Abstract
This study analyzed the effectiveness of active case detection (ACD) for new visceral leishmaniasis (VL) cases. ACD detection was carried out using house to house screening in Bangladesh and India and by neighborhood screening around index cases in Nepal. The percent increase of new VL cases through ACD compared to PCD was 6.7–17.1% in India; 38.8% in Nepal; and 60% in Bangladesh. The screening effort was high in India and Bangladesh (house to house screening) compared to Nepal (index case screening). The additional cost per new VL case detected varied: $50 to $106 in India; $172 in Bangladesh; $262 in Nepal depending on the type of screening staff, transport and training costs. The estimated annual VL incidence in the ACD arm ranged from 315–383 in India; 109 in Bangladesh, and 43 per 100,000 in Nepal. The additional effort and cost rises as disease incidence declines or PCD improves.
Introduction
Visceral leishmaniasis (VL) continues to be a major public health concern in the Indian subcontinent affecting the poorest of the poor. The endemic region including Northern India, Southern Nepal, and Bangladesh with 60% of the world's VL disease burden has a population of over 150 million people in 109 districts at risk from infection1 with more than 40,000 new VL cases reported in 2006.2–4 However, these estimates on the basis of passive reporting in the routine surveillance system (called throughout this work passive case detection; PCD) are likely to underestimate the actual burden of VL disease by up to five times.5,6 The VL disease is a prime candidate for elimination from the Indian subcontinent as the disease in this area is anthroponotic with no known animal reservoir and the female sandfly (Phlebotomous argentipes) is the only known vector transmitting VL disease. The discovery of leishmaniacidal activity of hexadecylphosphocholine (Miltefosine) offers a safe and effective oral drug alternative for home treatment of VL, improved treatment adherence, and cure.7–9 The development of an affordable field-based rapid diagnostic test—rK39 immuno-chromatographic test (rK39 ICT) with high sensitivity and specificity10–13 allows early diagnosis avoiding the need for parasite confirmation in spleen or bone marrow. The Indian, Bangladesh, and Nepal Governments agreed in 2005 to reduce VL burden of disease to below 10 per 100,000 by 2015.14 Current VL elimination efforts, however, face major challenges including inadequate surveillance, treatment failure with sodium stibogluconate (SAG),15 delays in diagnosis and treatment, poor treatment adherence,16 and persistence of perennial transmission through post kala-azar dermal leishmaniasis (PKDL) cases. The aim of this study was to analyze two active case detection (ACD) strategies in terms of their yield (ability to detect new VL patients), incremental program costs, and efforts as well as patient benefits in terms of reduced delay in diagnosing and treating their disease.
Materials and Methods
Study design and timing.
The study design had two arms: One arm included ACD through blanket screening of all households in villages in India and Bangladesh or focal screening of households in the neighborhood of an index case in Nepal. The other arm included passive case detection through routine surveillance. The study was conducted from January to May 2008.
Study area profile.
In India, the study was conducted in Saran and Muzaffarpur districts. In Nepal, the study was conducted in Saptari, Sunsari, and Morang districts of the Terai region and in Bangladesh in the Mymensingh district (Figure 1). These regions were selected for their high endemicity where the reported annual VL incidence per 100,000 was 200–250 in India; 53–184 in Nepal, and 130–310 in Bangladesh (Table 1).
Figure 1.
Study area map.
Table 1.
Study area profile*
| India 1 | India 2 | Nepal | Bangladesh | |
|---|---|---|---|---|
| Study area (district) | Saran district, India | Muzaffarpur district, India | Saptari, Sunsari, Morang districts, Nepal | Mymensinh district, Bangladesh |
| Active case detection (ACD) area | Parsa PHC (Primary Health Center) | Kurhani PHC | Kanchanapur PHC | Kanthal Union |
| Passive case detection (PCD) area | Amnaur PHC | Musahari PHC | Kalyanpur PHC | Moshakhali Union |
| Salient characteristics of study area | Highly endemic; few public or private initiatives for VL prevention and control | Highly endemic; well served by NGO (better public awareness activities and access to VL care) | Low endemic; civil unrest limiting public health interventions | Highly endemic; lower public awareness; poor PCD |
| Average family size | 5.87 | 5.73 | 5.25 | 4.4 |
| HH head without formal education (%) | 45.2% | 41.3% | 56.3% | 51.9% |
| Mean age in years (SD) | 24.8 (18.7) | 23.6 (18.5) | 25.3 (17.8) | 25.1 (18.7) |
| Sex distribution | ||||
| Male (%) | 53.6 | 53.4 | 50.9 | 51.4 |
| Female (%) | 46.4 | 46.6 | 49.1 | 48.6 |
| Estimated VL prevalence (per 100,000) in district (surveillance data) | 200–250 | 200–250 | 53–184 | 130–310 |
PHC = Primany Health Center; VL = visceral leishmaniasis; NGO = nongovernmental organization; HH = household head.
In India, sporadic indoor residual spraying (IRS) has been conducted for the past several years and SAG continues to be the mainstay of treatment. Amphotericin B and Miltefosine, though available in the private health sector, has recently been introduced at Primary Health Centers (PHCs) in VL endemic areas. In Nepal, IRS has been conducted sporadically in previous years and Miltefosine was introduced recently in the study area as a first line drug In Bangladesh, there has been no insecticide spraying in recent years and SAG was the only available drug against VL.
ACD strategies.
In the study sites of India and Bangladesh, after initial listing, all households were screened by health workers and accredited social health activists/community volunteers using a short screening questionnaire. All known VL cases (diagnosed in the last year) were recorded. Individuals with fever for more than 14 days were examined for spleen enlargement and, if positive, serologically tested for antibodies against Leishmania-specific rK39 ICT. Additionally, all individuals with maculopapular skin lesions with a past history of VL treatment were referred to the PHC/Upazila Health Complex for rK39 diagnosis.
In Nepal, known VL patients undergoing treatment or having been treated in the last year (index case), were identified and traced to their residence. House to house screening around the index case household was carried out by health assistants/technician in a progressive centrifugal manner in all directions until no new VL cases were identified in at least 50 consecutive households. Spleen examination and rK39 diagnosis were conducted at the household level for patients with fever longer than 14 days and suspected cases of PKDL. Patients testing positive were referred to the Zonal Hospital for treatment.
PCD strategy.
The yield of PCD in terms of detecting VL cases and patient characteristics were analyzed in adjacent endemic subdistricts in the following way: Researchers visited the PHCs, hospitals in the PCD arm on a weekly basis for 2 months to identify VL patients newly registered for diagnosis and treatment within this period. The VL patients registered in the past 12 months in these facilities before the start of this study were also documented and information recorded included the start of symptoms, date of diagnosis, and treatment initiation.
Definitions and treatment strategies.
The World Health Organization (WHO) definition of suspected VL (fever > 15 days with splenomegaly in a VL endemic area and a positive rK39) was taken as a basis for initiating treatment.17 Patients with PKDL-like skin lesions with a history of VL treatment in the last few years and testing positive for rK39 were treated for PKDL. At all sites, detailed information on diagnosis and treatment was collected, including delays in seeking care.
Statistical and sample size considerations.
A sample of 35,000 population screened at each site would detect a 5-fold increase in yield of new cases assuming an annual baseline VL incidence of 100/100,000 population with 80% power at 5% significance level. A further comparison of the yield of 18 new VL patients expected from ACD with cases reported by passive surveillance (PCD) would detect a difference of 2 weeks reduction in the delay in diagnosis with 90% power and 5% significance level, assuming that the lag time to diagnosis is 4 weeks. Data were entered into EpiInfo (CDC, Atlanta, GA) and analyzed using STATA (Stata Corp., College Station, TX).
Cost analysis.
The cost analysis focused at additional program costs of ACD, which will have to be covered by the control program if ACD, as described in this work, would be adopted. Training costs—including costs of training materials, per diem, salaries of trainers—were extrapolated to biannual training rounds (see Table 3). Household screening costs included additional cost for staff time (salary components), per diem, travel, and other related costs of supervision, screening forms, registers, performing diagnostic tests, and in some cases incentives. Diagnostic costs included costs of rK39 test strips. All costs were converted from local currency into US$ using the official exchange rate on January 31, 2008. Additional costs for identifying one new VL case were compared across sites. The costing of PCD was not attempted because the reporting of VL cases belongs to the routine activities of health staff and does not imply any additional costs for the program. No economic analysis of direct and indirect costs for patients and their families has been attempted and no assessment of the economic benefits of ACD has been done as this is subject to another study.
Table 3.
Incremental efforts and costs (in US$) of active case detection (ACD) through house to house screening
| Saran, India | Muzaffarpur India | Sunsari et al. Nepal | Mymensingh, Bangladesh | |
|---|---|---|---|---|
| New visceral leishmaniasis cases detected | 19 | 8 | 7 | 12 |
| Houses screened | 5776 | 7025 | 1143 | 6566 |
| Houses to screen for detecting 1 new VL case | 300 | 880 | 160 | 550 |
| Costs: | ||||
| Training costs ($) | 411 | 83 | 1262* | 1000* |
| Diagnostics cost (rK39 ICTs etc.) ($) | 94 | 138 | 93 | 696† |
| House screening survey costs–per diem, travel, etc. ($) | 634 | 668 | 1111 | 875 |
| Cost per house surveyed | 11 cents | 9.5 cents | 97 cents | 13.3 cents |
| Total direct costs of ACD (adjusted‡) ($) | 934 | 848 | 1836 | 2071 |
| Incremental cost per new VL case detected ($) | ~50 | 106 | ~262 | ~172 |
Reflects higher per diem and differences in number of training sessions.
Includes cost of study physician recruited exclusively for study.
Training costs assumed for two cycles of house surveys, hence half of training costs used here.
Ethical approval.
The research program was approved by the Ethics Committees of all participating sites and WHO.
Results
Background information.
All study populations showed characteristics of poverty; young people with a mean age of roughly 25 years had low literacy levels (Table 1). Almost half of household heads were without formal education. The family size was large, typically 4.4 to 5.9 persons per household. There was a high burden of VL in the test sites with 130 to 310 reported annual cases per 100,000 population with only one district in Nepal being lower and there was poor access to health care with roughly 13 km distance to primary care centers.
Yield (“effectiveness”) of ACD and estimated annual VL incidence.
In total, 161,184 persons were screened (Table 2). The number of reported (“known”) past or current VL cases was 268, being particularly high in the two Indian districts with 111 and 119 cases, respectively. The house to house screening (“blanket approach”) yielded 19 and 8 new cases in the Indian sites, which is an increase of 17% and 6.7% (Figure 2). A higher yield was observed in Bangladesh where 12 new cases were detected against 20 known VL cases (60% increase). The Nepal site using focal screening in neighborhoods of 18 “index” cases detected 7 new VL patients (increase of 38.8%). Considering the cases detected by PCD (268 in total) together with those identified by ACD (46), the overall annual VL incidence is estimated at 194.8/100,000 population, (315–383.1 in India; 109.4 in Bangladesh; and 43.3/100,000 in Nepal).
Table 2.
Improved visceral leishmaniasis (VL) case detection in the active case detection (ACD) arm by site
| Saran, India | Muzaffarpur, India | Sunsari et al., Nepal | Mymensingh, Bangladesh | Overall | |
|---|---|---|---|---|---|
| Population screened (a) | 33,928 | 40,317 | 57,713 | 29,226 | 161,184 |
| VL cases diagnosed in last 1 year and reported in screening (b) | 111 | 119 | 18 | 20 | 268 |
| New VL cases actively detected by screening (c) | 19 | 8 | 7 | 12 | 46 |
| % Increase caused by active detection (c/b*100) | 17.1% | 6.7% | 38.8% | 60% | 17.1% |
| Estimated VL incidence in 1 year (b + c)† | 130 | 127 | 25 | 32 | 314 |
| VL 1-year incidence rate per 100,000 ([b + c]/a*100,000) | 383.1 | 315 | 43.3 | 109.4 | 194.8 |
| PKDL cases newly detected | 0 | 14 | 0 | 18 | 32 |
| PKDL prevalence per 100,000 | – | 34.7 | – | 61.6 | 19.9 |
The few reported VL deaths were not included.
PKDL = post kala-azar dermal leishmaniasis.
Figure 2.
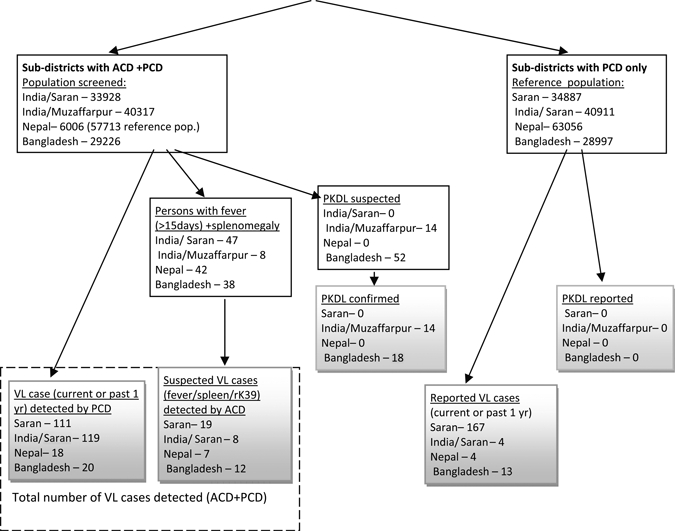
Study design and visceral leishmaniasis (VL)/ post kala-azar dermal leishmaniasis (PKDL) cases detected.
Effort and cost of ACD compared with PCD.
The additional efforts required to detect one new VL case by ACD varied from 300 to 880 houses to be screened in Indian sites, whereas in Bangladesh 550 households had to be screened (Table 3). In contrast, the focal screening in the neighborhood of “index” cases in Nepal required the visit of 160 houses to detect one new VL case.
The cost per house screened varied between 9.5 and 13.3 cents in India and Bangladesh (Table 3). In Nepal the cost per house screened was 97 cents reflecting high travel costs of researchers to reach distant villages. The additional cost for each new VL case identified varied from $50 to $106 in India. The costs were substantially higher in Bangladesh ($172) because of hiring a physician for supervising the screening and in Nepal ($262) caused by higher per diem for screeners and travel costs to the villages (information not included in the table).
Patient characteristics and treatment delays in the ACD and PCD group.
A total of 64 new VL cases detected in the ACD arm were compared with 28 VL cases detected in the PCD arm. Overall, the PCD arm had a lower proportion of females (39.29%; 95% confidence interval [CI]: 20–58%) and also of children < 15 years of age (35.7%) compared with the ACD arm with 50% females (95% CI: 37–62%) and 46.8% children (Figure 3). Although not statistically significant, this was consistent at all sites. Overall, treatment delays from onset of symptoms to diagnosis to start of treatment were significantly higher in the PCD arm (47.3 days; 95% CI: 25 to 70 days) compared with the ACD arm (26.8 days; 95% CI: 14 to 40 days; P value = 0.043).
Figure 3.
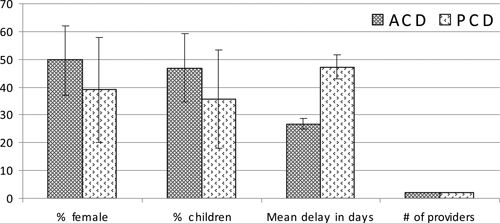
Visceral leishmaniasis (VL) patient and treatment characteristics by study arm.
Use of multiple health care providers.
In our study, patients sought care from an average of two providers (2.04 in the ACD arm; 95% CI: 1.8 to 2.3 providers and 2.03 in the PCD arm; 95% CI: 1.7 to 2.3 providers) (Figure 3). The first visit was usually to the nearest and most accessible provider and later visits included providers at far distances (not shown in the figure). The mean travel time taken to seek VL treatment at the final provider was similar in both groups; 59 minutes in the ACD group compared with 63 minutes in the PCD group.
PKDL detection.
The ACD yielded 14 and 21 new cases of PKDL corresponding to a point prevalence of 34.7 in Muzaffarpur, India and 71.8 per 100,000 population in Bangladesh (Table 2). The public sector had not reported PKDL cases for several years in the study areas.
Discussion
The ACD or screening on the basis of simple diagnostic tests has been an invaluable public health tool used for a number of diseases, including small pox, leprosy, guinea worm, and some childhood disorders, particularly in eradication or elimination programs.18,19 In the context of VL elimination, ACD strategies may be efficient (cost-effective) when the disease burden is high and passive surveillance is poor but may require higher efforts and costs to sustain for long periods as the disease burden falls. In this study, ACD with a simple questionnaire, skilled health workers able to diagnose spleen enlargement and a highly sensitive and specific rapid diagnostic test (rK39) detected undiagnosed VL cases. The study also estimated the overall annual VL incidence in the highly endemic districts. This was found to be about 19 times higher than the elimination target of 10 cases per 100,000 population. The VL deaths were not included, which would slightly increase the estimated rate. Clearly, it is essential for VL Control Programs to have such disease burden estimates to monitor progress in the elimination program.16
The largely unrecognized burden of PKDL disease remains an important source of VL transmission. The PKDL occurs in 5–10% of treated VL cases in India20 and develops 1–3 years post antimonial treatment of VL.21 The PKDL was only identified by ACD but not by PCD, as patients tend not to use health services for this condition.
Our study suggests that ACD results in decreased delays in diagnosis and start of treatment, particularly in vulnerable groups such as females and children. The number of providers from whom care is sought by VL patients in the ACD and PCD arms was not significantly different. This was probably because patients in the ACD arm were detected at different stages of their illness with some already having sought care for their illness, although without a VL diagnosis. Because of the small number of VL patients analyzed at each site, it is not possible to ascertain definitively if the ACD strategy encouraged a shift in provider preference from unqualified to qualified services.
The additional efforts and costs for ACD strategies in our study were variable. The effort of house to house screening was high in the Muzaffarpur study area (880 houses screened to detect one new VL case). This was possibly a result of greater awareness of VL and good access to VL diagnostic and treatment facilities resulting in fewer undiagnosed VL cases in the community. In contrast, fewer houses need to be screened when there are more undiagnosed VL cases in the community caused by poor availability of VL diagnostic and treatment facilities. As expected, the screening effort to detect new VL cases increases as endemicity levels decline; in this situation it is more efficient and sustainable to adopt a focal screening in the neighborhood of “index” cases as done in Nepal.
Finally, the large variation in estimated program cost incurred to detect one new VL case reflects the differing screening strategies adopted by each site. The cost per new VL case detected was higher in Muzafurpur, India because household screening was conducted by field workers employed by researchers. In contrast, in Saran India, screening was performed by community volunteers and accredited social health activists (ASHAs). Additionally, the differential effort and yield in the adjoining Indian sites could also be attributed to increased awareness and better VL care seeking behavior in Muzaffarpur. The cost was substantially higher in Bangladesh, which used full-time study physicians and in Nepal, which carried out an extensive training program and extra cost for travel of field supervisors to the villages of index cases. It may well be possible to design a standardized less costly ACD system.
Conclusions
To attain VL elimination goals, country programs need to be flexible and adapt innovative case detection strategies on the basis of epidemiological and operational needs. The study findings suggest that ACD may be a cost-effective complementary approach to PCD, especially in high endemic areas with poor access. Further research is needed to determine the periodicity of such strategies and to estimate the cost-effectiveness of alternative approaches, such as ACD using the “camp approach” (through mobile teams) or the “incentives-based approach.” Furthermore, the direct and indirect costs for the patient and their families have to be included in the equation to estimate the full costs and benefits of ACD from a societal perspective and not only from a program perspective. As the burden of disease declines and we move closer to VL elimination, it is important to identify a combination of approaches that are cost-effective, sustainable, and adaptable to the epidemiological situation.
Acknowledgments
The study was funded by the Special programme for Research and Training in Tropical Diseases (TDR-WHO). The authors are grateful to all Country, State and District VL Control Program Managers for guiding the development of the research proposal. We also thank InBios International, Inc., USA for providing free samples of the rK39 tests (KalazarDetect) and to patients who consented to participate in the study. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Authors' addresses: Siddhivinayak Hirve, Vadu Rural Health Program, KEM Hospital Research Center, Rasta Peth, Pune 411011 India, E-mail: sidbela@vsnl.com. Shri Prakash Singh, Department of Community Medicine, Institute of Medical Sciences, Benares Hindu University, Varanasi, India, E-mail: drspsingh_vns@yahoo.com. Narendra Kumar, Rajendra Memorial Research Institute of Medical Sciences, Patna, India, E-mail: narendra54in@yahoo.co.in. Megha Raj Banjara, Institute of Medicine, Tribhuvan University, Kathmandu, Nepal, E-mail: megharajbanjara@yahoo.com. Pradeep Das, Rajendra Memorial Research Institute of Medical Sciences, Patna, India, E-mail: drpradeep.das@gmail.com. Shyam Sundar, Department of Medicine, Institute of Medical Sciences, Benares Hindu University, Varanasi, India, E-mail: drshyamsundar@hotmail.com. Suman Rijal, Department of Internal Medicine, BP Koirala Institute of Health Sciences, Dharan, Nepal, E-mail: sumanrijal2@yahoo.com. Anand Joshi, Institute of Medicine, Tribhuvan University, Kathmandu, Nepal, E-mail: abjoshi2018@yahoo.com. Axel Kroeger, World Health Organization, Special Programme for Research and Training in Tropical Diseases, WHO, Geneva, Switzerland, and School of Tropical Medicine, Liverpool, UK, E-mail: kroegera@who.int. Beena Varghese, Public Health Foundation of India, New Delhi, India, E-mail: dr_b_varghese@yahoo.co.in. Chandreshwar Prasad Thakur, Balaji Utthan Sanstha, Patna, India, E-mail: cpthakur1@rediffmail.com. M. Mamun Huda, Parasitology, Laboratory Sciences Division, International Center for Diarrheal Diseases Research, Bangladesh, Mohakhali, Dhaka 1212, Bangladesh, E-mail: mhuda83@icddrb.org. Dinesh Mondal, International Center for Diarrheal Diseases Research, Bangladesh, Mohakhali, Dhaka 1212, Bangladesh, E-mail: din63d@icddrb.org.
References
- 1.World Health Organization . Report of the first meeting, Manesar, Haryana, 20–23 December 2004. New Delhi: Regional Office for South-East Asia; 2005. (Regional Technical Advisory Group on Kala-azar Elimination). [Google Scholar]
- 2.World Health Organization . Regional Strategic Framework for Elimination of Kala-azar from the South-East Asia Region (2005–2015) New Delhi: Regional Office for South-East Asia; 2004. [Google Scholar]
- 3.Bern C, Chowdhury R. The epidemiology of visceral leishmaniasis in Bangladesh: prospects for improved control. Indian J Med Res. 2006;23:275–288. [PubMed] [Google Scholar]
- 4.Joshi A, Narain JP, Prasittisuk C, Bhati R, Hashim G, Jorge A, Banjara M, Kroeger A. Can visceral leishmaniasis be eliminated from Asia? J Vector Borne Dis. 2008;45:105–111. [PubMed] [Google Scholar]
- 5.Desjeux P. Leishmaniasis. Public health aspects and control. Clin Dermatol. 1996;14:417–423. doi: 10.1016/0738-081x(96)00057-0. [DOI] [PubMed] [Google Scholar]
- 6.Singh SP, Reddy DC, Rai M, Sundar S. Serious underreporting of visceral leishmaniasis through passive case reporting in Bihar, India. Trop Med Int Health. 2006;11:899–905. doi: 10.1111/j.1365-3156.2006.01647.x. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya SK, Sinha PK, Sundar S, Thakur CP, Jha TK, Pandey K, Das VR, Kumar N, Lal C, Verma N, Singh VP, Ranjan A, Verma RB, Anders G, Sindermann H, Ganguly NK. Phase 4 trial of miltefosine for the treatment of Indian visceral leishmaniasis. J Infect Dis. 2007;196:591–598. doi: 10.1086/519690. [DOI] [PubMed] [Google Scholar]
- 8.Sundar S, Jha TK, Thakur CP, Bhattacharya SK, Rai M. Oral miltefosine for the treatment of Indian visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2006;100((Suppl 1)):S26–S33. doi: 10.1016/j.trstmh.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Sundar S, Chartterjee M. Visceral leishmaniasis–therapeutic modilities. Indian J Med Res. 2006;9:26–39. [PubMed] [Google Scholar]
- 10.Sundar S, Reed SG, Singh VP, Kumar PC, Murray HW. Rapid accurate field diagnosis of Indian visceral leishmaniasis. Lancet. 1998;351:563–565. doi: 10.1016/S0140-6736(97)04350-X. [DOI] [PubMed] [Google Scholar]
- 11.Sundar S, Pai K, Sahu M, Kumar V, Murray HW. Immunochromatographic striptest detection of anti-rK39 antibody in Indian visceral leishmaniasis. Ann Trop Med Parasitol. 2002;96:19–23. doi: 10.1179/000349802125000466. [DOI] [PubMed] [Google Scholar]
- 12.Sarker CB, Momem A, Jamal MF, Siddiqui NF, Chrowdhury KS, Rahman S, Talukder SI. Immunochromatographic (rK39) strip test in the diagnosis of visceral leishmaniasis in Bangladesh. Mymensingh Med J. 2003;12:93–97. [PubMed] [Google Scholar]
- 13.Bern C, Jha SN, Joshi AB, Thakur GD, Bista MB. Use of the recombinant K39 dipstick test and the direct agglutination test in a setting endemic for visceral leishmaniasis in Nepal. Am J Trop Med Hyg. 2000;65:153–157. doi: 10.4269/ajtmh.2000.63.153. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization . Regional Technical Advisory Group on kala-azar elimination. Report of the Second Meeting, Kathmandu, Nepal, 20 October–2 November 2006. New Delhi: WHO SEARO, WHO Project: CCP CPC 050; 2007. [Google Scholar]
- 15.Sundar S, Mondal D, Rijal S, Bhattacharya S, Ghalib H, Kroeger A, Boelacrt M, Desjeux P, Richter-Airijoki H, Harms G. Implementation research to support the initiative on the elimination of kala-azar from Bangladesh, India and Nepal–the challenges for diagnosis and treatment. Trop Med Int Health. 2008;13:2–5. doi: 10.1111/j.1365-3156.2007.01974.x. [DOI] [PubMed] [Google Scholar]
- 16.Mondal D, Singh SP, Kumar N, Joshi A, Sundar S, Das P, Siddivinayak H, Kroeger A, Boelaert M. Visceral leishmaniasis elimination program in India, Bangladesh and Nepal: reshaping the case finding/case management strategy. PLoS Negl Trop Dis. 2009;3:e355. doi: 10.1371/journal.pntd.0000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chappuis F, Rijal S, Soto A, Menten J, Boelaert M. A meta-analysis of the diagnostic performance of the direct agglutination test and rk39 dipstick for visceral leishmaniasis. BMJ. 2006;333:723–726. doi: 10.1136/bmj.38917.503056.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson JM, Juenger G. Principles and practice of screening for disease. Geneva: World Health Organization, Public Health Papers No. 34; 1968. [Google Scholar]
- 19.World Health Organization Leprosy elimination campaigns: impact on case detection. Wkly Epidemiol Rec. 2003;78:9–16. [PubMed] [Google Scholar]
- 20.Napier LE, Das Gupta CR. A clinical study of post kala-azar dermal leishmaniasis. Ind Med Gaz. 1930;63:249–257. [PMC free article] [PubMed] [Google Scholar]
- 21.Thakur CP. Epidemiological, clinical and therapeutic features of Bihar kala-azar (including post kala-azar dermal leishmaniasis) Trans R Soc Trop Med Hyg. 1984;78:391–398. doi: 10.1016/0035-9203(84)90131-7. [DOI] [PubMed] [Google Scholar]


