Abstract
Guaroa virus (GROV) was first isolated from humans in Colombia in 1959. Subsequent isolates of the virus have been recovered from febrile patients and mosquitoes in Brazil, Colombia, and Panama; however, association of the virus with human disease has been unclear. As part of a study on the etiology of febrile illnesses in Peru and Bolivia, 14 GROV strains were isolated from patients with febrile illnesses, and 3 additional cases were confirmed by IgM seroconversion. The prevalence rate of GROV antibodies among Iquitos residents was 13%; the highest rates were among persons with occupations such as woodcutters, fisherman, and oil-field workers. Genetic characterization of representative GROV isolates indicated that strains from Peru and Bolivia form a monophyletic group that can be distinguished from strains isolated earlier in Brazil and Colombia. This study confirms GROV as a cause of febrile illness in tropical regions of Central and South America.
Introduction
Guaroa virus (GROV) was first isolated in Guaroa, Meta Department, Colombia in 1959 from people without overt illness.1 Subsequently, isolates of GROV have been made from febrile persons in Brazil and mosquitoes in Colombia, Panama, and Brazil.2–4 Epidemiological investigations conducted in Colombia from 1956–1961 revealed that a significant number of people living in the Middle Magdalena Valley (especially adults) had GROV antibodies.5 Ecological investigations conducted thereafter have repeatedly isolated GROV from Anopheles (Kerteszia) neivai; consequently, this species has been implicated as the putative mosquito vector of GROV, which constitutes a rare event for arboviruses.4,6 Other arboviruses potentially transmitted by Anopheles mosquitoes include Breu Branco, Kadipiro, and Getah.7,8
Follow-up serologic studies in the town of Guaroa (Colombia) in 1956 indicated that 49 of 69 (75%) residents of the community had neutralizing antibodies to GROV.1 Another serosurvey in northern Brazil (Para state) found that 18% of residents had hemagglutination-inhibition (HI) antibodies to GROV.3 Low titers of HI antibodies to GROV have also been reported in sera of residents of Argentina, Sao Paulo state in Brazil, Peru, and Guatemala.9 Collectively, the results of these studies suggest that GROV is widely distributed in Central and South America; however, its association with a specific human illness or disease syndrome remains unclear.
The virus is a member of the family Bunyaviridae, genus Orthobunyavirus; it contains a segmented negative-strand RNA genome of three segments (S, M, and L). The L segment encodes the L protein (RNA polymerase); the M segment encodes the polyprotein precursor of the virion glycoproteins, G1 and G2, and the nonstructural protein NSm, and the S segment encodes for the N and NSs proteins.10–12 The taxonomic status of GROV has been controversial. Initially, GROV was considered to be a member of the California antigenic group (family Bunyaviridae) based on the results of hemagglutination-inhibition HI tests.13,14 Subsequently, Whitman and Shope14 showed that GROV was antigenically related to viruses in both the California and Bunyamwera serogroups. Based on the results of complement-fixation (CF) tests, GROV could be placed in the Bunyamwera group, but based on neutralization tests, it was more closely related to the California group. Because the two tests measure different gene products (nucleocapsid and glycoproteins, respectively), Bishop15 suggested that GROV represented a reassortant virus that possesses RNA segments originally derived from Bunyamwera and California virus groups. Subsequent sequence data for the S RNA of GROV indicated that it should be classified in the Bunyamwera serogroup rather than the California serogroup.16 In the Eighth Report of the International Committee on Taxonomy of Viruses,10 GROV is currently classified as a unique species within the genus Orthobunyavirus, and it is considered distinct from viruses included in the Bunyamwera and California species complexes.
In 2000, the United States Naval Medical Research Center Detachment (NMRCD) in Lima, in collaboration with the Ministries of Health of Bolivia and Peru, initiated a passive surveillance study to investigate etiology of febrile illnesses. As part of the surveillance program in Peru and Bolivia, 17 confirmed cases of GROV infection were diagnosed in patients with acute, self-limited febrile illnesses. Nine of seventeen confirmed cases were reported in 2007 in Peru (between January and November), and two cases were also confirmed in 2007 and 2009 in febrile patients from Bolivia, providing the first evidence that GROV also circulates in that country.
In the present study, we investigated the epidemiology of GROV infection, the prevalence of GROV antibodies and risk factors for infection among residents of the Amazonian city of Iquitos, Peru, and the phylogenetic relationship among the GROV strains isolated in Peru, Bolivia, and other South American countries.
Materials and Methods
Study sites.
The confirmed GROV human cases reported in this study lived in several distinct areas of Peru, including the cities of Iquitos in the Department of Loreto, Puerto Maldonado in the Department of Madre de Dios, La Merced in the Department of Junin, and Tumbes on the coast. Iquitos is a city of about 380,000 inhabitants located 120 m above sea level in the Amazon Basin in northeastern Peru. Puerto Maldonado is a city of approximately 56,000 habitants located in southeastern Peru about 256 m above sea level on the banks of the Madre de Dios river near the border with Brazil and Bolivia. La Merced is a city of about 50,000 persons located 751 m above sea level in the Department of Junin. Tumbes is a Pacific coastal city located in the north near the border with Ecuador; according to the 2007 census, it had a population of 139,811 inhabitants. Two GROV isolates were also obtained from febrile patients living in Cochabamba Department in Bolivia. Figure 1 shows the approximate geographic locations of the study sites where GROV infections were confirmed.
Figure 1.
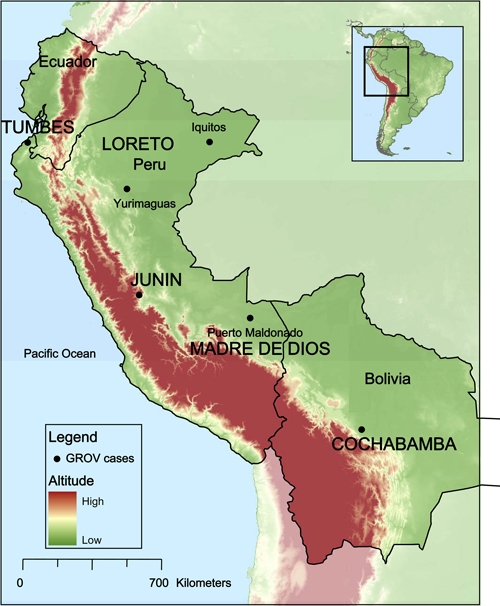
Geographic distribution of the Guaroa virus human cases identified as part of the febrile disease surveillance program. This figure appears in color at www.ajtmh.org.
Passive febrile surveillance study population.
The study protocols were approved by the Ministries of Health of Peru and Bolivia and the Naval Medical Research Center Institutional Review Board (protocols NMRCD.2000.0006, NMRCD.2000.0008, and NMRCD.2008.0002). The study subjects were patients (> 5 years of age) who presented with a diagnosis of an acute, febrile undifferentiated illness in their home or at military or civilian outpatient clinics at the study sites. Demographic and clinical information was obtained from each patient at the time of voluntary enrollment, and a signed consent form was obtained from each subject. The criteria for inclusion in the program were fever ≥ 38°C of no more than 5 days in duration, headache, myalgia, and other nonspecific symptoms. Two paired blood samples were collected, one during the acute phase of illness and the second sample 2–4 weeks after onset of symptoms. Acute samples were tested for virus by cell culture, and both acute and convalescent samples were assayed for IgM antibodies to a variety of arboviruses (including GROV) by an enzyme-linked immunosorbent assay (ELISA), as described previously.17 Diagnosis of a confirmed GROV infection (case) was based on isolation of the virus and/or a 4-fold or greater increase in IgM antibody titer between the acute and convalescent serum samples. A case was considered as presumptive when IgM antibodies were detected in a single acute sample or in both acute and convalescent samples without a 4-fold increase in titer.
Antibody prevalence studies.
The antibody prevalence of GROV was determined in Iquitos by testing a total of 1,124 human serum samples for IgG antibodies to GROV by an ELISA, as previously described.17 The samples were collected in 2006 as part of a cross-sectional antibody prevalence study carried out in Iquitos after an outbreak of febrile illness associated with Venezuelan equine encephalitis virus (VEEV) infection. Samples were collected in three Iquitos neighborhoods where Venezuelan equine encephalitis (VEE) cases were reported as well as in a control neighborhood where VEE cases were not reported.18 Thus, the selected population represented a suitable population to test the prevalence of GROV and other arboviral diseases. Serum samples from a subset of the original study participants, who agreed to the future use of their samples, were tested. All ELISA IgG antibody-positive samples were further evaluated using an 80% plaque-reduction neutralization assay (PRNT) for GROV. Briefly, sera were heat-inactivated at 56°C for 30 minutes, and two 2-fold serum dilutions were prepared, mixed with 100 plaque forming units (PFUs) of GROV (prototype Peruvian strain OBS 0069), and incubated at 4°C overnight. The virus–serum dilutions mixtures were inoculated onto confluent monolayer of Vero cells propagated in microplates and incubated at 37°C for 1 hour before adding an overlay of 0.4% of agarose in Eagle's minimum essential medium (EMEM). After 72 hours of incubation at 37°C, the plates were stained with 0.25% crystal violet in 20% methanol, and plaques were counted. All IgG-positive samples were tested at an initial concentration of 1:20, and all positive sera were further titrated to the endpoint. Neutralization titers were considered as the highest serum dilution that reduced plaque formation by ≥ 80%.
Virus isolation.
Patient's serum specimens were diluted 1:5 in EMEM, supplemented with 2% fetal bovine serum, 200 μg streptomycin, and 200 U/mL penicillin. Two hundred microliters of diluted samples were then inoculated into flasks with confluent monolayers of African green monkey kidney cells (Vero) and Aedes albopictus mosquito (C6/36) cells. Vero cell cultures were examined daily for evidence of viral cytopathic effect (CPE). Spot slides of C6/36 and Vero cells were subsequently prepared, and an immunofluorescence assay (IFA) was done using polyclonal antibodies against arboviruses endemic in Peru.17,19–23 A variety of arboviruses were isolated from these samples and will be reported elsewhere. The 14 GROV isolates are listed in Table 1.
Table 1.
Guaroa confirmed cases included in the study
| Strain | Study | Location | Year | Host | Age | Sex | Occupation | Laboratory diagnostic |
|---|---|---|---|---|---|---|---|---|
| CoH 352111 | NA | Colombia | 1956 | Human | NA | NA | NA | Virus isolation* |
| CoAr 2526 | NA | Colombia | 1964 | Mosquito | NA | NA | NA | * |
| BeH 22063 | NA | Para, Brazil | 1960 | Human | NA | NA | NA | Virus isolation* |
| 31498 | NA | NA | NA | NA | NA | NA | NA | * |
| FVB 0546 | Febrile surveillance | Cochabamba, Bolivia | 2007 | Human | 17 | Female | Student | Virus isolation |
| FVB 2032 | Febrile surveillance | Cochabamba, Bolivia | 2009 | Human | 24 | Male | Agricultural worker | Virus isolation |
| OBS 0069 | Outbreak investigations | Iquitos, Loreto, Peru | 1995 | Human | 38 | Male | Agricultural worker | Virus isolation |
| IQU 1091 | Febrile surveillance | Iquitos, Loreto, Peru | 1999 | Human | 30 | Male | Air Force | Virus isolation |
| IQD 8537 | Febrile surveillance | Iquitos, Loreto, Peru | 2004 | Human | 31 | Male | Agricultural worker | Virus isolation |
| FSJ 1266 | Febrile surveillance | La Merced, Junin, Peru | 2006 | Human | 32 | Male | Agricultural worker | Seroconversion |
| FSJ 1318 | Febrile surveillance | La Merced, Junin, Peru | 2007 | Human | 30 | Male | Driver | Virus isolation |
| FSJ 1335 | Febrile surveillance | La Merced, Junin, Peru | 2007 | Human | 35 | Male | Agricultural worker | Virus isolation |
| FSJ 1340 | Febrile surveillance | La Merced, Junin, Peru | 2007 | Human | 43 | Male | Driver | Virus isolation |
| FST 1122 | Febrile surveillance | Zarumilla, Tumbes, Peru | 2007 | Human | 27 | Female | Seller | Seroconversion |
| FMD 1553 | Febrile surveillance | Iberia, Madre de Dios, Peru | 2007 | Human | 17 | Female | House wife | Virus isolation |
| OBT 5637 | Outbreak investigations | Puerto Maldonado, Madre de Dios, Peru | 2007 | Human | 21 | Female | House wife | Virus isolation |
| OBT 5655 | Outbreak investigations | Puerto Maldonado, Madre de Dios, Peru | 2007 | Human | 30 | Male | Local healer | Virus isolation |
| OBT 5667 | Outbreak investigations | Puerto Maldonado, Madre de Dios, Peru | 2007 | Human | 27 | Male | Miner | Virus isolation |
| FMD 1720 | Febrile surveillance | Madre de Dios, Peru | 2007 | Human | 17 | Female | Miner | Seroconversion |
| FMD 1806 | Febrile surveillance | Tambopata, Madre de Dios, Peru | 2008 | Human | 37 | Male | Health worker | Virus isolation |
| MIS 0239 | Other | Puerto Maldonado, Madre de Dios, Peru | 2008 | Human | NA | Male | NA | Virus isolation |
NA = not applicable.
Samples provided by the World Health Organization Reference Collection, University of Texas Medical Branch (UTMB).
Extraction of RNA, reverse transcription, and PCR amplification of S, M, and L segments.
Viral RNA was extracted using the QIAamp viral RNA mini kit (Qiagen, Valencia, CA) or Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. The reverse transcription reaction (RT) was done using 1× RT buffer, 0.2 mM deoxyribonucleotide triphosphate (dNTPs), 1 μM primers, 80 units RNAsin ribonuclease inhibitor (Promega, Madison, WI), 1 mM dithiothreitol, 200 U SuperScript reverse transcriptase (Invitrogen), and 5 μL RNA. The reactions were incubated at 42°C for 1 hour. The PCR included 1× PCR buffer, 0.25 mM dNTPs, 1 μM primers, 3 mM MgCl2, 2.5 U GoTaq DNA polymerase (Promega), and 5 μL cDNA. The conditions for the PCRs included incubation at 94°C for 2 minutes, 35 cycles of 94°C for 30 seconds, 50°C for 1 minute, 72°C for 1.5 minutes, and a final extension of 72°C for 10 minutes to ensure complete double-stranded DNA synthesis. The primers used for the PCR amplification have been previously described and included Bunya 1 (GTCACAGTAGTGTACTCCAC) and Bunya 2 (CTGACAGTAGTGTGCTCCAC), which amplifies the S segment, M14C (CGGAATTCAGTAGTGTACTACC) and M619R (GACATATG(CT)TGATTGAAGCAAGCATG) that amplifies the M segment, and M13CBunL1C (TGTAAAACGACGGCCAGTAGTGTACTCCT) and BunL605R (AGTGAAGTCICCATGTGC), which amplifies the L segment.24
Sequencing and phylogenetic analyses.
To genetically characterize the GROV strains isolated in Peru, partial sequences of the S, M, and L segments were obtained and compared with those of GROV isolates from Brazil, Colombia, and Bolivia using a previously described methodology.24 Purified PCR products were sequenced directly, and sequencing analyses of the PCR products were performed using an Applied Biosystems (Foster City, CA) Prism automated DNA sequencing kit according to the manufacturer's protocol. Sequences were aligned using the Clustal program in the MacVector (MacVector Inc., Cary, NC) software package, and phylogenetic analyses were performed using the maximum parsimony, neighbor-joining, and maximum likelihood methods implemented in the phylogenetic analysis using parsimony (PAUP) software (Sinauer Associates, Sunderland, MA).25,26 For the neighbor-joining analyses, the HKY85 distance was used. Bootstrap values to place confidence values on groupings within trees were calculated based on 1,000 replicates.
Statistical analyses.
Proportions were compared using a χ2 test using the FREQ procedure in SAS (SAS version 8; SAS Institute Inc., Cary, NC). Risk factors for infection with GROV were evaluated by logistic regression using LOGISTIC in SAS. Models were constructed with the dichotomous dependent variable: PRNT positive for GROV antibody at a titer of ≥ 20 and the following independent variables: gender, age (adult or child), occupation, type of house, travel history, and neighborhoods.
Results
Description of GROV cases detected through febrile surveillance.
In 1995, GROV was isolated for the first time in Peru from a patient presenting with an undifferentiated febrile illness. Between 2000 and 2009, 16 additional confirmed GROV cases and 30 presumptive cases were identified in patients presenting with febrile illness. The most common symptoms among these patients were headache, chills, malaise, myalgia, arthralgia, and bone pain (Table 2). The majority of patients with confirmed GROV infection were males (12 of 17, 70.6%) with a mean age of 28.5 years (range = 17–43) (Table 1). These patients were mainly miners, wood cutters, agricultural workers, and students living in areas with high levels of arbovirus circulation.17,19–23,27–30 Fifteen of the cases were detected in Peru, whereas two GROV cases were confirmed in Cochabamba, Bolivia, providing the first evidence of circulation in that country. The Bolivian patients were a 17-year-old female student and a 24-year-old male agricultural worker. Year-round GROV activity was observed in Peru.
Table 2.
Principal clinical manifestations for 13 patients with confirmed Guaroa virus infection
| Sign and symptom | Patients (%) |
|---|---|
| Headache | 92 |
| Malaise | 92 |
| Chills | 85 |
| Myalgia | 77 |
| Arthralgia | 69 |
| Bone pain | 62 |
| Retro-orbital pain | 54 |
| Nausea | 46 |
| Asthenia | 46 |
| Abdominal pain | 23 |
| Conjunctival injection | 23 |
| Vomiting | 23 |
| Rash | 15 |
| Rhinorrhea | 15 |
| Cough | 15 |
| Weight Loss | 8 |
| Petechiae | 8 |
| Ear pain | 8 |
| Arthritis | 8 |
| Expectoration | 8 |
The largest number of GROV cases were detected in 2007 and 2008 from samples collected in La Merced, Junin, and Puerto Maldonado, Madre de Dios. One GROV case was detected in the coastal city of Tumbes in Peru in 2007 (Tables 1 and 3). A slight increase in GROV activity was observed in 2007 in La Merced and in 2008 in Madre de Dios (Table 3). The majority of patients with GROV infection (44% of the cases) in Puerto Maldonado reported having recently visited or worked in Bajo Puquiri, a gold-prospecting and extraction area in Madre de Dios Department that is currently undergoing intensive environmental modification.
Table 3.
Cases of Guaroa virus infection among febrile patients residing in Peru and Bolivia
| Guaroa virus cases | Madre de Dios | Junin | Iquitos, Loreto | Tumbes | Cusco | Yurimaguas | Bolivia | Total | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | 2008 | 2009 | 2006 | 2007 | 2008 | 2009 | 1995 | 1999 | 2004 | 2005 | 2007 | 2008 | 2009 | 2007 | 2008 | 2008 | 2007 | 2009 | 2007 | 2009 | ||
| Virus isolations | 4 | 2 | 0 | 0 | 3 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 14 |
| Seroconversion | 1 | 0 | 0 | 1 | 0 | 0 | 0 | ND | ND | ND | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Presumptive cases | 3 | 8 | 2 | 0 | 3 | 1 | 0 | ND | ND | ND | 1 | 2 | 3 | 2 | 0 | 0 | 3 | 1 | 1 | 0 | 0 | 30 |
| Total confirmed and presumptive GROV cases | 8 | 10 | 2 | 1 | 6 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 3 | 2 | 1 | 0 | 3 | 1 | 1 | 1 | 2 | 50 |
| Total number febrile cases seen at site | 593 | 301 | 355 | 120 | 105 | 133 | 111 | 867 | 810 | 2,308 | 1,459 | 1,197 | 1,885 | 1,104 | 193 | 264 | 121 | 309 | 280 | 645 | 537 | 13,697 |
| Percentage GROV cases/total febrile cases | 1.3 | 3.3 | 0.6 | 0.8 | 5.7 | 0.8 | 0 | 0.1 | 0.1 | 0.04 | 0.07 | 0.2 | 0.2 | 0.2 | 0.5 | 0 | 2.5 | 0.3 | 0.4 | 0.2 | 0.4 | 0.37 |
ND = not done.
Risk factors for GROV infection in the city of Iquitos, Peru.
Antibody prevalence and risk-factor data were obtained from blood samples collected as part of a cross-sectional antibody prevalence study carried out in four neighborhoods in Iquitos after an outbreak of febrile illness associated with VEEV infection.18 Iquitos was selected for study, because GROV was first detected in this city in 1995 and thus, there was evidence that the virus had been circulating in the area for more than 10 years.
In Iquitos, the overall antibody prevalence of GROV was 13% (144/1,124). The prevalence of GROV antibodies in the Iquitos population increased with age after adulthood (> 19 years of age), suggesting endemic circulation of the virus in this Amazon region of Peru (4.4% in 5- to 9-year-olds to 35.9% in 60- to 69-year-olds) (Figure 2). The antibody prevalence in adults was 16% compared with 5.6% in children [odds ratio (OR) = 3.3; 95% confidence interval (CI) = 1.992–5.467]. Persons who reported overnight travel had higher antibody prevalence rates than those who did not (P < 0.05).
Figure 2.
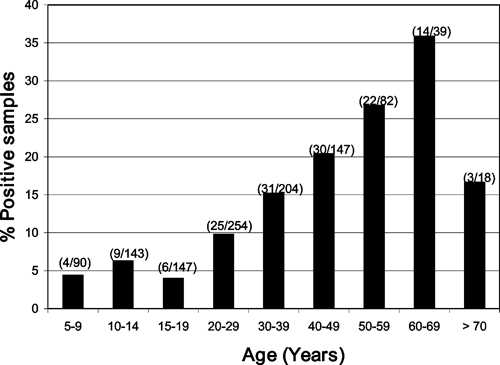
Guaroa virus antibody prevalence among residents by age groups in Iquitos.
Persons with high-risk occupations were fishermen, wood cutters, and oil workers; these groups had a higher prevalence of GROV antibodies than people with other occupations [7/23 (30%) versus 137/1,101 (12%)]. In addition, persons living in concrete/brick houses had significantly lower antibody prevalence rates than those living in wood houses (OR = 0.312; 95% CI = 0.179–0.546), and persons living in neighborhoods closer to the rivers surrounding the city (Belen, Bellavista, and San Juan) also had a higher antibody prevalence than those in the north-central parts of Iquitos where socio-economic conditions are higher (Figure 3). The univariate logistic regression analysis did not detect an association between gender and GROV antibody prevalence.
Figure 3.
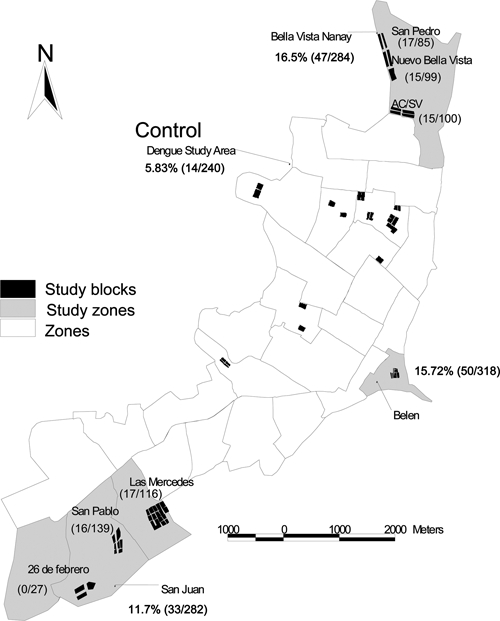
Guaroa virus antibody prevalence among residents by neighborhood in Iquitos.
Genetic characterization of the GROV isolates from Peru and other regions of South America.
Phylogenetic analyses using maximum parsimony, neighbor-joining, and maximum likelihood methods all generated similar tree topologies. Only the neighbor-joining phylogenetic trees are shown for simplicity reasons. The neighbor-joining tree based on the partial S and L segment sequences revealed a single genotype within the isolates from Peru and Bolivia. In contrast, strains from Colombia and Brazil (isolated between 1956 and 1964) differed by 4% at the amino acid level compared with the more recent (1995–2008) Peruvian and Bolivian isolates, and thus, they group within different genotypes in the phylogenetic tree (Figures 4 and 5). The neighbor-joining tree based on the M segment produced a similar tree topology as the S and L phylogenetic tree; however, the strains isolated from Junin, Peru grouped within a distinct genotype from the other isolates from different geographical regions in Peru (Figure 6).
Figure 4.
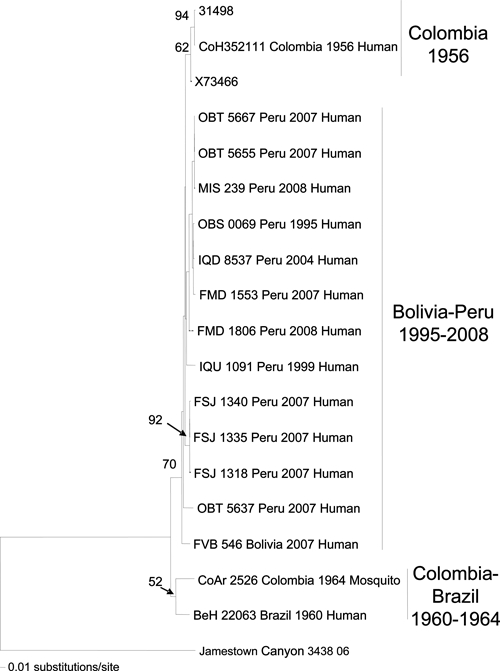
Neighbor-joining phylogenetic tree for Guaroa virus generated based on partial sequences of the S segment. The tree was rooted using Jamestown Canyon virus as the outgroup. Viruses are labeled by code designation, country name, year of isolation, and source. Numbers indicate bootstrap values.
Figure 5.

Neighbor-joining phylogenetic tree for Guaroa virus generated based on partial sequences of the M segment. The tree was rooted using Jamestown Canyon virus as the outgroup. Viruses are labeled by code designation, country name, and year of isolation. Numbers indicate bootstrap values.
Figure 6.
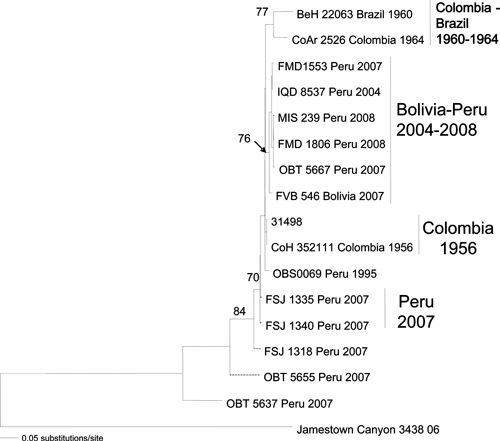
Neighbor-joining phylogenetic tree for Guaroa virus generated based on partial sequences of the L segment. The tree was rooted using Jamestown Canyon virus as the outgroup. Numbers indicate bootstrap values.
Discussion
GROV was initially isolated in Colombia from asymptomatic individuals as well as from persons exhibiting mild fever, raising the question of whether the virus consistently causes disease.1 Later, GROV cases identified in Brazil included individuals with fever and other symptoms, such as headache, myalgia, and prostration; however, several of these subjects also had Plasmodium falciparum infections.31,32 GROV was also isolated from the liver biopsy of a Brazilian patient with paralysis.2 In the present study of febrile illnesses in Peru and Bolivia, additional evidence for the disease potential of GROV was obtained. Seventeen patients who presented with undifferentiated febrile illnesses were diagnosed with GROV infection.
The most common clinical symptoms in the patients with confirmed GROV infection were chills, malaise, bone pain, headache, retro-orbital pain, myalgia, and arthralgia. Given the non-specific clinical manifestations in these cases, it would be very difficult to differentiate GROV infection clinically from other endemic arboviral illnesses. Because our surveillance activities included only eight sites in Peru and three in Bolivia (Figure 1), it is very likely that GROV infections occur in other areas of these countries but remain undiagnosed. Additional studies are needed to determine the ratio of apparent to unapparent cases and to fully measure the burden and the public-health impact of GROV infection in Peru, Bolivia, and other regions of South America.
Previous epidemiologic studies conducted in South America revealed a high antibody prevalence to GROV in selected populations in Colombia (43%) and Brazil (18%).1,31 Low HI antibody titers to GROV antigen were also found in sera of residents from Argentina, Peru, and Guatemala.9 However, further testing of the positive samples, using a confirmatory PRNT to GROV, was not performed. Because of the cross-reactivity of HI antibodies among orthobunyaviruses, it is uncertain whether those antibodies were specific to GROV or to other related members of this genus that are known to circulate in the area.31,33
To further investigate the frequency of GROV infection in Peru and the risk factors associated with infection in an endemic area of transmission, human serum samples collected in Iquitos in 2006 were assayed for GROV antibodies. An overall antibody prevalence rate of 13% was found among the Iquitos population. These results are consistent with previous serological studies done in Peru in 1965.29 Factors associated with infection in Iquitos included living in neighborhoods located near surrounding rivers that are seasonally flooded and occupations such as agriculture, fishing, and mineral prospecting and extraction. Results from our study also suggested that GROV transmission probably occurs in the forest or away from home.
Although GROV was first detected in Iquitos in 1995, the number of confirmed infections identified in our current surveillance activity was relatively low (~0.04–0.2%), despite the 13% overall antibody prevalence rate. In contrast, the presence of GROV in Madre de Dios was not detected until 2007, when evidence of GROV infection was observed in about 1% of the febrile cases in the region. By 2008, 3% of the febrile cases at the site had evidence of recent GROV infection. Eight of eighteen patients with evidence of GROV infection (2007–2008) reported recent work or travel in the area. It seems likely that environmental and land-use changes as well as human migration have played an important role in the emergence of this human pathogen in Madre de Dios. Improper and unregulated mining activities have been implicated as a factor responsible for the emergence of other vector-borne diseases such as malaria and yellow fever in Brazil.34 Further research is needed to determine more precisely the impact of these activities on the emergence of GROV and other arboviral diseases in Madre de Dios.
Despite our current knowledge of GROV as a human pathogen, little is known about its reservoir hosts and vectors, especially in Peru. Earlier investigations in Colombia, Panama, and Brazil detected the virus in Anopheles mosquitoes4,31; however, studies to evaluate the susceptibility and transmission potential of this mosquito genus have not been done. During previous ecological studies conducted in the Amazon region of Peru, many arboviruses were isolated from other mosquito genera,23 but GROV was never isolated from these other mosquitoes, suggesting that they are not involved in GROV transmission. Additional ecological studies are needed in Peru and Bolivia to identify the mosquito vectors and reservoir hosts involved in GROV transmission. Likewise, previous serological investigations in Brazil suggested that birds may act as reservoir hosts for GROV; however, these results remain to be confirmed.9
In summary, results of this study further confirm that GROV is a cause of febrile illness among humans in tropical regions of Central and South America.
Acknowledgments
The authors thank Roxana Caceda, Alfredo Huaman, Roger Castillo, and Juan Sulca for invaluable support. We also thank the Peruvian and Bolivian Ministries of Health for supporting the study and the physicians at the study sites for their participation and help. This study was funded by the United States Department of Defense Global Emerging Infections Systems Research Program Work Unit Number 847705.82000.25GB.B0016. The study protocol was approved by the Naval Medical Research Center Institutional Review Board (Protocols NMRCD.2000.0009, NMRCD.2000.0006, and NMRCD.2008.0002) in compliance with all applicable federal regulations governing the protection of human subjects. R.B.T. was supported by National Institutes of Health contract N01-AI30027.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government. None of the authors has a financial or personal conflict of interest related to this study. The corresponding author had full access to all data in the study and final responsibility for the decision to submit this publication. Some authors are military service members. This work was prepared as part of their official duties. Title 17 USC §105 provides that “[c]opyright protection under this title is not available for any work of the United States Government.” Title 17 USC §101 defines a US Government work as a work prepared by military service members or employees of the US Government as part of those person's official duties.
Footnotes
Authors' addresses: Patricia V. Aguilar, United States Naval Medical Research Center Detachment, Washington, DC, E-mail: patricia.aguilar@med.navy.mil. Douglas M. Watts, Office of Research and Sponsored Projects, University of Texas at El Paso, El Paso, TX. Amy C. Morrison, Naval Medical Research Center Detachment, Iquitos, Peru and Department of Entomology, University of California, Davis, CA, E-mail: amy.aegypti@gmail.com. Claudio Rocha, Naval Medical Research Center Detachment, Iquitos, Peru, E-mail: claudiorochacalderon@gmail.com. Cristhopher Cruz, Naval Medical Research Center Detachment, Lima, Peru, E-mail: cristhopher.cruz@med.navy.mil. Carolina Guevara, Naval Medical Research Center Detachment, Lima, Peru, E-mail: carolina.guevara@med.navy.mil. Joel M. Montgomery, Naval Medical Research Center Detachment, Lima, Peru, E-mail: joel.montgomery@med.navy.mil. Tadeusz J. Kochel, Naval Medical Research Center Detachment, Lima, Peru, E-mail: tad.kochel@med.navy.mil. Luis Beingolea, Office of Epidemiology, Lima, Peru, E-mail: lbeingolea@conhu.org.pe. Victor Suarez, National Institute of Health, Lima, Peru, E-mail: vjsuarezm@yahoo.com. Jorge Vargas, Centro de Enfermedades Tropicales, Santa Cruz, Bolivia, E-mail: drjvargasf@hotmail.com.
References
- 1.Groot H, Oya A, Bernal C, Barreto-Reyes P. Guaroa virus, a new agent isolated in Colombia, South America. Am J Trop Med Hyg. 1959;8:604–609. doi: 10.4269/ajtmh.1959.8.604. [DOI] [PubMed] [Google Scholar]
- 2.Causey OR, Shope RE, Rodrigues Filho A. Isolamento do virus Guaroa do figado por biopsia percutanea de um caso humano com paralisia. Rev Serv Espec Saude Pub. 1962;12:55–59. [Google Scholar]
- 3.Causey OR, Causey CE, Maroja OM, Macedo DG. The isolation of arthropod-borne viruses, including members of two hitherto undescribed serologic groups, in the Amazon region of Brazil. Am J Trop Med Hyg. 1961;10:227–249. doi: 10.4269/ajtmh.1961.10.227. [DOI] [PubMed] [Google Scholar]
- 4.Lee VH, Sanmartin C. Isolations of Guaroa virus from Anopheles (Kerteszia) Neivai in the Pacific lowlands of Colombia. Am J Trop Med Hyg. 1967;16:778–781. [PubMed] [Google Scholar]
- 5.Groot H. Estudios sobre virus transmitidos por artropodos en Colombia. Rev Acad Colomb. 1964;12:197–217. [Google Scholar]
- 6.Peralta PH, Shelokov A. Isolation and characterization of arboviruses from Almirante, Republic of Panama. Am J Trop Med Hyg. 1966;15:369–378. doi: 10.4269/ajtmh.1966.15.369. [DOI] [PubMed] [Google Scholar]
- 7.Sun X, Fu S, Gong Z, Ge J, Meng W, Feng Y, Wang J, Zhai Y, Wang H, Nasci R, Tang Q, Liang G. Distribution of arboviruses and mosquitoes in northwestern Yunnan Province, China. Vector Borne Zoonotic Dis. 2009;9:623–630. doi: 10.1089/vbz.2008.0145. [DOI] [PubMed] [Google Scholar]
- 8.Vieira Cde M, Nunes MR, da Silva EV, Carvalho VL, Nunes Neto JP, Cruz AC, Casseb SM, Vasconcelos HB, Quaresma JA, Vasconcelos PF. Full-length sequencing and genetic characterization of Breu Branco virus (Reoviridae, Orbivirus) and two related strains isolated from Anopheles mosquitoes. J Gen Virol. 2009;90:2183–2190. doi: 10.1099/vir.0.010165-0. [DOI] [PubMed] [Google Scholar]
- 9.Theiler M, Downs WG. The Arthropod-Borne Viruses of Vertebrates. New Haven, CT: Yale University Press; 1973. [Google Scholar]
- 10.Nichol ST, Beaty BJ, Elliott RM, Goldbach R, Plyusnin A, Schmaljohn CS, Tesh RB. In: Virus Taxonomy. Eighth Report of the International Committee on Taxonomy of Viruses. Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. San Diego, CA: Elsevier Academic Press; 2005. pp. 695–716. (Family Bunyaviridae). [Google Scholar]
- 11.Gentsch JR, Bishop DH. Small viral RNA segment of bunyaviruses codes for viral nucleocapsid protein. J Virol. 1978;28:417–419. doi: 10.1128/jvi.28.1.417-419.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentsch JR, Bishop DHL. M viral RNA segment of bunyaviruses codes for two glycoproteins, G1 and G2. J Virol. 1979;30:767–770. doi: 10.1128/jvi.30.3.767-770.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calisher CH. In: The Bunyaviridae. Elliot RM, editor. New York: Plenum Press; 1996. pp. 1–17. (History, classification and taxonomy of viruses in the family Bunyaviridae). [Google Scholar]
- 14.Whitman L, Shope RE. The California complex of arthropod-borne viruses and its relationship to the Bunyamwera group through Guaroa virus. Am J Trop Med Hyg. 1962;11:691–696. doi: 10.4269/ajtmh.1962.11.691. [DOI] [PubMed] [Google Scholar]
- 15.Bishop DH. In: International Symposium on Tropical Arboviruses and Haemorrhagic Fevers. Pinheiro FP, editor. Rio de Janeiro, Brazil: Academia Brasileira de Ciencias; 1982. pp. 117–133. (The molecular properties of bunyaviruses including Group C isolates and the genetic capabilities of bunyaviruses to form recombinants). [Google Scholar]
- 16.Dunn EF, Pritlove DC, Elliott RM. The S RNA genome segments of Batai, Cache Valley, Guaroa, Kairi, Lumbo, Main Drain and Northway bunyaviruses: sequence determination and analysis. J Gen Virol. 1994;75:597–608. doi: 10.1099/0022-1317-75-3-597. [DOI] [PubMed] [Google Scholar]
- 17.Watts DM, Callahan J, Rossi C, Oberste MS, Roehrig JT, Wooster MT, Smith JF, Cropp CB, Gentrau EM, Karabatsos N, Gubler D, Hayes CG. Venezuelan equine encephalitis febrile cases among humans in the Peruvian Amazon River region. Am J Trop Med Hyg. 1998;58:35–40. doi: 10.4269/ajtmh.1998.58.35. [DOI] [PubMed] [Google Scholar]
- 18.Morrison AC, Forshey BM, Notyce D, Astete H, Lopez V, Rocha C, Carrion R, Carey C, Eza D, Montgomery JM, Kochel TJ. Venezuelan equine encephalitis virus in Iquitos, Peru: urban transmission of a sylvatic strain. PLoS Negl Trop Dis. 2008;2:e349. doi: 10.1371/journal.pntd.0000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguilar PV, Greene IP, Coffey LL, Medina G, Moncayo AC, Anishchenko M, Ludwig GV, Turell MJ, O'Guinn ML, Lee J, Tesh RB, Watts DM, Russell KL, Hice C, Yanoviak S, Morrison AC, Klein TA, Dohm DJ, Guzman H, Travassos da Rosa AP, Guevara C, Kochel T, Olson J, Cabezas C, Weaver SC. Endemic Venezuelan equine encephalitis in northern Peru. Emerg Infect Dis. 2004;10:880–888. doi: 10.3201/eid1005.030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oberste MS, Weaver SC, Watts DM, Smith JF. Identification and genetic analysis of Panama-genotype Venezuelan equine encephalitis virus subtype ID in Peru. Am J Trop Med Hyg. 1998;58:41–46. doi: 10.4269/ajtmh.1998.58.41. [DOI] [PubMed] [Google Scholar]
- 21.Watts DM, Lavera V, Callahan J, Rossi C, Oberste MS, Roehrig JT, Cropp CB, Karabatsos N, Smith JF, Gubler DJ, Wooster MT, Nelson WM, Hayes CG. Venezuelan equine encephalitis and Oropouche virus infections among Peruvian army troops in the Amazon region of Peru. Am J Trop Med Hyg. 1997;56:661–667. doi: 10.4269/ajtmh.1997.56.661. [DOI] [PubMed] [Google Scholar]
- 22.Travassos da Rosa AP, Turell MJ, Watts DM, Powers AM, Vasconcelos PF, Jones JW, Klein TA, Dohm DJ, Shope RE, Degallier N, Popov VL, Russell KL, Weaver SC, Guzman H, Calampa C, Brault AC, Lemon AP, Tesh RB. Trocara virus: a newly recognized Alphavirus (Togaviridae) isolated from mosquitoes in the Amazon Basin. Am J Trop Med Hyg. 2001;64:93–97. doi: 10.4269/ajtmh.2001.64.93. [DOI] [PubMed] [Google Scholar]
- 23.Turell MJ, O'Guinn ML, Jones JW, Sardelis MR, Dohm DJ, Watts DM, Fernandez R, Travassos da Rosa A, Guzman H, Tesh R, Rossi CA, Ludwig V, Mangiafico JA, Kondig J, Wasieloski LP, Jr, Pecor J, Zyzak M, Schoeler G, Mores CN, Calampa C, Lee JS, Klein TA. Isolation of viruses from mosquitoes (Diptera: Culicidae) collected in the Amazon Basin region of Peru. J Med Entomol. 2005;42:891–898. doi: 10.1603/0022-2585(2005)042[0891:IOVFMD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Bowen MD, Trappier SG, Sanchez AJ, Meyer RF, Goldsmith CS, Zaki SR, Dunster LM, Peters CJ, Ksiazek TG, Nichol ST. A reassortant bunyavirus isolated from acute hemorrhagic fever cases in Kenya and Somalia. Virology. 2001;291:185–190. doi: 10.1006/viro.2001.1201. [DOI] [PubMed] [Google Scholar]
- 25.Swofford DL. PAUP* Phylogenetic Analysis Using Parsimony (*and Other Methods) Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- 26.Wilgenbusch JC, Swofford D. Current Protocols in Bioinformatics, Chapter 6: Unit 6.4. Somerset, NJ: John Wiley & Sons, Inc; 2003. (Inferring evolutionary trees with PAUP*). [DOI] [PubMed] [Google Scholar]
- 27.Aguilar PV, Robich RM, Turell MJ, O'Guinn ML, Klein TA, Huaman A, Guevara C, Rios Z, Tesh RB, Watts DM, Olson J, Weaver SC. Endemic eastern equine encephalitis in the Amazon region of Peru. Am J Trop Med Hyg. 2007;76:293–298. [PubMed] [Google Scholar]
- 28.Kondig JP, Turell MJ, Lee JS, O'Guinn ML, Wasieloski LP., Jr Genetic analysis of South American eastern equine encephalomyelitis viruses isolated from mosquitoes collected in the Amazon Basin region of Peru. Am J Trop Med Hyg. 2007;76:408–416. [PubMed] [Google Scholar]
- 29.Madalengoitia J, Flores W, Casals J. Arbovirus antibody survey of sera from residents of eastern Peru. PAHO Bull. 1973;7:25–34. [Google Scholar]
- 30.Yanoviak SP, Aguilar PV, Lounibos LP, Weaver SC. Transmission of a Venezuelan equine encephalitis complex Alphavirus by Culex (Melanoconion) gnomatos (Diptera: Culicidae) in northeastern Peru. J Med Entomol. 2005;42:404–408. doi: 10.1093/jmedent/42.3.404. [DOI] [PubMed] [Google Scholar]
- 31.Pinheiro FP, Travassos da Rosa APA. In: Handbook of Zoonoses. Beran GW, editor. Boca Raton, FL: CRC Press; 1994. pp. 217–218. (Guaroa and other Bunyamwera group fevers). [Google Scholar]
- 32.Pinheiro FP, Travassos da Rosa APA, Freitas RB, Travassos da Rosa JFS, Vasconcelos PF. Aspectos clinico-epidemiologicos dos arbovirus. Instituto Evandro Chagas: 50 anos de contribuicao as ciencias biologicas e a medicina tropical. Belem, Brazil: Fundacao Servicos de Saude Publica; 1986. pp. 375–408. [Google Scholar]
- 33.Beaty BJ, Calisher CH, Shope RE. In: Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections. Schmidt NJ, Emmons EW, editors. Washington, DC: American Public Health Association; 1989. pp. 797–855. (Arboviruses). [Google Scholar]
- 34.Vasconcelos PF, Travassos da Rosa AP, Rodrigues SG, Travassos da Rosa ES, Degallier N, Travassos da Rosa JF. Inadequate management of natural ecosystem in the Brazilian Amazon region results in the emergence and reemergence of arboviruses. Cad Saude Publica. 2001;17((Suppl)):155–164. doi: 10.1590/s0102-311x2001000700025. [DOI] [PubMed] [Google Scholar]


