Abstract
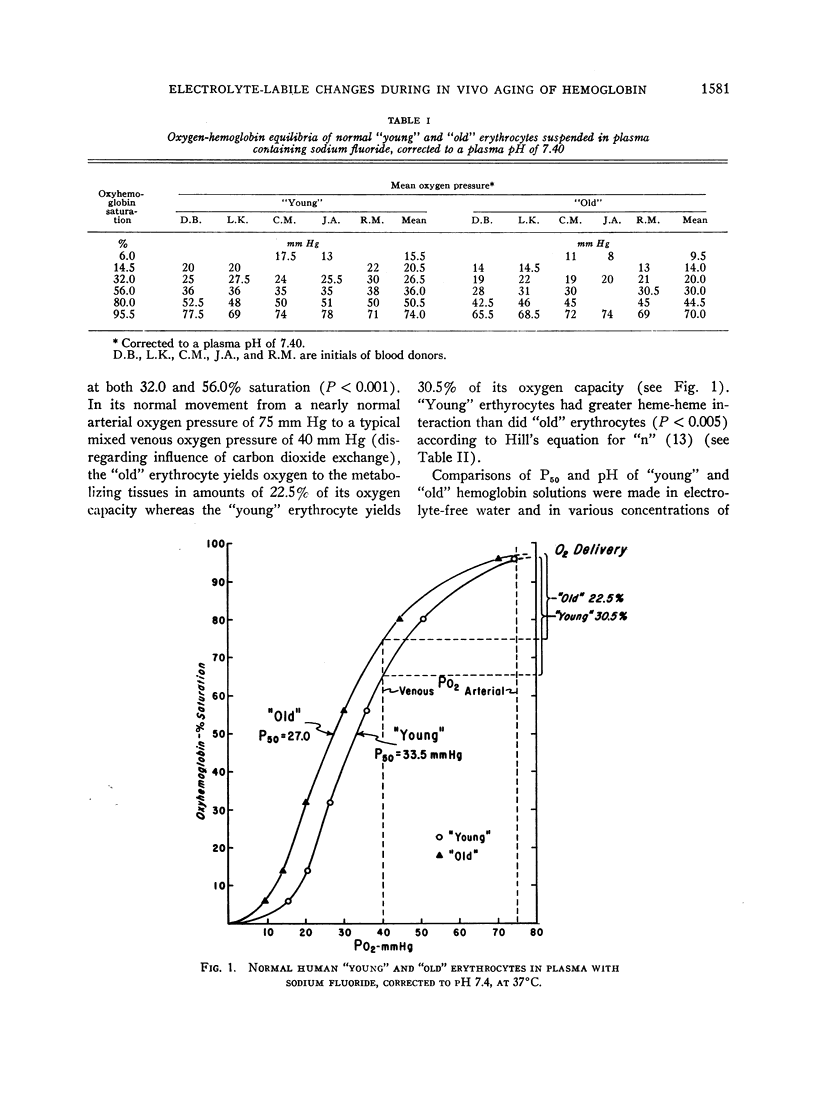
Normal human erythrocytes were separated according to in vivo age by ultracentrifugation. The “young” and “old” erythrocytes had mean cell ages of approximately 40 and 79 days, respectively. “Young” erythrocytes had a lower oxygen affinity and a higher heme-heme interaction than did “old” erythrocytes. This indicates an impairment of the oxygen-carrying function of erythrocyte hemoglobin with age.
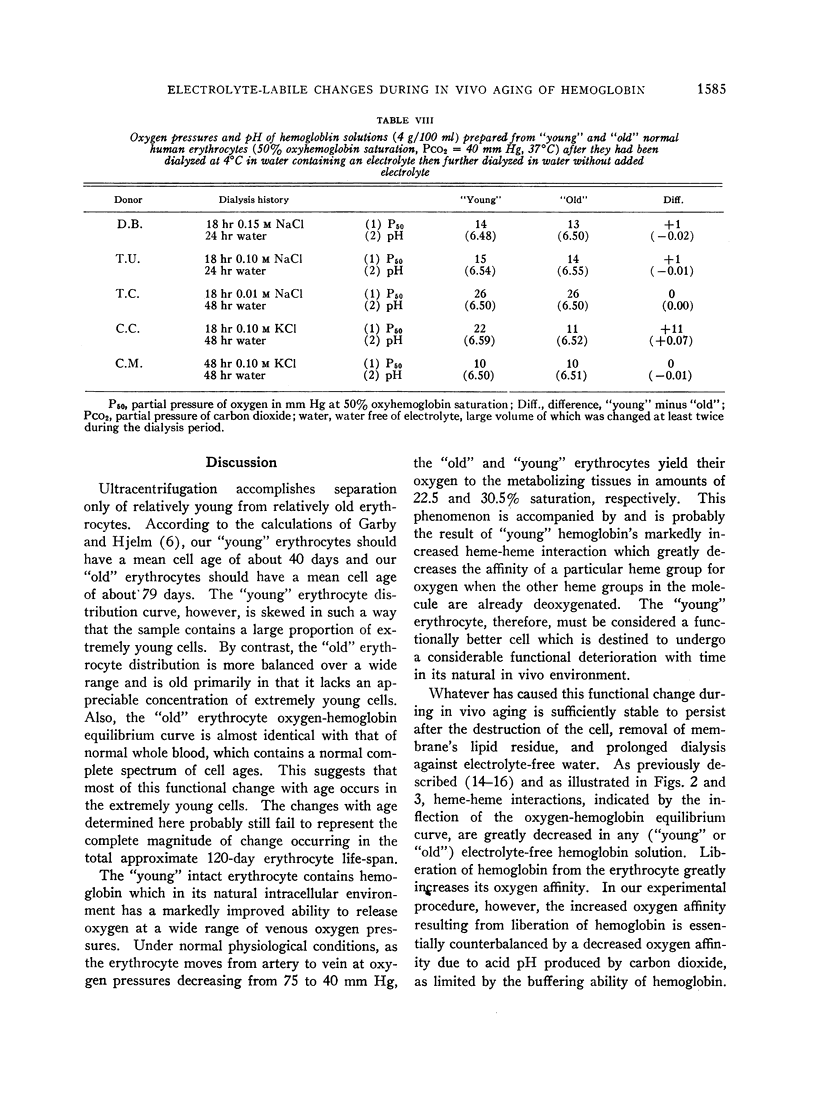
“Young” and “old” erythrocytes were hemolyzed yielding “young” and “old” hemoglobins. “Young” hemoglobin had a comparably lower oxygen affinity than did “old” hemoglobin when the hemolysates were dialyzed against electrolyte-free water.
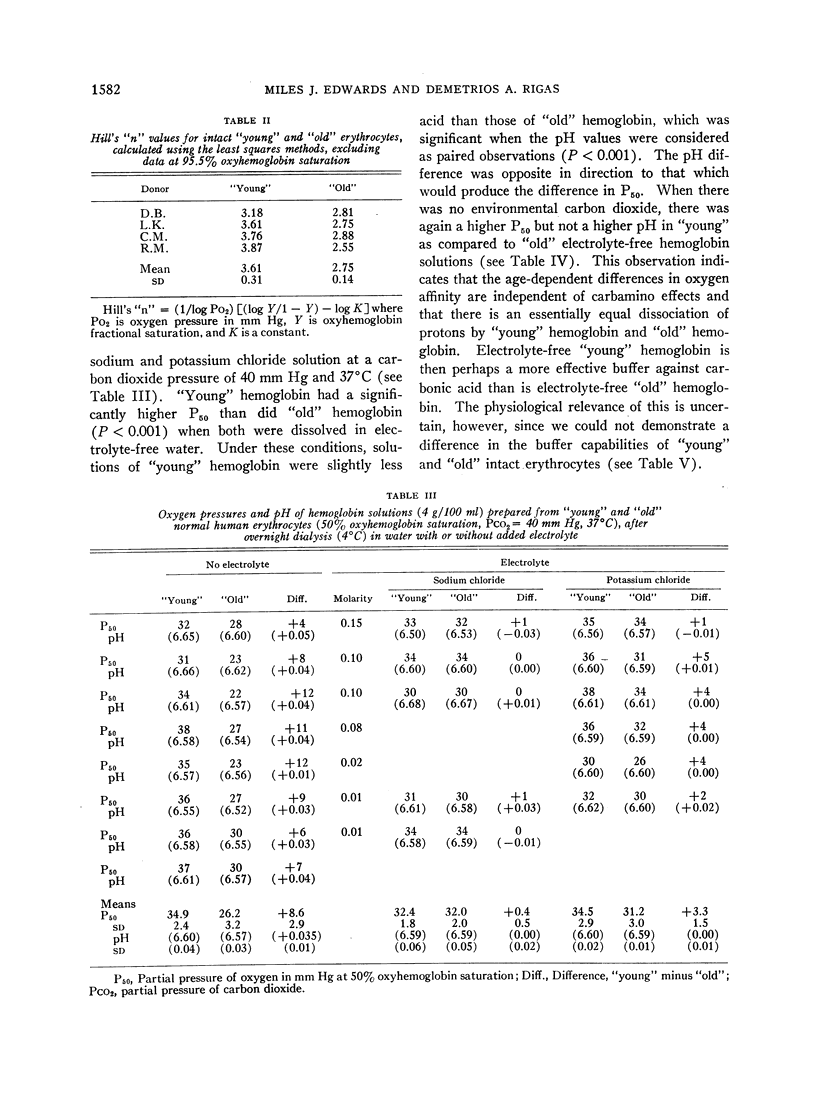
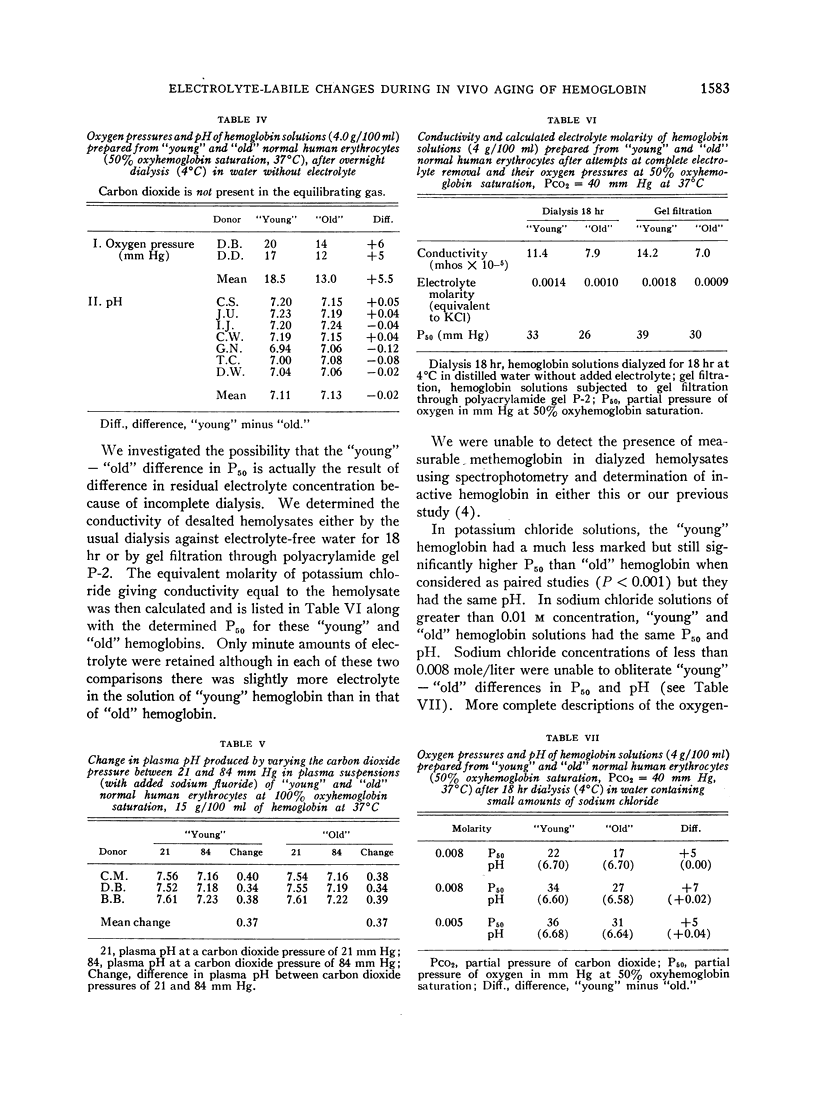
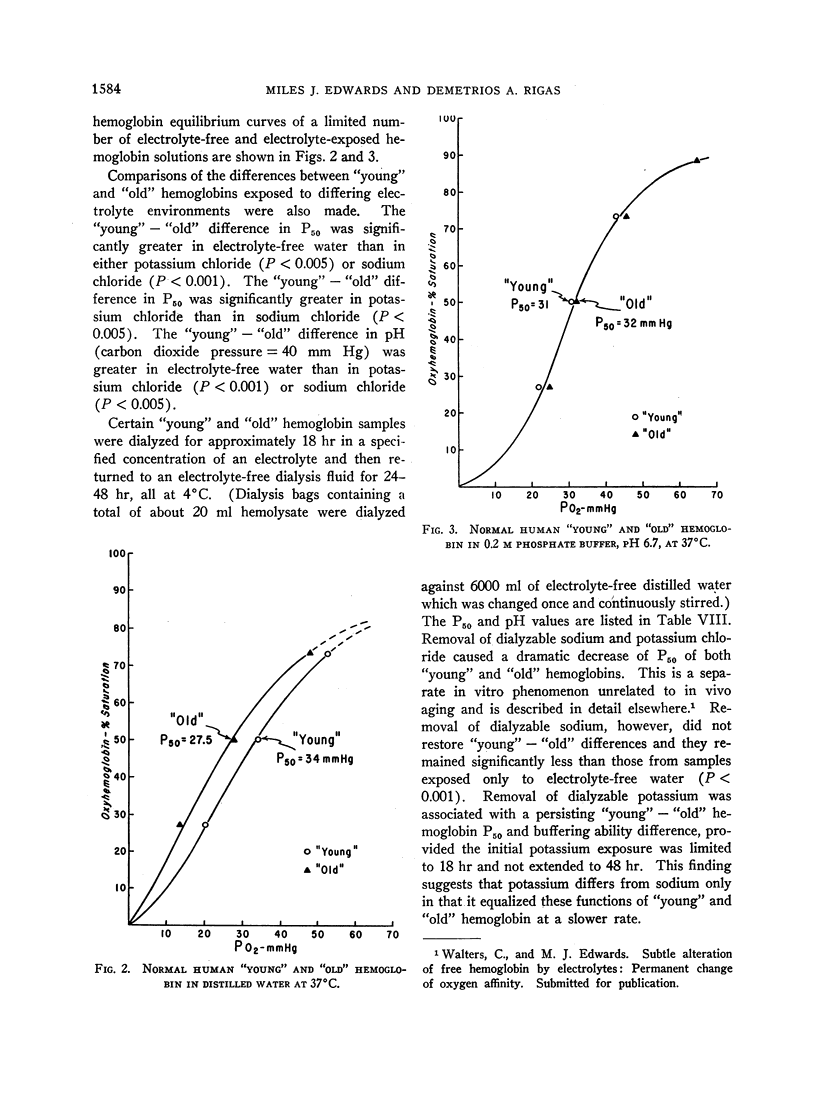
Exposure to sodium chloride completely obliterated this difference between the oxygen affinities and buffer values of “young” and “old” free hemoglobin. Similar exposure to potassium chloride resulted in partial obliteration of the difference between the oxygen affinities of “young” and “old” hemoglobin. Subsequent removal of sodium chloride by dialysis did not restore the pre-electrolyte differences between the oxygen affinities of “young” and “old” hemoglobin.
This evidence indicates that in vivo aging is accompanied by a conformational change of the hemoglobin molecule, which is probably due to an alteration of electrostatic interactions involving the hemoglobin molecule and which is retained after hemolysis and dialysis against water but is obliterated by addition of electrolyte. It is not possible, however, to decide from the available evidence whether this molecular change occurs independently or as a result of influences by other substances, such as 2,3-diphosphoglycerate, which also change during in vivo aging of the erythrocyte.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., BURN G. P. Enzyme activity as a function of age in the human erythrocyte. Br J Haematol. 1955 Jul;1(3):291–303. doi: 10.1111/j.1365-2141.1955.tb05511.x. [DOI] [PubMed] [Google Scholar]
- ANDERSEN O. S., ENGEL K., JORGENSEN K., ASTRUP P. A Micro method for determination of pH, carbon dioxide tension, base excess and standard bicarbonate in capillary blood. Scand J Clin Lab Invest. 1960;12:172–176. doi: 10.3109/00365516009062419. [DOI] [PubMed] [Google Scholar]
- BARTELS H., BETKE K., HILPERT P., NIEMEYER G., RIEGEL K. [The so-called standard O2 dissociation curve in normal adults]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1961;272:372–383. [PubMed] [Google Scholar]
- BERNSTEIN R. E. Alterations in metabolic energetics and cation transport during aging of red cells. J Clin Invest. 1959 Sep;38:1572–1586. doi: 10.1172/JCI103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcroft J., Roberts F. The dissociation curve of haemoglobin. J Physiol. 1909 Aug 26;39(2):143–148. doi: 10.1113/jphysiol.1909.sp001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- ENOKI Y., TYUMA I. FURTHER STUDIES ON HEMOGLOBIN-OXYGEN EQUILIBRIUM. Jpn J Physiol. 1964 Jun 15;14:280–298. doi: 10.2170/jjphysiol.14.280. [DOI] [PubMed] [Google Scholar]
- Edwards M. J., Koler R. D., Rigas D. A., Pitcairn D. M. THE EFFECT OF IN VIVO AGING OF NORMAL HUMAN ERYTHROCYTES AND ERYTHROCYTE MACROMOLECULES UPON OXYHEMOGLOBIN DISSOCIATION. J Clin Invest. 1961 Apr;40(4):636–642. doi: 10.1172/JCI104295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. J., Martin R. J. Mixing technique for the oxygen-hemoglobin equilibrium and Bohr effect. J Appl Physiol. 1966 Nov;21(6):1898–1902. doi: 10.1152/jappl.1966.21.6.1898. [DOI] [PubMed] [Google Scholar]
- GARBY L., HJELM M. ULTRACENTRIFUGAL FRACTIONATION OF HUMAN ERYTHROCYTES WITH RESPECT TO CELL AGE. Blut. 1963 Aug;9:284–291. doi: 10.1007/BF01678992. [DOI] [PubMed] [Google Scholar]
- Havinga E. Comparison of the Phosphorus Content, Optical Rotation, Separation of Hemes and Globin, and Terminal Amino Acid Residurs of Normal Adult Human Hemoglobin and Sickle Cell Anemia Hemoglobin. Proc Natl Acad Sci U S A. 1953 Feb;39(2):59–64. doi: 10.1073/pnas.39.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KACHMAR J. F., BOYER P. D. Kinetic analysis of enzyme reactions. II. The potassium activation and calcium inhibition of pyruvic phosphoferase. J Biol Chem. 1953 Feb;200(2):669–682. [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- MUIRHEAD H., PERUTZ M. F. STRUCTURE OF HAEMOGLOBIN. A THREE-DIMENSIONAL FOURIER SYNTHESIS OF REDUCED HUMAN HAEMOGLOBIN AT 5-5 A RESOLUTION. Nature. 1963 Aug 17;199:633–638. doi: 10.1038/199633a0. [DOI] [PubMed] [Google Scholar]
- RIGAS D. A., KOLER R. D. Ultracentrifugal fractionation of human erythrocytes on the basis of cell age. J Lab Clin Med. 1961 Aug;58:242–246. [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E., CAPUTO A. Studies on the relations between molecular and functional properties of hemoglobin. II. The effect of salts on the oxygen equilibrium of human hemoglobin. J Biol Chem. 1961 Feb;236:397–400. [PubMed] [Google Scholar]
- SEVERINGHAUS J. W. Oxyhemoglobin dissociation curve correction for temperature and pH variation in human blood. J Appl Physiol. 1958 May;12(3):485–486. doi: 10.1152/jappl.1958.12.3.485. [DOI] [PubMed] [Google Scholar]
- Schroeder W. A., Jones R. T. Some aspects of the chemistry and function of human and animal hemoglobins. Fortschr Chem Org Naturst. 1965;23:113–194. doi: 10.1007/978-3-7091-7139-4_4. [DOI] [PubMed] [Google Scholar]
- Warren J. C., Peterson D. M. Structure-disrupting ions: detection of qualitative change in an enzyme. Science. 1966 May 27;152(3726):1245–1246. doi: 10.1126/science.152.3726.1245. [DOI] [PubMed] [Google Scholar]
- Warren J. C., Stowring L., Morales M. F. The effect of structure-disrupting ions on the activity of myosin and other enzymes. J Biol Chem. 1966 Jan 25;241(2):309–316. [PubMed] [Google Scholar]



