Abstract
Studies evaluating radiologic aspects, local complications, and structural alterations of the paranasal sinus in patients with mucosal leishmaniasis (ML) are lacking. The aim of this study was to analyze alterations of the paranasal sinuses in patients with ML by using computed tomography (CT) scans. This prospective study evaluated 26 patients in Brazil with ML from December 2008 through June 2009. All patients underwent CT scans of the paranasal sinuses. Paranasal thickening was observed in 25 patients (96%). Nasal perforation was observed in 17 patients (65%). Those patients who received re-treatment showed more abnormalities on CT scan than cured patients (P < 0.05). Complications of ML are not limited to the nasal mucosa but extend to the paranasal sinuses. Mucosal thickening, opacified air cells, bony remodeling, and bony thickening caused by inflammatory osteitis of the sinus cavity walls are CT findings suggestive of chronic sinusitis.
Introduction
The global incidence of tegumentary leishmaniasis is increasing. Nevertheless, leishmaniasis is a neglected disease. Financial donors, public-health authorities, and medical professionals have shown little interest in implementing activities to investigate, prevent, or control the disease.1 Mucosal leishmaniasis (ML) is the most severe form of this disease because of its progressive lesions that can destroy cartilage and osseous structures of the face, pharynx, and larynx.2,3
The natural history of this disease and its complications were well described by Phillip Marsden in 1986.4 However, there have been no studies that evaluated the structural alterations of the face and paranasal sinuses by using radiologic methods. Moreover, the incidence of sinusitis is not described, although the most common symptoms of patients are related to nasal discharge, epistaxis, posterior drainage, and rhinorrhea.5 The absence of studies that evaluated radiologic aspects, local complications, and structural alterations of the paranasal sinus led us to perform a study to determine the radiologic alterations of the paranasal sinuses in patients with ML by using computed tomography (CT).
Materials and Methods
Patients.
In this prospective study, we evaluated 26 patients with ML who were admitted to the Leishmaniasis Ambulatory at the Hospital das Clinicas (University of São Paulo) in São Paulo from December 2008 through June 2009. The diagnostic approach for ML included clinical examination, the Montenegro skin test, serologic analysis, and mucosal biopsy. A fiberoptic examination of the nose, nasopharynx, oropharynx, and larynx was performed in all patients. Patients with active mucosal involvement (one or more of hyperemia, edema, erosion, or a granulomatous aspect at the palatal, pharyngeal mucosa, and laryngeal mucosa) were classified as having the active mucosal form of the disease. The disease was confirmed by biopsy that showed Leishmania at the mucosal site as determined by immunohistochemical analysis or Giemsa staining of amastigotes.6 Grocott- and Ziehl-Nielsen–stained smears were prepared to exclude fungal or mycobacterial infection.
After diagnostic confirmation, all patients were treated according to the clinical protocol as described, which considers N-methyl-glucamine as the drug of first choice.5 Cure was defined as total healing of mucosal lesions as determined by otorhinolaryngologic and fiberoptic examinations up to one year after the end of therapy. Failure was defined as the absence of improvement of a lesion after therapy or the return of the treated lesion before one year. Lesions that showed an improvement after therapy but did not heal completely were also considered to be failures. Recurrence was considered to be new lesions or the return of the treated lesion after one year of the therapy.
This study was reviewed and approved by the Ethics Board of the Faculty of Medical Sciences, São Paulo.
Clinical findings.
The epidemiologic and clinical findings of all patients were evaluated according to age, sex, race, region of origin, scar from previous cutaneous leishmaniasis, comorbidities, symptoms, and lesion location.
Laboratory tests.
All patients were subjected to a Montenegro skin test and a mucosal biopsy. The Montenegro skin test was performed with an intradermal injection of Leishmania amazonensis OMS MHOM/BR/73/M2269; Centro de Produção e Pesquisa de Imunobiologia, Piraquara, Paraná, Brazil), and the size of the skin response was measured after 48 hours. The diameter of induration was measured in millimeters (> 5 mm was considered a positive result).
Immunohistochemical analysis for detection of Leishmania antigens was performed as described by using antibodies from L. chagasi (provided by Heitor Andrade Jr., Instituto de Medicina Tropical, São Paulo, Brazil).7
Radiologic aspects.
Computed tomography scans of the paranasal sinuses were obtained for all patients. The examinations were performed by using a 16-channel multidetector scanner (IDT16; Philips Medical Systems, Best, The Netherlands) without contrast media with the following parameters: 120 kV, 100 mA, a rotation time of 0.75 seconds in the axial plane, a slice thickness of 1 mm, and an increment of 0.5 mm with bone and standard algorithms of reconstruction. Extra-sinusal abnormalities were observed in nine patients; for these patients, another CT scan was obtained by using intravenous contrast media (iobitridol, Henetix 300; Guerbet, Rio de Janeiro, Brazil) at a dose of 1.5 mL/kg.
The volumetric data was transferred to a workstation, and multiplanar reconstructions (axial, coronal, and sagittal planes) were obtained. All CT scans were analyzed by a radiologist who was unaware of the clinical condition of the patient (treated or untreated). The radiologist evaluated the grade of opacification of the paranasal sinuses (sinusopathy) and ostio-meatal complexes, and the presence of any abnormality that could be related to leishmaniasis such as foci of erosion of the nasal septum; deformity of the nasal pyramid; or cutaneous, subcutaneous, or mucosal lesions.
Sinusopathy was graded according to the Lund-McKay system, which establishes a value for the grade of opacification for each sinusal system and for the ostio-meatal complexes (Table 1).8
Table 1.
Radiologic grading of the sinusal system according to the Lund-Mackay system*
| Sinusal system | Left | Right |
|---|---|---|
| Maxillary | ||
| Anterior ethmoid | ||
| Posterior ethmoid | ||
| Sphenoid | ||
| Frontal | ||
| Osteomeatal complex | ||
| Total score for each side | ||
Scores: Sinuses 0 = no alterations; 1 = partial opacification; 2 = total opacification; Ostiomeatal complex: 0 = not occluded; 2 = obstructed.
Statistical analysis.
This study was essentially descriptive. Continuous data are expressed as means or medians with SD values or ranges. Frequencies are expressed as percentages. A cross-table analysis using chi-square and Fisher tests was performed to compare patients with recurrent disease and those who were cured. All tests were performed using Epi-Info software (Centers for Disease Control and Prevention, Atlanta, GA). A P value < 0.05 was considered statistically significant.
Results
General data.
The mean age of the 26 patients was 57.8 years (range = 25–80 years). Of the 26 patients, 12 were men and 14 were women. Twelve patients were Caucasian and 14 patients were of African descendent. Twelve (46.2%) patients were from northeastern Brazil. Fourteen (53.8%) patients had scars from previous cutaneous lesions (Table 2).
Table 2.
General data from 26 patients in São Paulo, Brazil with mucosal leishmaniasis
| Characteristic | No. | % |
|---|---|---|
| Median age, years (range) | 26 | 57.8 (25–80) |
| Sex | ||
| M | 12 | 46.2 |
| F | 14 | 53.8 |
| Race | ||
| African descendent | 4 | 15.3 |
| Caucasian | 12 | 46.2 |
| Region | ||
| Northeast | 12 | 46.2 |
| Southeast | 8 | 30.8 |
| North | 1 | 3.8 |
| South | 3 | 11.5 |
| Center-west | 2 | 7.7 |
| Comorbidities | ||
| Hypertension | 15 | 57.6 |
| Diabetes | 1 | 3.8 |
| Previous cutaneous lesion | 14 | 53.8 |
The most common symptoms were nasal obstruction (88.5%), epistaxis (65.4%), and rhinorrhea (50%) (Table 3). The nasal septum was the most common site of mucosal lesions (92.3%), but 10 patients (38.4%) showed lesions in other sites (pharynx and larynx).
Table 3.
Symptoms of patients with mucosal leishmaniasis in São Paulo, Brazil
| Symptoms/mucosal leishmaniasis site | No. | % |
|---|---|---|
| Nasal obstruction | 23 | 88.5 |
| Epistaxis | 17 | 65.4 |
| Rhinorrhea | 13 | 50.0 |
| Odynophagia | 11 | 42.3 |
| Coryza | 7 | 26.9 |
| Itching | 7 | 26.9 |
| Facial pain | 5 | 19.2 |
| Headache | 3 | 11.5 |
| Hyposmia | 3 | 11.5 |
| Posterior drainage | 1 | 3.8 |
| Anatomic form of mucosal leishmaniasis | ||
| Septal/nasal | 14 | 53.8 |
| Septal/nasal with palatal | 10 | 38.5 |
| Palatal | 2 | 7.7 |
| Total | 26 | 100.0 |
Radiologic findings.
Thickening of the paranasal sinus was observed in 25 patients (96%). Nasal perforation was described in 17 patients (65%), and the CT scan showed collapse of the nasal pyramid in 3 patients (11.5%). Other findings included alterations of the nasal conchae (n = 8, 30%), retention cyst/polyp of the maxillary sinus (n = 4, 15%), and bilateral mastoidopathy (n = 2, 8%). Less frequent alterations included erosion of the nasolacrimal duct, erosion of the nasal bone, osteitis of the paranasal sinuses, and thickening of the soft palate and the nasal wings (each alteration corresponding to n = 1, 4%). More details are shown in Table 4. Examples of radiologic findings are shown in Figure 1 and Figure 2.
Table 4.
Radiologic findings of patients with mucocutaneous leishmaniasis in São Paulo, Brazil
| Finding | No. | % |
|---|---|---|
| Paranasal thickness from paranasal sinus | 25 | 96 |
| Nasal perforation | 17 | 65 |
| Alterations of the nasal conchae | 8 | 30 |
| Retention cyst/polyp of the maxillary sinus | 4 | 15 |
| Collapse of the nasal pyramid | 3 | 11.5 |
| Erosion of the lacrimal duct | 1 | 4 |
| Erosion of the nasal bone | 1 | 4 |
| Bilateral mastoidopathy | 2 | 8 |
| Osteitis of paranasal sinuses | 1 | 4 |
| Thickening of the soft palate | 1 | 4 |
| Thickening of the nasal wings | 1 | 4 |
Figure 1.
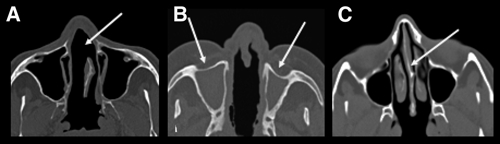
A, Axial computed tomography (CT) scan of a patient showing erosion of the nasal septum (arrow). B, Axial CT scan of another patient showing destruction of the nasal septum associated with mucous thickening of the nasal fossae and complete obliteration of the maxillary sinuses with thickening of their bony walls. C, Axial CT scan showing integrity of the nasal septum.
Figure 2.
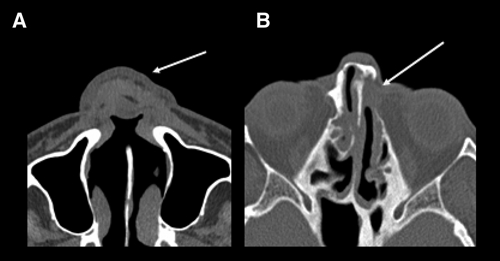
A, Axial computed tomography (CT) scan of a patient showing diffuse thickening of the nasal wings with collapse of the nasal pyramid (arrow) and erosion of the nasal septum. B, Axial CT scan of another patient showing erosion of the nasal septum and obliteration of the left nasolacrimal duct (arrow) and thickening of the bony walls of the paranasal sinuses.
Lund-Mackay scoring differed for patients given re-treatment compared with those without previous treatment: scores of 6.0 (95% confidence interval = 5.29–6.70) and 4.3 (95% confidence interval = 3.58–5.12), respectively) (P < 0.05).
Discussion
We evaluated radiologic findings for patients with ML in São Paulo, Brazil by using CT. In our study, CT scans showed mucosal thickening of at least one of the paranasal sinuses in all but one patient. This finding suggests that the disease in patients with ML is not limited to the nasal mucosa but extends to the paranasal sinuses. Mucosal thickening, opacified air cells, bony remodeling, and bony thickening caused by inflammatory osteitis of the sinus cavity walls are CT findings suggestive of chronic sinusitis. Bony erosion can also occur in severe cases.9
The pathophysiology of paranasal involvement remains unclear. Mucosal thickening may be caused by direct infection of the sinus mucosa by the Leishmania protozoan, which can live in the mucosa despite scar cicatrisation.10 Another hypothesis is chronic inflammation with continuous expression of tumor necrosis-α in situ after cicatrization of the mucosal lesion, as described.6 A third hypothesis is chronic sinusitis secondary to obstruction of the natural drainage pathways of the paranasal sinuses. Direct infection of the sinus mucosa by the parasite, which would require surgical procedures to obtain sinus mucosal biopsy specimens, was not investigated in the present study. Most of our patients did not satisfy the formal indication for sinus surgery. We believe that these three hypotheses are simultaneously present and contribute to paranasal thickening.
Obstruction of the sinus ostium or drainage pathways may occur in some patients with mucosal leishmaniasis who have inflammatory or cicatricial lesions that extend to the lateral nasal wall in the region of the middle meatus or the esphenoethmoidal recess. Obstruction of these key drainage regions may lead to stasis of secretions inside the sinuses, resulting in chronic sinusitis. In some patients in our study, massive destruction of the nasal structures led to the formation of a large nasal cavity, scarring and thickening nasal mucosa including the lateral nasal wall, and opacification of the paranasal sinuses.
It is important to comment on the relevance of mucosal thickening in the interpretation of sinus disease. Mucosal thickening may occur after a virus infection in an upper airway, even in asymptomatic patients.11 It may also be secondary to surgical intervention in the paranasal sinuses. In our study, none of the patients had a virus infection in an upper airway, acute worsening of nasal symptoms by the time the CT scan was obtained, or a history of previous sinus surgery.11,12 The mucosal thickening described is a CT finding that suggests chronic rhinosinusitis. This entity occurs in 16% of the American population, and is the second most common chronic disease in this population.13,14
We did not include a control group to determine if ML is a risk factor for chronic rhinosinusitis. However, mucosal thickening in the patients (96%) was superior to results of any epidemiologic studies.
Although we cannot exclude the possibility that the paranasal sinus changes found in our patients were caused by other factors or were even incidental, the high prevalence of these findings in our study suggests the need to further investigate the pathogenesis of sinus involvement in such patients.
Footnotes
Authors' addresses: Raphael A. Camargo, Rui Imamura, and Antonio C. Nicodemo, Department of Infectious Diseases, University of São Paulo, Medical School, São Paulo, Brazil. Felipe F. Tuon, Department of Infectious Diseases, University of São Paulo, Medical School, São Paulo, Brazil and Division of Infectious and Parasitic Diseases, Hospital Universitário Evangélico de Curitiba, Curitiba, Paraná, Brazil. Daniel V. Sumi, Eloisa M. Gebrim, and Giovanni G. Cerri, Institute of Radiology, Hospital das Clínicas, University of São Paulo, Medical School, São Paulo, Brazil. Valdir S. Amato, Infectious and Parasitic Diseases Clinic, Hospital das Clínicas, University of São Paulo, Medical School, São Paulo, Brazil.
References
- 1.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 2.Amato VS, Boulos MI, Amato Neto V, Filomeno LT. The use of a silicone T tube for the treatment of a case of American mucocutaneous leishmaniasis with tracheomalacia. Rev Soc Bras Med Trop. 1995;28:129–130. doi: 10.1590/s0037-86821995000200008. [DOI] [PubMed] [Google Scholar]
- 3.Amato VS, Tuon FF, Bacha HA, Neto VA, Nicodemo AC. Mucosal leishmaniasis: current scenario and prospects for treatment. Acta Trop. 2008;105:1–9. doi: 10.1016/j.actatropica.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Marsden PD. Mucosal leishmaniasis (“espundia” Escomel, 1911) Trans R Soc Trop Med Hyg. 1986;80:859–876. doi: 10.1016/0035-9203(86)90243-9. [DOI] [PubMed] [Google Scholar]
- 5.Amato VS, Tuon FF, Imamura R, Abegão de Camarago R, Duarte MI, Neto VA. Mucosal leishmaniasis: description of case management approaches and analysis of risk factors for treatment failure in a cohort of 140 patients in Brazil. J Eur Acad Dermatol Venereol. 2009;23:1026–1034. doi: 10.1111/j.1468-3083.2009.03238.x. [DOI] [PubMed] [Google Scholar]
- 6.Amato VS, Andrade HF, Amato Neto V, Duarte MI. Short report: persistence of tumor necrosis factor-alpha in situ after lesion healing in mucosal leishmaniasis. Am J Trop Med Hyg. 2003;68:527–528. doi: 10.4269/ajtmh.2003.68.527. [DOI] [PubMed] [Google Scholar]
- 7.Amato VS, Tuon FF, de Andrade HF, Bacha H, Pagliari C, Fernandes ER, Duarte MI, Neto VA, Zampieri RA, Floeter-Winter LM, Celeste BJ, Oliveira J, Quiroga MM, Mascheretti M, Boulos M. Immunohistochemistry and polymerase chain reaction on paraffin-embedded material improve the diagnosis of cutaneous leishmaniasis in the Amazon region. Int J Dermatol. 2009;48:1091–1095. doi: 10.1111/j.1365-4632.2009.04099.x. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins C, Browne JP, Slack R, Lund V, Brown P. The Lund-Mackay staging system for chronic rhinosinusitis: how is it used and what does it predict? Otolaryngol Head Neck Surg. 2007;137:555–561. doi: 10.1016/j.otohns.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Emedicine Web site CT Scan, Paranasal Sinuses. 2009. http://emedicinemedscapecom/article/875244-overview Available at. Accessed October 11, 2009.
- 10.Amato VS, de Andrade HF, Duarte MI. Mucosal leishmaniasis: in situ characterization of the host inflammatory response, before and after treatment. Acta Trop. 2003;85:39–49. doi: 10.1016/s0001-706x(02)00260-7. [DOI] [PubMed] [Google Scholar]
- 11.Havas TE, Motbey JA, Gullane PJ. Prevalence of incidental abnormalities on computed tomographic scans of the paranasal sinuses. Arch Otolaryngol Head Neck Surg. 1988;114:856–859. doi: 10.1001/archotol.1988.01860200040012. [DOI] [PubMed] [Google Scholar]
- 12.Moser FG, Panush D, Rubin JS, Honigsberg RM, Sprayregen S, Eisig SB. Incidental paranasal sinus abnormalities on MRI of the brain. Clin Radiol. 1991;43:252–254. doi: 10.1016/s0009-9260(05)80249-1. [DOI] [PubMed] [Google Scholar]
- 13.Collins JG. Prevalence of Selected Chronic Conditions: United States, 1986–88. Vital Health Statistics 101-87. Atlanta, GA: National Center for Health Statistics; 1993. [PubMed] [Google Scholar]
- 14.Collins JG. Prevalence of Selected Chronic Conditions: United States, 1990–1992. Vital Health Statistics 101-89. Atlanta, GA: National Center for Health Statistics; 1997. [PubMed] [Google Scholar]


