Abstract
In the United States, the American dog tick, Dermacentor variabilis (Say) is considered an important biological vector of Francisella tularensis, the etiologic agent of tularemia. In this study, we evaluated the vector efficiency of nymphal D. variabilis infected as larvae with differing clades and subspecies (A1b, A2, and type B) of F. tularensis. In all cases, D. variabilis larvae were able to acquire, maintain, and transstadially transmit F. tularensis. Significant replication of the bacteria also occurred in infected nymphs. Transmission of F. tularensis to Swiss Webster mice was not observed with A1b, and low rates were observed with A2 (8.0%) and type B (13.5%). Negative effects on tick survivorship were also observed for A1b, A2, and type B infections. Our results provide evidence of a high fitness cost and low transmission rates during the immature stages, suggesting that D. variabilis may play a limited role in enzootic maintenance of F. tularensis.
Introduction
Ticks have historically been considered important biological vectors of Francisella tularensis, the etiological agent of tularemia, because they can transmit the bacterium to humans as well as propagate the organism in zoonotic cycles.1,2 In the United States, the American dog tick, Dermacentor variabilis (Say), is suspected to be among the most important species for bridging F. tularensis from zoonotic cycles to humans.3 Tularemia outbreaks associated with D. variabilis have been reported,4,5 and the geographic distribution of this tick species is concordant with states reporting the highest incidence of tick-borne tularemia (Missouri, Arkansas, and Oklahoma).6 Adult D. variabilis has been found to be infected with F. tularensis,4,5,7–9 and immature life stages (larvae and nymphs) are thought to be associated with enzootic transmission.
F. tularensis is categorized into two subspecies, F. tularensis tularensis (hereafter called type A) and F. tularensis holarctica (hereafter called type B), and type A is subdivided further into clades, A1a, A1b, and A2.10,11 The subspecies and clades differ with respect to the severity of illness in humans as well as geographic distribution. A1b strains are considered to cause the most severe infections; among culture-confirmed human cases in the United States, infection with an A1b strain resulted in significantly higher mortality (24%) compared with infection with an A1a (4%), A2 (0%), or type B (7%).10,11 Type B strains have been recovered throughout the United States, with a higher concentration along the northern Pacific Coast and the Mississippi River. A1 strains (both A1a and A1b) are typically found in the central southeastern states of Arkansas, Missouri, and Oklahoma and along the Atlantic Coast as well as California, whereas A2 strains are found from the Rocky Mountains west to the Sierra Nevada Mountains.10 D. variabilis is widely distributed east of the Rocky Mountains and in parts of California, and it shows a geographic concordance with the distribution of A1 (A1a and A1b) and type B but not A2 strains.12–14
Both type A and type B have been identified in naturally infected D. variabilis adults. Outbreaks of tick-borne tularemia attributed to type B occurred in South Dakota in 1966 and 1984. Environmental investigations after the outbreaks revealed minimum infection rates of approximately 1.4% in D. variabilis adults collected from vegetation and local dogs.4,5 Prevalence of type A infections in D. variabilis collected between 2004 and 2009 ranged from 1% to 5% on Martha's Vineyard, an island that reported an outbreak of tularemia in 2000 and continues to report yearly cases.7,9 On Martha's Vineyard, the infecting type A clade is likely to be A1b, because this is the only clade identified to date from culture-confirmed human and animal cases on the island.11
In this study, we compared transmission rates among nymphal D. variabilis infected as larvae with wild-type strains of A1b, A2, and type B. Although historical studies evaluated vector competence of D. variabilis, these studies occurred before identification of F. tularensis subspecies (type A and type B) and clades (A1a, A1b, and A2). Moreover, these studies used methodologies that did not allow for precise estimates of tick-borne transmission. For example, many observed mass-action transmission at the nymphal stage when the hosts were infested with numerous infected nymphs, but accurate counts of the nymphs placed on the host and recovered were not recorded.15,16 As a result, precise estimates of transmission efficiency could not be made. Furthermore, in those studies, it remains unclear whether F. tularensis was transmitted to sentinel animals through infectious tick bites, consumption of infectious ticks, mass-action transmission, or other modes.
The aims of this study were to (1) compare nymphal transmission rates of D. variabilis infected as larvae with wild-type strains of A1b, A2, and type B, (2) evaluate the fitness cost by comparing A1b-, A2-, and type B-infected nymphs to uninfected nymphs, and (3) describe the bacterial kinetics from time of acquiring infection as larvae through feeding to repletion as nymphs. Based on the geographic concordance of D. variabilis with A1b and type B, we hypothesized that we would see high transmission rates and minimal fitness cost with A1b- and type B-infected nymphs but minimal transmission and high fitness cost with A2-infected nymphs. Our results provide evidence for high fitness costs and low transmission rates for A1b-, A2-, and type B-infected nymphs.
Materials and Methods
Bacterial strains, ticks, and mice.
The bacterial strains used in these experiments included F. tularensis tularensis clade A1b (strain: MA00-2987), clade A2 (strain: WY96-3418), and F. tularensis holarctica (type B; strain: KY99-3387) originating from source patients in Massachusetts in 2000, Wyoming in 1996, and Kentucky in 1999, respectively. Larval D. variabilis were obtained from the Oklahoma State University tick-rearing facility. The colony originated with ticks collected from Stillwater, OK and has been maintained since 1972. Six-week-old female Swiss Webster mice were obtained from a specific pathogen-free colony maintained at the Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, Fort Collins, CO.
Infecting larval D. variabilis with F. tularensis A1b, A2, and type B.
The strains were subcultured from stocks stored at −70°C onto cysteine heart agar containing 9% chocolatized sheep blood (CHAB) and incubated at 35°C for 48 hours. After an additional subculture for 24 hours, bacteria were suspended in 0.85% NaCl and diluted to a final concentration of ~1.25 × 103 colony-forming units (cfu)/mL. A target inoculating dose of 100 cfu was selected based on a previous time-course study.17 The concentrations of the inocula were confirmed by plating bacteria in duplicate on CHAB and counting colony forming units after 48–72 hours of incubation at 35°C.
Six female Swiss Webster mice were inoculated subcutaneously with 100–150 cfu suspended in 100 μL of 0.85% saline solution. For larval infestation, the mice were anesthetized with a ketamine HCl (50–75 mg/kg) and medetomidine (1 mg/kg) mixture diluted in phosphate buffered saline at 0.01 μL/g body weight and infested with approximately 300 larval D. variabilis (~17 weeks old). To hasten the recovery from anesthesia, the effects of the medetomidine were reversed with a subcutaneous administration of atipamezole (5 mg/kg; 0.01 μL/g body weight).18 For the A1b and A2 trials, the inoculation and infestation occurred simultaneously, which corresponded with 3.5 days before peak bacteremia and the period of rapid engorgement.17 Because type B commonly takes longer to reach peak bacteremia,17 mice were inoculated 4.5 days before rapid engorgement and then, were infested with larvae 16 hours later.
Infested mice were housed individually in microisolator cages (21°C and 50% relative humidity [RH]). To facilitate collection of replete larvae, mice were suspended over a small amount of water on a wire-mesh cage bottom until 3.5–4.5 days post-infestation (dpi). At this time, bacteremia was expected to exceed 109 cfu/mL for A1b and A2 and 1010 cfu/mL for type B; mice were euthanized, and whole blood was collected by cardiac puncture. The blood was 10-fold serially diluted to 10−8 in 0.85% NaCl solution, and 100 μL of 10−7 and 10−8 dilutions were plated in duplicate on CHAB and placed in a 35°C incubator for 48–72 hours.
The replete larvae were placed in 8-mL sample vials (Wheaton, Millville, NJ) with a piece of mesh covering the opening and were stored in a dessicator jar maintained for 17–21 days post–drop-off at 24°C and 95% RH with a photoperiod of 16:8 (light:dark) hours in a bioclimatic chamber (Revco Inc., Deerfield, MI). After the larvae molted to nymphs between days 17 and 21, the nymphs were moved to a dessicator jar in a 22°C bioclimatic chamber with 95% RH and a photoperiod of 16:8 (light:dark) hours for approximately 44 days.
To confirm that larvae and nymphs acquired and maintained infections from feeding on bacteremic mice, individual tick bacterial loads were determined for specific time points (24 hours post-larval drop-off, just after molt at 21 dpi, just before the nymphal feed at 65 dpi, and just after the nymphal blood meal). Larvae and nymphs were harvested and stored individually in Eppendorf tubes at −70°C for > 1 hour before they were triturated with a pestle (Scienceware, Pequannock, NJ) in 100 μL (500 μL for the replete nymphs) of bovine heart infusion broth (BHI; Becton, Dickinson, and Company, Sparks, MD) and 10% glycerol (Fisher Scientific, Pittsburgh, PA). Tick extracts were 10-fold serially diluted to 10−6 in 0.85% NaCl, and 100 μL were plated on CHAB supplemented with 80,000 units polymyxin B, 2.5 mg amphotericin B, 4 mg cefepime, 100 mg cyclohexamide, and 4 mg vancomycin per liter (CHAB + PACCV);19 this was incubated at 35°C for 72–96 hours. At 1, 21, and 66 dpi, five dilutions (undiluted and 10−1 to 10−4) were tested. The 10−5 and 10−6 dilutions were tested for replete nymphs. Bacterial loads were estimated based on cfu counts. The detection limit was 40 cfu/mL.
Evaluation of the ability of nymphal D. variabilis to transmit F. tularensis.
Approximately 65 dpi, infected nymphal ticks were placed onto susceptible Swiss Webster mice (25 males and 25 females, age: ~6 weeks). Mice were anesthetized following the methods described above, and a small area on the dorsal surface between the shoulders was shaved. A feeding capsule (18 mm in diameter; Nalgene, Rochester, NY) was glued to the skin using a mixture of three parts colophony (Kramer Pigments Inc., New York, NY) and one part beeswax.20 One nymph was immediately placed into each capsule, and a small piece of mesh was glued to the top of the capsule. Similar to the conditions described for the larval feed, mice were housed individually in microisolator cages on wire floor bottoms suspended over water to collect replete nymphs as they drop off of the host.
Forty-eight to seventy-two hours after the nymph was placed into the capsule on the mouse, the piece of mesh was removed, and tick attachment was confirmed; 4–11 days post-nymphal infestation, replete nymphs were collected from the water. Nymphs that were not actively feeding by 11 days post-infestation were removed with jeweler's forceps. After collection, all nymphs were stored individually in Eppendorf tubes at −70°C. After nymphs were collected, mice were housed individually on standard bedding and were observed for 14–19 days post–drop-off or tick removal for signs of infection (e.g., hunched posture, ruffled fur, rapid respiration, or lethargy). If these signs were observed, mice were euthanized; carcasses were frozen at –70°C and later necropsied. All animal methods were approved by Division of Vector-Borne Infectious Diseases Institutional Animal Care and Use Committee (protocol 08-003) and were conducted in animal biosafety level 3 (ABL3) facilities.
Mice surviving 14–19 days post–drop-off or tick removal were euthanized, and 0.60–0.75 mL of blood were collected by cardiac puncture. A portion of the blood was used for detection of live bacteria, and the other portion was used for microagglutination testing. Approximately 100 μL of blood were plated on CHAB and incubated at 35°C for 48–72 hours. The remaining blood was collected into Microtainer serum-separator tubes (Becton Dickinson, Franklin Lakes, NJ). The serum was separated, heat-inactivated for 30 minutes at 56°C, and tested for F. tularensis-specific antibodies using a standard microagglutination assay.21 Briefly, serial dilutions of serum were incubated overnight with safranin-stained, formalin-killed F. tularensis subsp. tularensis (SchuS4) cells at room temperature, and a titer was assigned reflecting the last well-demonstrating full agglutination. Samples with a titer of 1:128 or greater were considered positive.21
Mice showing signs of tularemia were euthanized, and spleen and liver tissues were surgically removed. Touch preps of spleen and liver tissues were prepared and heat fixed. The slides were incubated with fluorescein isothiocyanate (FITC)-labeled rabbit anti-F. tularensis subsp. tularensis (SchuS4 strain) antibodies (CDC, Fort Collins, Colorado) for 30 minutes at room temperature. Slides were washed two times in phosphate-buffered saline followed by a final rinse with dH2O, and they were viewed with a fluorescent microscope using the 63× objective and a 490-nm filter. Samples scored as positive by direct immunofluorescence assay were confirmed by isolation of F. tularensis from liver and spleen on CHAB plates incubated at 35°C for 48 hours.21
Four different transmission rates were calculated: overall transmission rate (number of transmission events observed per number of ticks attached), tick-borne transmission rate (number of transmission events observed in mice that had nymphs that fed per number of nymphs that fed), consumption transmission rate (number of transmission events observed in mice that consumed nymphs per number of nymphs consumed), and incomplete feeding transmission rate (number of transmission events observed in mice with nymphs attached that did not feed and were not consumed per number of nymphs attached 48 hours post-infestation that did not feed and were not consumed).
Evaluation of F. tularensis-induced mortality and feeding success in immature D. variabilis.
Survivorship and nymphal feeding rates were compared between groups of uninfected and infected immature D. variabilis. As negative controls, four uninfected Swiss Webster mice were infested with ~17-week-old larvae; replete larvae were collected and maintained as described above. These procedures were replicated with five infected mice for A1b and six infected mice for A2 and type B. For each group (i.e., uninfected, A1b-, A2-, and type B-infected), at approximately 44 days post-molt, nymphs were placed in a capsule (N = 50 nymphs derived from four to six larval pools for each group), as previously described, on 10 6-week-old Swiss Webster mice (5 nymphs per mouse) for uninfected and 50 6-week-old Swiss Webster mice (1 nymph per mouse) for A1b-, A2-, and type B-infected.
Tick survival rates were determined per individual mouse from the larval feed by dividing the number of surviving nymphs 65 dpi by the total number of nymphs analyzed. A mean survivorship for each treatment (uninfected, A1b-, A2-, and type B-infected nymphs) was then determined by averaging the survivorship values for 4–6 replicates per treatment. Daily mortality rates were calculated for uninfected and infected nymphs. The percentage of nymphs expected to die each day was determined by dividing the number of nymphs that died between the larval molt and the nymphal feed by the total number of nymphs in the population. This number was then divided by number of days between the molt and the feed (44 days) to determine the daily mortality rate.
Other fitness factors were compared between uninfected and infected nymphs (A1b, A2 and type B). These included nymphal attachment success (number of nymphs attached divided by the total number applied), nymphal feeding success (number of nymphs that fed to repletion divided by total number attached), length of nymphal feed (days from application to drop-off), feeding-induced mortality (proportion of nymphs that died during the 11-day blood meal), and weight and scutal index (measured for only A1b-infected and uninfected nymphs). For feeding-induced mortality, the daily mortality rate was used to calculate the number of nymphs expected to die during the 11-day blood meal. The scutal index used in this study is the ratio between the length of the alloscutum and the width of the scutum. As a tick feeds, the scutum measurement remains constant, whereas the alloscutum increases during feeding.22 The replete nymphs were viewed using a SteREO Discovery V.12 stereoscope (Carl Zeiss Microimaging, Thornwood, NY), and scutum and alloscutum measurements were calculated using AxioVision Rel. 4.7 software (Carl Zeiss Microimaging, Thornwood, NY).
Statistical analyses.
Rates of F. tularensis transmission were determined for nymphs infected with A1b, A2, and type B. The proportion of ticks transmitting F. tularensis to mice was compared between A1b, A2, and type B using contingency table analyses and Fisher's exact tests. Shapiro–Wilk tests were used to evaluate normality of the data for each of the fitness factors described above. For normally distributed variables (i.e., infection prevalence, survivorship, nymphal attachment, nymphal feeding success, length of nymphal feed, nymphal weight, and scutal index), analysis of variance (ANOVA) was used to determine if the variances were greater among treatments than within treatments. Tukey–Kramer post-hoc tests were used for pairwise comparisons of mean values. Within each treatment (uninfected, A1b, A2, and type B), 4–6 larval pool replicates were used, as described above. Median bacterial loads were not normally distributed, and thus, these were compared between A1b-, A2-, and type B-infected ticks and between 1 dpi, 21 dpi, 65 dpi, and replete nymphs using Kruskal–Wallis or Wilcoxon rank sum tests. All statistical comparisons were run using JMP statistical software (SAS Institute, Cary, NC), and comparisons were considered to be statistically significant when P ≤ 0.05. Transmission efficiency for individually infected nymphs was estimated using maximum likelihood estimates based on the number of infected nymphs that fed on an individual mouse or were consumed and whether transmission was observed for that recipient mouse using Microsoft Excel Add-In PooledInfRate version 3.0.23
Results
Larval infection.
To infect larval D. variabilis with F. tularensis, mice were infested with larvae either the day before (type B) or the day of infection (A1b and A2) with F. tularensis, and ticks were allowed to feed to repletion. In all cases, replete larvae dropped off the infected mice 3–5 days post-infestation. Groups of four to six mice were each infected with A1b, A2, or type B, and the larvae were pooled per each individual mouse. Analysis of bacterial burdens in the blood of infected mice indicated that replete larvae were obtained on the day before and the day of peak bacteremia (Table 1). All larvae efficiently acquired F. tularensis infections (Table 1), and no significant differences were observed in bacterial acquisition rates between A1b [mean = 0.640, standard deviation (SD) = 0.261, N = 5 pools of 5 larvae], A2 (mean = 0.800, SD = 0.179, N = 6 pools of 5 larvae), and type B (mean = 0.933, SD = 0.103, N = 6 pools of 5 larvae; ANOVA: F = 3.38, degrees of freedom (df) = 2 and 14, P = 0.0632) infections.
Table 1.
Acquisition, transstadial transmission, and maintenance of F. tularensis types A1b, A2, and type B in D. variabilis
| Infection groups* | Replicates | Average mouse peak bacteremia (log10 cfu/mL) | Larvae that fed to repletion 1 day before peak bacteremia | Larvae that fed to repletion the day of peak bacteremia | Total replete larvae | Acquisition rate | Transstadial transmission rate† | Maintenance infection rate |
|---|---|---|---|---|---|---|---|---|
| A1b | 5 | 9.56 (SD = 0.29) | 221 | 731 | 952 | 64.00% (SD = 26.10%) | 93.30% (SD = 11.50%) | 97.1% (SD = 6.40%) |
| A2 | 6 | 9.48 (SD = 0.39) | 742 | 109 | 851 | 80.00% (SD = 17.90%) | 96.70% (SD = 8.20%) | NA‡ |
| Type B | 6 | 10.59 (SD = 0.27) | 755 | 251 | 1,006 | 93.30% (SD = 10.30%) | 100.00% (SD = 0.00%) | 93.3% (SD = 16.30%) |
Sample size for acquisition rate, transstadial transmission rate, and maintenance infection rate for A1b was 25 ticks derived from five groups and for A2 and type B was 30 ticks derived from six groups.
The A1b transstadial transmission rate was obtained from an independent experiment.
Maintenance transmission rate could not be determined because of high mortality in the ticks at this time point.
Transstadial transmission.
To determine if F. tularensis was transmitted from the larval to the nymphal life stage, transstadial transmission rates (number of infected nymphs divided by total number nymphs tested) were determined just after the larval molt (21 dpi) (Table 1). A1b, A2, and type B infections were all found to be passed efficiently from infected D. variabilis larvae to nymphs, with no difference in transstadial transmission rates observed between A1b- (mean = 0.933, SD = 0.115, N = 5 pools of 6 larvae), A2- (mean = 0.967, SD = 0.082, N = 6 pools of 5 larvae), and type B- (mean = 1.00, SD = 0, N = 6 pools of 5 larvae) infected ticks.
To confirm that infections were maintained in nymphs at 44 days post-larval molt (the day of the nymphal blood meal for A1b and type B), the infection rate was measured in A1b- and type B-infected nymphs. Because of high mortality rates and the need to use 50 nymphs for transmission studies, insufficient numbers of A2-infected nymphs survived for these comparisons. No difference was seen in infection rates between A1b- (mean = 0.971, SD = 0.064, N = 5 pools of 7 nymphs) and type B-infected nymphs (mean = 0.933, SD = 0.163, N = 6 pools of 5 nymphs; ANOVA: F = 0.2376, df = 1 and 9, P = 0.6376), with the majority of all ticks maintaining F. tularensis infections throughout the nymphal stage.
Survivorship of uninfected and A1b-, A2-, and type B-infected D. variabilis.
The survival rate was measured 65 dpi (44 days post-larval molt) for A1b-, A2-, and type B- infected nymphs (the number of live nymphs divided by the total number of nymphs analyzed) (Figure 1) and compared with survival rates of uninfected nymphs to determine if fitness costs were associated with F. tularensis infection in ticks. Significant differences in survivorship were detected compared with uninfected, A1b-, A2-, and type B-infected nymphs (ANOVA: F = 11.14, df = 3, 21, P = 0.0002). Overall, survivorship was significantly higher for uninfected nymphs (mean = 0.585, SD = 0.127, N = 368 ticks derived from four larval pools) compared with A2- (mean = 0.116, SD = 0.072, N = 764 ticks derived from six larval pools) and type B-infected nymphs (mean = 0.298, SD = 0.190, N = 917 ticks derived from six larval pools). The highest mortality (88.0%) was observed among A2-infected nymphs. Of note, survivorship of A1b-infected nymphs (mean = 0.353, SD = 0.157, N = 912 ticks derived from five larval pools) did not differ significantly from uninfected groups. Survivorship of the A2-infected nymphs was significantly lower than the A1b- and type B-infected groups. The daily mortality rate for uninfected, A1b-, A2-, and type B-infected nymphs was 0.9%, 1.5%, 2.0%, and 1.6%, respectively.
Figure 1.
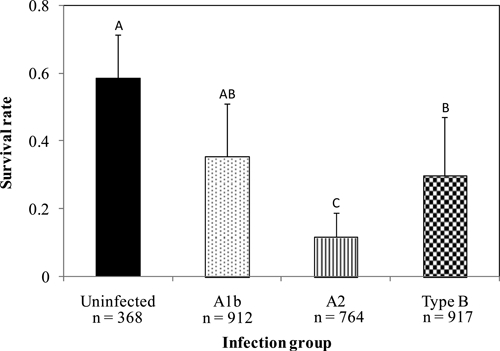
Mean survival-rate comparison on 65 dpi for uninfected, A1b-, A2-, and type B-infected D. variabilis. Error bars are the standard deviation in survivorship of group replicates (N = 4–6 groups). Sample size represents the number of ticks that were analyzed. Significant differences among F. tularensis determined by post-hoc pairwise Tukey–Kramer tests (P < 0.05) are indicated by differing letters above the columns.
Overall feeding success of uninfected, A1b-, A2-, and type B-infected nymphs.
Attachment rates, length of the nymphal feed, percentage of nymphs that fed to repletion, and feeding-induced mortality were compared between uninfected and infected ticks (Figure 2A and B). Attachment rates did not differ between uninfected (mean = 0.783, SD = 0.212, N = 4 pools of 50 nymphs) and A1b- (mean = 0.770, SD = 0.164, N = 5 pools of 50 nymphs), A2- (mean = 0.635, SD = 0.272, N = 6 pools of 50 nymphs), and type B-infected ticks (mean = 0.776, SD = 0.209, N = 6 pools of 50 nymphs; ANOVA: F = 0.580, df = 3, 20, P = 0.6361). By contrast, significantly prolonged feeding was observed for A1b-infected nymphs compared with uninfected or type B-infected nymphs (ANOVA: F = 73.95, df = 2, 12, P < 0.0001) (Figure 2A). The uninfected and type B-infected nymphs fed to repletion in approximately 5.38 days (SD = 0.334, N = 4 pools of 25 nymphs) and 5.63 days (SD = 0.277, N = 6 pools of 21 nymphs), respectively, compared with 9.83 days for A1b- (SD = 0.979, N = 4 pools of 9 nymphs) and 11 days for A2-infected nymphs (N = 1) (Figure 2A).
Figure 2.
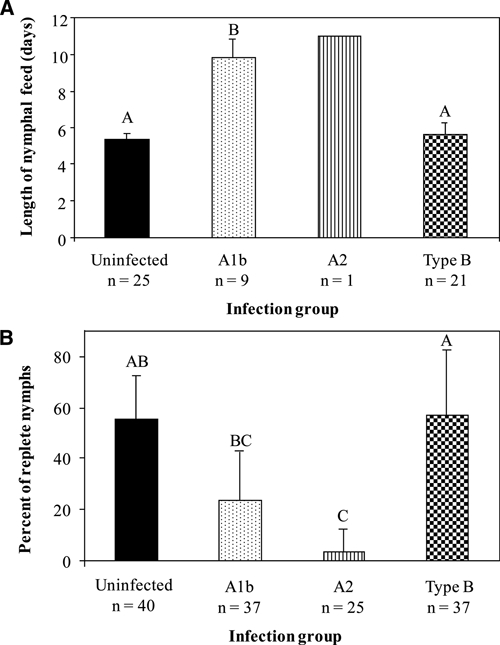
Overall feeding success was compared for uninfected, A1b-, A2-, and type B-infected nymphal D. variabilis by examining (A) the mean number of days to feed to repletion and (B) the mean percentages of nymphs that fed to repletion. Error bars are the standard deviation in the length of feed for the group replicates (N = 4–6 groups). Sample size represents the number of ticks that were analyzed. Significant differences among F. tularensis infection groups determined by post-hoc pairwise Tukey–Kramer tests (P < 0.05) are indicated by differing letters above the columns.
Significant differences were also observed in the percentage of nymphs feeding to repletion, with more uninfected nymphs feeding to repletion (mean = 55.23%, SD = 17.10%, N = 4 pools of 40 nymphs) than A2- (mean = 3.7%, SD = 9.1%, N = 6 pools of 25 nymphs) infected nymphs (Figure 2B). No significant differences were observed between the percentage of uninfected nymphs compared with both type B- (mean = 56.7%, SD = 25.82%, N = 6 pools of 37 nymphs) and A1b- (mean = 23.6%, SD = 19.19%, N = 5 pools of 37 nymphs) infected nymphs. Among infected nymphs, significant differences in the percentages feeding to repletion were observed between type B-infected compared with A1b- and A2-infected nymphs. Overall, the A2-infected nymphs exhibited the lowest percentage of nymphs to feed to repletion.
Within replete nymphs, a pronounced effect of A1b infection was observed, where even after prolonged feeding, the A1b-infected replete nymphs were very small compared with the uninfected replete nymphs (Figure 3). The average weight of the A1b-infected nymphs (mean = 6.0 mg, SD = 2.55 mg, N = 9) was significantly lower than that of the uninfected nymphs (mean = 11.0 mg, SD = 4.18 mg, N = 24; t = 4.22, P = 0.0002, pre-hoc one-tailed t test). Also, the scutal index was lower for the A1b-infected nymphs (mean = 4.79, SD = 1.06) compared with uninfected nymphs (mean = 6.99, SD = 0.806; t = 5.64, P < 0.0001, pre-hoc one-tailed t test). There was no visible difference in size of replete nymphs observed between the type B-infected and uninfected controls (data not shown).
Figure 3.

Physical effects of A1b infection on replete D. variabilis nymphs (Top) compared with uninfected D. variabilis replete nymphs (Bottom). This figure appears in color at www.ajtmh.org.
Additionally, both A1b- and A2-infected nymphs were subject to feeding-induced mortality. A large percentage of the A1b- and A2-infected nymphs died while attached to the host (A1b-infected nymphs: 75.0%; A2-infected nymphs: 94.4%) compared with type B-infected (19.4%) and uninfected (2.5%) nymphs. Therefore, the expected mortality rate was compared with the observed mortality rate for the length of the feed (11 days). Based on the calculated daily mortality rates, we expected to lose approximately 16.2% (N = 6 nymphs) of the A1b-infected population, 22.0% (N = 5 nymphs) of the A2-infected population, 16.2% (N = 5 nymphs) of the type B-infected population, and 10.0% (N = 4 nymphs) of the uninfected population over 11 days of feeding. The observed and expected mortality were statistically similar for type B-infected and uninfected nymphs; however, observed mortality was significantly higher than expected for the A1b- and A2-infected nymphs. For the A1b- and A2-infected nymphs, 75.0% (N = 36 nymphs; χ2 = 26.38, P < 0.0001, df = 1, Fisher's exact test) and 94.4% (N = 18 nymphs; χ2 = 25.82, P < 0.0001, df = 1, Fisher's exact test) died on the host, respectively. For the type B-infected nymphs, only 19.4% (N = 31; χ2 = 0.111, P = 0.739, df = 1, Fisher's exact test) died on the host. For the uninfected population, 2.5% (N = 1 nymph; χ2 = 2.05, P = 0.153, df = 1, Fisher's exact test) mortality was observed.
Comparison of transmission efficiency among A1b, A2, and type B.
For analysis of transmission efficiency, three possible modes of transmission were considered: incomplete feeding, tick-borne, and consumption of the tick. Incomplete feeding refers to incidents where a nymph attached to the mouse but failed to feed to repletion. Tick-borne refers to the classical concept of biological transmission whereby a nymph attaches to the mouse, feeds to repletion, and drops off of the host. In instances where the tick was not recovered because it could not be found in the water or on the mouse, it was assumed that the tick was consumed by the mouse (e.g., consumption form of transmission).
Overall, transmission of F. tularensis by nymphal D. variabilis was observed for type B and A2, whereas no transmission was observed for A1b (Table 2). Comparisons of individual rates of transmission indicated that 0% [N = 9; 95% confidence interval (CI) = 0.00–29.91] of A1b-, 0% (N = 1; 95% CI = 0.00–79.35) of A2-, and 14.3% (N = 21; 95% CI = 3.91–30.06) of type B-infected nymphs transmitted by classical biological transmission. Transmission by tick consumption occurred at a rate of 0% (N = 1, 95% CI = 0.00–79.35) for A1b-, 28.6% (N = 7, 95% CI = 5.57–65.85) for A2-, and 33.3% (N = 5, 95% CI = 6.61–72.59) for type B-infected nymphs. Transmission by incomplete feeding was observed for 0% of A1b- (N = 27, 95% CI = 0.00–12.46), 0% of A2- (N = 17, 95% CI = 0.00–18.43), and 10.0% (N = 10, 95% CI = 0.51–40.43) of type B-infected nymphs. Although we did not observe transmission for A1b, maximum likelihood estimates indicated that transmission could be as high as 30%, 80%, and 65% for classical, consumption, and incomplete feeding, respectively. In all cases, the absence of bacterial growth and seroconversion against F. tularensis in mice surviving 14–19 days post-tick attachment confirmed the lack of transmission by ticks. Similarly, all mice showing signs of infection after being fed on by F. tularensis-infected ticks were F. tularensis antigen- and culture-positive.
Table 2.
Comparison of transmission rates among D. variabilis nymphs infected with F. tularensis A1b, A2, and type B
| Infection groups* | Estimated overall transmission rate (95% CI)† | Estimated tick-borne transmission rate (95% CI)‡ | Estimated consumption transmission rate (95% CI)§ | Estimated incomplete feeding transmission rate (95% CI)¶ |
|---|---|---|---|---|
| A1b | 0.00% (0.00−9.41) (N = 37) | 0.00% (0.00−29.91) (N = 9) | 0.00% (0.00−79.35) (N = 1) | 0.00% (0.00−12.46) (N = 27) |
| A2 | 8.00% (1.46−23.66) (N = 25) | 0.00% (0.00−79.35) (N = 1) | 28.60% (5.57−65.85) (N = 7) | 0.00% (0.00−18.43) (N = 17) |
| Type B | 13.51% (5.21−27.28) (N = 37) | 14.29% (3.91−30.06) (N = 21) | 33.33% (6.61−72.59) (N = 6) | 10.00% (0.51−40.42) (N = 10) |
One nymph was applied per mouse (N = 50 nymphs per F. tularensis infection group).
Overall transmission rate is the number of transmission events per total number of mice exposed with nymphs attached.
Tick-borne transmission rate is the number of transmission events by bite of an infected tick per total number of infected replete nymphs.
Consumption transmission rate is the number of transmission events by consumption of nymph per total number of consumed nymphs. Infection status in ticks could not be determined.
Incomplete feeding transmission rate is the number of transmission events from infected nymphs that attached but did not feed to repletion per total number of infected nymphs that attached but failed to feed to repletion or be consumed.
Bacterial kinetics of infection—bacterial acquisition through nymphal blood meal.
To determine if bacterial loads changed in infected ticks over the entire time course of the experiment, colony-forming units were determined at various time points: replete larvae 1 day after drop-off (1 dpi), just after larval molt (21 dpi), just before nymphal feed (65 dpi), and after nymphal feed and drop-off (Figure 4). A similar trend was observed in A1b-, A2-, and type B-infected ticks; replication of the bacteria occurred between initial acquisition of infection at the larval stage and the completion of the nymphal blood meal. Measuring from 1 dpi to when the nymphs were replete, we observed a 5.39-log increase for A1b- (χ2 = 15.37, df = 1, P < 0.0001, Mann–Whitney U test with χ2 approximation), a 2.35-log increase for type B- (χ2 = 32.83, df = 1, P < 0.0001, Mann–Whitney U test with χ2 approximation), and a 2.76-log increase for A2-infected nymphs (no statistical analyses performed because only one infected nymph fed to repletion). Replication of F. tularensis occurred throughout most stages (Figure 4), with the most significant changes in bacterial numbers occurring during the blood meal. For A1b-infected nymphs, a 2.66-log increase was seen between 65 dpi and replete nymphs (χ2 = 18.61, df = 1, P < 0.0001, Mann–Whitney U test with χ2 approximation), and for type B-infected nymphs, a 1.67-log (χ2 = 26.09, df = 1, P < 0.0001, Mann–Whitney U test with χ2 approximation) increase was observed for the same time period.
Figure 4.
Bacterial loads within A1b-, A2-, and type B-infected ticks 1 day after larval feeding (1 dpi), shortly after the larval molt (21 dpi), 1 day before the nymphal feed (65 dpi), and the day of nymphal drop-off. Each data point represents the bacterial load of one tick. The median of each group of measurements is represented by the black line.
Bacterial loads in infected ticks were also compared among F. tularensis infections at 65 dpi. A1b-infected nymphs had significantly higher bacterial loads (median = 6.38 log10 cfu/mL, range = 5.78–6.80, N = 31) compared with type B-infected nymphs (median = 5.73 log10 cfu/mL, range = 4.11–6.56, N = 23; χ2 = 28.10, df = 1, P < 0.0001, Mann–Whitney U test with χ2 approximation).
Discussion
Vector efficiency is typically evaluated based on an arthropod's ability to acquire infection, survive and maintain the infection until the next blood meal, and transmit the disease agent to a susceptible host. Results from this study revealed that D. variabilis larvae were able to efficiently acquire and maintain the infection as well as transstadially transmit A1b, A2, and type B infections. Additionally, all three F. tularensis strains replicated within infected D. variabilis. Despite these findings, fitness costs to ticks were clearly associated with all F. tularensis infections. Transmission was found to vary with respect to A1b, A2, and type B infection; these differences correlated directly with the fitness costs associated with A1b, A2, or type B infections. The observed low survivorship coupled with low transmission efficiency suggest that D. variabilis nymphs may be relatively inefficient vectors of A1b, A2, and type B and thus, only play a limited role in enzootic transmission of F. tularensis. Furthermore, the reluctance of the nymphs to bite humans12 implies that nymphal D. variabilis poses little risk of transmitting F. tularensis to humans.
A significant effect on tick survivorship was observed for A1b, A2, and type B infections, although at differing points during the time course of the experiments. At 65 dpi, a significant decrease in survivorship was observed for A2- and type B-infected nymphs but not for A1b-infected nymphs. This difference was not readily attributed to variations in bacterial burdens, because A1b-infected nymphs displayed significantly higher bacterial loads compared with type B-infected nymphs 65 dpi. Negative effects were also readily observed on tick feeding, including prolonged feeding, feeding-induced mortality, and smaller size of replete nymphs, but in these cases, negative effects were observed for A1b infections compared with type B infections. A2-infected nymphs also suffered negative effects during feeding, but because the vast majority of A2-infected nymphs (88.0%) had died before tick feeding, these effects could not be directly connected to feeding. In both A1b- and type B-infected nymphs, replication of bacteria was shown during the nymphal feed, although in the case of A1b-infected ticks, the increase in numbers of bacteria was 1.5-fold higher than for type B-infected ticks.
Impaired feeding could be a result of amplification of the bacteria in the midgut epithelial cells during the blood meal. Other studies have observed that the feeding success of ticks is impaired by infection with F. tularensis.16,24 When a tick feeds, it undergoes three phases: preparatory phase (i.e., attachment), slow feeding (growth phase), and rapid feeding (repletion).25 It is during the slow-feeding phase that ticks start ingesting blood, and intracellular digestion commences. As blood enters the midgut lumen, epithelial cells begin to proliferate and differentiate into secretory or digestive cells. Because F. tularensis has previously been shown to invade and replicate in alveolar epithelial cells, proliferation of midgut epithelial cells could trigger amplification of F. tularensis,26 with bacterial amplification potentially linked to epithelial cell death or impairment of epithelial cell differentiation.
The level of transmission observed for A1b-, A2-, and type B-infected nymphs was found to vary. Type B-infected nymphs were capable of transmitting F. tularensis (observed transmission efficiency = 14.29%) through the bite of an infected tick, whereas transmission of A1b and A2 by tick bite was not observed, although at 65 dpi, A1b-infected nymphs contained higher levels of bacteria than type B-infected nymphs. All nymphs that fed to repletion were confirmed to be infected with F. tularensis. The lack of observed transmission of both A1b and A2 by tick bite under the conditions of the current study is not necessarily indicative of D. variabilis nymphs being incapable of transmitting A1b and A2 by tick bite. Maximum likelihood estimates indicated that the tick-borne transmission efficiency by A1b-infected nymphs could be as high as 30%. Thus, with sufficiently high infestation rates, transmission may be observed. The transmission rates reported here are subject to the limitations of the described experiments. Whether transmission efficiency might be higher at shorter time periods after larval molt (< 44 days) or with lower numbers of bacteria to initiate infections are questions to be addressed in future studies.
In these experiments, we also observed effective transmission through consumption (i.e., when mice groomed off the capsule containing the attached tick) of A2- (observed transmission efficiency = 28.6%) and type B- (observed transmission efficiency = 33.33%) infected nymphs. If the capsule was groomed from the mouse and a nymph was not recovered in the water pan traps, we assumed that the nymph was consumed by the mouse. However, not all ticks consumed by mice transmitted F. tularensis. This could be caused by either the tick not actually being consumed or the high observed mortality among feeding ticks. Ticks that died on the host were not found to contain viable bacteria. Comparisons of transmission efficiency by consumption between type B-, A2-, and A1b-infected nymphs were not made, because observation of this mode of transmission was strictly fortuitous.
In addition to the two modes of transmission analyzed in this study, other unconventional modes of transmission could also occur for F. tularensis, such as exposure to infected feces or interrupted feeding. Studies have shown the presence of F. tularensis in excreta of nymphal and adult ticks.24 During the blood meal, defecation occurs, and feces accumulate around the feeding tick.25 Through grooming and scratching, F. tularensis could potentially be transmitted through infected feces. Transmission through interrupted feeding has also been observed with D. marginatus and Rhipicephalus rossicus infected with F. tularensis.24 Partially replete ticks will drop off from a dead host, continue to host seek, feed on a new host,25 and in the process, potentially transmit the pathogen.
Transmission efficiency estimates based on single tick per mouse experiments have been generated for another tick-borne bacterial pathogen, Borrelia burgdorferi. In this case, a transmission efficiency of 93.8% was observed with the tick Ixodes scapularis.27 In contrast to F. tularensis infections, fitness costs are not associated with infection of ticks by B. burgdorferi. Thus, in the case of B. burgdorferi, relatively high infections can be established in naturally infected host-seeking I. scapularis nymphal populations (29.6%), contributing to the overall efficiency of this vector.28 The high mortality observed among A2- and type B-infected nymphs and low feeding success of A1b- and A2-infected nymphs is consistent with the low infection prevalence rates reported in nature for D. variabilis ticks infected with F. tularensis (1–5%).9 Fitness costs coupled with low transmission efficiency suggest that transmission of F. tularensis by bite of D. variabilis nymphs is relatively inefficient. Nonetheless, we showed that F. tularensis could also be transmitted by consumption. The possibility exists that vector efficiency for F. tularensis in nature is dependent on several different modes of transmission (i.e., tick bite, consumption, interrupted feeding, and fecal–oral route).
We initially hypothesized, based on the geographic concordance of D. variabilis with A1b and type B, that we would see high transmission rates and minimal fitness costs with A1b- and type B-infected nymphs but minimal transmission and high fitness costs with A2-infected nymphs. Indeed, fitness costs in non-feeding nymphs seemed highest for A2-infected nymphs and lowest for A1b-infected nymphs; this is consistent with A1b being better adapted for survival in D. variabilis. With respect to transmission by the bite of D. variabilis nymphs, we observed that type B was transmitted more efficiently than A2 or A1b, and significant feeding-induced mortality was observed in A1b-infected ticks, suggesting that pathogenicity of the infecting strain may be directly linked with transmission efficiency. The role that other tick species, Haemaphysalis leporis-palustris and Ambylomma americanum, may play in enzootic maintenance of F. tularensis is an important area for future research. Additionally, because there have been human cases of tick-borne tularemia in the United States associated with D. variabilis, it is also imperative to explore transmission efficiency and cost of fitness of F. tularensis infections at the adult stage.
Acknowledgments
The authors thank John Young for his technical assistance, Jennifer Holmes for her assistance with bacterial-load determination, Claudia Molins for her scientific discussions, and Martin Schriefer for his scientific and logistical support. We also like to thank the Division of Vector-Borne Infectious Diseases Animal Care personnel for their assistance in these studies, especially Andrea Peterson. Sara Reese was funded by the Association of Public Health Laboratories and Centers for Disease Control and Prevention Emerging Infectious Diseases postdoctoral fellowship program.
Footnotes
Authors' addresses: Rebecca J. Eisen, Sara M. Reese, Gabrielle Dietrich, Marc C. Dolan, Sarah W. Sheldon, Joseph Piesman, and Jeannine M. Petersen, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Fort Collins, CO, E-mails: dyn2@cdc.gov, Sreese@smtpgate.dphe.state.co.us, eid7@cdc.gov, mcd4@cdc.gov, sara.sheldon@cdc.gov, jfp2@cdc.gov, and nzp0@cdc.gov.
References
- 1.Hopla CE. The ecology of tularemia. Adv Vet Sci Comp Med. 1974;18:25–53. [PubMed] [Google Scholar]
- 2.Morner T. The ecology of tularaemia. Rev Sci Tech. 1992;11:1123–1130. [PubMed] [Google Scholar]
- 3.Petersen JM, Mead PS, Schriefer ME. Francisella tularensis: an arthropod-borne pathogen. Vet Res. 2009;40:7. doi: 10.1051/vetres:2008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saliba GS, Harmston FC, Diamond BE, Zymet CL, Goldenbe M, Chin TDY. An outbreak of human tularemia associated with American dog tick, Dermacentor variabilis. Am J Trop Med Hyg. 1966;15:531–538. doi: 10.4269/ajtmh.1966.15.531. [DOI] [PubMed] [Google Scholar]
- 5.de la Cruz P, Cummings L, Harmon D, Mosier D, Johannes P, Lawler J, Pintz F, Senger K, Dosch T. Outbreak of tick-borne tularemia—South Dakota. MMWR Morb Mortal Wkly Rep. 1984;33:601–602. [PubMed] [Google Scholar]
- 6.Eisen L. A call for renewed research on tick-borne Francisella tularensis in the Arkansas–Missouri primary national focus of tularemia in humans. J Med Entomol. 2007;44:389–397. doi: 10.1603/0022-2585(2007)44[389:acfrro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Goethert HK, Shani I, Telford SR. Genotypic diversity of Francisella tularensis infecting Dermacentor variabilis ticks on Martha's Vineyard, Massachusetts. J Clin Microbiol. 2004;42:4968–4973. doi: 10.1128/JCM.42.11.4968-4973.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green RG. The occurrence of Bacterium tularense in the eastern wood tick, Dermacentor variabilis. Am J Trop Med Hyg. 1931;14:600–613. [Google Scholar]
- 9.Goethert HK, Telford SR. Nonrandom distribution of vector ticks (Dermacentor variabilis) infected by Francisella tularensis. PLoS Pathog. 2009;5:e1000319. doi: 10.1371/journal.ppat.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staples JE, Kubota KA, Chalcraft LG, Mead PS, Petersen JM. Epidemiologic and molecular analysis of human tularemia, United States, 1964–2004. Emerg Infect Dis. 2006;12:1113–1118. doi: 10.3201/eid1207.051504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kugeler KJ, Mead PS, Janusz AM, Staples JE, Kubota KA, Chalcraft LG, Petersen JM. Molecular epidemiology of Francisella tularensis in the United States. Clin Infect Dis. 2009;48:863–870. doi: 10.1086/597261. [DOI] [PubMed] [Google Scholar]
- 12.Smith CN, Cole MM, Gouck HK. Biology and Control of the American Dog Tick. Washington, DC: United States Department of Agriculture; 1946. pp. 1–74. [Google Scholar]
- 13.Bishop FC, Trembley HL. Distribution and hosts of certain North American ticks. J Parasitol. 1945;31:1–54. [Google Scholar]
- 14.Farlow J, Wagner DM, Dukerich M, Stanley M, Chu MC, Kubota KA, Petersen JM, Keim P. Francisella tularensis in the United States. Emerg Infect Dis. 2005;11:1835–1841. doi: 10.3201/eid1112.050728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell JF. The infection of ticks (Dermacentor variabilis) with Pasteurella tularensis. J Infect Dis. 1945;72:83–95. [Google Scholar]
- 16.Philip CB, Jellison WL. The American dog tick, Dermacentor variabilis, as a host of Bacterium tularense. Public Health Rep. 1934;49:386–392. [Google Scholar]
- 17.Eisen RJ, Yockey B, Young J, Reese SM, Piesman J, Schriefer ME, Ben Beard C, Petersen JM. Short report: time course of hematogenous dissemination of Francisella tularensis A1, A2, and type B in laboratory mice. Am J Trop Med Hyg. 2009;80:259–262. [PubMed] [Google Scholar]
- 18.Hahn N, Eisen RJ, Eisen L, Lane RS. Ketamine-medetomidine anesthesia with atipamezole reversal: practical anesthesia for rodents under field conditions. Lab Anim (NY) 2005;34:48–51. doi: 10.1038/laban0205-48. [DOI] [PubMed] [Google Scholar]
- 19.Petersen JM, Carlson J, Yockey B, Pillai S, Kuske C, Garbalena G, Pottumarthy S, Chalcraft LG. Direct isolation of Francisella spp. from environmental samples. Lett Appl Microbiol. 2009;48:663–667. doi: 10.1111/j.1472-765X.2009.02589.x. [DOI] [PubMed] [Google Scholar]
- 20.Soares CAG, Zeidner NS, Beard CB, Dolan MC, Dietrich G, Piesman J. Kinetics of Borrelia burgdorferi infection in larvae of refractory and competent tick vectors. J Med Entomol. 2006;43:61–67. doi: 10.1093/jmedent/43.1.61. [DOI] [PubMed] [Google Scholar]
- 21.Petersen JM, Schriefer ME, Carter LG, Zhou Y, Sealy T, Bawiec D, Yockey B, Urich S, Zeidner NS, Avashia S, Kool JL, Buck J, Lindley C, Celeda L, Monteneiri JA, Gage KL, Chu MC. Laboratory analysis of tularemia in wild-trapped, commercially traded prairie dogs, Texas, 2002. Emerg Infect Dis. 2004;10:419–425. doi: 10.3201/eid1003.030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meiners T, Hammer B, Gobel UB, Kahl O. Determining the tick scutal index allows assessment of tick feeding duration and estimation of infection risk with Borrelia burgdorferi sensu lato in a person bitten by an Ixodes ricinus nymph. Int J Med Microbiol. 2006;296:103–107. doi: 10.1016/j.ijmm.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 23.Biggerstaff BJ. PooledInfRate, Version 3.0: A Microsoft Excel Add-In to Compute Prevalence Estimates from Pooled Samples. Fort Collins, CO: Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 24.Petrov VG. Experimental study of Dermacentor marginatus Sulz and Rhipicephalus rossicus Jak. Et K. Jak. ticks as vectors of tularemia. J Parasitol. 1960;46:877–884. [PubMed] [Google Scholar]
- 25.Sonenshine DE. Biology of Ticks. volume 2. New York: Oxford University Press; 1991. [Google Scholar]
- 26.Hall JD, Craven RR, Fuller JR, Pickles RJ, Kawula TH. Francisella tularensis replicates within alveolar type II epithelial cells in vitro and in vivo following inhalation. Infect Immun. 2007;75:1034–1039. doi: 10.1128/IAI.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hojgaard A, Eisen RJ, Piesman J. Transmission dynamics of Borrelia burgdorferi s.s. during the key third day of feeding by nymphal Ixodes scapularis (Acari: Ixodidae) J Med Entomol. 2008;45:732–736. doi: 10.1603/0022-2585(2008)45[732:TDOBBS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Maupin GO, Fish D, Zultowsky J, Campos EG, Piesman J. Landscape ecology of Lyme disease in a residential area of Westchester County, New York. Am J Epidemiol. 1991;133:1105–1113. doi: 10.1093/oxfordjournals.aje.a115823. [DOI] [PubMed] [Google Scholar]


