Abstract
Immunity to saliva of Phlebotomus papatasi protects against Leishmania major infection as determined by co-inoculation of parasites with salivary gland homogenates (SGHs) of this vector. These results were obtained with long-term colonized female P. papatasi. We investigated the effect of pre-immunization with SGH of long-term colonized P. papatasi against L. major infection co-inoculated with SGH of wild-caught P. papatasi. Our results showed that pre-exposure to SGH of long-term, colonized P. papatasi do not confer protection against infection with L. major co-inoculated with SGH of wild-caught P. papatasi. These preliminary results strongly suggest that the effectiveness of a vector saliva-based vaccine derived from colonized sand fly populations may be affected by inconsistent immune response after natural exposure.
Several studies have shown that pre-immunization of mice with needle-injected saliva or pre-exposure to uninfected bites of Phlebotomus papatasi provided protection against infection with Leishmania major, the etiologic agent of zoonotic cutaneous leishmaniasis (ZCL).1–3 These studies were performed by using long-term laboratory colonized sand flies mainly because of the difficulty of working with wild-caught flies. A puzzling fact is that people in leishmaniasis-endemic areas succumb of ZCL despite the high frequency of uninfected bites compared with infected ones.4 We showed in a previous study that pre-immunization of mice with salivary gland homogenate (SGH) of wild-caught P. papatasi does not confer protection against L. major co-inoculated with the same type of SGH compared with a significant protection obtained when pre-immunization and challenge were performed with SGH of long-term colonized flies.5 It was reported that colonized and wild-caught Lutzomyia longipalpis differ in the composition and the amount of salivary proteins and these differences may account for the lower effect observed on the modulation of experimental Leishmania infection by wild-caught SGH.6,7 Thus, these studies provide good evidence that the outcome of Leishmania infection differs significantly between colonized and wild-caught salivary gland proteins. Our aim in this study was to assess the protective effect of pre-immunization with SGH of long-term colonized P. papatasi on experimental L. major challenge co-inoculated with SGH of wild-caught P. papatasi.
Phlebotomus papatasi (Tunisian strain that originated in the Governorate of Sidi Bouzid) have been reared at the Vector Ecology Laboratory of the Institut Pasteur de Tunis since 2003.8 This colony is maintained without being supplemented periodically with wild-caught P. papatasi. Generation F39 was used in this study. Wild sand flies were collected using CDC light traps from animal shelters located in the village of Felta (Governorate of Sidi Bouzid), a focus highly endemic for ZCL.9 Preparation of SGH from laboratory-reared and wild-caught P. papatasi was performed as described.5 A highly virulent strain of L. major, MHOM/TN/95/GLC94, isolated from a patient in Tunisia was used in this study.5 We used female BALB/c mice (6–8 weeks of age) bred in the animal facility of the Institut Pasteur de Tunis.
Mice were immunized intradermally in the right ear with the equivalent of two pairs of salivary glands in 10 μL of phosphate-buffered saline (PBS). Two groups of 10 mice each were pre-immunized with SGH of long-term colonized female P. papatasi (F39), once a week for two weeks. In the fourth week, the first [CSGH (F39)-L.m+CSGH (F39)] and the second [CSGH (F39)-L.m+WSGH] groups were challenged with 106 stationary phase L. major promastigotes in 50 μL of PBS co-inoculated subcutaneously in the right hind footpads with SGH of long-term, colonized and SGH of wild-caught, female P. papatasi, respectively. Three control groups of 10 mice each were used in this study. The third [PBS-L.m+CSGH (F39)] and the fourth group [PBS-L.m+WSGH] (control groups) were injected with PBS instead of SGH and challenged with 106 stationary phase L. major promastigotes in 50 μL of PBS co-inoculated subcutaneously in the right hind footpads with SGH of long-term colonized, and SGH of wild-caught female P. papatasi, respectively. The fifth group [PBS-L.m+PBS] (control group) was injected with PBS instead of SGH and challenged with only 106 stationary phase L. major promastigotes in 50 μL of PBS inoculated subcutaneously in the right hind footpads. All experiments were replicated three times. The footpad swelling at the site of inoculation was monitored at weekly intervals by using a vernier caliper. Lesion size was defined as the increase in the footpad thickness after subtracting the size of the contralateral uninfected footpad.
Using a linear mixed-effects model for longitudinal data10 but allowing for nested random effects, and where the within-group errors are permitted to be correlated and/or have unequal variances, we tested for difference in trends (group effect) and time-group interaction between curves illustrating the variation of the lesion size through time for each group of mice immunized and challenged differently as described above. In addition, for specific time point analysis (post-challenge week) the Wilcoxon test11 and Student t-test were used to test for median and mean difference of lesion size between groups. Holm's correction for multiple testing12 of the reported P values was used when appropriate. All statistical analyses were performed with R software for statistical computing (version 2.7).
After challenge with L. major co-inoculated with SGH of long-term, colonized, female P. papatasi (F39), mice pre-immunized with the same type of SGH developed footpad lesions that were significantly smaller in size and grew more slowly than in the control groups (P < 0.0001) (Figure 1). In contrast, mice pre-immunized with SGH of long-term colonized P. papatasi and challenged with L. major co-inoculated with SGH of wild-caught P. papatasi developed lesions that grew as rapidly and as large in size as the control groups (P = 0.7389) (Figure 1). Lesion size differed significantly in mice pre-immunized with SGH of long-term colonized P. papatasi and then challenged with L. major co-inoculated with the same type of SGH compared with lesion size observed in the group of mice pre-immunized with SGH of long-term colonized P. papatasi and then challenged with L. major co-inoculated with SGH of wild-caught female P. papatasi (P < 0.0001). Overall, no significant difference was observed in lesion size among three control groups (P = 0.18) (Figure 1).
Figure 1.
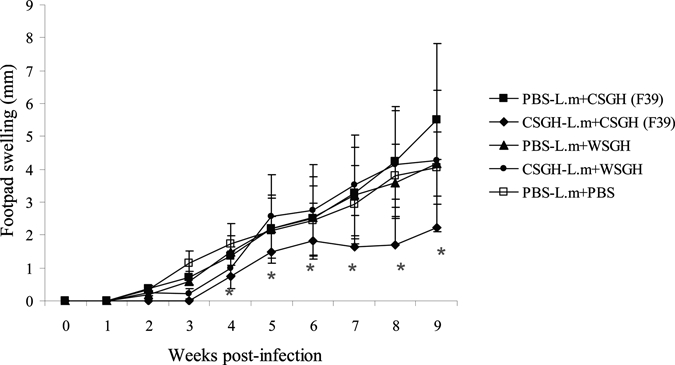
Course of lesion development in vaccinated and control BALB/c mice after challenge with 106 Leishmania major metacyclic promastigotes. Results are expressed as increases in infected footpad thickness (in millimeters) and are means + SD per group. Data are representative of three experiments combined. CSGH (F39)-L.m+ CSGH (F39) = mice pre-immunized with salivary gland homogenate (SGH) of long-term laboratory-colonized female Phlebotomus papatasi (F39) and challenged with L. major co-inoculated with the same type of SGH; CSGH (F39)- L.m+ WSGH = mice pre-immunized with SGH of long-term colonized female P. papatasi (F39) and challenged with L. major co-inoculated with SGH of wild-caught P. papatasi; PBS-L.m+ CSGH (F39) (control group) = mice pre-immunized with phosphate-buffered saline (PBS) only and challenged with L. major co-inoculated with SGH of long-term colonized female P. papatasi (F39); PBS-L.m +WSGH (control group) = mice pre-immunized with PBS only and challenged with L. major co-inoculated with SGH of wild-caught P. papatasi; PBS-L.m+PBS (control group) = mice pre-immunized with PBS only and challenged with L. major co-inoculated with PBS. Symbols represent the mean + SD curve for each group. Asterisks indicate a significant statistical difference in lesion size (by t-test or Wilcoxon test, adjusted P < 0.05) for specific time point (week) between the CSGH-L.m+CSGH (F39) group and the remaining groups (three control groups and the CSGH+L.m+WSGH group).
Our results confirmed the finding of previous studies showing that pre-immunization with SGH of long-term laboratory-colonized female P. papatasi induced significant protection against L. major co-inoculated with the same type of SGH.1–3 However, all aforementioned studies were performed with long-term laboratory colonized sand flies. Because persons in leishmaniasis-endemic areas are exposed to bites of wild populations of P. papatasi, we investigated the effects of sand fly saliva as close as possible to natural transmission. Our results showed that pre-immunization of mice with SGH of long-term, laboratory-colonized, female P. papatasi did not protect mice against L. major co-inoculated with SGH of wild-caught female P. papatasi.
Previous studies using small numbers of parasites (500–1,000 promastigotes) reported that lesion size is exacerbated in the presence of SGH compared with parasites alone.1,3 In our study, SGH of either colonized or wild-caught P. papatasi did not exacerbate L. major infection compared with lesion sizes observed when parasites were injected alone. This finding is probably caused by the high number of parasites (106 promastigotes) used in our study.
Among nine identified salivary gland proteins of long-term, colonized, female P. papatasi, one with an apparent molecular weight of 15 kD (SP-15) provided significant protection of mice when challenged with L. major.3 Wild-caught P. papatasi exhibited higher genetic variation in SP-15 compared with colonized flies of the same species.13 Many variants of SP15 were found in natural field populations of P. papatasi.13 It was hypothesized that the development of a vaccine based on SP-15 will not be affected by an inconsistent immune response because of genetic variation in natural populations of P. papatasi.13 However, the protective ability of one variant of SP15 against exposure to another variant needs to be studied.
The fact that pre-immunization of mice with SGH of long-term, laboratory-colonized, female P. papatasi did not protect mice against L. major co-inoculated with SGH of wild-caught female P. papatasi strongly suggests that the effectiveness of a sand fly saliva–based vaccine will be affected by the antigenic diversity of sand fly salivary proteins that results from the genetic variation in natural populations of P. papatasi. To clarify this hypothesis, studies concerning salivary proteins of wild populations of P. papatasi are needed. Similarly, among natural field populations of Lu. longipalpis, extensive amino acid sequence variation (up to 23%) was observed in maxadilan peptides.14 Natural selection may favor the polymorphism observed in maxadilan peptides to escape host immune responses.14,15 This hypothesis is corroborated by the fact that although maxadilan exacerbates infection with L. major, vaccination against one variant of maxadilan protected mice against L. major infection.16 Therefore, the protective effect observed with one variant of maxadilan may differ when exposed to another variant.16 Although SP-15 is protective against L. major, immunization with another salivary gland protein (SP-44) from the same colony of P. papatasi induced disease exacerbation.17 Thus, proper selection of a vector-based vaccine candidate is of major importance.17,18
Laboratory colonies of insects are often accepted as being representative of field populations from which they have been derived, but this assumption may be challenged because colonies frequently incorporate only a fraction of the genetic variability present in the original populations.19 It has been reported that laboratory colonization of sand flies reduces natural genetic variability, and might foster selection for certain traits that are normally suppressed in field populations.20–22The loss of genetic variation as a result of colonization might figure prominently in the protection observed in mice pre-immunized with SGH of long-term colonized P. papatasi and challenged with L. major co-inoculated with the same type of SGH.5 Antigenic variation of salivary gland proteins of field populations of P. papatasi similar to that observed in Lu. longipalpis might explain the absence of protection observed in mice pre-immunized with SGH of long-term colonized P. papatasi and challenged with L. major co-inoculated with SGH of wild-caught P. papatasi.5 Thus, our preliminary results strongly suggest that the development of a vaccine based on salivary gland proteins needs to include consideration of variability in natural populations of P. papatasi.
Acknowledgments
We thank Chokri Bahloul for providing L. major isolates used in this study and Howard Ginsberg and Jose Marcelo Ramalho-Ortigao for critically reviewing the manuscript. This study was part of the post-doctoral research program of Sami Ben Hadj Ahmed.
Footnotes
Financial support: This study was supported by World Health Organization/Tropical Diseases Research grant A60291.
Authors' addresses: Sami Ben Hadj Ahmed, Laboratory of Vector Ecology, Institut Pasteur de Tunis, 13 Place Pasteur, BP 74, 1002 Tunis, Tunisia, and Department of Biology, University of Gafsa, Gafsa, Tunisia. Belhassen Kaabi, Laboratory of Epidemiology and Ecology of Parasites, Institut Pasteur de Tunis, 13 Place Pasteur, BP 74, 1002 Tunis, Tunisia. Ifhem Chelbi, Mohamed Derbali, Safedine Cherni, and Elyes Zhioua, Laboratory of Vector Ecology, Institut Pasteur de Tunis, 13 Place Pasteur, BP 74, 1002 Tunis, Tunisia. Dhafer Laouni, Laboratory of Immuno-Pathology, Vaccinology, and Molecular Genetic, Institut Pasteur de Tunis, 13 Place Pasteur, BP 74, 1002 Tunis, Tunisia.
References
- 1.Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, Ribeiro J, Sacks DL. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva pre-exposure on the long-term outcome of Leismania major infection in the mouse ear dermis. J Exp Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science. 2000;290:1351–1354. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- 3.Valenzuela J, Belkaid Y, Garfiela MK, Mendez S, Kamhawi S, Rowton E, Sacks D, Ribeiro JMC. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp Med. 2001;194:331–342. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Handman E.2001Protective saliva: a novel approach to a Leishmania vaccine Trends Parasitol 17513–514.11872384 [Google Scholar]
- 5.Ben Hadj Ahmed S, Chelbi I, Kaabi B, Cherni S, Derbali M, Zhioua E. Differences in the salivary effects of wild-caught versus colonized Phlebotomus papatasi (Diptera: Psychodidae) on the development of zoonotic cutaneous leishmaniasis in BALB/c mice. J Med Entomol. 2010;47:74–79. doi: 10.1603/033.047.0110. [DOI] [PubMed] [Google Scholar]
- 6.Laurenti MD, Silveira VM, Secundino NF, Corbett CE, Pimenta PP. Saliva of laboratory-reared Lutzomyia longipalpis exacerbates Leishmania (leishmania) amazonensis infection more potently than saliva of wild-caught Lutzomyia longipalpis. Parasitol Int. 2009;58:220–226. doi: 10.1016/j.parint.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Laurenti MD, da Matta VL, Pernichelli T, Secundino NF, Pinto LC, Corbett CE, Pimenta PP. Effects of saliva gland homogenate from wild-caught and laboratory-reared Lutzomyia longipalpis on the evolution and immunomodulation of Leishmania (leishmania) amazonensis infection. Scand J Immunol. 2009;70:389–395. doi: 10.1111/j.1365-3083.2009.02310.x. [DOI] [PubMed] [Google Scholar]
- 8.Chelbi I, Zhioua E. Biology of Phlebotomus papatasi (Diptera: Psychodidae) in the laboratory. J Med Entomol. 2007;44:597–600. doi: 10.1603/0022-2585(2007)44[597:boppdp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Chelbi I, Derbali M, AL-Ahmadi Z, Zaafouri B, El Fahem A, Zhioua E. Phenology of Phlebotomus papatasi (Diptera: Psychodidae) relative to the seasonal prevalence of zoonotic cutaneous leishmaniasis in central Tunisia. J Med Entomol. 2007;44:385–388. doi: 10.1603/0022-2585(2007)44[385:poppdp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Laird NM, Ware JH. Random-effect models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 11.Bauer DF. Constructing confidence sets using rank statistics. J Am Stat Assoc. 1972;67:687–690. [Google Scholar]
- 12.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 13.Elnaiem DEA, Menesses C, Slotman M, Lanzaro GC. Genetic variation in the sand fly salivary protein, SP-15, a potential vaccine candidate against Leishmania major. Insect Mol Biol. 2005;14:145–150. doi: 10.1111/j.1365-2583.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 14.Lanzaro GC, Lopes AHCS, Riberiro JMC, Shoemaker CB, Warburg A, Soares M, Titus RG. Variation in the salivary peptide, maxadilan from species in the Lutzomyia longipalpis complex. Insect Mol Biol. 1999;8:267–275. doi: 10.1046/j.1365-2583.1999.820267.x. [DOI] [PubMed] [Google Scholar]
- 15.Milleron RS, Mutebi JP, Valle S, Montoya A, Yin H, Soong L, Lanzaro GC. Antigenic diversity in maxadilan, a salivary protein from the sand fly vector of American visceral leishmaniasis. Am J Trop Med. 2004;70:278–293. [PubMed] [Google Scholar]
- 16.Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus R. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol. 2001;167:5226–5230. doi: 10.4049/jimmunol.167.9.5226. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira F, Lawyer PG, Kamhawi S, Valenzuela JG. Immunity to distinct sand fly salivary proteins primes the anti-Leishmania immune response towards protection or exacerbation of disease. PLOS Neg Trop Dis. 2008;2:e226. doi: 10.1371/journal.pntd.0000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomes R, Teixeira C, Teixeira MJ, Olivera F, Menezes MJ, Silva C, Miranda JC, Kamhawi S, Valenzuela J, Brodskyn CI. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc Natl Acad Sci USA. 2008;105:7845–7850. doi: 10.1073/pnas.0712153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenz L, Beaty BJ, Aitken THG, Wallis GP, Tabachnik WJ. The effect of colonization upon Aedes aegypti susceptibility to oral infection with yellow fever virus. Am J Trop Med Hyg. 1984;33:690–694. doi: 10.4269/ajtmh.1984.33.690. [DOI] [PubMed] [Google Scholar]
- 20.Kassem HA, Fryauff DJ, Shehata MG, Sawaf BM. Enzyme polymorphism and genetic variability of one colonized and several field populations of Phlebotomus papatasi (Diptera: Psychodidae) J Med Entomol. 1993;30:407–413. doi: 10.1093/jmedent/30.2.407. [DOI] [PubMed] [Google Scholar]
- 21.Mukhopadhyay J, Rangel E, Ghosh K, Munstermann LE. Patterns of genetic variability in colonized strains of Lutzomyia longipalpis (Diptera: Psychodidae) and its consequences. Am J Trop Med Hyg. 1997;57:216–221. doi: 10.4269/ajtmh.1997.57.216. [DOI] [PubMed] [Google Scholar]
- 22.Lanzaro GC, Warburg A. Genetic variability in phlebotomine sand flies: possible implication for leishmaniasis epidemiology. Parasitol Today. 1995;4:151–154. [Google Scholar]


