Abstract
Dengue is an acute emerging infectious disease transmitted by Aedes mosquitoes and has become a serious global public health problem. In mainland China, a number of large dengue outbreaks with serious consequences have been reported as early as 1978. In the three decades from 1978 to 2008, a total of 655,324 cases were reported, resulting in 610 deaths. Since the 1990s, dengue epidemics have spread gradually from Guangdong, Hainan, and Guangxi provinces in the southern coastal regions to the relatively northern and western regions including Fujian, Zhejiang, and Yunnan provinces. As the major transmission vectors of dengue viruses, the biological behavior and vectorial capacity of Aedes mosquitoes have undergone significant changes in the last two decades in mainland China, most likely the result of urbanization and global climate changes. In this review, we summarize the geographic and temporal distributions, the serotype and genotype distributions of dengue viruses in mainland China, and analyze the current status of surveillance and control of vectors for dengue transmission.
Introduction
Dengue fever (DF) is an acute infectious disease caused by dengue viruses and transmitted by Aedes mosquitoes. This disease is endemic or epidemic in more than 100 countries and regions in Asia, Oceania, America, and Africa, and it is especially prevalent in Southeast Asia, the west Pacific Ocean regions, and southern Africa. The rapid increase of DF incidence in recent years has become a serious public health threat to nearly half of the world's population.1 The World Health Organization (WHO) estimates that approximately 2.5 billion people worldwide are at risk and 50 million people are infected by dengue viruses each year. Half a million people suffer from dengue hemorrhagic fever (DHF), which results in more than 25,000 deaths (http://www.who.int/tdr/publications/publications/dengue). Dengue is the second-most serious vector-borne disease in the world, only behind malaria in terms of morbidity and mortality.
The first outbreak of DF in mainland China occurred in Guangdong province in 1978. Since then, dengue outbreaks were recorded sequentially in Hainan, Guangxi, Fujian, and Zhejiang provinces.2 These epidemics have had significant negative impact on the affected population, the society in general, and the development of the economy. The rapid urbanization in China changed the characteristics of DF epidemics. Dengue epidemics have spread from Guangdong, Hainan, and Guangxi provinces in the southern coastal regions to the relatively northern and western regions including Fujian, Zhejiang, and Yunnan provinces, with shorter epidemic intervals as compared with those experienced before the 1990s.
Prevalence
Geographic distribution.
Dengue fever in mainland China is still characterized as an imported epidemic disease, and so far has not been confirmed to be an endemic.3 Sporadic cases and outbreaks of DF in the southeast coast region, the middle and lower reaches of the Yangzi River were documented in the early 1940s,4,5 but since then, no cases were reported. In May, 1978, a sudden outbreak of DF occurred in Foshan, Guangdong province, and it was spread to seven adjacent counties and cities where a total of 22,122 cases, including 16 fatalities were reported.6 In the past 30 years (1978 to 2008), DF outbreaks in varying scales have occurred in China, and a total of 655,324 cases were documented, resulting in 610 deaths. Figure 1 presents the annual cases of DF in the mainland from 1978 to 2008. In Hainan province, the two most severe outbreaks of DF and DHF occurred in 1980 and 1986, resulting in > 600,000 cases with 475 deaths overall. The 1980 outbreak alone caused 454,205 cases.2 However, no additional DF outbreaks have been reported from Hainan province since 1991.7 In recent years, Guangdong province has the highest incidence of DF epidemics (Figure 2), with cases reported every year since 1997.8,9
Figure 1.
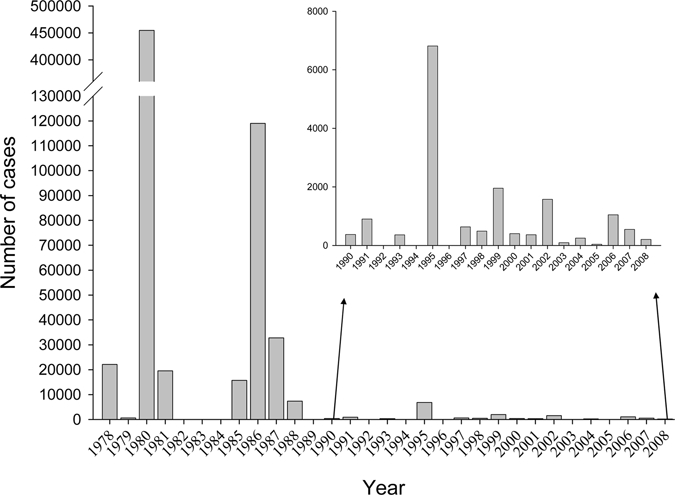
Annual number of dengue fever cases in mainland China from 1978 to 2008. Data for 1978–2002 are summarized in Refs. 7–10. Data for 2003–2008 are derived from reports of the State Ministry of Public Heath (http://www.moh.gov.cn/publicfiles/business/htmlfiles/zwgkzt/pyq/index.htm).
Figure 2.
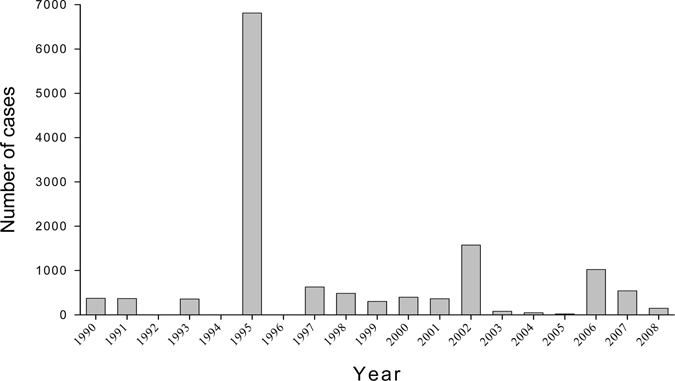
Annual number of dengue fever cases in Guangdong Province from 1990 to 2008. The 1990–2000 and 2001–2006 data are from Refs. 8 and 9. The 2007–2008 data are from reports of the State Ministry of Public Health (http://www.moh.gov.cn/publicfiles/business/htmlfiles/zwgkzt/pyq/index.htm).
Before the 1990s, large-scale epidemics of DF were characterized by a sudden outbreak, fast progression, quick transmission, and were under control within 2 or 3 years of onset (Figure 1). After the major outbreaks, the following DF epidemics would usually become weak either in the incidence or in transmission speed, but the affected area was more extensive. The DF epidemic now spreads gradually from Guangdong, Hainan, and Guangxi provinces in the Southern coastal regions to the relatively Northern regions, including Fujian, Zhejiang, and Yunnan provinces (Figure 3). In Fujian province in particular, a major outbreak with 1,549 cases was reported in 1999 in Fuzhou.14 In 2004, a DF outbreak with 83 cases reported in Zhejiang province.11 An outbreak with 56 cases was reported in 2008 in Yunnan province (State Ministry of Public Heath in October 2008, http://www.moh.gov.cn/publicfiles/business/htmlfiles/zwgkzt/pyq/index.htm), which was the first outbreak of the disease in this region since 1949.
Figure 3.
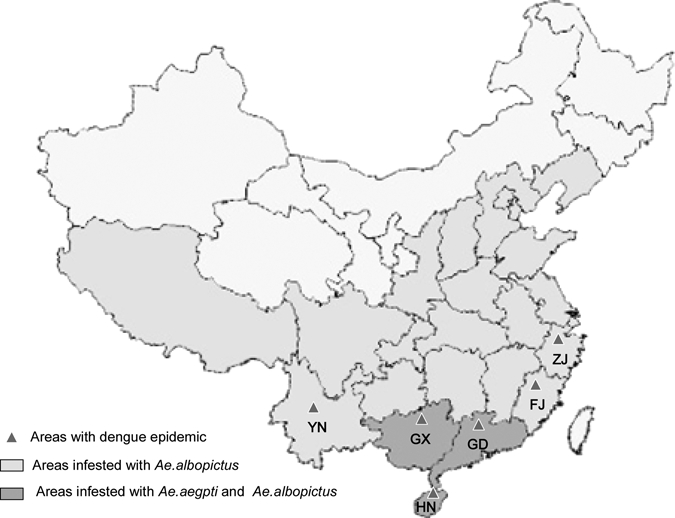
Approximate distribution of dengue and Aedes mosquitoes in mainland China. ZJ: Zhejiang Province; FJ: Fujian Province; GD: Guangdong Province; GX: Guangxi Province; HN: Hainan Province, YN: Yunnan Province. Data are summarized in Refs. 7, 8, 10–13.
Currently, no evidence is available to support the presence of epidemic foci in China, and most of the researchers attributed the prevalence of DF to the imported cases.15–17 However, with the rapid growth of the Chinese economy, international exchanges are more frequent than ever. In the meantime, more than 10% of China's population has moved away from their original residences, mainly from poor rural areas to urban centers, searching for better working and living opportunities. This migration promotes the transmission of infectious diseases and creates major challenges to prevent and control them.18 It is possible that DF is transforming from an imported to an endemic infectious disease, especially in Guangdong province where the climate favors the survival and transmission of Dengue virus. Although DF in mainland China has not resulted in large epidemics since 1990 (> 10,000 cases), frequent small outbreaks of DF (1,000–7,000 cases) ensure long-term viral circulation in local regions, which has the potential of making DF endemic if no effective intervention is implemented.
Temporal distribution.
In mainland China, DF is prevalent mostly in the tropical and subtropical regions (south of 29° north latitude), where the mosquito vectors of dengue viruses breed throughout the year. Figure 4 shows the total number of DF cases reported monthly from 2002 to 2008 (http://www.moh.gov.cn/publicfiles//business/htmlfiles/zwgkzt/pyq/index.htm). January to May is identified as the period of sporadic occurrence, while June to December is recognized as the prevalent period of DF. In general, July is the early stage of the epidemic, which then increases from August to October with 14,487 cases, accounting for 92.34% of the total cases reported within these years. It is documented that the prevalence of virus is associated highly with the breeding activity of the Aedes mosquitoes. Aedes albopictus is the predominant species in South China. It can breed in various small containers or plants that hold accumulated water (such as tree holes, bamboo stems, or leaf axils) that are found in gardens or backyards. The large amount of rainfall from July to September increases the breeding places of the mosquitoes. The emergence of large numbers of larvae causes drastic expansion of the mosquito populations and greatly increases the probability of DF epidemic.19 Yi and others20 analyzed DF cases in Guangdong province from 1990 to 2001 and Aedes surveillance and climate from 1995 to 2001. They showed a clear seasonal pattern of DF occurrence, with a high frequency occurring mostly in hot and humid seasons. Geographic distribution of dengue epidemic has spread gradually from southern (Guangdong, Guangxi, Hainan) to relatively northern and western regions including Fujian, Zhejiang, and Yunnan provinces (Figure 3), which may be associated with the intensifying global warming.19,21,22 However, the impact of global warming on the spread of vector-borne diseases in tropical and sub-tropical regions in Asia is a subject of debate.23 Countries or regions experiencing increasing DF should establish as part of their disease-control efforts surveillance that allows an evaluation of the variation in DF incidence and prevalence as the climate in the regions change.24
Figure 4.
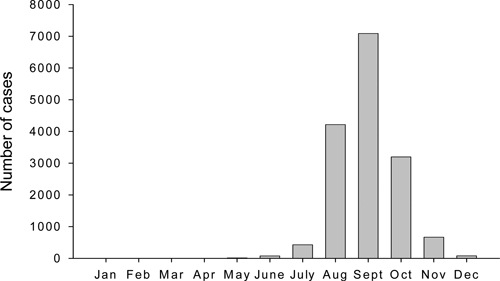
Total number of dengue fever cases reported monthly from 2002 to 2008. Data are summarized from (http://www.moh.gov.cn/publicfiles/business/htmlfiles/zwgkzt/pyq/index.htm).
Serotype and genotype distribution of dengue viruses.
Four serotypes (DV1, DV2, DV3, and DV4) of dengue viruses have been identified and they all are capable of causing DHF and dengue shock syndrome.12,25 Although the association between the serotype and the severity of the disease is not clear, the DV2 serotype, particularly in the event of superinfection following infection of another serotype, has been shown to correlate with dengue shock syndrome.25 All four serotypes have caused epidemics in the mainland. Figure 5 shows the geographic distribution of dengue epidemics reflecting a gradual spread from southern to northern regions. Interestingly, no DF epidemics outbreaks have been reported in Guangxi and Hainan province since 1986 and 1991, respectively. The outbreaks in Guangdong province, where the latitude and weather conditions are similar to Guangxi and Hainan, have been reported more frequently since 1990. In the mainland, DV3 was the principal serotype of the viruses reported early (1978), and then DV1 and DV4 were recorded. However, DV1 has become the main serotype since the 1990s. The outbreak of DV4 DF was first reported in Foshan, Guangdong province in 197812 and it reappeared here in 1990.8 The epidemic of DV3 DF was first reported in north Shanbei County of Hainan province in October, 1979; the epidemic then spread north along the west coast to compromise Zhanjiang, Foshan, Guangzhou, Shantou, and Shaoguan of Guangdong province, and then to Beihai and Hepu of Guangxi province, lasting for 3 years.12 The epidemic caused by DV2 was reported in north Shanbei County, Hainan province in September, 1985, and by the following year it had compromised the entire Island (e.g., Hainan province) and spread further to Guangzhou of Guangdong province and Beihai of Guangxi province, and persisted until 1988.7 The DV2 caused DF epidemics in Guangdong province in 1993, 1998, 2001, and in the Fujian province in 1999.10 In the 1993 epidemic, several cases were found to consist of both DV2 and DV4, which represented the rare cases of concurrent infections in mainland China.6 The DV1 was responsible for the outbreaks of DF in Guangdong province in 1979 and 1985, respectively,12 and then several outbreaks caused by the same virus were reported in 1991, and from 1995 to 2006.8,9
Figure 5.
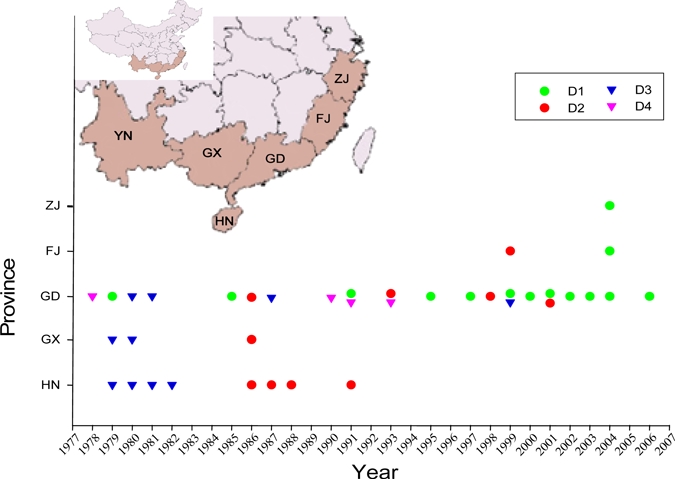
The epidemics of four serotypes of dengue virus in mainland China. ZJ: Zhejiang Province; FJ: Fujian Province; GD: Guangdong Province; GX: Guangxi Province; HN: Hainan Province, YN: Yunnan Province. Data are summarized in Refs. 7, 8, 10–13. There were 56 cases of DF in Yunnan Province (2008) according to the report from the Department of Health [http://www.moh.gov.cn/publicfiles/business/htmlfiles/zwgkzt/pyq/index.htm], but no publication regarding the serotype of this virus caused this outbreak was found. This figure appears in color at www.ajtmh.org.
According to the full-length sequence of the E/NS1 gene, the four serotypes of the virus can be divided into different genotypes. The DV1 virus has five genotypes,26 DV2 has six,27 DV3 has four,28 and DV4 has two genotypes, I and II.29 Genotyping of the virus strains isolated from different outbreaks has provided some insight into the variation and transmission of the prevalent strains.26 Analysis of the genotypes of the virus strains in mainland China show complex origins consistent with the interpretation that the viruses might be imported from more than one country (Table 1). A molecular epidemiological analysis comparing the viral gene sequences of the DV1 strains isolated from four DF outbreaks (1991, 1995, 1997, and 1999) in Guangdong province revealed extensive nucleotide variation over the whole genome. The strain GD03/91, isolated in 1991, shows 97% nucleotide identity with the strains GD23/95 isolated in 1995, 93% identity with GD14/97 isolated in 1997, and only 93% identity was identified between strains GD23/95 and GD14/97, indicating that GD03/91 has a closer genetic relationship to GD23/95 than GD14/97. The phylogenetic tree is consistent with the hypothesis that strains GD03/91 and GD23/95 derive from Southeast Asia or the Pacific islands.15 Strains GD14/97 and GD05/99 isolated in 1999 had sequences similar to the Cambodia strain, showing nucleotide and amino acid identities of 98% and 99%, respectively. Phylogenetic tree analysis indicated that the strain GD14/97, GD05/99, and GD23/95 belong to two different genotypes.30 Fang and others38 compared the nucleotide sequences of a number of genes between the DV2 strains GD06/93 and GD01/98 isolated from the outbreak in 1993 and 1998, respectively, in Guangdong province and found that these two strains and the strain (04) isolated in Hainan province in 1985 belong to different genotypes. However, GD01/98 shows high similarity to the strain ThNH2P28/93 from Thailand with nucleotide and amino acid sequences at 98% and 100% identity, respectively. Comparison of the sequences among the strain GD01/98, GD06/93, and HN04 was consistent with the interpretation that these three strains originate from different regions. Yao and others31 analyzed the NS2 gene in DV4 virus isolated in 1978 and 1990 in mainland China, and they found a 96% identity between the 1990 strain and the Philippine strain, and a 96% identity between the strain 7856B2 (isolated in 1978 in mainland China) and the Caribbean strain 814669. Therefore, they presumed that these two strains isolated in mainland China might come from these two regions. These results are consistent with the conclusion that DF epidemics in Guangdong province may be primarily the result of the virus imported from multiple regions. Molecular epidemiological analysis of DF in the last three decades did not identify new variants of dengue viruses in mainland China (Table 1). Therefore, we propose that the rapid expansion of the urban population and the great migration of citizens as well as frequent international travel may result in an increase in the frequency of DF epidemics.
Table 1.
Genotype of dengue virus recognized in mainland China
| Serotype | Year | Region | Genotype | Origin (homology) | References |
|---|---|---|---|---|---|
| D1 | 1985 | Guangzhou, Guangdong Province | V | Thailand | 32 |
| 1991 | Guangzhou, Guangdong Province | IV | Southeast Asia, Australia | 15,32 | |
| 1995 | Guangzhou, Guangdong Province | IV | Southeast Asia, Australia | 32 | |
| Chaozhou, Guangdong Province | IV | Southeast Asia | 15,30 | ||
| 1997 | Chaozhou, Guangdong Province | I | Cambodia | 15,30 | |
| 1999 | Zhongshan, Guangdong Province | I | Cambodia | 30 | |
| 2002 | Guangzhou, Guangdong Province | IV | Australia TI4 strain | 33 | |
| 2003 | Guangzhou, Guangdong Province | I | Cambodia | 34 | |
| 2006 | Guangzhou, Guangdong Province | V | Thailand, Taiwan ThD1 strain | 35 | |
| 2004 | Fujian Province | I | Thailand | 36 | |
| 2004 | Zhengjiang Province | I | Thailand | 12 | |
| D2 | 1989 | Hainan Province | III | Jamaican or Brazil 90 strain | 37 |
| 1998 | Nanhai, Guangdong Province | III | Thailand ThNH81/93 strain | 15,38 | |
| 1993 | Fanshan, Guangdong Province | I | Australia TSV01 strain | 38,39 | |
| 1999 | Fujian Province | IV | Indonesia, Sri Lanka | 40 | |
| 2001 | Jiangmen, Guangdong Province | I | Australia TSV01 strain | 38 | |
| D4 | 1978 | Fanshan, Guangdong Province | II | Indonesia | 41 |
| 1990 | Guangdong Province | I | Philippines | 31 |
Vectors
Behaviors.
Aedes aegypti and Ae. albopictus are the two most important mosquito species for transmission of dengue viruses in mainland China. Aedes aegypti, a domestic mosquito, can breed inside and outside houses, especially in water-containing vessels in the kitchen or in jars with accumulated rain water. This species is found mostly in the regions south of the 22° north latitude, including the coastal areas in West Guangdong province and the Beibu Gulf of Guangxi Province (Figure 3). Aedes albopictus breeds mostly in the wild and depends on accumulated water in various utensils or plants. It is found in nearly one-third of China ranging from Shenyang in the North, Longxian County and Baoji in the Northwest, South Tibet in the Southwest to the South Changjiang regions, where this mosquito is most common (Figure 3).42 However, the environment and ecology of these mosquitoes has changed greatly because of the rapid urbanization, which may cause significant alteration in the number and types of vector breeding sites. Su and others43 showed following close monitoring that the Breteau index of Ae. aegypti in Haiko city, Hainan province, dropped from 32.81 to 1.73, and the number of Ae. aegypti-positive communities reduced from 23 to 2, from 1987 to 2002. Conversely, the Breteau index of Ae. albopictus increased gradually from 13.15 to 21.84, and the number of positive communities increased from 19 in 1987 to 23 in 2002. Aedes albopictus is considered to be only semi-domestic, but according to a survey by Lin and others,44 the density of this mosquito inside houses was higher than outside in all of the four monitoring sites. They proposed that the indoor environment provides a suitable breeding place for this mosquito and it has adapted to being a domestic mosquito like Ae. aegypti.
Efficiency for virus transmission.
The transmission cycles of arboviruses result from horizontal (by adult mosquitoes) and vertical (transovarial) transmission of the mosquito vectors with the significance of each of these components varying for the specific virus. Furthermore, the transmission dynamics of the viruses depend largely on the efficiency of the vector in terms of the susceptibility of mosquitoes to the virus, its subsequent transmissibility, and the transovarial transmission ability of the virus. All of these factors may be affecting Aedes transmission of dengue viruses. It is clear that the susceptibility and transmission efficiency of mosquitoes to dengue viruses is affected by the species and strains of mosquito44–46 and the virus serotypes,47,48 in addition to the other factors, such as temperature of the environment and the nutrition status of the vector.49,50
Temperature may cause alterations in the length of the extrinsic incubation period by affecting viral replication in vivo. Within the optimal range (26–35°C), relatively higher temperature (35°C) can promote viral replication in vivo, shorten the extrinsic incubation period, and enhance the transmissibility of the virus. Exposure to a relatively lower temperature (≤ 10°C) alone may reduce the physiological activities of the mosquito, decrease the viral replication rate, thus prolong the extrinsic incubation period and attenuate the transmissibility of the virus.51 The question then arises over the preservation of the virus in winter and dry seasons when the mosquito hosts are present in low abundance. A possible answer is that the virus can survive in a vector in functional diapause, or has been transmitted to the next generation through the eggs. Lin and others52 orally infected Ae. albopictus with DV1 and DV2 and homogenates prepared from parental and F1–F3 generations. Dengue viruses in these samples were identified following inoculation of C6/36 cell cultures and specific viral antigen was detected by indirect immunofluoresence assay (IFA). These results provide compelling evidence that Ae. albopictus could transmit virus to the F2 generation by the eggs. Zhao and others53 used the same method to infect three geographic strains of Ae. albopictus with dengue viruses and the viruses in following generations were detected by IFA and reverse transcription-polymerase chain reaction (RT-PCR). Their result also confirmed that four serotypes of dengue virus are capable of vertical transmission by Ae. albopictus. However, the virus can be seldom detected in the vector mosquitoes collected from the field. Fang and others54 performed RT-PCR of over 6,000 Ae. albopictus specimens collected between 1996 and 1998 from three monitoring sites in the suburbs of Guangzhou, Guangdong province, and found none positive for dengue viruses. Similarly, Duan and others55 collected > 300 Ae. albopictus from three monitoring sites in Jieyang, Foshan, and Guangzhou, and they also failed to detect the presence of virus using a TaqMan MGB real-time PCR method. In contrast, many studies from other countries have reported a high infective rate of dengue virus in wild-derived Aedes mosquitoes. For example, the minimum infective rate (MIR) in Ae. albopictus and Ae. aegypti collected in Singapore, from April 1995 to July 1996, was 50 and 57.6, respectively.56 Kow and others57 reported that they used a single-step RT-PCR to assay the male vector (adults) collected from the field of Singapore and found that 1.33% and 2.15% of Ae. aegypti and Ae. albopictus, respectively, were positive for dengue viruses. The serotypes detected in male Ae. aegypti were DV1 (44%), followed by DV2 (22.2%) and DV3 (22.2%) and DV4 (11.1%). In Ae. albopictus males, the serotypes were DV4 (38.9%), followed by DV2 (33.3%), DV3 (16.7%), and DV1 (11.1%). These results emphasize the need for sensitive methods for the identification of the viruses that would allow us to address questions about the presence and absence of infectious virus, the origins of the strains in specific outbreaks, and whether viral genotypes in one epidemic are related to those causing previous or subsequent epidemic.
Prevention and Control
In mainland China, the primary goals of DF control should be timely epidemic detection, prevention, and control of new cases to prevent large-scale outbreaks and to minimize the damage of the epidemic. On the basis of the global DF/DHF prevention and control strategies drafted by WHO in 1995,2 and considering the actual domestic condition, China has instituted its DF control strategy with close monitoring as the key, accompanied by control of mosquito vector density, high vigilance for imported cases, improvement of diagnosis and treatment, enhancing the cooperation among the related different public health departments, and mobilizing the social effort to combat the epidemic.
Mosquito vector monitoring.
The DF is characterized by periodical and sudden occurrence with rapid transmission, often resulting in an unexpected large-scale epidemic.7 A sensitive monitoring system that allows prediction and early detection of an epidemic is critical to the reduction and arresting of the epidemic. This monitoring system involves examination of vector species, their distribution, breeding places, density, sensitivity to pesticides, and viral subtype identification. China currently monitors the major vector mosquitoes of the genus Aedes, primarily by examining mosquito larvae using the Breteau index, house index, and container index. This approach has produced encouraging results in the high-risk regions for DF (such as Hainan and Guangxi).43,58,59 However, the larval and pupal density of mosquitoes in a defined environment may not represent the exact density of the adult mosquitoes, therefore such an approach can be laborious and the results subjected to influences by various factors. Because Ae. albopictus is generally not domestic, Lin and others60 designed a mosquito trap based on the U.S. Centers for Disease Control and Prevention (CDC, Atlanta, GA) trap. This new trap allows close observation of the seasonal variation in the number of adult Ae. albopictus, and complete data sets on mosquito densities in the larval, pupal, and adult stages. Adult mosquitoes trapped also are useful as specimens for virus detection, which can be of great significance in the prediction and control of an DF outbreak.61–63 The preliminary testing results of this new mosquito trap showed its greater advantages over the Breteau index for Ae. albopictus monitoring in the urban areas.64
Vector control.
Because no DF vaccine is currently available, the only effective means for DF prevention is mosquito vector control. The key to this control relies on the maintenance of environmental hygiene and elimination of the breeding places of the mosquitoes.65 Pesticide application remains the conventional means for eliminating the vectors in the breeding sites. However, these activities are associated with the emergence of pesticide resistance and this is evident already in some regions. The use of pesticides is by no means an environmentally friendly solution to the mosquito problem. More environmentally friendly methods therefore are urged for controlling the breeding places of the mosquitoes. Some cost-effective biocontrol agents such as Bacillus thuringiensis var. israelensis and predatory cyclops that do not cause environmental damage or produce resistance in the mosquitoes after long-term use are promising.66,67 However, these slow-acting agents may not seem appropriate in emergency settings,66 and pesticides are still necessary for mosquito control during an DF or DHF outbreak. The WHO68 recommends the ultra-low volume (ULV) spray or thermospray of malathion and sumithion to eliminate the mosquitoes around breeding sites. However, because of its poor effect against female mosquitoes and the larvae,69,70 UVL aerosol is seldom used for outdoor mosquito control during the epidemic of DF or DHF.68
Future Challenge
Accelerated urbanization, expanding urban populations, frequent international travel, and perhaps global warming, all may contribute to increasing the frequencies of DF/DHF epidemics. The absence of effective vaccines and robust measures for the vector management make DF and DHF control difficult, and they will remain a major public health issue in the tropical and subtropical regions. The challenges in DF control depend on the following four aspects: 1) vector control; much effort is needed to establish a monitoring system for early prediction of the occurrence and distribution of the mosquitoes on the basis of their chemical, biological and environmental control. Chinese researchers71–73 have made preliminary but meaningful attempts at DF control using a geographic information system (GIS),74 which allows prediction of the occurrence and distribution of the vectors as well as estimation of the probability of DF epidemic. 2) The establishment of a rapid virus detection system to provide early prediction of the epidemic, which necessitates effective international communication and cooperation. At the same time, the deciphering of the genome of Aedes mosquito75 and the application of transgenic technology76,77 have provided many platforms for dengue prevention and control. 3) Community-based education also is critical. The full knowledge of DF and dengue viruses among the community residents may help greatly to achieve the goal of controlling the monitoring indices within the safe limits, and the participation by the community can be of great importance in winning the battle against vector mosquitoes. 4) Elimination of the technical obstacles to develop safe and effective vaccines. Rapid progress is being made in research of the immune and pathogenic mechanisms of dengue fever, and better understanding of these mechanisms will facilitate the development of the safe and effective vaccines against dengue virus.
Acknowledgments
We thank Zhijian Tu for his critical reading and suggestions to this manuscript.
Footnotes
Financial support: The authors' laboratories are supported in part by grants from the National Natural Science Foundation of China (no. 30771871 and no. U0832004) and GDUPS (2009) to X. G. Chen.
Authors' addresses: Jin-Ya Wu and Xiao-Guang Chen, School of Public Health and Tropical Medicine, Southern Medical University, Guangzhou, China, E-mails: airsing@163.com and xgchen@fimmu.com. Zhao-Rong Lun, Key Laboratory of Tropical Diseases Control (the Ministry of Education), Zhongshan Medical College and State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-Sen (Zhongshan) University, Guangzhou, China, E-mail: lsslzr@mail.sysiu.edu.cn. Anthony A. James, Departments of Microbiology and Molecular Genetics and Molecular Biology and Biochemistry, University of California, Irvine, CA, E-mail: aajames@uci.edu.
References
- 1.Rigau-Pérez JG, Clark GG, Gubler DJ, Reiter P. Dengue and dengue haemorrhagic fever. Lancet. 1998;352:971–977. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 2.Yi BT, Zhang ZY. Epidemic and control of dengue fever in China. Chin Public Health. 2002;18:1128–1130. [Google Scholar]
- 3.Luo HM. A big challenge for prevention and control of dengue fever in China. South Chin J Prev Med. 2007;33:1–3. [Google Scholar]
- 4.Mao XH, Zhang ZX. The status of epidemic of dengue fever in China. J Pathog Biol. 2007;2:385–388. [Google Scholar]
- 5.Han WB, Yang XY. Overview of dengue fever in China. Clin J Med Officer. 2003;31:103–104. [Google Scholar]
- 6.Guan YQ, Huang ZX, Liao HL, Chen XB, Cai DS. Epidemiological analysis of dengue fever in Foshan city, 1978 to 1998. Guangdong Health Prev. 2000;26:7–10. [Google Scholar]
- 7.Kan B. Proposition for prevalence and monitor of dengue fever in China. Chin J Vector Bio Control. 1997;8:152–156. [Google Scholar]
- 8.Liang WJ, He JF, Luo HM, Zhou HQ, Yang F, Zheng K. Epidemiological analysis of dengue fever in Guangdong province, 2001–2006. South Chin J Prev Med. 2007;33:4–7. [Google Scholar]
- 9.Luo HM, He J, Zheng K, Li L, Jiang L. Analysis on the epidemiologic features of dengue fever in Guangdong province, 1990–2000. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23:427–430. [PubMed] [Google Scholar]
- 10.Liu MH, Hung SY, Pan C. Clinical analysis on 1649 cases of dengue fever breakout in Fuzhou city in 1999. Chin J Zoonoses. 2002;18:132–133. [Google Scholar]
- 11.Xu G, Dong H, Shi N, Liu S, Zhou A, Cheng Z, Chen G, Liu J, Fang T, Zhang H, Gu C, Tan X, Ye J, Xie S, Cao G. An outbreak of dengue virus serotype 1 infection in Cixi, Ningbo, People's Republic of China, 2004, associated with a traveler form Thailand and high dengsity of Aedes albopictus. Am J Trop Med Hyg. 2007;76:1182–1188. [PubMed] [Google Scholar]
- 12.Qiu FX, Gubler DJ, Liu JC, Chen QQ. Dengue in China: a clinical review. Bull World Health Organ. 1993;71:349–359. [PMC free article] [PubMed] [Google Scholar]
- 13.Yan TS, Hong RS, Shen XN, Weng YW, Cai SK, Xu BH, Li SQ, He JX, Xu LS, Lin YQ, Zheng NX, Lin M, Lin SH. Study on the epidemiology and etiologic agent of Dengue fever outbreaks in FUzhou in 2004. Chin J Epidemiol. 2006;27:371–374. [PubMed] [Google Scholar]
- 14.Zheng NX, Wang ZH, Lin YQ, Zhang XY, Zheng G, Chen HH, Wu HH, Chen MH. Analysis on the result of epidemiological detection of dengue in Fuzhou city from 1999 to 2001. Modern Prev Med. 2002;29:495–497. [Google Scholar]
- 15.Fang MY, Zhao WZ, Jiang LH, Chen CH, Liu JW, Bai ZJ, Tian XD, Lin LH. Molecular epidemiological study of dengue fever in Guangdong province. Chin J Microbiol Immunol. 2001;21:326–329. [Google Scholar]
- 16.Liu JW, Tian XD, Fang MY, Jiang LH, Ren RW, Hong WY, Chen GF. Gene sequence of NS1 of three strain dengue 1 virus isolated from Guangdong in China. J Prev Med Chin PLA. 2003;21:64. [Google Scholar]
- 17.Ren RW, Fang MY, Hong WY, Huang BM, Jiang LH, Tian XD, Chen GF. Isolation, identification and sequence analyses of dengue virus type 2 strain GD19/2001. Chin J Epidemiol. 2003;24:288–290. [PubMed] [Google Scholar]
- 18.Wang L, Wang Y, Jin S, Zunyou Wu ZY, Chin DP, Koplan JP, Wilson ME. Emergence and control of infectious diseases in China. Lancet. 2008;372:1598–1605. doi: 10.1016/S0140-6736(08)61365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jetten TH, Focks DA. Potential change in the distribution of dengue transmission under climate warming. Am J Trop Med Hyg. 1997;57:285–297. doi: 10.4269/ajtmh.1997.57.285. [DOI] [PubMed] [Google Scholar]
- 20.Yi BT, Zhang ZY, Xu DZ, Xi YZ, Fu JG, Lou J, Yuan MH, Liu SQ, Kuang Q. Correlation between dengue fever epidemic and climate factors in Guangdong Province. J Fourth Mil Med Univ. 2003;24:143–146. [Google Scholar]
- 21.Brower V, Report Vector-borne diseases and global warming: are both on an upward swing. Eur Mol Biol Organ. 2001;21:755–757. doi: 10.1093/embo-reports/kve193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi BT, Zhang ZY, Xu DZ. Study on influence of climate factors on dengue vector Aedes density. Chin J Publ Health. 2003;19:129–131. [Google Scholar]
- 23.Reiter P. Climate change and mosquito-borne disease: knowing the horse before hitching the cart. Rev Sci Tech. 2008;27:383–398. [PubMed] [Google Scholar]
- 24.Benitez MA. Climate change could affect mosquito-borne diseases in Asia. Lancet. 2009;373:1070. doi: 10.1016/s0140-6736(09)60634-6. [DOI] [PubMed] [Google Scholar]
- 25.Shao Y, Jiang LF, Ye CY. Molecular epidemiology of dengue fever. Chin J Microecol. 2000;12:365–368. [Google Scholar]
- 26.Rico-Hesse R. Molecular evolution and distribution of dengue viruses type 1 and 2 in nature. Virology. 1990;174:479–492. doi: 10.1016/0042-6822(90)90102-w. [DOI] [PubMed] [Google Scholar]
- 27.Rico-Hesse R, Lisa M, Aannda N, Vaughn DW, Kalayanarooj S, Green S, Rothman AL, Ennis FA. Molecular evolution of dengue type 2 virus in Thailand. Am J Trop Med Hyg. 1998;58:96–101. doi: 10.4269/ajtmh.1998.58.96. [DOI] [PubMed] [Google Scholar]
- 28.Lanciotti R, Lewis JG, Gubler JD, Trent DW. Molecular evolution and epidemiology of dengue-3 viruses. J Gen Virol. 1994;75:65–75. doi: 10.1099/0022-1317-75-1-65. [DOI] [PubMed] [Google Scholar]
- 29.Lanciotti R, Gubler D, Trent DW. Molecular evolution and phylogeny of dengue-4 viruses. J Gen Virol. 1997;78:2279–2286. doi: 10.1099/0022-1317-78-9-2279. [DOI] [PubMed] [Google Scholar]
- 30.Tian XD, Liu JW, Fang MY, Jiang LH, Ren RW, Hong WY, Cheng GF. Analysis of the E gene sequences of three dengue virus type 1isolates in Guangdong province. J First Mil Med Univ. 2005;25:54–57. [PubMed] [Google Scholar]
- 31.Yao HJ, Liu JW, Fang MY, Zhao WZ, Liao YH, Lin LH, Jiang LH, Wu SM, Lei LB. Sequence analysis of NA2a-NS2b of dengue 4 viruses in Guangdoang province. J Prev Med Chin PLA. 2005;23:23–25. [Google Scholar]
- 32.Wang ZG, Tang Q, Zhao XQ. Preliminary study on variation of Guangdong dengue-1 virus strains. Chin J Microbiol Immunol. 2000;20:160–163. [Google Scholar]
- 33.Zhang JL, Rui X, Wan YJ, Peng T, An J, Zhang FC, Tang XP. Identification and phylogenetic analysis of virulence of dengue-1 virus isolated from strains prevalent in Guangzhou in 2002. Acta Acad Med Mil Tertiae. 2005;27:282–286. [Google Scholar]
- 34.Lu YC, Jiang ZY, Chen WS, Ren RW, Fang MY, Wang SZ, Cheng GF, Tian XD, Liu JW, Yin ZB. Isolation and sequence analysis of dengue virus type 1 from suspected dengue virus infected patients in Guangzhou in 2003. J Trop Med. 2004;4:666–669. [Google Scholar]
- 35.Han JF, Yu M, Chen SP, Jiang T, Qin CF, Qin ED. Genomic characteristics and phylogenetic analysis of dengue virus type 1 isolated from Guangzhou in 2006. Med J Chin PLA. 2008;33:1055–1058. [Google Scholar]
- 36.Weng YW, Chen D, Zhang ZS, Zhao ZY, Xie JF, Shen XN, Yan TS. Complete genome sequencing and phylogenetic analysis of a Dengue virus type I strain isolated in Fujian province. Chin J Zoonoses. 2006;22:1013–1016. [Google Scholar]
- 37.Zhou JM, Jiang LF, Gao Y, Xue YH, Fang DY. Gene sequencing and analysis of the envelope protein of dengue 2 virus isolated in Hainan China. Chin J Microbiol Immunol. 2003;23:304–306. [Google Scholar]
- 38.Fang MY, Ren RW, Hong WY, Liu JW, Jiang LH, Tian XD, Lin LH. Sequencing and structural analysis of structural gene of three dengue virus type 2 isolated from Guangdong province. Chin J Microbiol Immunol. 2003;23:300–303. [Google Scholar]
- 39.Li JJ, Ren RW, Ma AD, Xu XL, Shen M, Jiang ZJ. Sequencing and analysis of the structural gene of dengue virus type 2 strain GD06 /93 isolated from Foshan, Guangdong province. J Trop Med. 2006;8:877–880. [Google Scholar]
- 40.Geng LQ, Qin ED, Zhao W, Hu ZJ, Yuan XT, Yu M, Li XY, Yang PY. Genomic sequence determination of a new dengue 2 virus Fujian strain. Chin J Microbiol Immunol. 2001;21:330–333. [Google Scholar]
- 41.Liu ZW, Yu YX, Jia LL, Dong GM, Wang ZW. Sequence determination and phylogenetic analysis of two dengue virus type 4 strains isolated in the first outbreak of dengue fever after the foundation of the People's Republic of China. Chin J Zoonoses. 2007;23:678–683. [Google Scholar]
- 42.An JY, Yan G, Zhang XW, Yan BS. Aedes albopictus: the important transmission vector. Med Ani Prev. 2003;17:449–452. [Google Scholar]
- 43.Su AF, Pei ZC, Fu JC, Li JG, Feng F, Zhu Q, Huang SP. Analysis of distribution and population density changes of Aedes egypti the transmission vector of dengue fever in Haikou city. China Trop Med. 2005;5:1394–1395. [Google Scholar]
- 44.Lin LH, Chen WJ, Ma YH, Bai ZJ, Yang JQ, Fang MY. Analysis on relationship between characteristic of bleeding in house and dengue epidemic. Chin Public Health. 2000;16:610. [Google Scholar]
- 45.Song XL, Huang JL, Zheng XY, Wu Y. Advancement study on dengue virus transmission efficacy with adult mosquito. J Trop Med. 2005;5:251–253. [Google Scholar]
- 46.Tang SY, Li GA, Zhang QE. Study on transmission efficacy spreading dengue virus by several mosquito in China. Bull Acad Mil Med Sci. 1987;30:458–462. [Google Scholar]
- 47.Zhou GZ, Zhao TY, Guo XX, Zhu LH, Xue J. Study among different strains of Aedes aegypti in susceptibility for oral infection with DEN-2 virus. Chin J Vector Bio Control. 2004;15:99–101. [Google Scholar]
- 48.Zhang S, He GM. Different geographical strains Aedes albopictus susceptibility to dengue virus in China. Zhonghua Liu Xing Bing Za Zhi. 1989;10:348–351. [PubMed] [Google Scholar]
- 49.Zhou GZ, Zhao TY, Xue J. The impact of the media mosquito susceptibility to dengue virus research. Chin J Vector Bio & Control. 2003;14:237–239. [Google Scholar]
- 50.Zhang S, He GM. Observation of barrier Aedes mosquito infected with dengue virus in vivo. Chin J Zoonoses. 1989;5:35–36. [Google Scholar]
- 51.Xi GL. Study on the influence of meteorological factors upon density of mosquito. Chin J Vector Bio Control. 2000;11:21–23. [Google Scholar]
- 52.Lin LH, Fang MY, Chen CH, Peng YF. Study on vectorial capacity spreading dengue virus by Aedes albopictus. Chin J Vector Bio Control. 2000;11:173–176. [Google Scholar]
- 53.Zhao X, Zuo L, Shu LP, Wei LH. Study on vertical transmission with dengue virus in Aedes albopietus. Chin J Publ Health. 2006;22:1185–1187. [Google Scholar]
- 54.Fang MY, Chen CH, Li JQ, Li RB, Wen WD, Peng YF, Lin LH, Chen Z, Tian XD, Jiang LH, Bai ZJ, Chen WJ. Epidemiological surveillance of dengue fever in Guangdong province. Chin Public Health. 1999;15:990–991. [Google Scholar]
- 55.Duan JH, Lin LF, Cai SW, Lu WC, Zheng K, Yi JR. Detection of dengue virus from Aedes albopitus by TaqMan MGB real-time polymerase chain reaction. Chin J Vector Bio Control. 2006;17:86–89. [Google Scholar]
- 56.Chow VT, Chan YC, Yong R, Lee KM, Lim LK, Chung YK, Lam-Phua SG, Tan BT. Monitoring of dengue viruses in field-caught Aedes aegypti and Aedes albopictus mosquitoes by a type-specific polymerase chain reaction and cycle sequencing. Am J Trop Med Hyg. 1998;58:578–586. doi: 10.4269/ajtmh.1998.58.578. [DOI] [PubMed] [Google Scholar]
- 57.Kow CY, Koon LL, Yin PF. Detection of dengue viruses in field caught male Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Singapore by type-specific PCR. J Med Entomol. 2001;38:475–479. doi: 10.1603/0022-2585-38.4.475. [DOI] [PubMed] [Google Scholar]
- 58.Xie ZS, Fu JC. Results of monitoring and control of transmission vectors of dengue fever in Haikou City in 2005–2006. China Trop Med. 2007;7:1416–1417. [Google Scholar]
- 59.Tan Y, Feng XY, Jiang JH. Analysis on the dengue vector surveillance results during 2002–2003 in Guangxi. Chin J Hyg Insecticides Equipment. 2004;10:154–156. [Google Scholar]
- 60.Lin LF, Lu WC, Cai SW, Duan JH, Yi JR, Deng F, Chen Q, Chen XG. The design and efficacy observation of new mosq-ovitrap for monitoring of vector of dengue fever. Chin J Vector Biol Control. 2005;16:26–28. [Google Scholar]
- 61.Yu DX, Ren XQ, Zhou W, Liao YX, Chen Q, Yu SY. Efficacy observation of mosq-ovitrap for monitoring Aedes albopictus in field. J Trop Med. 2008;8:309–312. [Google Scholar]
- 62.Xu RQ, Jiang LY, Ren WJ, Hong M, Leng PE, Wang SZ. Efficacy observation of mosq-ovitrap for monitoring Aedes albopictus in field. Chin J Hyg Insecticides Equipment. 2006;12:345–348. [Google Scholar]
- 63.Lin LF, Cai SW, Duan JH, Zhou H, Lu WC, Feng Q, Chen Q. Application of mosq-ovitrap on vector surveillance during dengue fever outbreak. Chin J Publ Health. 2005;21:1459–1461. [Google Scholar]
- 64.You JR, Zheng SH, Li JQ, Ye ZY, Li RB, Lin LF, Huang Y, Lai PH. Study on new method of mosq-ovitrap for monitoring of vector of dengue fever. Chin J Vector Bio Control. 2008;19:368–369. [Google Scholar]
- 65.Luo HM. Manual for Prevention and Control of Dengue Fever. Beijin: Chinese Standard Publishing; 2003. pp. 1–6. [Google Scholar]
- 66.Zeng TM. Mosquito biological control and its role in integrated control. Chin J Hygienic Insecticides Equipment. 1997;3:5–7. [Google Scholar]
- 67.Wang HW, Wang XG. Population surveillance and prevention for dengue fever vector–Aedes mosquito. Chin Med Ani Prev. 2000;16:389–391. [Google Scholar]
- 68.Pan American Health Organization . Dengue and Dengue Hemorrhagic Fever in the Americas: Guidelines for Prevention and Control. Washington, DC: PAHO; 1994. pp. 3–20. 49–58, 69–70. [Google Scholar]
- 69.Gubler D. Aedes aegypti and Aedes aegypti-borne disease control in the 1990s: top down or bottom up. Am J Trop Med Hyg. 1989;40:571–578. doi: 10.4269/ajtmh.1989.40.571. [DOI] [PubMed] [Google Scholar]
- 70.Newton E, Reiter P. A model of the transmission of dengue fever with an evaluation of the impact of ultra-low volume (ULV) insecticide applications on dengue epidemics. Am J Trop Med Hyg. 1992;47:709–720. doi: 10.4269/ajtmh.1992.47.709. [DOI] [PubMed] [Google Scholar]
- 71.Yi BT, Xu DZ, Zhang ZY, Zhang B, Xi YZ, Fu JG, Luo J, Yuan MH, Liu SQ, Kuang Q. Study on the distribution of dengue fever and vector in Guangdong province combined application of SRS/GIS/ PCA. Chin J Dis Control Prev. 2003;7:509–514. [Google Scholar]
- 72.Yi BT, Xu DZ, Zhang ZY, Zhang B, Xi YZ, Fu JG, Luo J, Yuan MH, Liu SQ. Development and application of geographic information system of Aedes vector in Chaozhou city Guangdong province. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:134–137. [PubMed] [Google Scholar]
- 73.Yi BT, Xu DZ, Zhang ZY, Zhang B, Xi YZ, Fu JG, Luo J, Yuan MH, Liu SQ. Study on the relationship between NDVI of NOAA-AVHRR image and the Aedes density in Guangdong Province. J Fourth Mil Med Univ. 2003;24:1720–1724. [Google Scholar]
- 74.Su M, Chang N. Framework for application of GTS on the monitoring of dengue vectors: Kaohsiung. Med Sci. 1994;10:94–101. [PubMed] [Google Scholar]
- 75.Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M, Debruyn B, Decaprio D, Eiglmeier K, Eisenstadt E, El-Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, Labutti K, Lee E, Li S, Lovin DD, Mao C, Mauceli E, Menck CF, Miller JR, Montgomery P, Mori A, Nascimento AL, Naveira HF, Nusbaum C, O'leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JM, Vanzee JP, Verjovski-Almeida S, Werner D, White O, Wyder S, Zeng Q, Zhao Q, Zhao Y, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser-Liggett CM, Severson DW. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1722. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adelman ZN, Sanchez-Vargas I, Travanty EA, Carlson JO, Beaty BJ, Blair CD, Olson KE. RNA silencing of dengue virus type 2 replication in transformed C6/36 mosquito cells transcribing an inverted-repeat RNA derived from the virus genome. J Virol. 2002;76:12925–12933. doi: 10.1128/JVI.76.24.12925-12933.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Franz AW, Sanchez-Vargas I, Adelman ZN, Blair CD, Beaty BJ, James AA. Engineering RNA interference-based resistance to dengue virus type-2 in genetically-modified Aedes aegypti. Proc Natl Acad Sci USA. 2006;103:4198–4203. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]


