Abstract
Traditional study designs do not identify acute asymptomatic or pre-symptomatic dengue virus (DENV) infections, thus limiting our understanding of immunologic and viral factors that modulate infection outcome. In the 2006 and 2007 dengue seasons, we conducted a pilot index cluster study in Managua, Nicaragua, in which 442 persons living within 50 meters of 22 index cases identified through an ongoing pediatric cohort study were evaluated for DENV infection. Post-enrollment and pre-enrollment DENV infections were confirmed in 12 (2.7%) and 19 (4.3%) contacts, respectively. Five (42%) post-enrollment infections were asymptomatic, and DENV-2 was identified in 9 (75%) infections. Phylogenetic analysis with full-length DENV genomic sequence from contacts, index cases, and cohort dengue cases indicated focal transmission and infection outside the local area. We demonstrate the feasibility of identification of acute asymptomatic and pre-symptomatic cases in urban Latin America, the first report of such a study in the Americas, and identify age and concomitant immunity to DENV of contacts as a key factor in index cluster study design.
Introduction
Dengue is a mosquito-borne viral illness that causes a spectrum of clinical disease ranging from dengue fever to the more severe, life-threatening dengue hemorrhagic fever and dengue shock syndrome. However, data from several studies around the world,1–3 including Nicaragua,4,5 indicate that 50–90% of dengue virus (DENV) infections are inapparent. An ongoing pediatric cohort study in Managua, Nicaragua, enables detection of the number of inapparent DENV infections that occur each year by analyzing titers of antibodies to DENV in yearly blood samples, but does not permit their identification at the moment that they occur. Identification of acute asymptomatic DENV infections and cases immediately before symptom onset has proven impossible through traditional study designs. Therefore, genetic, immunologic, and virologic determinants of why certain persons remain asymptomatic whereas others progress to disease of varying severity are incompletely understood, and characterization of the immune response immediately prior to the onset of clinical symptoms in dengue disease has remained elusive. However, the index cluster study design, in which household and neighborhood contacts of viremic dengue cases are recruited, enables analysis of these questions and refined spatio-temporal analysis of DENV transmission.
To enable investigation of these critical questions, we assessed the feasibility of an index cluster study in Nicaragua, where persons living near a dengue index case were evaluated for DENV infection to enable identification of asymptomatic and pre-clinical symptomatic DENV infections. Although two such index cluster studies in Asia have now been reported, this pilot study tested the methods in the Americas, where different cultural acceptance of frequent blood draws and distinct DENV transmission patterns may impact the feasibility and outcome of such a design. In addition, we wanted to investigate the possible impact of study design (e.g., site, age) on observed incidence of pre-enrollment and post-enrollment DENV infections in contacts, as one published study was conducted in the homes of schoolchildren from 20 rural villages in Thailand such that dengue cases were geographically separated6 and because another study was conducted in urban Indonesia with a population consisting of children and adults,7 more similar to our study that included children and adults in a single urban district of Managua. Finally, the pattern of DENV transmission has distinct characteristics in Asia versus the Americas, with more concurrent circulation of different DENV serotypes, more severe disease, and in general higher incidence of DENV infection in Asia.8 In our study, we also included full-length sequencing of several viral isolates from contacts and phylogenetic comparison to DENV isolated from their index cases, the results of which suggest focal transmission and infection outside the household area.
Materials and Methods
Study population, location, and design.
The study was conducted in the capital of Nicaragua, Managua, which has a population of approximately 1.4 million. Participants were recruited in District II of Managua, a low-to-middle income area bordering Lake Managua, in which approximately 30% of the population is less than 15 years of age and 53% is female.9 Most of the 62,000 inhabitants in the district live in dense housing conditions, with an average of 8–10 persons living in cement block and zinc roof homes of ~10 m2 without screened windows or doors. Most households have potable water and sewage service, but water service is sporadic, necessitating water storage within homes. The population of District II is served by the Health Center Sócrates Flores Vivas (HCSFV), a public clinic run by the Ministry of Health. Baseline seroprevalance is high in the population, as observed in the concurrent pediatric cohort study, in which prevalence of antibodies to DENV reached > 90–95% by 10–12 years of age.5 All four serotypes have circulated in Nicaragua since the 1990s, with one serotype dominating each season: e.g., in Managua DENV-3 predominated in 1995 and 1998; DENV-2 in 1999–2001; DENV-1 in 2003–2004; DENV-2 in 2005–2007; and DENV-3 in 2008–2009.
The study was conducted within the Pediatric Dengue Cohort Study (PDCS), which is based at the HCSFV and has been on-going since August 2004.5,10 In 2006 and 2007, the PDCS was composed of approximately 3,750 persons 2–12 years of age residing in District II. Active DENV infections were identified through enhanced passive surveillance by study physicians and nurses at the HCSFV and periodic home visits for follow-up.10 Participants were followed closely for all illnesses, and children who had fever were screened for signs and symptoms of dengue. Suspected dengue cases and cases of undifferentiated fever were tested for acute infection and followed-up closely while symptoms persisted. Once a year in July, prior to the onset of the dengue season, blood samples were obtained from healthy participants for serologic testing of paired annual samples to identify inapparent DENV infections.
Active participants in the cohort study who came to the HCSFV within 48 hours after onset of symptoms of dengue and met World Health Organization criteria (acute febrile illness with two or more of the following symptoms or signs: headache, retro-orbital pain, myalgia, arthralgia, rash, hemorrhagic manifestations, or leukopenia) and who were confirmed as having a DENV infection by reverse transcription–polymerase reaction (RT-PCR) within 36 hours were eligible to enroll as index cases. To maximize the number of contact cases identified, index case enrollment occurred during the peak of the dengue season, which typically occurs between August and January, and was initiated once transmission began to increase each year (October 16, 2006–January 12, 2007 and September 8, 2007–November 29, 2007).5
In the first year of the cluster study, four DENV-negative index cases were included for comparison of focal transmission patterns. Cluster contacts consisted of persons ≥ 2 years of age who were not participating in the PDCS and who lived in the same household as the index case or neighbors living within 50 meters of the index household. Fifty meters was selected because it is within the flight range of Aedes aegypti in urban settings,11,12 minimizes potential spatial overlap with cohort participants given the occasionally close distance between homes of cohort participants, and was a large enough radius to enroll the target number of contact persons. Once an index case was identified and consented to participate in the study, research field staff visited the area surrounding the home of the index case to invite possible contacts to participate in the study. Field staff screened contacts to ensure that they lived within 50 meters of the index case (which was verified by a global positioning system) and briefly described the study procedures and goals. Subsequently, Ministry of Health teams visited the home of the index case for vector control activities. All participants and their legal guardians provided informed consent and/or assent prior to enrollment. The study was reviewed and approved by the Institutional Review Boards of the University of California, Berkeley, and the Nicaraguan Ministry of Health. Participants were given a temporary identification card that enabled them 24-hour access to consults with the PDCS study physicians and use of the clinical laboratory at the HCSFV.
Upon enrollment (day 1), field staff recorded each participant's temperature and administered a short questionnaire to evaluate the presence of any dengue-like symptoms in the previous 48 hours. On days 1 and 14, 5 mL of blood was obtained from each participant for virologic and serologic testing (4 mL) and complete blood count (1 mL). Participants who were confirmed with a current DENV infection or who experienced fever during the 14-day duration of the study were encouraged to attend the HCSFV for care during their illness, where study physicians provided care and used a standardized data collection instrument to document clinical signs and symptoms.
Laboratory assays.
DENV-specific IgM was detected by using a single-dilution IgM-capture enzyme-linked immunosorbent assay (ELISA),13 and total antibodies to DENV were measured by using an inhibition ELISA4,14,15 and analyzed using the method of Reed and Muench16 to confirm serologic diagnosis and to distinguish primary and secondary DENV infections. Both serologic assays were performed on paired day 1 and day 14 serum samples. In addition, day 1 serum samples from persons who were dengue positive by either IgM ELISA or inhibition ELISA analysis of paired day 1 and day 14 samples, or who were positive for DENV-specific IgM antibodies in both samples, were subjected to RNA extraction (QIAamp® Viral RNA Mini Kit; Qiagen, Valencia, CA) and RT-PCR amplification with primers specific for the capsid gene.17 Viral isolation in C6/36 Ae. albopictus cells18 was performed on samples positive for DENV by RT-PCR, and the serotype of the isolated virus was confirmed by RT-PCR. All virologic and serologic testing was performed at the Ministry of Health's National Virology Laboratory, and complete blood counts were obtained at the HCSFV.
Sequencing and phylogenetic analysis.
RNA was extracted using the QIAamp® Viral RNA Mini Kit from viral isolates from index cases and their contacts. RNA was also extracted from viruses isolated from cohort study participants who experienced a DENV infection two weeks prior, during, or two weeks after the period of the cluster study in the 2007 dengue season (August 25, 2007–December 13, 2007). The RNA was reverse transcribed with Superscript III RT (Invitrogen, Carlsbad, CA), primed with random hexamers and a single primer directed to the conserved 3′ end of the DENV genome (5′-AGAACCTGTTGATTCAACAGCAC-3′, DENV-2 16681 position 10701–10723), and sent to the Broad Institute in Cambridge, Massachusetts. At the Broad Institute, 96 overlapping amplicons of 500–900 nucleotides were generated and sequenced bidirectionally by using the ABI3730xl DNA sequencer (Applied Biosystems, Foster City, CA), resulting in ~8-fold sequence coverage per genome.
Sequences were then assembled and annotated by using the Broad Institute's viral consensus assembly and reference annotation algorithms. Phylogenetic trees were inferred for DENV-2 sequences from PDCS participants during the 2007 dengue season, including cluster index cases, and four contacts of three index cases using whole-genome nucleotide data. Related American/Asian DENV-2 strains were used as an outgroup. Sequences were aligned using Muscle version 4.0,19 and the best-fit model of nucleotide substitution was determined by using MODELTEST.20 Maximum likelihood trees were generated by using PhyML version 3.021 with subtree pruning and regrafting branch swapping under the general time reversible + I model of nucleotide substitution where the proportion of invariable sites was 0.6811.
Definitions.
A post-enrollment DENV infection was defined as a person who demonstrated seroconversion of DENV-specific IgM or a ≥ 4-fold increase in DENV-specific antibodies by inhibition ELISA in paired samples, with or without detection of DENV by RT-PCR or virus isolation. A pre-enrollment DENV infection was defined as a person whose day 1 and day 14 samples were positive for DENV-specific antibodies by IgM ELISA or whose day 1 sample was IgM positive and whose day 14 sample was IgM negative. A primary DENV infection was defined when the antibody titer was < 10 in the day 1 sample and/or < 2,560 in the day 14 sample as determined by inhibition ELISA.4 Secondary DENV infection was defined by inhibition ELISA as an antibody titer ≥ 10 in the day 1 sample and/or ≥ 2,560 in the day 14 sample.15,22 Symptomatic cases were those meeting the World Health Organization definition (as described above) or with fever without a defined focus (undifferentiated febrile illness), as determined by signs and symptoms collected by field teams at enrollment and follow-up and by study physicians at the HCSFV, together with complete blood count results. Asymptomatic cases were afebrile and showed no symptoms during the study period.
Results
In the 2006 and 2007 seasons of the PDCS, 875 initial medical consultations to the HCSFV for possible DENV infections by cohort participants occurred, of which 13 in 2006 and 64 in 2007 were laboratory-confirmed dengue cases.5 Dengue virus type 2 was responsible for 70% and 95% of symptomatic cases in the 2006 and 2007 seasons, respectively, as determined by RT-PCR and viral isolation. A total of 22 index participants were selected from the cohort study during the peak of dengue transmission, 7 in the 2006 season and 15 in the 2007 season. Of these index cases 18 had laboratory-confirmed DENV-2 symptomatic infections (all dengue fever) and 4 were DENV-negative. Index case participants ranged in age from 4 to 12 years and 10 were female (Table 1). Clusters were distributed throughout the catchment area of the HCSFV (Figure 1). At each cluster site, 18–25 contacts were enrolled, totaling 495 contacts. Females comprised 70% (346) of the contacts, and 94 (19%) were less than 15 years of age, with a mean age of 30 years (Table 1).
Table 1.
Demographic characteristics and dengue virus infection in index cases and contacts, Nicaragua*
| Characteristic | Index cases (n = 22) no. (%) | All contacts (n = 495) no. (%) | Pre-enrollment dengue (n = 19) no. (%) | Post-enrollment dengue (n = 12) no. (%) |
|---|---|---|---|---|
| Age, years | ||||
| < 15 | 22 (100) | 94 (19) | 1 (5) | 6 (50) |
| ≥ 15 | 0 (0) | 401 (81) | 18 (95) | 6 (50) |
| Mean | 8 | 30 | 34 | 23 |
| Sex | ||||
| F | 10 (46) | 346 (70) | 11 (58) | 10 (83) |
| M | 12 (54) | 149 (30) | 8 (42) | 2 (17) |
| Index case | ||||
| DENV negative | 4 (18) | 81 (16) | 1 (5) | 2 (17) |
| DENV positive | 18 (82); DENV-2 | 414 (84) | 18 (95) | 10 (83) |
| Clinical classification | ||||
| Dengue fever | 18 (100) | – | – | 4 (33) |
| Undifferentiated fever | – | – | – | 3 (25) |
| Asymptomatic | – | – | – | 5 (42) |
| Immune response | ||||
| Primary | 3 (17) | – | – | 2 (17) |
| Secondary | 15 (83) | – | – | 10 (83) |
| Fever onset | (All index cases febrile at first consultation) | |||
| Prior to enrollment | – | – | 2 (29) | |
| At follow-up | – | – | 5 (71) | |
DENV = dengue virus.
Figure 1.
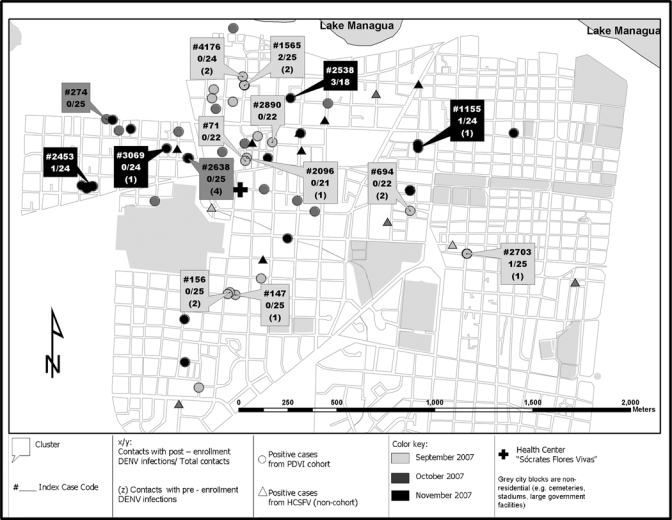
Geographic distribution of laboratory-confirmed dengue among cluster contacts, participants in the Pediatric Dengue Cohort Study, and patients at Health Center Sócrates Flores Vivas, Nicaragua, September–November 2007.
Serologic analysis was completed for 442 contacts (89%) from whom day 1 and day 14 blood samples were obtained, leaving 53 indeterminate cases that were not further analyzed. Of those with complete serologic results, 19 (4.3%) were positive for pre-enrollment DENV infections (18 positive for DENV-specific IgM antibodies on days 1 and 14, and 1 positive on day 1 and negative on day 14), and post-enrollment DENV infections developed in 12 (2.7%) (Table 2). Overall, nine of the 22 clusters (41%) had a positive post-enrollment contact, ranging from 1 to 3 positive contacts per cluster. As in the index cases, all contact infections were caused by DENV-2. Nine participants were positive by RT-PCR, of which six were also positive by virus isolation (Tables 2 and 3).
Table 2.
Laboratory results of cluster contacts, Nicaragua*
| Result | IgM ELISA (n = 442) no. (%) | Inhibition ELISA (n = 442) no. (%) | RT-PCR† (n = 22) no. (%) | Virus isolation‡ (n = 9) no. (%) | Final result§ (n = 442) no. (%) |
|---|---|---|---|---|---|
| Negative | 415/442 (93.9) | 433/442 (98.0) | 13/22 (59.1) | 3/9 (33.3) | 430/442 (97.3) |
| Positive | 27/442 (6.1)¶ | 9/442 (2.0) | 9/22 (40.1) DENV-2# | 6/9 (66.7) DENV-2# | 12/442 (2.7) |
Of 495 contacts enrolled, 53 were indeterminate, missing the day 14 sample. ELISA= enzyme-linked immunosorbent assay; RT-PCR = reverse transcription–polymerase chain reaction; DENV = dengue virus.
RT-PCR was only performed on day 1 samples from those contacts who had a positive result for IgM and/or for inhibition ELISA.
Virus isolation was only attempted among those day 1 samples positive by RT-PCR.
Positive post-enrollment.
Serostatus of paired day 1 and 14 samples were as follows: negative-positive: 27 (1.6%) of 442; positive-positive: 19 (4.3%) of 442; and positive-negative: 1 (0.2%) of 442. One of the 19 positive-positive samples was classified as post-enrollment because of a > 4-fold increase in antibodies to DENV as determined by inhibition ELISA (ID# 20002, Table 3).
DENV-2 was the only serotype identified by RT-PCR or virus isolation among study participants.
Table 3.
Characteristics of post-enrollment dengue cases, Nicaragua*
| Study year | Study code | Index case | Age, years | Sex | Clinical classification | IgM1 | IgM2 | InhE1titer | InhE2 titer | Result InhE | Primary/secondary infection | RT-PCR | Virus isolation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006–2007 | 20002 | 276 | 30 | F | AS | + | + | 5 | 1,443 | + | 1 | – | NP |
| 20032 | 2510† | 13 | M | AS | – | – | 200 | 7,563 | + | 2 | DENV-2 | DENV-2 | |
| 20065 | 3325 | 24 | F | AS | – | + | 1,550 | 3,933 | – | 2 | – | NP | |
| 20131 | 3555† | 40 | F | AS | – | – | 15 | 211 | + | 1 | – | NP | |
| 2007–2008 | 20170 | 1565 | 14 | F | AS | – | – | 2,862 | 100,001 | + | 2 | DENV-2 | DENV-2 |
| 20172 | 1565 | 11 | F | UFI | – | + | 2,011 | 36,547 | + | 2 | DENV-2 | DENV-2 | |
| 20213 | 2703 | 14 | F | DF‡ | – | + | 345 | 100,001 | + | 2 | DENV-2 | DENV-2 | |
| 20410 | 2453 | 25 | F | DF | – | + | 296 | 16,099 | + | 2 | DENV-2 | DENV-2 | |
| 20443 | 1155 | 67 | F | DF | – | + | 12,724 | 33,942 | – | 2 | DENV-2 | – | |
| 20497 | 2538 | 12 | F | DF‡ | – | + | 316 | 18,634 | + | 2 | DENV-2 | DENV-2 | |
| 20498 | 2538 | 14 | F | UFI | – | + | 1,561 | 5,092 | – | 2 | DENV-2 | – | |
| 20502 | 2538 | 15 | M | UFI‡ | – | – | 4,154 | 20,180 | + | 2 | DENV-2 | – |
InhE = inhibition enzyme-linked immunosorbent assay; RT-PCR = reverse transcription–polymerase chain reaction; AS = asymptomatic; NP = not processed; DENV = dengue virus; UFI = undifferentiated febrile illness; DF= dengue fever.
Negative index case.
Febrile at enrollment.
Contacts with post-enrollment DENV infections ranged in age from 11 to 67 years old, and 10 (83%) of the 12 were female (Table 1). Dengue virus infections were confirmed among contacts of negative (2 contact infections) and positive (10 contact infections) index cases. Most infections were secondary (10 of 12, 83%); two primary infections occurred during the 2006 season. Seven symptomatic (58%) and five asymptomatic (42%) DENV infections were recorded. In 2006, all four infections were asymptomatic, and all of the symptomatic cases occurred during the 2007 season in secondary DENV infections (7 of 8 infections) (Table 3). In the concurrent pediatric cohort in the same district, 16 asymptomatic DENV infections were identified through serologic testing of paired annual samples for every 1 symptomatic case identified in 2006, whereas in 2007 only 3 asymptomatic infections were recorded for every symptomatic DENV infection.5 During the 2006 study period, there were 8 symptomatic dengue cases in the cohort (including those serving as index cluster cases). During the 2007 season, 53 symptomatic cohort cases were identified. National Surveillance data on confirmed dengue cases showed similar temporal trends throughout Managua compared with the cohort study and the index cluster study during both years.
Dengue virus type 2 was isolated from three cases prior to symptom onset, from one case of undifferentiated fever, and from two of the five asymptomatic infections, both of which were secondary infections. The two primary DENV infections were asymptomatic and negative by RT-PCR. In both study years, infections were observed among cohort participants and other HCSFV patients who lived near positive cluster contacts (Figure 1).
Phylogenetic analysis of full-length DENV-2 sequences from three index cases and four contacts (DENV-2 isolates were obtained from two contacts of the same index case) was performed together with sequences from 50 PDCS participants who had confirmed dengue during the period of the 2007 cluster study (Figure 2). In two of the three instances, viral sequences from the index cases and their contacts were identical (index 1565 and contacts 20170 and 20172, both with 0 substitutions/site) or nearly identical (index 2453 and its contact 20410 with 0.00174 substitutions/site), which indicated a close evolutionary relationship (including identity) and likely transmission. However, the sequence from contact 20213 is substantially more divergent from that of its index case (2703), with 0.01583 substitutions per site, and it appears in a different branch of the tree with sequences from a few other cohort members from different neighborhoods.
Figure 2.
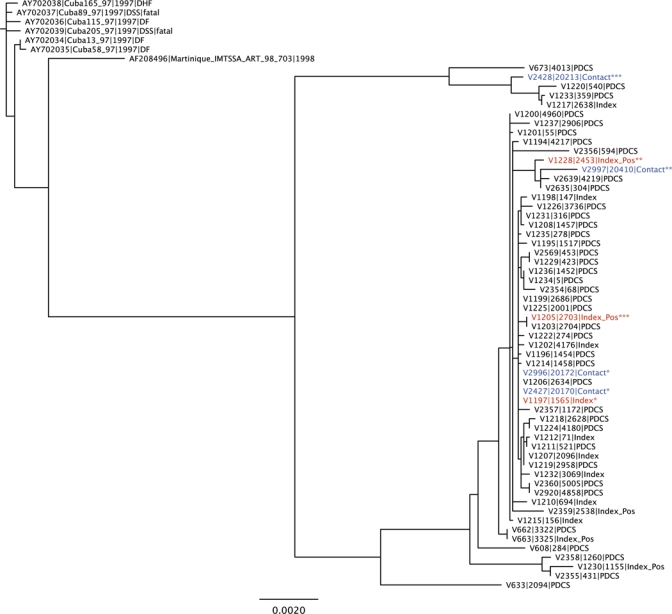
Phylogenetic analysis of dengue virus type 2 (DENV-2) sequences from index cases, cluster contacts, and cohort dengue cases during the 2007 index cluster study in Nicaragua. Complete DENV-2 genomes from Pediatric Dengue Cohort Study participants during the 2007 dengue season, including cluster index cases and four contacts of three index cases, were used to generate a maximum likelihood phylogenetic tree using PhyML v3.0, as described in the Materials and Methods. Other full-length DENV-2 genomes from the Asian-American genotype were used to root the tree. PDCS = dengue cases from the Pediatric Dengue Cohort Study; index = index case; index pos = index case with a dengue-positive contact. *, **, and *** denote paired index cases and their contacts. Red = index case with paired contact in tree; blue = cluster contact. This figure appears in color at www.ajtmh.org.
Discussion
In this pilot study, we have shown the feasibility of using an index cluster design to identify DENV infections prior to symptom onset as well as acute asymptomatic DENV infections. To our knowledge, this is the first report of an effective index cluster study in Latin America. The study, which was conducted by community health workers from the local health center within an established cohort study, had high acceptance in the community, and a day 14 blood sample was collected from more than 89% of the participants. In addition, we were able to obtain full-length sequence of viral isolates from several contacts and perform phylogenetic analysis to investigate the evolutionary relationship with sequences from their index cases and dengue cases in the cohort study during the same period.
The results of this study provide additional information about silent DENV transmission in the Americas. The much higher proportion of asymptomatic to symptomatic cases in 2006 as compared with 2007 paralleled the data obtained in the cohort study,5 which indicated that the cluster method successfully identified asymptomatic DENV infections in similar proportions to the cohort study, but during the acute phase of disease. Likewise, the frequency of DENV infections in the cluster study followed similar trends as in the concurrent large-scale cohort study, in which many more symptomatic cases were identified in the 2007 dengue season compared with 2006.5 In addition, temporal trends in dengue cases paralleled National Dengue Surveillance reports. Interestingly, DENV infections occurred among contacts of negative and positive index cases in our study, suggesting that DENV transmission is widespread throughout urban Managua.
The incidence proportion of post-enrollment DENV infections (2.7%) in this index cluster study in Nicaragua is similar to that observed in the adult and pediatric population study that used the index cluster method in urban Indonesia (2.2% post-enrollment DENV infections).7 In contrast, an index cluster study in rural Thailand among children less than 15 years of age observed post-enrollment DENV infections among 12.4% of contacts of DENV-positive index cases.6 A possible explanation is that the study in Thailand was in children (more susceptible to DENV infection), whereas our study and the study in Indonesia, which had comparable post-enrollment incidence rates, included a substantial adult population (mean age = 30 years in Managua and 23 years in Indonesia, where more children were included). In addition, in Managua, the population has a high level of immunity against DENV, as documented in the concurrent cohort study.5 Thus, the age and concommitent immunity to DENV of contacts seemed to have a major impact on observed incidence of post-enrollment infections, although other geographic, environmental, virologic, and host genetic differences between the studies may also have influenced the outcome.
In Nicaragua, where one serotype typically predominates each year, phylogenetic analysis of viral isolates best enables investigation of the focal nature of transmission. We were able to obtain full-length genomic sequence from DENV isolated from several cluster contacts, their index cases, and dengue cases from the cohort study during the same period. Although the small number precludes definitive conclusions, in two instances, the sequence of the virus isolated from the index case and its contact(s) was identical or nearly identical, which suggested focal transmission. In the third case, the sequence of the virus from the index case was different from that of the positive contact (20213), a 14-year-old girl who could well have been infected with DENV in another part of Managua or via her guardian (20212), a 43-year old woman who was identified as a pre-enrollment DENV infection. Thus, phylogenetic analysis suggested focal transmission and possible infection outside the local area. This finding is consistent with the known focal nature of DENV transmission5,23–26 and with DENV infection in mobile populations.
This study, although useful in its demonstration of the feasibility and utility of the cluster method in the Americas, had too small a sample size to permit definitive conclusions to be drawn. Therefore, we have presented descriptive data and do not attempt to generalize the observations. Rather, these data suggest areas for further study and future application of the index-cluster method. The pediatric population eligible for our study was reduced significantly because approximately one-third of the children in the study district were already under surveillance for dengue as participants in the cohort study and could not be enrolled because of limits on numbers of blood drawings in a given period. Many of the index cases had siblings or other household members and neighbors who were enrolled in the cohort study (13 of 22 index cases), which limited the number of potential contact participants. Five DENV infections were identified in cohort participants living within 50 meters of an index case during the 14 days of cluster study follow-up. Another limitation is that because of severe resource constraints of this pilot study, not all samples were processed by PCR, but rather only those that were positive for antibodies to DENV in paired serologic samples, which resulted in loss of 53 initially enrolled participants who were not located on day 14. The resource limitations also precluded inclusion of additional index cases. Seventy percent of the contacts were female, possibly because women are more likely to be in the home during the daytime when study enrollment was performed. Although no strong sex-related differences in dengue fever have been reported, this finding may have affected identification of infections if household members not enrolled spent more daytime hours, when Ae. aegypti are active, outside the study area.
We show by using an index cluster study that the incidence of post-enrollment DENV infections was similar in Managua and in a comparable population in urban Indonesia. Sequencing of samples from cluster contacts and their index cases, performed here for the first time, improves our understanding of focal and non-localized transmission of DENV strains. This successful implementation of an index-cluster study in the Americas sets the stage for larger studies addressing variations in manifestations of DENV infection and characterizing the immune response prior to symptom onset by identification of pre-symptomatic infections and investigation of the nature of focal transmission and introduction of new DENV strains through phylogenetic analysis of cluster index cases and contacts. In addition, host gene expression analysis and immune profiling of asymptomatic infections in a larger study will provide critical information about natural control of DENV infection and what a vaccine-induced immune response should look like. Finally, future comparisons with results of index cluster studies in Asia will enable more definitive comparison of factors affecting disease severity, silent transmission, and patterns of transmission in the two regions.
Acknowledgments
We give special thanks to Aubree Gordon and Samantha Hammond for help with initial study design and preparation of institutional review board protocols, Patrick Charlebois for technical support with the comparative bioinformatic analysis of the complete genome data, Molly OhAinle for assistance with phylogenetic analysis and tree preparation, and Edward Holmes for support. We also thank the phenomenal study team based at the Centro de Salud Sócrates Flores Vivas and the National Virology Laboratory in the Centro Nacional de Diagnóstico y Referencia for their dedication and high-quality work. Finally, we are extremely grateful to the study participants and their families.
Footnotes
Financial support: This study was supported by the Pediatric Dengue Vaccine Initiative (grant #VE-1) and was supported in part with federal funds from the National Institute of Allergy and Infectious Disease, National Institutes of Health, Department of Health and Human Services, under contracts HHSN266200400001C (Broad) and HHSN272200900006C (Broad).
Authors' addresses: Miguel Reyes and Guillermina Kuan, Centro de Salud Sócrates Flores Vivas, Barrio Monseñor Lezcano, Managua, Nicaragua. Juan Carlos Mercado, Juan Carlos Matute, Berman Moraga, and Angel Balmaseda, Departamento de Virología, Centro Nacional de Diagnóstico y Referencia, Ministerio de Salud, Complejo de Salud Dra. Concepcion Palacios, Primero de Mayo, Managua, Nicaragua. Katherine Standish, Oscar Ortega, and William Avilés, Sustainable Sciences Institute, c/o Centro de Salud Sócrates Flores Vivas, Barrio Monseñor Lezcano, Managua, Nicaragua. Matthew R. Henn, The Broad Institute of Harvard and MIT, Cambridge, MA. Eva Harris, Division of Infectious Diseases and Vaccinology, School of Public Health, University of California, Berkeley, CA.
References
- 1.Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA. Epidemiology of inapparent and symptomatice acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 2.Thein S, Aung MM, Shwe TN, Aye M, Zaw A, Aye K, Aye KM, Aaskov J. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–572. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 3.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 4.Balmaseda A, Hammond SN, Tellez Y, Imhoff L, Rodriguez Y, Saborio S, Mercado JC, Perez L, Videa E, Almanza E, Kuan G, Reyes M, Saenz L, Amador JJ, Harris E. High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua. Trop Med Int Health. 2006;11:935–942. doi: 10.1111/j.1365-3156.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 5.Balmaseda A, Mercado JC, Matute JC, Tellez Y, Saborío S, Hammond SN, Standish K, Nuñez A, Henn MR, Holmes EC, Gordon A, Coloma J, Kuan G, Harris E. Trends in patterns of dengue transmission in a pediatric cohort study in Nicaragua. J Infect Dis. 2010;201:5–14. doi: 10.1086/648592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mammen MP, Pimgate C, Koenraadt CJ, Rothman AL, Aldstadt J, Nisalak A, Jarman RG, Jones JW, Srikiatkhachorn A, Ypil-Butac CA, Getis A, Thammapalo S, Morrison AC, Libraty DH, Green S, Scott TW. Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med. 2008;5:e205. doi: 10.1371/journal.pmed.0050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckett CG, Kosasih H, Faisal I, Nurhayati, Tan R, Widjaja S, Listiyaningsih E, Ma'roef C, Wuryadi S, Bangs MJ, Samsi TK, Yuwono D, Hayes CG, Porter KR. Early detection of dengue infections using cluster sampling around index cases. Am J Trop Med Hyg. 2005;72:777–782. [PubMed] [Google Scholar]
- 8.Halstead SB. Dengue in the Americas and southeast Asia: do they differ? Rev Panam Salud Publica. 2006;20:407–415. doi: 10.1590/s1020-49892006001100007. [DOI] [PubMed] [Google Scholar]
- 9.Anonymous . Managua en Cifras. Instituto Nacional de Información de Desarrollo; Managua, Nicaragua: 2008. [Google Scholar]
- 10.Kuan G, Gordon A, Avilés W, Ortega O, Hammond SN, Elizondo D, Nuñez A, Coloma J, Balmaseda A, Harris E. The Nicaraguan Pediatric Dengue Cohort Study: study design, methods, use of information technology, and extension to other infectious diseases. Am J Epidemiol. 2009;170:120–129. doi: 10.1093/aje/kwp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuno G. In: Dengue and Dengue Hemorrhagic Fever. Gubler DJ, Kuno G, editors. New York: CAB International; 1997. pp. 61–88. (Factors influencing the tranmsission of dengue viruses). [Google Scholar]
- 12.Getis A, Morrison AC, Gray K, Scott TW. Characteristics of the spatial pattern of the dengue vector, Aedes aegypti, in Iquitos, Peru. Am J Trop Med Hyg. 2003;69:494–505. [PubMed] [Google Scholar]
- 13.Balmaseda A, Guzman MG, Hammond S, Robleto G, Flores C, Tellez Y, Videa E, Saborio S, Perez L, Sandoval E, Rodriguez Y, Harris E. Diagnosis of dengue virus infection by detection of specific immunoglobulin M (IgM) and IgA antibodies in serum and saliva. Clin Diagn Lab Immunol. 2003;10:317–322. doi: 10.1128/CDLI.10.2.317-322.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez R, Vasquez S. Serological diagnosis of dengue by an ELISA Inhibition method. Mem Inst Oswaldo Cruz. 1990;85:347–351. doi: 10.1590/s0074-02761990000300012. [DOI] [PubMed] [Google Scholar]
- 15.Harris E, Videa E, Perez L, Sandoval E, Tellez Y, Perez ML, Cuadra R, Rocha J, Idiaquez W, Alonso RE, Delgado MA, Campo LA, Acevedo F, Gonzalez A, Amador JJ, Balmaseda A. Clinical, epidemiologic, and virologic features of dengue in the 1998 epidemic in Nicaragua. Am J Trop Med Hyg. 2000;63:5–11. doi: 10.4269/ajtmh.2000.63.5. [DOI] [PubMed] [Google Scholar]
- 16.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 17.Lanciotti R, Calisher C, Gubler D, Chang G, Vorndam A. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balmaseda A, Sandoval E, Pérez L, Gutiérrez CM, Harris E. Application of molecular typing techniques in the 1998 dengue epidemic in Nicaragua. Am J Trop Med Hyg. 1999;61:893–897. doi: 10.4269/ajtmh.1999.61.893. [DOI] [PubMed] [Google Scholar]
- 19.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 21.Hordijk W, Gascuel O. Improving the efficiency of SPR moves in phylogenetic tree search methods based on maximum likelihood. Bioinformatics. 2005;21:4338–4347. doi: 10.1093/bioinformatics/bti713. [DOI] [PubMed] [Google Scholar]
- 22.Hammond SN, Balmaseda A, Perez L, Tellez Y, Saboría SI, Mercado JC, Videa E, Rodríguez Y, Perez MA, Cuadra R, Solano S, Rocha J, Idiaquez W, Gonzalez A, Harris E. Differences in dengue severity in infants, children, and adults in a three-year hospital-based study in Nicaragua. Am J Trop Med Hyg. 2005;73:1063–1070. [PubMed] [Google Scholar]
- 23.Halstead SB, Scanlon JE, Umpaivit P, Udomsakdi S. Dengue and chikungunya virus infection in man in Thailand, 1962–1964. IV. Epidemiologic studies in the Bangkok metropolitan area. Am J Trop Med Hyg. 1969;18:997–1021. doi: 10.4269/ajtmh.1969.18.997. [DOI] [PubMed] [Google Scholar]
- 24.Morrison AC, Getis A, Santiago M, Rigau-Perez JG, Reiter P. Exploratory space-time analysis of reported dengue cases during an outbreak in Florida, Puerto Rico, 1991–1992. Am J Trop Med Hyg. 1998;58:287–298. doi: 10.4269/ajtmh.1998.58.287. [DOI] [PubMed] [Google Scholar]
- 25.Neff JM, Morris L, Gonzalez-Alcover R, Coleman PH, Lyss SB, Negron H. Dengue fever in a Puerto Rican community. Am J Epidemiol. 1967;86:162–184. doi: 10.1093/oxfordjournals.aje.a120722. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber MJ, Holmes EC, Ong SH, Soh HS, Liu W, Tanner L, Aw PP, Tan HC, Ng LC, Leo YS, Low JG, Ong A, Ooi EE, Vasudevan SG, Hibberd ML. Genomic epidemiology of a dengue virus epidemic in urban Singapore. J Virol. 2009;83:4163–4173. doi: 10.1128/JVI.02445-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


