Abstract
A commercial Dengue Duo rapid test kit was evaluated for early dengue diagnosis by detection of dengue virus NS1 antigen and immunoglobulin M (IgM)/IgG antibodies. A total of 420 patient serum samples were subjected to real-time reverse transcription-polymerase chain reaction (RT-PCR), in-house IgM capture enzyme-linked immunosorbent assay (ELISA), hemagglutination inhibition assay, and the SD Dengue Duo rapid test. Of the 320 dengue acute and convalescent sera, dengue infection was detected by either serology or RT-PCR in 300 samples (93.75%), as compared with 289 samples (90.31%) in the combined SD Duo NS1/IgM. The NS1 detection rate is inversely proportional, whereas the IgM detection rate is directly proportional to the presence of IgG antibodies. The sensitivity and specificity in diagnosing acute dengue infection in the SD Duo NS1/IgM were 88.65% and 98.75%, respectively. The assay is sensitive and highly specific. Detection of both NS1 and IgM by SD Duo gave comparable detection rate by either serology or RT-PCR.
Introduction
The global prevalence of dengue has grown dramatically in recent decades. The disease is now endemic in more than 100 countries in Africa, the America, Eastern Mediterranean, Western Pacific, and particularly in South East Asia. The World Health Organization (WHO) estimates that more than 2.5 billion people are at risk of dengue infections with 50–100 million cases occurring annually. Among these infections, approximately 250,000–500,000 cases are dengue hemorrhagic fever (DHF), with 24,000 deaths that mostly occurred in children.1,2 One of the most important reasons for the increase in cases is most likely caused by rapid development and urbanization, which provide breeding sites for Aedes aegypti, a principal mosquito vector responsible for transmission of dengue virus (DENV). The spread of infection is also enhanced by modern air travel and international trade such as motor vehicle tires, which facilitates the transmission of infected individuals and mosquito larvae to non-infected areas, posing the treat of introducing both the virus and its vector.
Therefore, the emerging pattern and the increasing trend in the incidence of dengue infection are of great concern as there is no specific treatment of dengue, and most forms of therapy are supportive in nature. Furthermore, a licensed vaccine is not available yet.
There are four dengue serotypes (DENV-1, DENV-2, DENV-3, DENV-4), which can cause illnesses in humans ranging from the self-limiting to the life-threatening dengue hemorrhagic fever and dengue shock syndrome (DHF/DSS). Classical dengue fever (DF) is generally self-limited and is characterized by fever and a variety of non-specific signs and symptoms such as headache, malaise, weakness, rash and body aches. The DHF is distinguished from DF by the onset of plasma leakage, marked thrombocytopenia, and a bleeding diathesis. Severe plasma leakage can lead to shock, with the mortality rate for untreated patients varying between 10% and 20% but can reach as high as 40% with the involvement of shock. However, the figure can be minimized to 0.2% in hospitals with staff trained in managing the disease.3
With the increasing incidence of dengue infection, the early diagnostic confirmation of dengue infection in patients allows for timely clinical intervention, etiological investigation, and disease control. Hence, diagnosis of dengue disease during the acute phase should be a priority and is a public health concern. Several approaches have been applied for laboratory diagnosis of dengue virus infection. These methods include detection of the virus (by cell culture, immunofluorescence), detection of virus antigen (by enzyme-linked immunosorbent assay [ELISA]), detection of anti-dengue virus antibody (by hemagglutination inhibition [HI], complement fixation test [CF], neutralization tests, ELISA), and detection of virus nucleic acid (by real-time reverse transcription-polymerase chain reaction [RT-PCR]). However, for a confirmed dengue diagnosis, DENV should be identified by isolation or nucleic acid detection or there should be a 4-fold rise in antibody titer with paired sera in patients presenting with signs and symptoms that are consistent with dengue virus infection. Recently, commercially available kits have been developed for the rapid detection of dengue infections.4–8 These kits are designed based on the principle of detecting the presence of NS1 antigen and/or anti-dengue antibodies in the blood of suspected dengue patients.
The NS1 is a highly conserved glycoprotein that is present at high concentrations in sera of dengue-infected patients during the early clinical phase of disease, and is found from Day 1 and up to Day 9 after onset of fever in sample of primary or secondary dengue-infected patients.9,10 The IgM become detectable on Day 3 to 5 of illness in case of primary dengue infection and persist for 2 to 3 months, whereas IgG appear by the fourteenth day and persist for life. Secondary infection shows that IgG rises within 1 to 2 days after onset of symptoms, simultaneously with IgM antibodies. Therefore, patients with secondary infections will have a positive IgG result, usually, but not always with a positive IgM result.11–13
In this study, a commercially available rapid dengue diagnostic kit, the SD BIOLINE Dengue Duo combo device (Standard Diagnostic Inc., Korea), which is designed for the detection of dengue NS1 antigen and IgM/IgG antibodies was evaluated for it potential application for early diagnosis of acute dengue virus infection based on a single acute serum sample. The evaluation was performed with reference to the existing laboratory diagnostic test methods, such as dengue virus isolation, real-time RT-PCR, in-house IgM capture ELISA (MAC-ELISA), and HI.
Materials and Methods
Serum specimens.
A total of 420 sera were used in this evaluation. These blood samples were collected from patients admitted to University Malaya Medical Center (UMMC), Kuala Lumpur, Malaysia, for acute viral infection. These sera consist of 1) 30 samples from which dengue virus was isolated, 2) 50 samples positive for dengue virus RNA by RT-PCR, 3) 50 samples from which dengue NS1 antigen was detected positive by both the Platelia Dengue NS1 antigen (Bio-Rad Laboratories, Marnes-la-Coquette, France) and pan-E dengue early ELISA (Panbio, Queensland, Australia), 4) 50 samples that were IgM negative but sero-converted in convalescent sera, 5) 10 pairs of confirmed primary dengue, 6) 10 pairs of confirmed secondary dengue, 7) 100 random samples for which IgM to dengue was detected, 8) 20 samples that were diagnosed clinically as dengue but laboratory tested as negative, and 9) 80 samples from healthy donors and patients with infections other than dengue (Table 1A and B). All samples were subjected to virus isolation, in-house MAC-ELISA, in-house dengue real-time RT-PCR, and HI assay. Written informed consent was obtained from the patients. Ethical clearance was given by the Scientific and Ethical Committee at the UMMC, with procedures carried out in accordance with the Helsinki Declaration of 1975, as revised in 2000.14
Table 1A.
Serum samples used for the evaluation of SD BIOLINE Dengue Duo kit
| Category | Sample number (n) |
|---|---|
| (a) Acute serum from which dengue virus was isolated | 30 |
| (b) Dengue PCR-positive sera | 50 |
| (c) Dengue NS1 antigen positive sera | 50 |
| (d) Sero-negative acute samples (IgM negative), but convalescent samples sero-converted | 50 |
| (e) Primary dengue sera (10 pairs) | 20 |
| (f) Secondary dengue sera (10 pairs) | 20 |
| (g) IgM positive sera (varying P/N ratio)* | 100 |
| (h) Clinically dengue but laboratory tested negative | 20 |
| (i) Other disease agents and healthy donors (negative control) | 80 |
| Total | 420 |
P/N ratio (positive:negative ratio): A P/N ratio of greater than or equal to 2.0 was considered positive; a result with a P/N ratio of less than 2 was reported as negative.
Table 1B.
Panel of negative control*
| Other disease agents and healthy donors | Sample number (n) |
|---|---|
| (a) BK virus | 10 |
| (b) Cytomegalovirus (CMV) | 10 |
| (c) Measles | 2 |
| (d) Epstein-Barr virus | 10 |
| (f) Japanese encephalitis | 11 |
| (g) Chikungunya | 7 |
| (h) Herpes simplex virus (HSV) | 3 |
| (i) CMV & HSV dual infection | 1 |
| (j) Nipah IgG positive | 5 |
| (k) Leptospirosis | 1 |
| (l) Ricketsia | 1 |
| (m) S. typhi (n) West Nile | 36 |
| (o) Healthy donor | 10 |
| Total | 80 |
Table shows the 15 groups of negative controls and the number of the serum samples that were used for the evaluation in this study.
Virus isolation.
Virus isolation was carried out by inoculating 5 μL of acute sera into confluent C6/36 mosquito cell lines and incubated at 28°C for 7 to 10 days. Dengue antigens were then detected by immunofluorescence with dengue-specific monoclonal antibodies obtained from Center for Disease Control and Prevention (Foothills Campus, Fort Collins, CO).15,16
In-house hemagglutination inhibition assay.
The HI assay was carried out as described earlier.17 Briefly, 25 μL of 0.4% bovine albumin borate saline (BABS) was added into wells 2 to 12 of a 96-well ELISA microtiter plate. Fifty microliters and 25 μL of acetone-extracted serum were added into wells 1 and 12, respectively, and the serum was titrated from well 1 to well 12. Twenty-five microliters antigen was added into the first 11 wells while BABS was added into the last well (serum control), after which the plate was covered and incubated at 4°C for 18 to 24 hours. Freshly prepared Goose RBC (50 μL) was added into all the wells and the microtiter plate was further incubated at 37°C for 45 minutes, before interpreting the results. The results are interpreted according to WHO guidelines.18
In-house IgM capture ELISA (MAC-ELISA).
The in-house MAC ELISA was carried out as described earlier.19 Briefly, 100 μL of a 1:100 dilution of sample was added to human anti-IgM-coated 96-well U bottomed plates and incubated at 37°C for 1 hour. After washing three times with PBS-Tween 20 (0.05%), 100 μL of a 1:100 dilution of dengue antigen was added and then incubated at 37°C for another hour. The plates were then washed three times with PBS-Tween 20 (0.05%), followed by addition of 100 μL of a 1:5000 dilution of anti-dengue mouse monoclonal antibody and further incubation at 37°C for 1 hour. The plates were washed again with PBS-Tween 20 (0.05%) and 100 μL of a 1:50,000 dilution of goat anti-mouse IgG conjugated with horseradish peroxidase (HRP) was added, before incubation for 1 hour at 37°C hour. After three additional washes, 100 μL of OPD (o-phenylendiamine 2HCl) was added to all wells and incubated in the dark at room temperature. The reaction was stopped with 50 μL 4N sulphuric acid and the absorbance (OD) of each well was read at λ490 nm with a reference filter of 630 nm using an ELISA reader. The positive versus negative ratio was then calculated. The positive control/sample OD was divided by the mean of the negative OD to obtain a positive:negative ratio (P/N). A P/N ratio of equal or greater than 2.0 was considered positive. A result with a P/N ratio of less than 2.0 was reported as negative if the sample was collected two weeks after disease onset.
Real-time RT-PCR.
The one-step SYBR green I real-time RT-PCR was carried out in an iCycler (Bio-Rad Laboratories, Hercules, CA) thermocycler using an iScript one-step RT-PCR Kit with SYBR Green I (Bio-Rad Laboratories), as described by Yong and others.20 Briefly, 5 μL of the extracted sample RNA was added to a 25 μL reaction containing 1 × SYBR Green I, 75 nM of each primer, and 3 mM of MgCl2. The thermal cycling conditions consisted of a 30 minutes reverse transcription at 50°C, 15 minutes of Taq polymerase activation at 95°C, followed by 45 cycles of PCR at 95°C denaturation for 30 seconds, 60°C of annealing for 30 seconds, and 72°C extension for 1 minute. Following amplification, the melting curves were analyzed. This is to verify the specificity of the PCR product by looking at its Tm. Melting curve analysis consisted of a denaturation step at 95°C for 1 minute, lowered to 55°C for 30 seconds, and followed by 80 cycles of incubation in which the temperature is increased to 95°C at a rate of 0.5°C/10 seconds/cycle. The Tm of each specific PCR product was analyzed using iCycler iQ optical system software version 3.0a (Bio-Rad Laboratories). The Tm for each sample was used to identify the dengue serotype and the sample sharing the same Tm was interpreted as belonging to the same serotype.
SD BIOLINE Dengue Duo NS1 Ag and IgG/IgM test.
The SD BIOLINE Dengue Duo rapid test kit is produced by Standard Diagnostics and is a one-step immunochromatographic assay designed for the detection of both dengue virus NS1 antigen and differential IgM/IgG antibodies to dengue virus in human whole blood, serum, or plasma. The SD BIOLINE Dengue Duo rapid test contains two test devices; the left side is for dengue NS1 antigen test, whereas the right side is for dengue IgG/IgM test. These kits were designed based on the principle that when a specimen is added to the sample well, anti-dengue IgG and IgM in the specimen will react with recombinant dengue virus envelope proteins-colloidal gold conjugates and forms a complex of antibodies-antigen. This complex will be captured by the relevant anti-human IgG and/or anti-human IgM immobilized on the test device and generate a colored line when migrated along the length of the test device by capillary action. Similarly, dengue NS1 antigen captured by the anti-dengue NS1 Ag-colloid gold conjugate will migrate along the length of the device until being captured by the anti-dengue NS1 antigen immobilized on the membrane strips and generate a color line. All tests in this study were carried out in accordance with the manufacturer's instructions. Briefly, for SD BIOLINE dengue NS1 Ag device, 100 μL of the test sample was added into the sample well (S). Test results were interpreted at 15–20 minutes. Similarly, for SD BIOLINE dengue IgG/IgM device, 10 μL of test sample was added into the sample well (S). This was followed by the addition of 4 drops (90–120 μL) of assay diluent to the round shaped assay diluent well. Results were interpreted at 15–20 minutes. The test results were examined and interpreted according to the manufacturer instructions by three different readers to avoid biasness.
Data analysis.
Tabulation, management, and analysis of raw data were carried out using Microsoft Excel (Microsoft Inc., Redmond, WA). Statistical analysis was performed with Statistica version 18 (StatSoft, Inc., Tulsa, OK). The sensitivity, specificity, efficiency, positive predictive value (PPV), and negative predictive value for the assays were calculated based on “true positive dengue samples” (virus isolation/PCR positives, sero-negative acute sera, acute primary, acute secondary) using the following formula:
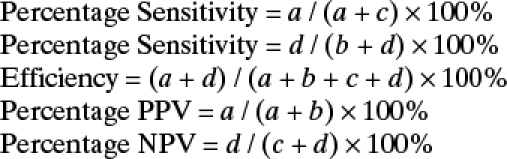 |
where
 |
Results
A total of 420 samples collected from year 2007 until year 2009 were selected for the evaluation of the SD BIOLINE Dengue Duo rapid test kit. The evaluation of the SD rapid test kit was assessed against a panel of samples including true positives by standard tests such as virus isolation and real-time RT-PCR, IgM positive sera, NS1 antigen positive sera, and sera derived from healthy donors and other disease agents. Results are summarized in Table 2. Of the 320 sera for dengue, 168 (52.5%) tested positive for SD Duo NS1 Ag, and 220 (68.75%) tested positive for SD Duo IgM. A diagnostic strategy combining SD Duo NS1 or IgM (NS1/IgM) gave a 289 (90.31%) positive detection. In comparison, dengue infection was detected by either MAC-ELISA or RT-PCR in 300 samples (93.75%), by MAC-ELISA in 229 samples (71.56%), and by RT-PCR in 162 samples (50.63%). Among the 30 samples that were virus isolation positive, the SD Duo NS1/IgM detected 21 (70%) as positive. One sample from the category that was clinically dengue but laboratory tested as negative, and another sample from the negative control panel were also tested positive by the SD Dengue Duo kit.
Table 2.
Comparison of SD BIOLINE Dengue Duo kit with in-house MAC-ELISA and RT-PCR*
| Category of samples | Number (n) | No. of samples that tested positive, n (%) | |||||
|---|---|---|---|---|---|---|---|
| MAC-ELISA | RT-PCR | SD DUO NS1 | SD DUO IgM | SD DUO IgG | SD DUO NS1/IgM | ||
| Dengue virus isolation positive | 30 | 9 (30) | 30 (100) | 18 (60) | 11 (36) | 9 (30) | 21 (70) |
| Dengue RT-PCR positive | 50 | 46 (92) | 50 (100) | 22 (44) | 40 (80) | 37 (74) | 44 (88) |
| Dengue NS1 positive† | 50 | 34 (68) | 35 (70) | 39 (78) | 33 (66) | 19 (38) | 47 (94) |
| Sero-negative acute sample but convalescent sero-converted | 50 | 0 (0) | 37 (74) | 43 (86) | 7 (14) | 9 (18) | 46 (92) |
| Dengue IgM-positive sera | 100 | 100 (100) | 1 (1) | 28 (28) | 92 (92) | 84 (84) | 93 (93) |
| Primary dengue | 20 | 20 (100) | 6 (30) | 17 (85) | 19 (95) | 4 (20) | 20 (100) |
| Secondary dengue | 20 | 20 (100) | 3 (15) | 1 (5) | 18 (90) | 20 (100) | 18 (90) |
| Clinically dengue but Laboratory tests negative | 20 | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 1 (5) |
| Non-dengue sera (negative panel) | 80 | 0 (0) | 0 (0) | 1 (1.7) | 0 (0) | 2 (3.4) | 1 (1.7) |
MAC-ELISA = in-house IgM capture-enzyme-linked immunosorbent assay; RT-PCR = reverse transcription-polymerase chain reaction.
NS1 positive sera were confirmed by both the Platelia Dengue NS1 antigen (Bio-Rad Laboratories, Marnes-la-Coquette, France) and pan-E dengue early ELISA test (Panbio, Queensland, Australia).
Analysis on the performance of SD Dengue Duo in detecting acute dengue has shown that SD Duo NS1 gave 100% positive detection of NS1 antigen in all primary dengue acute samples, whereas only 10% of secondary dengue acute samples were detected for NS1 antigen. On the other hand, 90% of each primary and secondary acute dengue was tested IgM positive by SD Duo IgM. Combining SD Duo NS1/IgM gave a positive detection rate of 100% and 90%, respectively, for primary and secondary acute dengue infections (Table 3).
Table 3.
Performance of SD BIOLINE Dengue Duo kit in primary and secondary dengue infections
| Category | Number (n) | Samples tested positive, n (%) | |||||
|---|---|---|---|---|---|---|---|
| RT-PCR | MAC-ELISA | SD DUO NS1 | SD DUO IgM | SD DUO IgG | SD DUO NS1/IgM | ||
| Primary dengue acute | 10 | 6 (60) | 10 (100) | 10 (100) | 9 (90) | 0 (0) | 10 (100) |
| Secondary dengue acute | 10 | 3 (30) | 10 (100) | 1 (10) | 9 (90) | 10 (100) | 9 (90) |
| Primary dengue convalescent | 10 | 0 (0) | 10 (100) | 7 (70) | 10 (100) | 4 (40) | 10 (100) |
| Secondary dengue convalescent | 10 | 0 (0) | 10 (100) | 0 (0) | 0 (0) | 10 (100) | 9 (90) |
Further analysis of the data indicated that the NS1 detection rate is inversely proportional, whereas the IgM detection rate is directly proportional to the presence of IgG antibodies as depicted by the HI value (Figure 1). At lower levels of antibodies (HI ≤ 320) the SD Duo NS1 gave better detection rates as compared with high levels of antibodies, whereas high levels of antibodies (≥ 640 HI ≤ 10 240) gave better detection rate for SD Duo IgM/IgG. For acute dengue diagnosis, a combined analysis of both SD Duo NS1 and IgM/IgG has allowed for a more sensitive early diagnosis of acute dengue infection.
Figure 1.
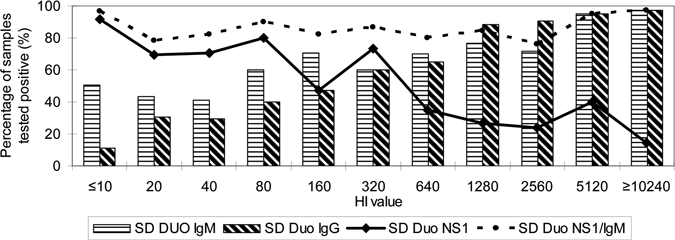
Hemagglutination inhibition (HI) value vs. positive detection rate for laboratory diagnosis of dengue. A total of 320 samples were used in the analysis. Samples were derived from the following category of samples: virus isolation positive (N = 30), dengue RT-PCR (N = 50), NS1 positive (N = 50), sero-negative acute sera but convalescent sero-converted (N = 50), random IgM positive samples (N = 100), primary dengue (N = 20), and secondary dengue (N = 20).
The data with regards to day of onset of disease symptoms in corresponding to the SD Dengue Duo kit were further determined. As seen in Figure 2, dengue NS1 antigen could be detected up to Day 12 from the onset of fever, whereas RT-PCR was only able to detect viral RNA up to Day 8 post-infection. The SD Dengue Duo rapid kit was also used to detect dengue positive samples comprising all four serotypes of dengue viruses (N = 162) and results are presented in Table 4. No significant difference in detection was observed between the four dengue serotypes (P > 0.05).
Figure 2.
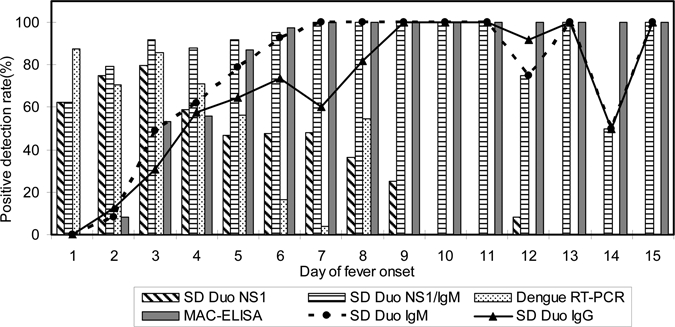
Comparison of SD BIOLINE Dengue Duo commercial kit and conventional laboratory methods in relation to day of onset of fever. A total of 320 samples were used in the analysis. These sample panels are virus isolation positive (N = 30), dengue reverse transcription-polymerase chain reaction (RT-PCR) (N = 50), NS1 positive (N = 50), sero-negative acute sera but convalescent sero-converted (N = 50), random IgM positive samples (N = 100), primary dengue (N = 20), and secondary dengue (N = 20).
Table 4.
Comparative performances of SD BIOLINE Dengue Duo in detecting dengue virus serotypes*
| Dengue diagnostics | Sensitivity %, [95% CI] | P value (between group) | |||
|---|---|---|---|---|---|
| DENV-1 (N = 101) | DENV-2 (N = 21) | DENV-3 (N = 23) | DENV-4 (N = 17) | ||
| SD Dengue Duo NS1 | 62.38 (63/101) [52.77–71.99] | 61.90 (13/21) [39.25–84.55] | 86.96 (20/23) [72.07–100] | 70.59 (12/17) [46.44–94.71] | 0.14 |
| SD Dengue Duo IgM | 57.43 (58/101) [47.62–67.24] | 57.14 (12/21) [34.06–80.22] | 43.48 (10/23) [21.56–65.40] | 41.18 (7/17) [15.10–67.26] | 0.44 |
| SD Dengue Duo NS1/IgM | 90.10 (91/101) [84.18–96.03] | 76.19 (16/21) [56.32–90.06] | 95.65 (22/23) [86.63–100] | 82.35 (14/17) [62.15–100] | 0.17 |
χ2 test was used for statistical analysis (StatSoft, Inc.). A P value > 0.05 indicating that the results were not significantly associated.
The total sensitivity and specificity of the SD Dengue Duo rapid kit were calculated based on samples that were considered true dengue (N = 185) by virus isolation, RT-PCR, or a rising titer in a paired samples (Table 5). The overall sensitivity and specificity of SD Duo NS1 is 65.41% (95% confidence interval [CI]: 58.49–72.32) and 98.75% (95% CI: 96.26–100) with an efficiency of 75.47%, whereas SD Duo IgM gave 53.51% (95% CI: 46.26–60.76) sensitivity and 100% specificity with an efficiency of 67.55%. By combining both tests for NS1 and IgM, the SD Dengue Duo NS1/IgM gave a better performance for detection of acute dengue, with an overall sensitivity of 88.65% (95% CI: 84.04–93.26) and specificity of 98.75% (95% CI: 96.26–100), and an assay efficiency of 91.70%.
Table 5.
Performance of SD BIOLINE Dengue Duo kit in the diagnosis of dengue
| Dengue diagnostics | Sensitivity, % (N = 185) (95% CI)* | Specificity, % (N = 80) (95% CI)† | Efficiency, % (N = 265) (95% CI) | Positive predictive value, % (95% CI) | Negative predictive value, % (95% CI) |
|---|---|---|---|---|---|
| SD Dengue Duo NS1 | 65.41 (121/185) [58.49–72.32] | 98.75 (79/80) [96.26–100] | 75.47 (200/265) [70.26–80.68] | 99.18 (121/122) [97.56–100] | 55.24 (79/143) [46.99–63.49] |
| SD Dengue Duo IgM | 53.51 (99/185) [46.26–60.76] | 100 (80/80) | 67.55 (179/265) [61.88–73.22] | 100 (99/99) | 48 (80/166) [40.32–55.68] |
| SD Dengue Duo NS1/IgM | 88.65 (164/185) [84.04–93.26] | 98.75 (79/80) [96.26–100] | 91.70 (243/265) [88.36–95.04] | 99.39(164/165) [91.68–100] | 79.0 (79/100) [71.29–86.71] |
True positive samples used in analysis were derived from the following category of samples: virus isolation positive (N = 30), dengue RT-PCR (N = 50), a dengue NS1 antigen positive sera, which is also positive for dengue RT-PCR (N = 35), and sero-negative acute sera but convalescent sero-converted (N = 50), acute primary samples (N = 10), and acute secondary samples (N = 10).
True negative samples were derived from non-dengue sera, which comprise patients with other infectious agents and healthy donors.
Discussion
Early laboratory diagnosis of acute dengue virus infection is important to provide appropriate treatment of the patients and to prevent potential dengue outbreak. At present, the most commonly used techniques in the laboratory for diagnosing of dengue virus infections are virus isolation, detection of viral nucleic acid by RT-PCR, and detection of dengue-specific IgM by MAC-ELISA. Although virus isolation is considered as gold standard for laboratory diagnosis of dengue, it is laborious and requires at least 6–10 days for the virus to replicate.11 On the other hand, RT-PCR and MAC-ELISA are expensive, and usually take at least half a day for running the assay. Moreover, these facilities are not widely available in the clinics and hospital settings. Hence, a rapid test kit would be useful to provide early diagnosis of acute dengue infection.
The advantage of this SD Dengue Duo rapid test kit is that it is designed to detect both dengue virus NS1 antigen and differential IgM/IgG antibodies to dengue virus in human blood. NS1 antigen is generally found during Day 1 and up to Day 9 after onset of fever. The detection of anti-NS1 is inhibited if anti-NS1 antibodies produced. Whereas IgM become detectable by Day 3 to Day 5 after onset of illness in primary dengue and by Day 1 to Day 2 after onset of illness in secondary infections.11–13 The uniqueness of this combo device has made diagnosis of dengue possible as early as Day 1 of dengue infection.
In this study, we evaluated the use of the SD Dengue Duo rapid test kit for the diagnosis of dengue infections. A diagnostic strategy combining both SD Duo NS1 and IgM were used in the interpretation of results, as this could increase the overall clinical sensitivity of both assays. The sensitivity of SD Duo NS1/IgM (88.65%) compares well with recent studies.4,5,21–23 The specificity (98.75%) observed in our study also correlated with all earlier studies.4,5,21–23 However, most used an ELISA-based assay except for Vajpayee and other21 and Dussart and other.22 In their studies, however, sample size was smaller but most conclusions made were similar. Analysis of all samples in groups revealed that the detection of true dengue was lowest in the RT-PCR group (88%), and virus isolation group (70%). Sensitivity was highest in the primary acute dengue group (100%), whereas with secondary acute dengue sera group a lower sensitivity of 90% was observed. A much lower sensitivity of detection rate was noted for the primary group when SD Duo NS1 (85%) and SD Duo IgM (95%) were used separately for detection of dengue. This has suggested that both NS1 and IgM are essential components in the early diagnosis of dengue. This was further supported by the observation that the presence of high antibodies gave better detection rate for SD Duo IgM/IgG device, whereas SD Dengue NS1 gave better detection rates at low levels of antibodies as shown in Figure 2.
The specificity for SD Dengue Duo assay was shown to be 98% with only one sample detected with NS1 antigen. However, the types of samples used to determine specificity are limited and more samples, especially from other flaviviral infections such as West Nile virus, St. Louis Encephalitis virus, Roccio virus, and related viruses in this group need to be included to ensure adequate coverage of possible cross-reactions. Obtaining such sera that is dengue antibody negative is sometimes not possible because of the high endemicity in the tropical belt and also to effects of globalization. Another reason is that more individuals have been exposed to multiple flaviviruses as they travel to various regions as tourists.
This evaluation shows that SD Dengue Duo rapid test kit is useful for the rapid, early diagnosis of dengue infections. The current evaluation of the SD Dengue Duo NS1/IgM shows that this assay has a sensitivity of 88.65% (95% CI: 84.04–93.26), a specificity of 98.75% (95% CI: 96.26–100) with an assay efficiency of 91.70%. By detecting both NS1 and IgM in dengue infection has shown that the SD Dengue Duo rapid test kit is useful, sensitive, and specific for the diagnosis of acute dengue infection.
Supplementary Material
Acknowledgments
We thank SD Diagnostics, Inc., Korea, for the generous provision of the SD Dengue Duo kits, the clinicians and the nurses at the University Malaya Medical Centre (UMMC), Kuala Lumpur, Malaysia, who assisted in collecting samples; and the patients for their participation in this study. All the monoclonal antibodies used in the immunofluorescence were provided by Centre for Disease Control and Prevention, Fort Collins, CO. We thank Kimberly Holloway from National Microbiology Laboratory, Public Health Agency of Canada, and Canadian Science Centre for Human and Animal Health, for providing us with West Nile serum samples. We also thank Sutee Yoksan from Center for Vaccine Development, Institute of Molecular Biosciences of Mahidol University at Salaya for providing us with Japanese encephalitis serum samples.
Disclaimer: We have no financial conflict of interest.
Note: Supplementary data appear at www.ajtmh.org.
Footnotes
Authors' addresses: Seok Mui Wang and Shamala Devi Sekaran, Department of Medical Microbiology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, E-mails: seokmuiwang@yahoo.com and shamalaya@yahoo.com.
Reprint requests: Shamala Devi Sekaran, Department of Medical Microbiology, Faculty of Medicine, University of Malaya, Kuala Lumpur, 50603, Malaysia, Tel: 603-79675755, Fax: 603-79676672, E-mail: shamalaya@yahoo.com.
References
- 1.Gubler DJ, Meltzer M. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv Virus Res. 1999;53:35–70. doi: 10.1016/s0065-3527(08)60342-5. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Handbook of the World Health Organization. Geneva: WHO; 2000. pp. 1–84. (Dengue haemorrhagic fever: diagnosis, treatment and control). [Google Scholar]
- 3.Innis BL. In: Exotic Viral Infections. Porterfield JS, editor. London: Chapman & Hall Medical; 1995. pp. 103–140. (Dengue and dengue haemorrhagic fever). [Google Scholar]
- 4.Blacksell SD, Mammen MP, Thongpaseuth S, Gibbons RV, Jarman RG, Jenjaroen K, Nisalak A, Phetsouvanh R, Newton PN, Day NP. Evaluation of the Panbio dengue virus nonstructural 1 antigen detection and immunoglobulin M antibody enzyme-linked immunosorbent assays for the diagnosis of acute dengue infections in Laos. Diagn Microbiol Infect Dis. 2008;60:43–49. doi: 10.1016/j.diagmicrobio.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Dussart P, Labeau B, Lagathu G, Louis P, Nunes MR, Rodrigues SG, Storck-Hermann C, Cesaire R, Morvan J, Flamand M, Baril L. Evaluation of an enzyme immunoassay for detection of dengue virus NS1 antigen in human serum. Clin Vaccine Immunol. 2006;13:1185–1189. doi: 10.1128/CVI.00229-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumarasamy V, Wahab AH, Chua SK, Hassan Z, Chem YK, Mohamad M, Chua KB. Evaluation of a commercial dengue NS1 antigen-capture ELISA for laboratory diagnosis of acute dengue virus infection. J Virol Methods. 2007;140:75–79. doi: 10.1016/j.jviromet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 7.McBride W. Evaluation of dengue NS1 test kits for the diagnosis of dengue fever. Diagn Microbiol Infect Dis. 2009;64:31–36. doi: 10.1016/j.diagmicrobio.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Sekaran SD, Lan EC, Mahesawarappa KB, Appanna R, Subramaniam G. Evaluation of a dengue NS1 capture ELISA assay for the rapid detection of dengue. J Infect Dev Ctries. 2007;1:182–188. [Google Scholar]
- 9.Young PR, Hilditch PA, Bletchly C. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J Clin Microbiol. 2000;38:1053–1057. doi: 10.1128/jcm.38.3.1053-1057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flammand M. Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in blood during the acute phase of disease in patient experiencing primary or secondary infection. J Clin Microbiol. 2002;40:376–381. doi: 10.1128/JCM.40.2.376-381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shu P, Huang J. Current advances in dengue diagnosis. Clin Diagn Lab Immunol. 2004;11:642–650. doi: 10.1128/CDLI.11.4.642-650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gubler DJ. Serological diagnosis of dengue haemorrhagic fever. Dengue Bull. 1996;20:20–23. [Google Scholar]
- 13.Innis B. In: Dengue and Dengue Haemorrhagic Fever. Gubler DJ, Kuno G, editors. New York: CAB International; 1997. pp. 221–243. (Antibody responses to dengue virus infections). [Google Scholar]
- 14.World Medical Association Declaration of Helsinki Ethical principles for medical research involving human subjects. JAMA. 2000;284:3043–3045. [PubMed] [Google Scholar]
- 15.Kuberski TT, Rosen L. A simple technique for detection of dengue antigen in mosquitoes by immunofluorescence. Am J Trop Med Hyg. 1977;26:533–537. doi: 10.4269/ajtmh.1977.26.533. [DOI] [PubMed] [Google Scholar]
- 16.Gubler DJ, Kuno G, Sather GE, Velez M, Oliver A. Use mosquitoes cell culture and specific monoclonal antibodies for routine surveillance of dengue viruses. Am J Trop Med Hyg. 1984;33:158–165. doi: 10.4269/ajtmh.1984.33.158. [DOI] [PubMed] [Google Scholar]
- 17.Clarke DH, Cassals J. Techniques for haemagglutination and haemagglutination inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization . Regional Guidelines on Dengue/DHF Prevention & Control. WHO/SEARO; 1997. (Management of dengue epidemic). [Google Scholar]
- 19.Lam SK, Devi S, Pang T. Detection of specific IgM in dengue infection. Southeast Asian J Trop Med Public Health. 1987;18:532–538. [PubMed] [Google Scholar]
- 20.Yong YK, Thayan R, Chong HT, Tan CT, Sekaran SD. Rapid detection and serotyping of dengue virus by multiplex RT-PCR and real-time SYBR green RT-PCR. Singapore Med J. 2007;448:662–668. [PubMed] [Google Scholar]
- 21.Vajpayee M, Singh UB, Seth P, Broor S. Comparative evaluation of various commercial assays for diagnosis of dengue fever. Southeast Asian J Trop Med Health. 2001;32:472–475. [PubMed] [Google Scholar]
- 22.Dussart P, Petit L, Labeau B, Bremand L, Leduc A, Moua D, Matheus S, Baril L. Evaluation of two new commercial tests for the diagnosis of acute dengue virus infection using NS1 antigen detection in human. PLoS Negl Trop Dis. 2008;2:e280. doi: 10.1371/journal.pntd.0000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapphra K, Sangsharaswichai A, Chokephaibulkit K, Tiengrim S, Piriyakarnsakul W, Chakorn T, Yoksan S, Wattanamongkolsil L, Thamlikitkul V. Evaluation of an NS1 antigen detection for diagnosis of acute dengue infection in patients with acute febrile illness. Diagn Microbiol Infect Dis. 2008;60:387–391. doi: 10.1016/j.diagmicrobio.2007.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


