi: 10.1128/MCB.21.23.8035-8044.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–63. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 35.Pandey UB, Batlevi Y, Baehrecke EH, Taylor JP. HDAC6 at the intersection of autophagy, the ubiquitin-proteasome system and neurodegeneration. Autophagy. 2007;3:643–5. doi: 10.4161/auto.5050. [DOI] [PubMed] [Google Scholar]
- 36.Ju JS, Miller SE, Hanson PI, Weihl CC. Impaired protein aggregate handling and clearance underlie the pathogenesis of p97/VCP-associated disease. J Biol Chem. 2008;283:30289–99. doi: 10.1074/jbc.M805517200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vesa J, Su H, Watts GD, Krause S, Walter MC, Martin B, et al. Valosin containing protein associated inclusion body myopathy: abnormal vacuolization, autophagy and cell fusion in myoblasts. Neuromuscul Disord. 2009;19:766–72. doi: 10.1016/j.nmd.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finnberg N, Gruber JJ, Fei P, Rudolph D, Bric A, Kim SH, et al. DR5 knockout mice are compromised in radiation-induced apoptosis. Mol Cell Biol. 2005;25:2000–13. doi: 10.1128/MCB.25.5.2000-2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blot analysis of lysates Mel Juso cells stably expressing the ubiquitin fusion degradation (UFD) substrate UbG76V-YFP that were untransfected (UT) or transiently transfected with expression vectors encoding GFP-tagged wild type VCP (wt), VCP-R155H (R155H), VCP-A232E (A232E) and dominant negative VCP (DN). As a control, untransfected UbG76V-YFP Mel Juso cells were treated for 6 hrs with 10 μM of the proteasome inhibitor MG132. The steady state levels of the GFP-tagged VCPs and UbG76V-YFP were determined using a GFP-specific antibody.
A. MEFs were co-transfected with RFP-LC3 and EGFP-VCP-wt (upper panel) or EGFP-VCP-DN (lower panel). WT and DN VCP are cytoplasmic molecules and do not co-localize with LC3 positive vesicles. B. Co-transfection efficiency control. Graph representing efficiency of co-transfection of EGFP-VCP and RFP-LC3.
Quantification of proportions of autophagosomes and autophagolysosomes under basal conditions (A) or in cells treated with epoxomycin (B) or starvation (C). Cells were transfected with the empty vector, or with VCP-wt, VCP-DN, VCP-R155H or VCP-A232E. Empty vector transfected cells were also treated with bafilomycin (ev +Baf). * indicates p<0.05; ** p<0.01; *** p<0.001.
Graph representing efficiency of co-transfection of EGFP-VCP and RFP-LC3.
Example of a very large LC3-positive vesicle from a R155H transfected cell immunostained with anti-ubiquitin (red). N indicates nucleus (with DAPI staining).
Control individual corresponds to control B from figure 6.