Abstract
The Aedes aegypti densovirus (AeDNV) has previously shown potential in mosquito control. To improve its efficacy as a biopesticide, the gene for an excitatory insect-specific toxin from Buthus martensii Karsch (BmK IT1) was inserted into the AeDNV genome and cloned into pUCA plasmid. The coding sequence for green fluorescent protein was ligated to the C-terminus of the BmK IT1 gene as a screening marker. Recombinant and helper plasmids were cotransfected into C6/36 cells; wild-type viruses were the controls. The recombinant viruses were identified and quantified by real-time polymerase chain reaction and exposed to Ae. albopictus larvae for the evaluation of its bioinsecticidal activity. LT50 and LD50 bioassays showed that the recombinant AeDNV had stronger and faster pathogenic effects on Ae. albopictus than the wild-type virus. This is the first report on the recombinant AeDNA containing the insect-specific toxin, BmK IT1, which may be used to develop a novel type of insecticide.
Introduction
Mosquito-borne diseases such as malaria, dengue, and yellow fever constitute a major public health problem, because they are responsible for a considerable amount of morbidity and mortality. Vector control is a key means of combating mosquito-borne diseases. However, the traditional method of the application of chemical pesticides has generated complex problems because of the high level of toxicity caused to the environment, consequent safety risks for humans, and insecticide resistance in mosquitoes.1 Growing concern over the environmental effects of pesticides has encouraged the development of valuable alternative approaches to control these diseases. Further research into the biology of the pathogens that infect mosquitoes may offer potential strategies. They could be used as biological control agents or foreign gene carriers that act either to reduce mosquito populations or the vectorial capacity of mosquitoes.2,3
Mosquito densoviruses (MDVs) are small, autonomous, non-enveloped DNA viruses with small single-stranded DNA genomes, and the majority of them belong to the Brevidensovirus genus in the subfamily Densovirinae within the Parvoviridae family.4 Since the first MDV, Aedes aegypti densovirus (AeDNV) was discovered at Kiev National University in 1972,5 a number of MDVs have been isolated from persistently infected mosquito cell lines, laboratory colonies, or natural populations.6–10 These viruses are often pathogenic to their hosts, they replicate in the nuclei of cells of mosquitoes and cause characteristic histopathological signs, which include hypertrophied, densely stained nuclei. Most importantly, MDVs have a high specificity for mosquitoes as a host, are relatively stable in the environment, and have the potential to spread and persist in mosquito populations. All of these features make them attractive agents that could be developed as bioinsecticides for the control of mosquitoes.6 However, like most wild-type (wt) microbial insecticides, there are several limitations for the use of wt MDVs as insecticides that have restricted their commercial use. Their major disadvantage is poor efficacy. They are slow to act and their killing efficiency operates in a dose-dependent manner, which means that a significant insecticide activity can only be achieved when the virus titer exceeds their native level. To solve this problem, insect viruses are often genetically modified to express insecticidal genes. As a result, the efficiency of recombinant viruses was enhanced and is able to incapacitate its host in a shorter period of time.11
Scorpion insect-specific toxin is strictly selective with a high insect toxicity, which means that they can be considered as an ideal candidate for pest control. It was reported previously that the insecticidal properties of Autographa california nuclear polyhedrosis virus (AcNPV) and Periplaneta fuliginosa densovirus (PfDNV) were enhanced effectively by introducing scorpion insect-specific toxin.12,13 An excitatory insect-specific toxin from Buthus martensii Karsch (BmK IT1) is an insect-selective single-chain neurotoxic polypeptide that is composed of 88 amino acid residues cross-linked by four disulfide bridges. It has been shown to affect insect neuronal sodium conductance by binding to excitable sodium channels.14 In this report, we constructed a series of recombinant AeDNV that expressed BmK IT1. A green fluorescent protein (GFP) gene was also ligated to the C-terminus of the BmK IT1 gene as a screening marker. Bioassays were carried out in vitro to measure the insecticidal effect of recombinant viruses on Ae. albopictus larvae.
Materials and Methods
Plasmid construction.
The DH5α strain of Escherichia coli was used for all cloning procedures and for plasmid preparations. The BmK IT1 gene (GenBank accession no. AF057555), which includes a 54-bp sequence that encodes the signal, was redesigned based on the codon usage frequency preferred by Ae. albopictus. The artificially synthesized optimized BmK IT1 complementary DNA (cDNA) was cloned into the pMD18-T vector (TaKaRa) and incorporation was then confirmed by sequencing. All BmK IT1 expression constructs were built using pUCA or p7NS1-GFP plasmids and only functional features of the plasmids are described here (Figure 1).
Figure 1.
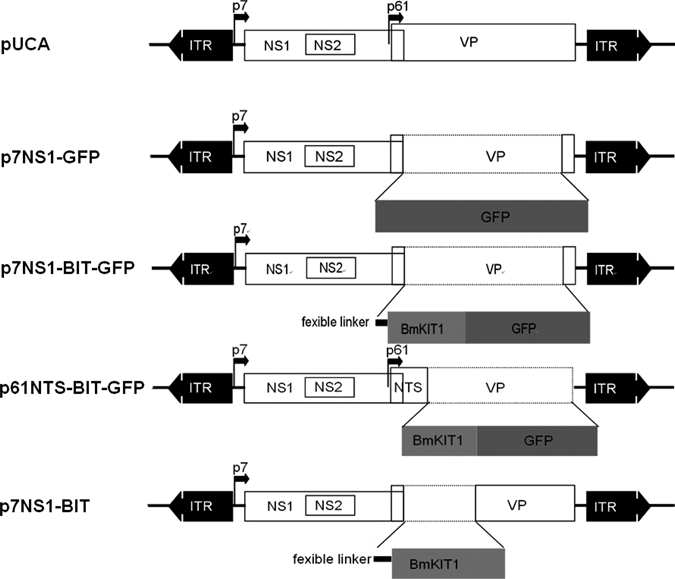
Schematic organization of recombinant Aedes aegypti densovirus (AeDNV) plasmids. The p7 and p61 viral promoters drive the expression of the NS genes and of the VP genes, respectively. In plasmids p7NS1-GFP, p7NS1-BIT-GFP, and p7NS1-BIT, the GFP, BIT-GFP, and BIT gene were fused to the NS1 gene, respectively. In plasmid p61NTS-BIT-GFP, the sequence of the BmK IT1-GFP is fused to the VP1 nuclear targeting signal and its expression is controlled by the p61 promoter.
The pUCA is the infectious clone that contained the AeDNV genome (3,981 nt) in pUC19; the non-structural 1 protein (NS1), and structural protein (VP) genes were transcribed from the P7 and P61 promoters in the genome, respectively, which has been described in detail previously.15
The p7NS1-GFP expresses an NS1-GFP fusion protein from the p7 promoter. The construction of p7NS1-GFP is described in detail elsewhere.15
The p7NS1-BIT-GFP was derived from p7NS1-GFP, and BmK IT1 coding sequences were inserted at the AgeI site of p7NS1-GFP. As a result, the BmK IT1-GFP construct was fused to the C-terminus of NS1. An additional flexible linker (GGGGS) was used to link these two fragments.16 The NS1-linker-BmK IT1-GFP fusion protein was expressed from the p7 promoter.
The p61NTS-BIT-GFP was derived from pUCA. The BmK IT1-GFP coding sequence was cloned into the structural protein, ORF (2,791–3,635 nt). As a result, the 77 aa of the N-terminal of the structural protein, VP1, which harbors a putative nuclear targeting signal (NTS) sequence,17 were fused to the N-terminus of the BmK IT1-GFP protein, and the fusion protein was expressed from the p61 promoter.
The p7NS1-BIT was derived from p7NS1-BIT-GFP by deleting GFP from C-terminus of BmK IT1. As a result, the NS1-linker-BmK IT1 fusion protein was expressed from the p7 promoter.
All of the constructions were confirmed by sequencing (data not shown). The plasmids that were used in this study are depicted in Figure 1.
Mosquito cell maintenance and transfection.
Aedes albopictus C6/36 cells (ATCC CRL-1660) were grown at 28°C in RPMI 1640 medium (Gibco BRL, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco BRL) and 1% antibiotics (Penicillin-Streptomycin; Gibco BRL). The C6/36 cells were grown to a 50–70% confluent monolayer in 24-well cell culture plates (Corning costar, USA) and washed once with 200 μL sterile phosphate buffered solution (PBS: 137 mM NaCl, 27 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4). Lipofect reagent transfection reagent (Tiangen, China) was used to transfect C6/36 cells according to the recommendations of the manufacturer. Supercoiled plasmids used for transfection were prepared using an OMEGA endo-free Plasmid Purification Kit (Omega, USA). The mosquito cells were collected at several time points post-transfection to detect the transcription and expression of BmK IT1 by reverse transcription-polymerase chain reaction (RT-PCR) and immunoblots.
Expression and intracellular localization of BmK IT1-GFP fusion proteins.
The C6/36 cells were transfected with p61NTS-BIT-GFP and p7NS1-BIT-GFP vectors, and pUCA was cotransfected as the helper plasmid (the cotransfection concentration ratio was 2:1). The expression of GFP in cells was monitored with a Nikon Eclipse TE2000-S inverted microscope (Nikon Corp.,Tokyo, Japan) at several time points post-transfection. The C6/36 cells that had been transfected with GFP expression vectors, p61NTS-BIT-GFP, p7NS1-BIT-GFP, and p7NS1-GFP, were fixed in 4% paraformaldehyde in PBS for 10 min, 4′,6-Diamidino-2-phenylindole (DAPI) staining was performed with 0.5 μg/mL of DAPI in PBS for 10 min at 37°C. After washing with PBS, a coverslip was mounted onto a glass slide. Fluorescent signals of the fusion protein were observed under an inverted fluorescence microscope, and photographs were made using a Nikon ACT-2U digital camera (Nikon Corp.). Data were processed and superimposed using Adobe Photoshop 7.0 software (Adobe Systems Inc., San Jose, CA).
Recombinant virus production.
Recombinant viruses (vAep7NS1-BIT-GFP, vAep61NTS-BIT-GFP, and vAep7NS1-BIT) were generated along with wt AeDNV by cotransfecting the infectious clones, p61NTS-BIT-GFP, p7NS1-BIT-GFP, and p7NS1-BIT, individually with helper plasmid pUCA into C6/36 cells according to the protocol of the manufacturer (the cotransfection concentration ratio was 2:1). After an incubation of 5 days, MDV-infected cells were harvested by using cell scrapers, lysed by freezing and thawing, and then centrifuged for 10 min at 3,750 rpm. The supernatants were kept as Recombinant Virus and wt AeDNV mix stocks.
Mosquito maintenance and transduction.
The Ae. albopictus strain used in this work was obtained from the Center for Disease Control and Prevention of Guangdong Province. Every batch of mosquitoes was examined by conventional PCR to ensure that the experimental mosquitoes were free from MDVs (data not shown). Mosquitoes were reared at a temperature of 27°C and a relative humidity of 70% to 80% with 14/10 h light/dark cycles. Larvae were reared in pans and fed on finely ground fish food mixed 1:1 with yeast powder. Mosquito adults were fed on a 10% sugar solution. Adult females were fed for between 3 and 4 days after eclosion on anesthetized mice when a blood meal was required.
To confirm the efficiency of recombinant viruses as gene delivery carriers in vivo, the main portal of entry, dissemination, and tissue tropisms of recombinant virus in Ae. albopictus larvae were investigated. Five hundred first-instar Ae. albopictus larvae were exposed to either vAep61NTS-BIT-GFP or vAep7NS1-BIT-GFP mix stocks by introducing them into the beaker that contained the virus mix stocks. After incubation for 48 h at 28°C, the larvae were transferred back to the pans and fed regularly. Once the fluorescent larvae were detected post-exposure, they were separated into an individual test plastic cup to facilitate the following continuous observation. The newly dead larvae were dissected to observe the presence of GFP in the body. In the other group, three kinds of recombinant viruses mix stocks with infected living larvae were collected at several time points post-transfection to detect the transcription and expression levels of BmK IT1 by RT-PCR and immunoblotting.
Reverse transcription–polymerase chain reaction.
The total RNA from cells of larvae was isolated at several time points post-transfection. One-step RT-PCR was performed on the total RNA using BmK IT1 gene-specific primers (pBmK IT1 F and pBmK IT1 R; Table 1) that amplify a product that was 264 bp in length. As an internal control the transcription product of the Ae. albopictus β-actin gene (GenBank accession no. CB367652) was amplified using another pair of primers (pβ-actin F and pβ-actin R; Table 1) that yielded a 911-bp fragment.
Table 1.
Primers used in the conventional polymerase chain reaction (PCR) and real-time qPCR
| Gene | Usage | Nucleotide sequences (5′–3′) | Product |
|---|---|---|---|
| AeDNV | Real-time qPCR | Sense primer: TGAACCAATTGATTTCGCAT | 179 bp |
| Antisense primer: TTGCCTGTGACCCGTTATTA | |||
| vAep7NS1- BIT-GFP | Real-time qPCR | Sense primer: GGTGGAGGTTCGATGAAGTT | 76 bp |
| Antisense primer: CGTTCTTCTTACCCAGCACA | |||
| vAep7NS1-BIT | Real-time qPCR | Sense primer: GGTGGAGGTTCGATGAAGTT | 76 bp |
| Antisense primer: CGTTCTTCTTACCCAGCACA | |||
| vAep61NTS-BIT-GFP | Real-time qPCR | Sense primer: CGTGCTACTGCTTCGGTCTA | 217 bp |
| Antisense primer: CCGTATGTTGCATCACCTTC | |||
| BmK IT1 | RT | pBmK IT1 F: ATGAAGTTCTTCCTGATCTTC | 264 bp |
| pBmK IT1 R: ACCGATGATC TGCACGTCGCA | |||
| β-actin | RT | pβ-actin F: CACCAGGGTGTGATGGTCGG | 911 bp |
| pβ-actin R: CCACCGATCCAGACGGAGT |
SDS-PAGE and immunoblots.
Proteins were extracted from mosquito larvae and C6/36 cells at several time points post-transfection by a Total Protein Extraction Kit (Keygen, China) according to the instructions of the manufacturer. Loading buffer (0.125 M Tris–HCl, pH 6.8, 10% glycerol, 2% β-mercaptoethanol, 2% SDS, and 0.1% bromophenol blue) was added to the sample and heated at 100°C for 5 min. The 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed for 30 min at 80 V and 90 min at 120 V, and the proteins were then transferred to a polyfinylidene fluoride (PVDF) membrane at 15 mA for 15 min with Bio-rad Trans-Blot SD Semi-Dry Transfer Cell (Bio-Rad, USA). The rabbit polyclonal IgG anti-BmK IT1 was used as the primary antibody (1:600 dilution) and goat anti-rabbit IgG-AP (1:100,000 dilution) was used as the secondary reagent. The BCIP/NBT Alkaline Phosphatase Color Development Kit (Tiangen, China) was used for visualization of the reactive bands.
Quantitative real-time PCR.
Non-encapsidated genomes DNA in mix stocks were removed by treatment with 1 U of DNase I (Takara, Japan) at 37°C for 1 h. The DNase was inactivated by incubation of the samples at 75°C for 30 min. Samples were allowed to cool to room temperature. The total encapsidated genome DNA was extracted using a Viral DNA Kit (Omega). The concentration of the total virus and recombinant virus in mix stocks were determined by real-time PCR using the DNA-binding dye technique (SYBR Green). A set of primers were designed within a conserved region of the viral NS1 gene and BmK IT1 (Table 1). The pUCA and p7NS1-BK were used as a standard control for the total and recombinant viruses, respectively. A standard curve was first constructed by making serial 10-fold dilutions of pUCA and p7NS1-BK at known concentrations, and these were used for the quantitative estimation of samples of unknown concentrations. Reactions were performed using a SYBR Premix Ex Taq Kit (Takara) and run on MX3005P Real-Time PCR System (Stratagene, USA), according to the instructions of the manufacturer. Each reaction contained 12.5 μL of enzyme mix, 0.2 μM of each primer, and 2 μL of DNA solution along with buffer in 25 μL of reaction volume. The real-time PCR thermocycler program follows: stage 1: 95°C 30 sec, stage 2: 95°C 5 sec, 55°C 30 sec, 72°C 30 sec for 40 cycles. Each sample was assessed in triplicate. The resulting data were analyzed using the software provided by the Mx3005P system (MxPro version 4.10 software; Stratagene).
Insecticidal efficacy of the recombionant densoviruses against Ae. albopictus.
The copy number of recombinant virus and AeDNV in three kinds of mix stocks were confirmed by real-time PCR, and then the copy number of recombinant virus and AeDNV in three kinds of mix stocks were adjusted to the same ratio (recombinant virus copy number; AeDNV copy number: 1:5) by applying different volumes of pure AeDNV.
One thousand newly hatched first-instar larvae were randomly divided into five groups: three recombinant virus experimental groups from Ito III (treated with recombinant virus mix stocks vAep7NS1-BIT-GFP/AeDNV, vAep61NTS-BIT-GFP/AeDNV, and vAep7NS1-BIT/AeDNV, respectively), a wt AeDNV treating group and a control group. In the three treatment groups, larvae were exposed to the same concentration recombinant virus mix or wt stocks in a total volume of 10 mL, and food was withheld for 24 h. The control group, which received no virus, was exposed to C6/36 cell culture medium in identical conditions to the treatment groups. After 24 h, mosquito larvae were transferred into dechlorinated tap water and fed regularly. Larval mortality was scored every 12 h for 20 days.
The median lethal concentration (LC50) of first-instar mosquito larvae was determined after being exposed to wt and three kinds of recombinant virus at a series of concentrations that ranged from 1 × 107 to 1 × 1011 copies/mL. The median survival time (LT50) was calculated from the time-mortality curve of larvae that were infected by recombinant virus and wt virus at the concentrations of 1.0 × 1010 and 1.0 × 1011 copies/mL, respectively. The sublethal effect was determined by the variances in time of pupation and emergence, the rate of pupation, and their emergence at the concentrations of 1.0 × 108 copies/mL. Each experiment was repeated three times.
Statistical analysis.
Survival curves were formulated using the Kaplan-Meier test. The log-rank test was used to analyze the differences between survival curves. The LC50 and LT50 values were determined by probit analysis. The LT50 values were compared between different treatments by a one-way analysis of variance (ANOVA) followed by the Fisher's least significant difference test (LSD). The rate of pupation and emergence were compared between the different treatments by the Kruscal-Wallis test. The SPSS computer software version 17.0 (SPSS Inc., Chicago, IL) was used for data analysis.
Results
Expression of BmK IT1 in mosquito cells.
To confirm that recombinant plasmids were expressed in mosquito cells, these constructs were transfected into Ae. albopictus C6/36 cells, and the expression of GFP reporter gene was analyzed by the direct visualization of green fluorescence. The GFP expression was observed in p61NTS-BIT-GFP and p7NS1-BIT-GFP transfected cells as early as 12 h post-transfection. The maximum levels of expression were detected approximately 48 h after transfection.
Furthermore, a similar pattern of intracellular GFP distribution was found among p61NTS-BIT-GFP and p7NS1-BIT-GFP transfected cells. The GFP fusion protein was detected throughout the cell but mainly in nuclei where it showed a punctate or uniform pattern of nuclear distribution (Figure 2); this was consistent with the pattern of p7NS1-GFP expression reported previously.18 This distribution indicates that functions that are vital to the intracellular localization of NS1 or NTS are preserved in the recombinant plasmid, which was an important premise to ensure a high level of expression of the target gene.
Figure 2.
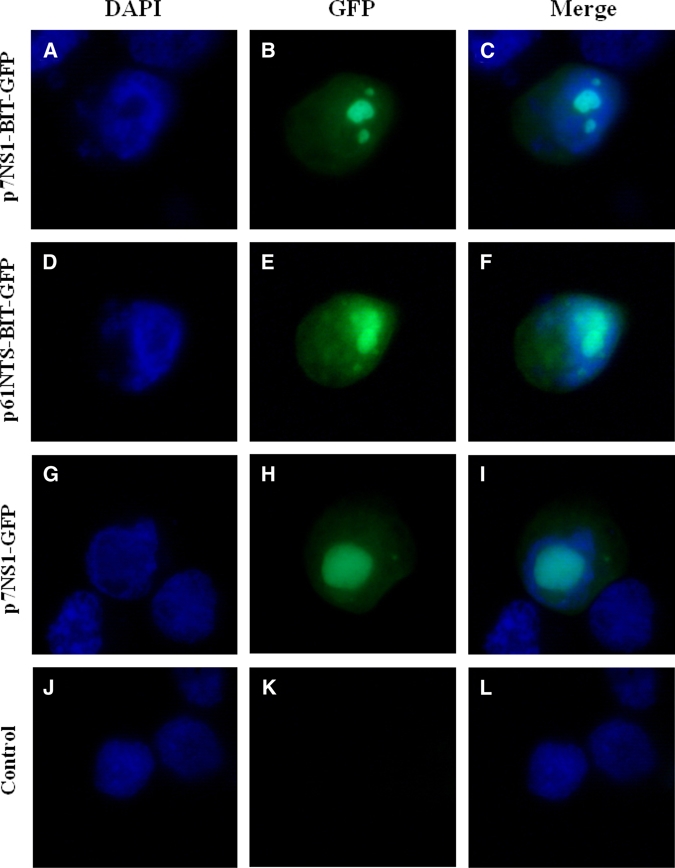
Localization of BmK IT1 fusion protein in recombinant plasmid-transfixed C6/36 cells: Expression of NS1-BIT-GFP (A-C), NTS-BIT-GFP (D-F), BIT-GFP (G-I), and control (J-K). Cells were counterstained with 4′,6-Diamidino-2-phenylindole (DAPI) to visualize the morphology of the nuclei, and green fluorescence shows the localization of the BmK IT1 fusion protein. This figure appears in color at www.ajtmh.org.
We identified the expression of BmK IT1 by RT-PCR and immunoblotting with the use of gene-specific primers and anti-BmK IT1 polyclonal immunoglobulin G (IgG), respectively. The RT-PCR revealed that BmK IT1 mRNA was detected in all cells that were transfected with BmK IT1 constructs at different time points from 12 h post-transfection, and this reached the highest accumulated levels at 48 h. Western blot analysis identified that the anti-BmK IT1 polyclonal IgG reacts specifically with approximately 136, 107, and 47 kDa proteins, respectively. Their molecular masses were consistent with their corresponding fusion proteins (NS1-BmK IT1-GFP, NTS-BmK IT1-GFP, and NS1-BmK IT1) (Figure 3).
Figure 3.
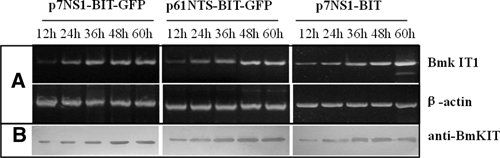
Detection of BmK IT1 expression in Aedes albopictus larvae transduced with recombinant Aedes aegypti densovirus (AeDNV) mixed stock. (A) Reverse transcription-polymerase chain reaction (RT-PCR) techniques were performed on total RNA samples extracted from Ae. albopictus larvae. BmK IT1 messenger RNA (mRNA) accumulation in larvae at 24, 36, 48, 60, and 72 h post-transfection. (B) Western blot analysis for BmK IT1 expressed in larvae. Proteins were extracted at 24, 36, 48, 60, and 72 h; proteins were probed with anti-BmK IT1.
When pUCA was used as a helper plasmid to supply the missing viral proteins of recombinant plasmids, the recombinant particles were generated along with wt virus. These were found with the use of electron microscopy using negative staining with phosphotungstic acid after purification from transfected cells, and their presence was confirmed by PCR (data not shown). These results confirm that BmK IT1 can be expressed successfully in C6/36 cells using a series of expression constructs.
Portal of entry and tissue tropisms of recombinant virus in Ae. albopictus larvae.
Aedes albopictus larvae were exposed to recombinant virus mix stocks. The GFP marker expression was examined by fluorescence microscopy. The new emerging fluorescent larvae ware separated continuously from 1 to 3 d post-exposure to the transducing particles. In group I, GFP expression was observed in 84.2% (421/500) larvae. Among those, 49.6% (248/500) larvae first expressed GFP in the anal papillae, 42.6% (213/500) within the bristle cell, and 12.6% (63/500) at the base of an anal papilla. Other tissue locations accounted for only 9.8% (49/500) of the primary infection sites. Twenty-seven percent of larvae (135/500) showed primary infection in more than one tissue site. In group II, 81.0% (405/500) of GFP-positive larvae were observed; the corresponding percentages for the site of initial GFP expression were 45.8% (229/500) in the anal papillae, 37.4% (187/500) in the bristle cell, 16.2% in the base of an anal papilla (81/500), and 4.2% (21/500) in other tissues. Infection was seen in more than one tissue in 28.8% (144/500) of larvae (Figure 4; Table 2).
Figure 4.
Primary infection site in Aedes albopictus larvae transduced with green fluorescent protein (GFP) marker recombinant Aedes aegypti densovirus (AeDNV); (A) anal papillae; (B) bristle cell; (C) base of anal papillae; (D) anal papillae and bristle cell. This figure appears in color at www.ajtmh.org.
Table 2.
Location and frequency of portal of entry in first-instar Aedes albopictus larvae
| Primary infection site | GFP larvae number infected with recombinant virus | ||
|---|---|---|---|
| vAep7NS1-BIT-GFP | vAep61NTS-BIT-GFP | Total | |
| Anal papillae (SA*) | 248 (157) | 229 (133) | 477 (290) |
| Bristle cell (SA) | 213 (104) | 187 (102) | 400 (206) |
| Base of anal papillae (SA) | 63 (14) | 81 (17) | 144 (31) |
| Other† (SA) | 49 (11) | 21 (9) | 70 (20) |
| Multiple sites | 135 | 144 | 279 |
SA = site alone, the location indicated was unique site of green fluorescent protein (GFP) expression observed.
All locations other than the three kinds of primary infection indicated in the table.
Investigation into the dissemination of recombinant virus in separated individual mosquitoes was based on daily monitoring of GFP expression. There were three major dissemination routes observed from the primary site of viral entry. The major dissemination route was from an infected bristle cell to adjacent tissues and then to other tissues. Nearly 94.7% (195/206) of larvae that only expressed GFP within a single bristle cell developed other infected tissues. The other two typical courses of dissemination were from infected anal papillae and their bases, which accounted for nearly 67.2% (195/290) and 87.1% (27/31) of larvae. Wide dissemination of the expression of GFP always led to death of the mosquito larvae. Only a few larvae with a low level of dissemination survived and went on to pupate; most of these survivors were infected in an area that was restricted to the anal papillae, or they lost their infected anal papillae, which delayed or prevented further dissemination (Table 3). Nearly all larval tissues are possible sites of infection, including muscle fibers, the midgut, salivary glands, nerves, the Malpighian tubule, the foregut and hindgut, and others (Figure 5).
Table 3.
Frequency of dissemination from the route of entry in Aedes albopictus larvae
| Primary infection site* | Green fluorescent protein (GFP) dissemination larvae number | ||
|---|---|---|---|
| vAep7NS1-BIT-GFP | vAep61NTS-BIT-GFP | Total | |
| Anal papillae | 126 | 101 | 207 |
| Bristle cell | 99 | 96 | 195 |
| Base of anal papillae | 12 | 15 | 27 |
Primary infection site larvae alone were included in the statistics for facilitating detected the dissemination route.
Figure 5.
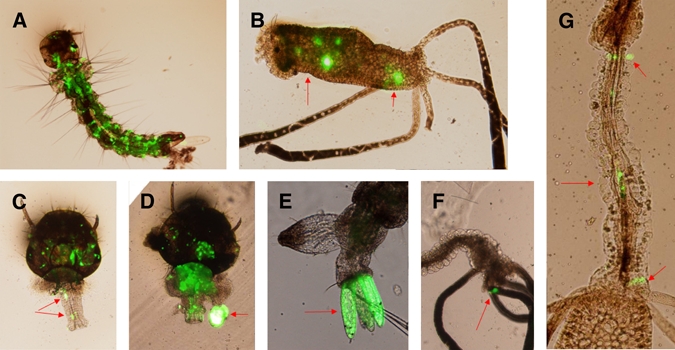
The distribution of recombinant Aedes aegypti densovirus (AeDNV) from the primary infection site in Aedes albopictus larvae; (A) the whole body; (B) hindgut; (C) foregut; (D) salivary gland; (E) multi-anal papillae; (F) Malpighian tubules; (G) hindgut. This figure appears in color at www.ajtmh.org.
Insecticidal efficacy of the recombinant viruses.
To confirm if the expressed BmK IT1 in recombinant vAep61NTS-BIT-GFP, vAep7NS1-BIT-GFP, and vAep7NS1-BIT was functionally active, the insecticidal efficacy of the mix stocks of these recombinant viruses on mosquito larvae was assessed by measuring the overall mortality, the LC50 and the LT50.
In a preliminary assessment, when the concentration of virus was raised to 1 × 108 copies/mL, the mortality of the virus treatment groups was significantly higher than the control group, and this increased in a dose-dependent manner with increasing concentrations of virus. Furthermore, different concentrations of the three kinds of recombinant virus mix stock in the treatment groups always displayed a higher mortality rate than that of wt virus (Figure 6).
Figure 6.
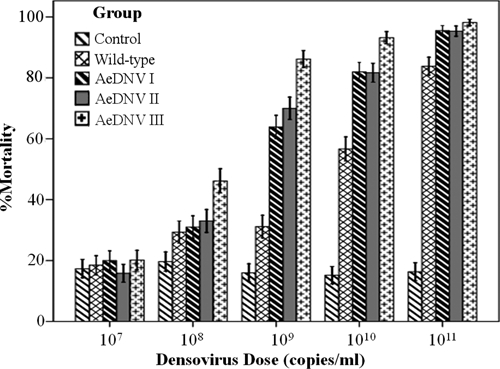
Mortality of Aedes albopictus after exposure to wt-type Aedes aegypti densovirus (AeDNV) and all three kinds of recombinant AeDNV mix stocks. The first-instar Ae. albopictus larvae were exposed to doses of densovirus from 107 to 1011 copies/mL. Bars indicate SDs.
The LC50 values of groups I, II, and III and wt controls were estimated to be 108.43, 108.39, 107.71, and 109.37 copies/mL, respectively (Table 4). These results indicate that the three kinds of recombinant virus mix stock were more virulent than the wt virus. Furthermore, the vAep7NS1-BK/AeDNV mix stock was the most virulent of each of the three kinds of recombinant virus mix stocks.
Table 4.
LC50 of Aedes albopictus larvea exposed to a wild-type and three recombinant Aedes aegypti densovirus (AeDNV)
| Group | LD50 (copies/mL) (95%CL) | Regression equation | Correlation coefficient |
|---|---|---|---|
| Wild-type | 109.37 (108.37–1010.45)a* | Y = 33.201–2.10X† | −0.43 |
| AeDNV I | 108.43 (107.31–109.45) b | Y = 38.61–2.10X | −0.54 |
| AeDNV II | 108.39 (107.26–109.41)b | Y = 39.00–3.02X | −0.55 |
| AeDNV III | 107.71 (106.42–108.75)c | Y = 38.34–3.11X | −0.58 |
Means within the same column with different letters are significantly different (P ≤ 0.05).
Y represents survival time and X represents lg10(concentration).
For both recombinant virus dosages at 1010 copies/mL and 1011 copies/mL the survival curves of the Ae. albopictus larvae exposed to recombinant virus showed a significant reduction in longevity compared with the control mosquitoes exposed to wt virus (Figure 7). The LT50 values of recombinant virus and wt virus were estimated at 1010 copies/mL and 1011 copies/mL, respectively. At a concentration of 1010 copies/mL, the LT50 of groups I, II, and III showed a decrease of 4.5, 4.5, and 6 d, respectively, when compared with the wt virus control group. This indicates that the recombinant strain killed the larvae 1.7, 1.7, and 2.2 times faster than the wt viral strain (P ≤ 0.05) (Table 5). At a concentration of 1011 copies/mL, the LT50 of groups I, II, and III showed a decrease of 2, 2, and 3 d, respectively, when compared with the wt viral strain, which indicated that the recombinant strain killed the larvae 1.4, 1.4, and 1.7 times faster than the controls (P ≤ 0.05) (Table 5). Some sublethal effects were estimated in Ae. albopictus larvae that were exposed to concentrations under the LC50 of the virus (Table 6); the age of pupation and the emergence of these larvae was delayed significantly when compared with control larvae (P ≤ 0.05). In particular, group III showed larval stages with longer periods compared with the control group, and experimental groups I and II (P ≤ 0.05). The pupation and emergence rates of larvae exposed to recombinant virus tended to decrease compared with the control; group III showed the most significant decrease in these rates when compared with larvae infected with the wt virus.
Figure 7.
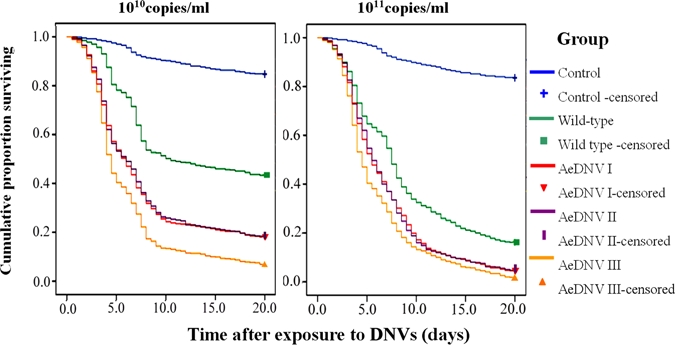
The cumulative proportion Aedes albopictus that survived after the first-instar larvae were exposed to wt-type Aedes aegypti densovirus (AeDNV) and all three kinds of recombinant AeDNV mix stocks at dosages of 1010 and 1011 copies/mL. This figure appears in color at www.ajtmh.org.
Table 5.
LT50 of Aedes albopictus larvae exposed to a wild-type and three recombinant Aedes aegypti densovirus (AeDNV)*
| Group | LT50 (days) (95% CI) | |
|---|---|---|
| 1010 copies/mL | 1011 copies/mL | |
| Wild-type | 10.50 (7.42–13.58)a | 7.50 (7.15–7.85)a |
| AeDNV I | 6.00 (5.42–6.58)b | 5.50 (5.07–5.93)b |
| AeDNV II | 6.00 (5.36–6.64)b | 5.50 (5.15–5.85)b |
| AeDNV III | 4.50 (4.31–4.70)c | 4.50 (4.25–4.75)c |
The LT50 values were calculated from 200 larvae with each tested virus in triplicate. Means within the same column with different letters are significantly different (P ≤ 0.05).
Table 6.
The sublethal effects were effect of the recombinant and wild-type densovirus on Ae. albopictus larvae*
| Group | Age at pupation (Days ± SE) | Pupation (% ± SE) | Age at emergence (Days ± SE) | Emergence (% ± SE) |
|---|---|---|---|---|
| Control | 5.95 ± 0.10a | 0.93 ± 0.10 | 7.83 ± 0.27a | 0.94 ± 0.01 |
| Wild-type | 6.85 ± 0.26b | 0.87 ± 0.20 | 8.57 ± 0.10b | 0.88 ± 0.04 |
| AeDNV I | 7.11 ± 0.19b | 0.85 ± 0.01 | 8.66 ± 0.06b | 0.88 ± 0.03 |
| AeDNV II | 6.90 ± 0.15b | 0.88 ± 0.01 | 8.73 ± 0.17b | 0.90 ± 0.04 |
| AeDNV III | 7.37 ± 0.34c | 0.80 ± 0.05 | 9.22 ± 0.14c | 0.77 ± 0.01 |
The mean values were calculated from 200 larvae per tested virus in triplicates. Means within the same column with different letters are significantly different (P ≤ 0.05).
Discussion
Mosquito-borne diseases are serious medical problems and are significant impediments to economic development for much of the developing world. The MDV as naturally occurring entomopathogens are important regulatory factors in mosquito populations. After being isolated and characterized extensively, MDV showed a great potential as a vector-borne disease control agent. However, their increased use will require increased virulence and efficacy with regards to the speed of mosquito killing. In this study, we evaluated the first use of a scorpion neurotoxin to improve the efficiency of recombinant AeDNV for Ae. albopictus.
As described previously, wherever ORFs of MDV were inserted or replaced by foreign genes, the virions would not be generated independently by the defective recombinant genome unless a helper plasmid was cotransfected to supply the essential viral proteins that were otherwise absent. Therefore, a recombinant genome plasmid, a helper plasmid, and C6/36 packaging cell lines are essential components of densovirus-based transducing systems.
There are two major limitations of these transducing systems when applied to the problem of vector control. The first is the strict size limit of the recombinant genome imposed by the icosahedral geometry of the viral particle. For efficient packaging, the length of the recombinant genome can be no more than 4,400 nucleotides (110% of the length of the wt densovirus genome), which leaves enough space for the foreign gene with an entirely independent expression cassette and still preserve the terminal inverse repeat sequence (IRS) that is necessary for replication. However, expression levels declined sharply when the NS1 gene was deleted from the recombinant genome, presumably because of the multiple activities that this densovirus replication initiator protein possesses. These activities include sequence- and strand-specific nicking activity,19 ATP-dependent helicase activity,20 and especially promoter transregulation21,22 that influences the expression of target genes. If NS1 is preserved in its entirety, the size of the inserted sequence is limited to approximately 1,000 bases; this limit excludes most genes for insecticidal proteins.
Scorpion insect-specific neurotoxins as a group of proteins are composed of between 30 and 80 amino acids. They show a high degree of neurotoxicity toward insects and are safe for use in humans and other animals.23,24 Their significance and potential for application in insect-pest control has been confirmed, and their relatively short length of nucleotide sequences are suitable for incorporation into densovirus-based transducing systems.
The second limitation of these systems is that although the pure recombinant virus can be generated by the Sindbis virus expression system,25 they would lose the ability for secondary transmission that takes place in vivo in mosquitoes with defective genome. Therefore, cotransduction with wt virus is necessary for developing this system for biological control.
It has been shown previously that the strategy of high levels of expression of target genes in mosquito cells were expressed under the control of the p7 or p61 promoters in the presence of NS1 protein, which leaves little space when transfected without the pUCA helper plasmid. The p7 promoter was slightly more efficient than p61.17,18,26 Therefore, in this study, we used different expression strategies for gene of the BmK IT1 toxin and evaluated which was the best for producing a high level of expression, and preserved wt viral function. For high levels of expression and packaging, all expression constructs require the preservation of the entire NS1 gene and a suitable length of the genome, as described previously. However, they lacked VP required for genome packaging. In p7NS1-BIT-GFP and p7NS1-BIT, BmK IT1 was fused to the N-terminal of NS1. In p61NTS-BIT-GFP, the BmK IT1 gene was cloned into the VP ORF and transcribed from the p61 promoter, and 77 aa of VP1 that contained a putative NTS sequence was fused to the N-terminal instead; it has been confirmed previously that an additional NTS can target the protein into the nucleus of mosquito cells and increase the expression level of protein.17,18,27
From a safety standpoint, it is important to track the genetic stability of recombinant densovirus and their presence in vitro and in vivo. For this purpose, GFP was expressed constitutively as a visible marker in p61NTS-BIT-GFP and p7NS1-BIT-GFP. The p7NS1-BIT was also used to compare whether insecticidal efficacy was influenced by additional GFP. However, according to previous reports, the functions of the NS1 fusion protein should be preserved and will be manifested by its nuclear localization.21,28,29 Under the control of the p61 promoter, the N-terminal fused NTS will target the protein into nucleus and its function was confirmed by its nuclear localization. Our results showed a similar pattern of intracellular GFP distribution between p61NTS-BIT-GFP and p7NS1-BIT-GFP compared with p7NS1-GFP, which confirmed that the NS1-BmK IT1-GFP and NTS-BmK IT1-GFP fusion proteins, NS1 and NTS, retained their functions well. On the other hand, our results did not show significant differences in the level of messenger RNA (mRNA) expression and reporter gene fluorescent brightness between the three different kinds of expression strategy in vitro and in vivo when they were cotransfected with either pUCA or were cotransducted with wt AeDNV. It seems that the helper plasmid gave the recombinant genome the ability for secondary transmission to uninfected cells and elevated expression levels, so slight differences in efficiency were neglected.
Aedes albopictus was selected as the target insect because it is a major vector of dengue fever in mainland China. Dengue fever, together with associated dengue hemorrhagic fever (DHF), is the fastest growing vector-borne disease in the world. Additionally, there have been no reports with regards to the infection rate and pathogenicity of AeDNV in Ae. albopictus to date.
In previous studies the portal of entry, dissemination, and tissue tropisms of AeDNV in Ae. aegypti and Anopheles gambiae have been reported.15 The anal papillae and bristle cells were major portals of entry in all three species of mosquito, but the base of anal papillae was only shown in Ae. aegypti. The other tissues, including muscle and body fat, were also observed as the initial site of GFP expression in Ae. aegypti, whereas the alimentary tract was excluded from GFP expression despite the alimentary tract of mosquitoes playing a central role in the digestion of pathogens and is the primary site of many pathogenic infections. Some studies proved that lepidopteran larvae are infected with Junonia coenia densovirus (JcDNV) through the ingestion of contaminated food. The JcDNV first traffics through polarized intestinal cells, although these cells do not replicate the virus.30 This observation does not explain the late dissemination and widespread expression of GFP in the alimentary that included the foregut, midgut, and hindgut. In Ae. albopictus, besides the anal papillae, infected bristle cells were inclined to lead to viral dissemination, whereas in Ae. aegypti the anal papillae were the only sites that tended to lead to a high level of viral dissemination. The GFP expression levels in vivo showed a focal distribution, and there were no obvious tissue tropisms with the exception of a slower infection rate being seen in the Malpighian tubules and trachea. Therefore, we can also draw the conclusion that infection with recombinant virus originates from only a few primary infection sites and then spreads throughout the larval body if wt virus is used as a helper.
It was shown previously that the efficacy of viruses as insecticides was enhanced by genetic engineering to express scorpion insect-specific toxin.31 Mosquitoes have been confirmed as being sensitive to a scorpion neurotoxin with the use of an infectious Sindbis virus as the expression vector.32 Our studies show that all three kinds of recombinant densovirus mix stocks (vAep7NS1-BIT-GFP/AeDNV, vAep61NTS-BIT-GFP/AeDNV, and vAep7NS1-BIT/AeDNV) were significantly more effective than wt AeDNA alone for decreasing the LC50 and LT50 in mosquitoes. The vAep7NS1-BIT/AeDNV mix stocks was shown to have the highest insecticidal effects, and it appears that additional GFP may be influenced partly by the interaction between the functional domains of BmK IT1 in NS1-BIT-GFP and NTS-BIT-GFP with its receptor. Under low concentrations, vAep7NS1-BIT/AeDNV mix stocks are more effective in delaying the age of pupation and emergence of mature mosquitoes, with an overall decreased rate of emergence rate, as compared with infection with wt virus alone. Pupal weight and larval length were also decreased (data not shown), which is likely to reduce the capacity of this mosquito to be a disease vector.
Different strains of MDV differ with regard to infectivity ratios and pathogenicity for different hosts. Infection rates of AeDNV for Ae. albopictus cannot reach 100% because some mosquitoes sometimes escape from infection by losing their infected papilla,4 and there is the possible influence of fusion expression on the function of scorpion toxin. All of these factors account for the observation that recombinant viruses are clearly more lethal than the wt virus, but not dramatically. However, the pathogenicity of the recombinant virus still has the potential to be improved by selecting high infection rate strains of the MDV for different target mosquitoes and the introduction of an internal ribosome entry site (IRES) for the insect virus to gain non-fusion expression of BmK IT1.
Although recombinant virus could be excreted into the water by infected larvae accompanied with the wt virus and allowing for horizontal spread of the virus to other larvae, the amount of recombinant virus was markedly decreased in the rearing water as the inability to self-package. However, the problem of the persistence of recombinant virus could be solved effectively by the construction of nondefective hypervirulent strains, and this appears to be feasible with the use of appropriate genetic methods in our ongoing studies.
In summary, we have showed that genetic engineering is an effective way to increase the virulence of wt AeDNV, and the recombinant virus may be valuable as a vector control agent in the future. However, whether this system will become a practical method for insect control remains to be seen. The main concern in the use of recombinant virus with toxin genes is safety, including the issue of toxin specificity and the risk of gene flow into non-target organisms through unintended infection. Even though MDV is highly specific for mosquitoes as hosts, the high selectivity of the toxin to insects together with the uniqueness of transcriptional activation of MDV promoters in permissive insect cells provides safety at multiple levels. Further data to support the environmental safety of genetically modified AeDNV are still required. Analogous research of insect viruses, including the genetic modification of baculovirus to express scorpion toxin, has yet to be translated into practice. This is largely a result of commercial and political issues rather than technical limitations. Although MDV has a number of attractive attributes as a microbial control agent, there are a number of questions that remain to be answered before its maximal value can be realized.
Acknowledgments
We gratefully acknowledge Jonathan Carlson (Colorado State University) for kindly providing us the plasmid pUCA and p7NS1-GFP.
Footnotes
Financial support: The research was supported by the National Natural Science Foundation of China (no. 30800957 and 30771871) and President Foundation of School of Public Health and Tropical Medicine, Southern Medical University (no. GW200829).
Authors' addresses: Jin-Bao Gu, Hong-Juan Peng, and Xiao-Guang Chen, School of Public Health and Tropical Medicine, Southern Medical University, Guangzhou, China, E-mails: gujinbao@fimmu.com, floriapeng@hotmail.com, and xgchen@fimmu.com. Yun-Qiao Dong, Institute of Genetic Engineering, Southern Medical University, Guangzhou, China, E-mail: yunqiao@fimmu.com.
References
- 1.Attaran A, Roberts DR, Curtis CF, Kilama WL. Balancing risks on the backs of the poor. Nat Med. 2000;6:729–731. doi: 10.1038/77438. [DOI] [PubMed] [Google Scholar]
- 2.Carlson JO. Genetic manipulation of mosquitoes: an approach to controlling disease. Trends Biotechnol. 1996;14:447–448. doi: 10.1016/S0167-7799(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 3.Corsini J, Traul DL, Wilcox CL, Gaines P, Carlson JO. Efficiency of transduction by recombinant Sindbis replicon virus varies among cell lines, including mosquito cells and rat sensory neurons. Biotechniques. 1996;21:492–497. doi: 10.2144/96213rr02. [DOI] [PubMed] [Google Scholar]
- 4.Ward TW, Kimmick MW, Afanasiev BN, Carlson JO. Characterization of the structural gene promoter of Aedes aegypti densovirus. J Virol. 2001;75:1325–1331. doi: 10.1128/JVI.75.3.1325-1331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebedeva PO, Zelenko AP, Kuznetsova MA, Gudzgorban AP. Studies on the demonstration of a viral infection in larvae of Aedes aegypti mosquitoes. Microbiol JSU. 1972;34:70–73. [PubMed] [Google Scholar]
- 6.Carlson J, Suchman E, Buchatsky L. Densoviruses for control and genetic manipulation of mosquitoes. Adv Virus Res. 2006;68:361–392. doi: 10.1016/S0065-3527(06)68010-X. [DOI] [PubMed] [Google Scholar]
- 7.Pattanakitsakul SN, Boonnak K, Auethavornanan K, Jairungsri A, Duangjinda T, Puttatesk P, Thongrungkiat S, Malasit P. A new densovirus isolated from the mosquito Toxorhynchites splendens (Wiedemann) (Diptera: Culicidae) Southeast Asian J Trop Med Public Health. 2007;38:283–293. [PubMed] [Google Scholar]
- 8.Ren X, Hoiczyk E, Rasgon JL. Viral paratransgenesis in the malaria vector Anopheles gambiae. PLoS Pathog. 2008;4:e1000135. doi: 10.1371/journal.ppat.1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhai YG, Lv XJ, Sun XH, Fu SH, Gong ZD, Fen Y, Tong SX, Wang ZX, Tang Q, Attoui H, Liang GD. Isolation and characterization of the full coding sequence of a novel densovirus from the mosquito Culex pipiens pallens. J Gen Virol. 2008;89:195–199. doi: 10.1099/vir.0.83221-0. [DOI] [PubMed] [Google Scholar]
- 10.Sivaram A, Barde PV, Kumar SR, Yadav P, Gokhale MD, Basu A, Mourya DT. Isolation and characterization of densonucleosis virus from Aedes aegypti mosquitoes and its distribution in India. Intervirology. 2009;52:1–7. doi: 10.1159/000210044. [DOI] [PubMed] [Google Scholar]
- 11.van Beek NA, Hughes PR. The response time of insect larvae infected with recombinant baculoviruses. J Invertebr Pathol. 1998;72:338–347. doi: 10.1006/jipa.1998.4814. [DOI] [PubMed] [Google Scholar]
- 12.McCutchen BF, Choudary PV, Crenshaw R, Maddox D, Kamita SG, Palekar N, Volrath S, Fowler E, Hammock BD, Maeda S. Development of a recombinant baculovirus expressing an insect-selective neurotoxin: potential for pest control. Biotechnology (NY) 1991;9:848–852. doi: 10.1038/nbt0991-848. [DOI] [PubMed] [Google Scholar]
- 13.Jiang H, Zhang JM, Wang JP, Yang B, Liu CF, Lu J, Hu YY. Genetic engineering of Periplaneta fuliginosa densovirus as an improved biopesticide. Arch Virol. 2007;152:383–394. doi: 10.1007/s00705-006-0844-6. [DOI] [PubMed] [Google Scholar]
- 14.Goudet C, Chi CW, Tytgat J. An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch. Toxicon. 2002;40:1239–1258. doi: 10.1016/s0041-0101(02)00142-3. [DOI] [PubMed] [Google Scholar]
- 15.Ward TW, Jenkins MS, Afanasiev BN, Edwards M, Duda BA, Suchman E, Jacobs-Lorena M, Beaty BJ, Carlson JO. Aedes aegypti transducing densovirus pathogenesis and expression in Aedes aegypti and Anopheles gambiae larvae. Insect Mol Biol. 2001;10:397–405. doi: 10.1046/j.0962-1075.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 16.Lu P, Feng MG. Bifunctional enhancement of a beta-glucanase-xylanase fusion enzyme by optimization of peptide linkers. Appl Microbiol Biotechnol. 2008;79:579–587. doi: 10.1007/s00253-008-1468-4. [DOI] [PubMed] [Google Scholar]
- 17.Afanasiev BN, Kozlov YV, Carlson JO, Beaty BJ. Densovirus of Aedes aegypti as an expression vector in mosquito cells. Exp Parasitol. 1994;79:322–339. doi: 10.1006/expr.1994.1095. [DOI] [PubMed] [Google Scholar]
- 18.Afanasiev BN, Ward TW, Beaty BJ, Carlson JO. Transduction of Aedes aegypti mosquitoes with vectors derived from Aedes densovirus. Virology. 1999;257:62–72. doi: 10.1006/viro.1999.9621. [DOI] [PubMed] [Google Scholar]
- 19.Walker SL, Wonderling RS, Owens RA. Mutational analysis of the adeno-associated virus Rep68 protein: identification of critical residues necessary for site-specific endonuclease activity. J Virol. 1997;71:2722–2730. doi: 10.1128/jvi.71.4.2722-2730.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson GM, Jindal HK, Yeung DE, Chen W, Astell CR. Expression of minute virus of mice major nonstructural protein in insect cells: purification and identification of ATPase and helicase activities. Virology. 1991;185:90–98. doi: 10.1016/0042-6822(91)90757-3. [DOI] [PubMed] [Google Scholar]
- 21.Yang B, Zhang J, Cai D, Li D, Chen W, Jiang H, Hu Y. Biochemical characterization of Periplaneta fuliginosa densovirus non-structural protein NS1. Biochem Biophys Res Commun. 2006;342:1188–1196. doi: 10.1016/j.bbrc.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 22.You H, Liu Y, Prasad CK, Agrawal N, Zhang D, Bandyopadhyay S, Liu H, Kay HH, Mehta JL, Hermonat PL. Multiple human papillomavirus genes affect the adeno-associated virus life cycle. Virology. 2006;344:532–540. doi: 10.1016/j.virol.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 23.Zlotkin E, Miranda F, Kupeyan C, Lissitzky S. A new toxic protein in the venom of the scorpion Androctonus australis Hector. Toxicon. 1971;9:9–13. doi: 10.1016/0041-0101(71)90038-9. [DOI] [PubMed] [Google Scholar]
- 24.Darbon H, Zlotkin E, Kopeyan C, van Rietschoten J, Rochat H. Covalent structure of the insect toxin of the North African scorpion Androctonus australis Hector. Int J Pept Protein Res. 1982;20:320–330. doi: 10.1111/j.1399-3011.1982.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 25.Allen-Miura TM, Afanasiev BN, Olson KE, Beaty BJ, Carlson JO. Packaging of AeDNV-GFP transducing virus by expression of densovirus structural proteins from a sindbis virus expression system. Virology. 1999;257:54–61. doi: 10.1006/viro.1999.9622. [DOI] [PubMed] [Google Scholar]
- 26.Kimmick MW, Afanasiev BN, Beaty BJ, Carlson JO. Gene expression and regulation from the p7 promoter of Aedes densonucleosis virus. J Virol. 1998;72:4364–4370. doi: 10.1128/jvi.72.5.4364-4370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dingwall C, Robbins J, Dilworth SM, Roberts B, Richardson WD. The nucleoplasmin nuclear location sequence is larger and more complex than that of SV-40 large T antigen. J Cell Biol. 1988;107:841–849. doi: 10.1083/jcb.107.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensen J, Tattersall P. Parvovirus initiator protein NS1 and RPA coordinate replication fork progression in a reconstituted DNA replication system. J Virol. 2002;76:6518–6531. doi: 10.1128/JVI.76.13.6518-6531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jindal HK, Yong CB, Wilson GM, Tam P, Astell CR. Mutations in the NTP-binding motif of minute virus of mice (MVM) NS-1 protein uncouple ATPase and DNA helicase functions. J Biol Chem. 1994;269:3283–3289. [PubMed] [Google Scholar]
- 30.Vendeville A, Ravallec M, Jousset FX, Devise M, Mutuel D, Lopez-Ferber M, Fournier P, Dupressoir T, Ogliastro M. Densovirus infectious pathway requires clathrin-mediated endocytosis followed by trafficking to the nucleus. J Virol. 2009;83:4678–4689. doi: 10.1128/JVI.02401-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zlotkin E, Fishman Y, Elazar M. AaIT: from neurotoxin to insecticide. Biochimie. 2000;82:869–881. doi: 10.1016/s0300-9084(00)01177-9. [DOI] [PubMed] [Google Scholar]
- 32.Higgs S, Olson KE, Klimowski L, Powers AM, Carlson JO, Possee RD, Beaty BJ. Mosquito sensitivity to a scorpion neurotoxin expressed using an infectious Sindbis virus vector. Insect Mol Biol. 1995;4:97–103. doi: 10.1111/j.1365-2583.1995.tb00013.x. [DOI] [PubMed] [Google Scholar]


