This work shows that PCR2 is a membrane protein implicated in two processes, namely, the detoxification of zinc in the presence of high concentrations of zinc and the transfer of zinc from the root to the shoot. This dual role is likely made possible by PCR2’s expression pattern that differs in different parts of the root.
Abstract
Plants strictly regulate the uptake and distribution of Zn, which is essential for plant growth and development. Here, we show that Arabidopsis thaliana PCR2 is essential for Zn redistribution and Zn detoxification. The pcr2 loss-of-function mutant was compromised in growth, both in Zn-excessive and -deficient conditions. The roots of pcr2 accumulated more Zn than did control plants, whereas the roots of plants overexpressing PCR2 contained less Zn, indicating that PCR2 removes Zn from the roots. Consistent with a role for PCR2 as a Zn-efflux transporter, PCR2 reduced the intracellular concentration of Zn when expressed in yeast cells. PCR2 is located mainly in epidermal cells and in the xylem of young roots, while it is expressed in epidermal cells in fully developed roots. Zn accumulated in the epidermis of the roots of pcr2 grown under Zn-limiting conditions, whereas it was found in the stele of wild-type roots. The transport pathway mediated by PCR2 does not seem to overlap with that mediated by the described Zn translocators (HMA2 and HMA4) since the growth of pcr2 hma4 double and pcr2 hma2 hma4 triple loss-of-function mutants was more severely inhibited than the individual single knockout mutants, both under conditions of excess or deficient Zn. We propose that PCR2 functions as a Zn transporter essential for maintaining an optimal Zn level in Arabidopsis.
INTRODUCTION
Zinc is a micronutrient involved in many cellular functions. Approximately 1230 Arabidopsis thaliana proteins are predicted to contain, bind to, or transport zinc (Wintz et al., 2003; Krämer and Clemens, 2006). Zinc has diverse roles in all organisms. For instance, zinc plays an important role in transcriptional and posttranscriptional processes, in detoxification of superoxide, in protein degradation, and in protein–protein interactions (Marschner, 1995; Krämer and Clemens, 2006; Broadley et al., 2007). Furthermore, about one-third of the world’s population suffers from mild zinc deficiency. Symptoms related to zinc deficiency include impaired brain development, dysfunction of the reproductive system, impaired immune response, hair loss, skin lesions, and loss of taste and smell (http://www.innvista.com/health/nutrition/minerals/zinc.htm; Hershfinkel, 2006).
In plants, leaf zinc concentrations below 15 to 20 μg per g dry mass lead to zinc deficiency syndromes, which are associated with early senescence and chlorotic leaves and often result in stunted growth (Marschner, 1995). Young, dividing organs require a higher zinc concentration for optimal development (Marschner, 1995). However, despite its importance in plant development, elevated concentrations of zinc have deleterious effects and lead to impaired growth and chlorosis (Schutzendubel and Polle, 2002). Therefore, precise control of zinc uptake, homeostasis, and allocation to the different plant organs and cellular organelles is a prerequisite for optimal plant growth. Homeostatic regulation mechanisms include control of uptake, intracellular binding to metal chelators, efflux from the cell, and sequestration into vacuoles (Clemens, 2001; Hall and Williams, 2003).
Members of the ZIP family transport zinc into the cytosol (Zhao and Eide, 1996; Pence et al., 2000). Several members of the ZIP family are upregulated in roots under zinc-deficient conditions, suggesting that they participate in zinc uptake (Wintz et al., 2003; Palmer and Guerinot, 2009). However, so far it is unknown which member plays the dominant role in zinc uptake in roots. In Arabidopsis, knockout of IRON TRANSPORTER1 (IRT1) leads to a reduction in zinc accumulation, suggesting that, at least in Arabidopsis, IRT1 participates in zinc nutrition (Henriques et al., 2002). Once absorbed into the root, zinc needs to be translocated to the shoot. Deletion mutants of the Arabidopsis HEAVY METAL TRANSPORTING ATPASES4 (HMA4) contain less zinc in their leaves and more in their roots. Furthermore, hma4 knockout plants are more sensitive to increased zinc concentrations than are the corresponding wild-type plants (Verret et al., 2004). The double mutant of HMA4 and its close homolog HMA2 is strongly impaired in growth due to the fact that zinc transport to the shoot is drastically reduced. Growth of hma2 hma4 mutant plants can, however, be restored by increasing the zinc concentration in the medium. This suggests that HMA2 and HMA4, while not the sole transporters for translocation of zinc from the roots to the shoot, are nevertheless very important. Interestingly, Hanikenne et al. (2008) showed that three Arabidopsis halleri homologs of HMA4, namely, HMA4-1, HMA4-2, and HMA4-3, are the transporters responsible for the increased root-to-shoot transfer of zinc in A. halleri and are important factors for hyperaccumulation and zinc tolerance in this Arabidopsis species.
Within cells, zinc has to be distributed to the different organelles. It is believed that the majority of zinc that is not associated with proteins is bound to various compounds, such as metal chaperones, nicotinamine, glutathione, or organic acids (Takahashi et al., 2003; Krämer et al., 2007; Palmgren et al., 2008). Excess zinc is exported from the cytosol and accumulated in the vacuoles by transporters of the Zn transporter of Arabidopsis thaliana or metal-ion transport protein 1 (MTP1) family (Blaudez et al., 2003; Kobae et al., 2004; Krämer, 2005; Gustin et al., 2009).
Besides playing an important role in the uptake of micronutrients, iron and zinc transporters are responsible for the uptake of nonessential heavy metals, such as cadmium. Hence, furthering our knowledge of zinc uptake and translocation will also increase our knowledge of the distribution of cadmium in plants (Eide et al., 1996; Rogers et al., 2000; Clemens 2006). In previous work, we identified a small plasma membrane protein with a Cys-rich domain, placenta-specific 8, involved in Cd resistance in plants, which we named Arabidopsis thaliana PLANT CADMIUM RESISTANCE1 (AtPCR1) (Song et al. 2004). PCR1 overexpressing Arabidopsis plants and yeast were more resistant to Cd(II) than was the corresponding wild type, whereas PCR1 antisense plants were more sensitive. These results suggest that PCR1 is involved in the efflux of heavy metals. In Arabidopsis and rice (Oryza sativa), PCRs form a multigene family consisting of 12 and 21 members (http://aramemnon.botanik.uni-koeln.de/), respectively. We hypothesized that, in addition to being involved in the detoxification of nonessential toxic heavy metals, such as Cd, PCRs may be involved in the transport and redistribution of essential heavy metals. To test this hypothesis, we investigated PCR2, which, in contrast with PCR1, is expressed not only in the aerial parts of Arabidopsis but also in the roots.
Here, we show that PCR2 is a membrane protein implicated in two processes, namely, the detoxification of zinc in the presence of high concentrations of zinc and the transfer of zinc from the root to the shoot. This dual role is possible because of the dual expression pattern of PCR2 in the xylem parenchyma of the young parts of the root and in the root epidermis in the differentiation zone of roots, where root hairs develop. These functions of PCR2 are most likely based on its activity as a transporter because, although a single PCR2 protein contains only two putative membrane-spanning α-helices, it can form homooligomers.
RESULTS
PCR2 Is Involved in Plant Survival under Conditions of Both Excess and Limiting Concentrations of Zinc
We previously performed a detailed analysis of Arabidopsis PCR1 and showed that PCR1 confers cadmium tolerance when heterologously expressed in yeast and when overexpressed in plants (Song et al., 2004). PCR2 also confers cadmium tolerance when heterologously expressed in yeast. In this work, we further investigated the role of PCR2. First, we sought to establish where PCR2 is expressed within the plant. Microarray data do not distinguish between PCR1 and PCR2, since the two genes are very similar (89% identity in nucleotide sequence). RT-PCR and PCR2 promoter-β-glucuronidase (GUS) analysis revealed that, in contrast with PCR1, which is expressed only in the aboveground parts of Arabidopsis (see Supplemental Figure 1 online), PCR2 is strongly expressed in the roots, in addition to being expressed in the leaves, stems, flowers, and siliques (Figure 1). In leaves, expression is restricted mainly to the vascular tissue. Our previous analysis of PCR1 (Song et al., 2004), which confers heavy metal tolerance when constitutively overexpressed in Arabidopsis under the control of the 35S promoter, suggested that PCR2 may be involved in the transport of heavy metals or divalent cations. To test this hypothesis, we searched for T-DNA insertion lines that are disrupted in the expression of PCR2. We identified a candidate mutant in the Wisconsin lines (http://www.biotech.wisc.edu/NewServicesAndResearch/Arabidopsis/) and verified the site of insertion of T-DNA in PCR2 (see Supplemental Figure 2 online). To confirm that homozygous lines of the pcr2 mutant do not contain PCR2 transcripts, RT-PCR was performed using total RNA extracted from the plants. No product was observed in pcr2 homozygous knockout plants (Figure 2A). Since during our previous work on PCR1 we did not find a T-DNA insertion line, we included PCR1 in our search and were able to identify a T-DNA insertion mutant (salk_106043) for this gene also (see Supplemental Figure 2 online).
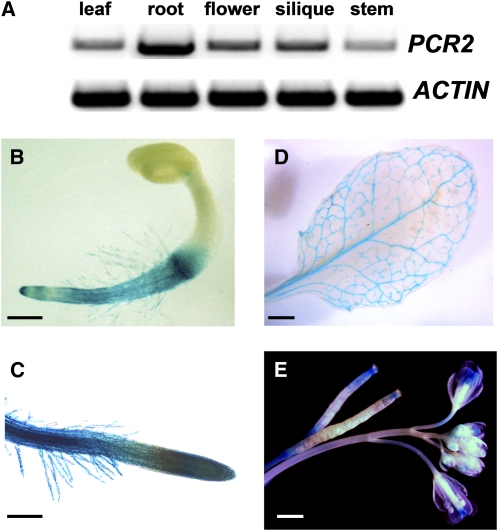
Figure 1.
Expression Pattern of PCR2.
(A) RT-PCR analysis of PCR2 using total RNA from various parts of the shoot and root of plants grown on half-strength MS plates for 3 weeks. ACTIN was used as a loading control.
(B) to (E) Histochemical GUS staining of PCR2pro:GUS plants. Five GUS-expressing lines were observed and all showed a similar pattern, although they differed slightly in the intensity of the staining. Pictures were taken with a stereomicroscope.
(B) Three-day-old seedling grown on half-strength MS agar plates.
(C) Root of a 10-d-old plant grown on half-strength MS agar plates.
(D) Leaf of a 5-week-old plant grown on soil.
(E) Flowers and siliques of a 6-week-old plant grown on soil.
Bars = 100 μm in (B) and (C) and 1 mm in (D) and (E).
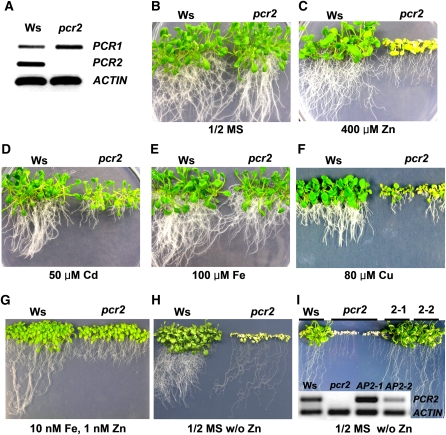
Figure 2.
The pcr2 Loss-of-Function Mutant Is Sensitive to Both an Excess and a Deficiency of Zinc.
(A) RT-PCR analysis confirmed the absence of PCR2 transcripts in the pcr2 mutant.
(B) to (I) Phenotypes of wild-type (Ws) and pcr2 knockout mutant plants grown under various conditions for 25 d.
(B) Control condition (half-strength MS [1/2 MS]).
(C) Excess zinc (1/2 MS supplemented with 400 μM ZnSO4).
(D) Excess cadmium (1/2 MS supplemented with 50 μM CdCl2).
(E) Excess iron (1/2 MS supplemented with 100 μM FeCl3).
(F) Excess copper (1/2 MS supplemented with 80 μM CuSO4).
(G) Zinc- and iron-limiting conditions (1/2 MS containing 10 nM FeCl3 and 1 nM ZnSO4).
(H) Zinc-limiting conditions (1/2 MS without ZnSO4).
(I) Growth of the wild type, the pcr2 knockout, and the knockout lines complemented with V5-tagged PCR2 under the control of the PCR2 promoter (2-1 and 2-2) on a plate without zinc. The inset shows the result from an RT-PCR assay of the PCR2 transcript levels in the same lines. All experiments have been performed at least three times. For details, see Methods.
To obtain information about the function of PCR2, we first compared Ca, Fe, Mg, Mn, and Zn concentrations in the mutant line and wild-type plants grown under control conditions (see Supplemental Table 1 online). The plants did not exhibit any major differences in growth or other phenotypes. While no difference could be observed for Ca, Mg, and Mn, increased Fe and Zn concentrations were found in the roots of pcr2 plants compared with those of wild-type plants (see Supplemental Table 1 online). These preliminary results led us to hypothesize that pcr2 plants exhibit an altered resistance to Zn and Fe, in addition to Cd. Indeed, a comparison of wild-type and pcr2 plants revealed that the growth of pcr2 plants was strongly impaired in the presence of 400 μM zinc (Figure 2C; see Supplemental Figure 3A online), 50 μM cadmium (Figure 2D; see Supplemental Figure 3A online), and 80 μM copper (Figure 2F), a heavy metal not measured in Supplemental Table 1 online. A weaker, but still visible, phenotype was observed in the presence of 100 μM Fe (Figure 2E; see Supplemental Figure 3A online). In contrast with the severe inhibition of growth observed in pcr2 plants, pcr1 knockout plants did not exhibit any difference in growth compared with the wild type when grown on medium containing excess Zn, Fe, or Cd (see Supplemental Figure 4 online). This underlines that the cadmium sensitivity reported for antisense-PCR1 plants (Song et al., 2004) is due to the cosuppression of PCR2, which has a similar sequence to PCR1 (Song et al., 2004). To verify that the phenotype is also visible under more physiological conditions, we grew wild-type plants and pcr2 mutants on clay irrigated with a solution containing limited amounts of zinc. As observed on agar plates, growth of pcr2 mutants was much more impaired under zinc deficiency than was that of wild-type or PCR2 overexpressing plants (see Supplemental Figure 5 online).
The determination of metal concentrations in the shoots and roots of wild-type and mutant plants revealed that zinc, cadmium, and iron are preferentially retained in the roots of pcr2 plants (Figure 3A; see Supplemental Figures 6A and 6B online), consistent with our preliminary results (see Supplemental Table 1 online). This result indicates that the mutant plants transferred less of the heavy metals to the shoot or excluded them less efficiently at the root level than did wild-type plants. To understand better the role of PCR2 in the transfer of heavy metals to the shoot, we calculated the shoot-to-root ratio of Zn, Fe, and Cd concentrations in wild-type and pcr2 plants exposed to different concentrations of the heavy metals. Increasing the zinc concentration in the medium resulted in a decreased shoot-to-root ratio of zinc in the wild-type plants as well as in the pcr2 mutants (Figure 3B). However, the ratio remained lower in the mutant than in the wild type. The shoot-to-root ratio of iron and cadmium concentrations was also lower in the pcr2 mutant than in the wild-type plants at low heavy metal concentrations (see Supplemental Figures 6C and 6D online). In contrast with zinc, increases in iron and cadmium concentrations resulted in a reduction of the shoot-to-root ratio only in wild-type plants and not in the mutants (see Supplemental Figures 6C and 6D online). Therefore, at elevated Fe and Cd concentrations, the shoot-to-root ratio of the concentrations of these metals did not differ much between wild-type and pcr2 plants. At the whole-plant level, Zn, Cd, and Fe concentrations of pcr2 plants did not differ from those of wild-type plants, except slightly at the 100 μM Fe condition (see Supplemental Figure 3B online). This suggests that the toxicity symptoms are not due to alterations in the total sum of metal contents in the plant but to a defect in xylem loading, leading to higher heavy metal contents in the root. Short-term experiments using 65Zn indicate that less 65Zn is translocated into the shoot of pcr2 plants than into those of wild-type plants (Figures 3C and 3D). No significant differences were detected in the roots. Together, these results indicate that Zn is taken up by the roots at similar rates in pcr2 and wild-type plants but that it is not efficiently transferred to the shoot by the mutant.
Figure 3.
pcr2 Retains Heavy Metals in the Root.
(A) Zn concentrations in the shoots and roots of pcr2 plants and the corresponding Ws wild type. Plants were grown on half-strength MS agar medium under control conditions or supplemented with various concentrations of Zn. Closed bars represent the Ws and open bars the pcr2 knockout mutant. DW, dry weight.
(B) Shoot-to-root ratios of Zn concentrations. Samples were prepared from plants grown vertically for 4 weeks on half-strength MS-agar medium in the presence of various concentrations of Zn. Zn contents were measured using atomic absorption spectrometry.
(C) and (D) Uptake of 65Zn in a short-term experiment (C) and the corresponding shoot-to-root ratio of 65Zn (D). Ten-day-old Arabidopsis seedlings were treated with 10 μM ZnCl2 spiked with a trace amount of 65Zn for 10 h, and their radioactivity was measured with a γ-ray counter. Arabidopsis seedlings were precultured under Zn-deficient conditions (half-strength MS plate containing 10 nM ZnSO47H2O) with a 16/8-h light/dark regimen for 10 d.
The data represent averages of six samples collected in two separate experiments (more than 30 plants per sample) ±se. (*P < 0.05; **P < 0.01 compared with the wild type by Student’s t test).
As the growth of pcr2 plants was impaired in the presence of elevated concentrations of several heavy metals, we expected that pcr2 plants would not be affected when micronutrients were present at limiting concentrations. Surprisingly, the growth of pcr2 was also strongly impaired under Zn- or Fe-limiting conditions (Figures 2G and 2H). Whereas during treatments with excess heavy metals, the reduction of root growth and the chlorotic phenotype appeared concomitantly, reduction of root length under zinc deficiency was often less pronounced. Some differences in root growth between seed batches could be observed, which indicates that zinc reserves in the seeds were slightly different. It is known that plants generally attempt to increase or at least not reduce root length under nutrient deficiency conditions to explore better the soil for the sparingly available nutrient.
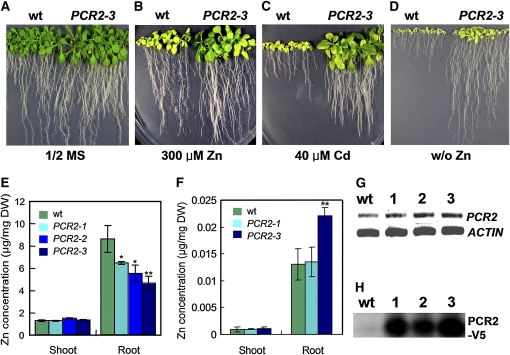
To test whether the phenotype observed was indeed due to lack of PCR2, we searched for a second allele of the knockout mutant but were unable to find one. Therefore, we produced a construct to express PCR2 tagged with V5 under the control of the PCR2 promoter (PCR2pro:PCR2-V5) and introduced it into the pcr2 knockout plant (Figure 2I, inset). Several lines rescued the pcr2 phenotype observed under Zn-limiting conditions to a level of growth comparable to that of the Wassilewskija (Ws) wild type (Figure 2I), indicating that the phenotype observed was due to lack of PCR2. To test whether increased expression of PCR2 confers heavy metal resistance and increased zinc use efficiency, we transformed Columbia (Col) wild-type plants with PCR2 driven by its own promoter and selected three lines of plants exhibiting the highest expression levels (Figures 4G and 4H). Indeed, all lines exhibiting enhanced PCR2 expression showed strong tolerance to excess Zn and Cd and grew better than the wild type when exposed to Zn-limiting conditions (Figures 4B to 4D for PCR2-3 and data not shown for other lines). This result indicates that the V5-tagged PCR2 is functional and that by expressing PCR2 at a high level, the capacity of Arabidopsis to cope with heavy metal excess and deficiency is improved.
Figure 4.
Expression of PCR2 Driven by Its Own Promoter in Wild-Type Plants Enhances Tolerance to Excess Heavy Metals as well as to Zinc Deficiency.
(A) to (D) Phenotypes of Col-0 wild-type plants (wt) and one representative PCR2pro:PCR2-V5 transgenic line (PCR2-3) grown under various conditions for 21 d.
(A) Control conditions (half-strength MS [1/2 MS]).
(B) Excess zinc (1/2 MS supplemented with 400 μM ZnSO4).
(C) Excess cadmium (1/2 MS supplemented with 50 μM CdCl2).
(D) Zinc-deficient conditions (1/2 MS without ZnSO4).
(E) and (F) Zinc concentrations determined for Col-0 wild-type and three PCR2-expressing lines grown on half-strength MS medium containing 0.2 mM zinc (E) or in hydroponic medium without zinc (F). Data are mean ± se of n = 6 (more than 30 plants per sample) (E) or n = 3 (four plants per sample) (F) (*P < 0.05; **P < 0.01 compared with the wild type by Student’s t test). DW, dry weight.
(G) RT-PCR analysis of PCR2-overexpressing lines.
(H) Protein immunoblot of PCR2pro:PCR2-V5–expressing lines. The same amount of protein (50 μg) was loaded on each lane. The transgenically introduced PCR2 was detected with the V5 antibody. No band is present in the wild-type lane due to the absence of the V5 tag in wild-type plants.
To learn more about the zinc resistance mechanism mediated by PCR2, we analyzed the zinc concentrations in roots and shoots of the lines PCR2-1, PCR2-2, and PCR2-3 grown on medium complemented with 0.2 mM ZnCl2 (Figure 4E). All three lines had lower zinc concentrations in the root than did the wild type. This result indicates that PCR2-overexpressing plants may enhance their zinc tolerance by excreting excess zinc from the roots. We also grew two of these lines, PCR2-1 and PCR2-3, hydroponically in the absence of Zn. In this case, PCR2-3, which exhibited the strongest phenotype when zinc was deficient, accumulated more Zn in the roots, whereas no differences could be observed for PCR2-1 (Figure 4F).
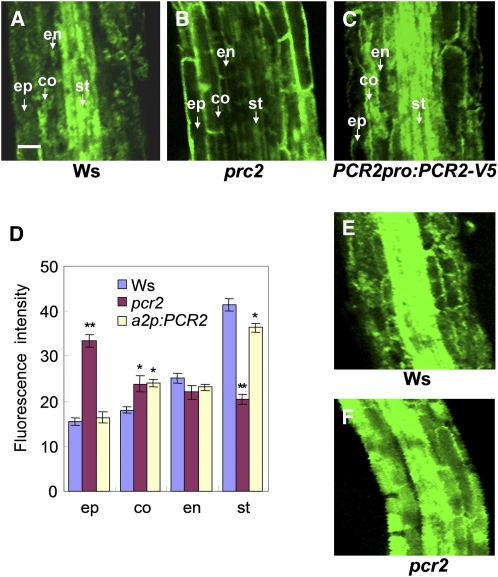
PCR2 Can Fulfill Its Dual Role Because of Its Dual Localization in the Epidermal Cells and the Vascular Tissue
To understand how PCR2 contributes to both zinc tolerance and plant survival under Zn-limiting conditions, two processes that appear to be exactly the opposite of each other, we further investigated the membrane- and tissue-specific localization of PCR2. Transgenic Arabidopsis lines expressing the PCR2pro:PCR2-GFP (for green fluorescent protein) and PCR2pro:GUS constructs were generated. Examination of root epidermal cells expressing the PCR2pro:PCR2-GFP construct revealed fluorescence at the plasma membrane (Figure 5A). To verify this localization, we used the PCR2pro:PCR2-V5 described above (Figure 4). The PCR2-V5 constructs were shown to be functional in Arabidopsis plants as well as in yeast cells (Figures 2, 4, and 7). We performed a density gradient centrifugation followed by protein immunoblotting with V5 antibody to localize PCR2-V5. As shown in Figure 5B, PCR2-V5 is present in the same fraction as the plasma membrane markers PIP and H+-ATPase. This result confirms the results obtained with the GFP fusion protein, which supported the plasma membrane localization of PCR2.
Figure 5.
Localization of PCR2 at the Cellular Level.
(A) PCR2-GFP expressed in roots localized to the plasma membrane. Root cells of PCR2pro:PCR2-GFP plants were treated with the fluorescent dye FM4-64 (red), which localizes to the vacuolar membrane, endosomes, and plasma membrane. The left panel is a light microscopy image, and the right panel is a merged image of two pictures of the same cells taken under the GFP (green) and FM-64 (red) fluorescence settings. v, vacuole, Bar = 10 μm.
(B) PCR2-V5 comigrates with plasma membrane markers H+-ATPase and PIP in sucrose gradients. Microsomes of PCR2pro:PCR2-V5 plants were subjected to linear sucrose density gradient fractionation. Fractions were immunoprobed against the indicated marker enzymes as described in Methods. PCR-V5 was visualized with anti-V5 antibody. Immunopositive peak fractions are highlighted by asterisks; sucrose concentrations of individual gradient fractions are indicated below.
(C) and (D) GUS staining of roots of PCR2pro:GUS transgenic plants in the meristematic/elongation zone (C) and in the root hair zone (D). Pictures were taken with a light microscope. Bars = 100 μm.
(E) and (F) GFP fluorescence in roots of PCR2pro:PCR2-GFP transgenic plants in the elongation (E) and the root hair zone (F). ep, epidermis; c, cortex; e, endodermis; p, pericycle; x, xylem. Bars = 10 μm.
(G) and (H) The PCR2-GFP fluorescence intensity is enhanced in roots of plants grown in Zn excess conditions (G) and reduced in roots of plants grown in Zn deficiency conditions (H). Bars = 10 μm.
(I) Immunoblot of protein samples prepared from roots of PCR2pro:PCR2-V5 transgenic plants grown on half-strength MS without zinc or supplemented with 0.3 mM ZnSO4 detected with the V5 or PIP antibody.
Figure 7.
Expression of PCR2 Inhibited Yeast Cell Growth under Zn-Deficient Conditions and Enhanced It under Zn-Excess Conditions.
(A) and (B) Growth of the zrt1 zrt2 mutant ZHY3 expressing either the empty vector [pYES2 NT/C (EV)] or Arabidopsis PCR2 [pYES2 NT/C-AtPCR2 (PCR2)]. ZHY3 is sensitive to zinc-limiting conditions due to the lack of the two zinc importers. Yeast was grown either on SG ura-agar medium with normal (control) or limiting levels of Zn (A) or in liquid medium with the same limiting level of Zn (B).
(C) Growth of the zinc-sensitive yeast strain, CM104 (cot1 zrc1), transformed with the empty vector pYEPGal (EV) or pYEPGal containing At-PCR2 on SG-leu agar medium under control or excess (0.15 mM) levels of zinc.
(D) Zinc concentrations in CM104 yeast transformed with the empty vector (EV) or AtPCR2 were measured after cultivation in SG-leu liquid medium or SG-leu liquid medium supplemented with 0.15 mM ZnSO4 for 12 h. The values are the mean (±se) of six cultures grown in two independent experiments. (*P < 0.05; **P < 0.01 compared with EV by Student's t test).
Detailed GUS analysis revealed that, at the root, PCR2 exhibited a dual localization. In the root tip, PCR2 was expressed in all cells (Figure 5C). However, in the elongation zone of the root, PCR2 was localized mainly in vascular tissues and the epidermis (Figure 5C), while in the root hair zone, PCR2 was present preferentially in epidermal cells (Figure 5D). Higher resolution images of PCR2-GFP fluorescence in the root of PCR2pro:PCR2-GFP transgenic plants revealed a very similar expression pattern as was observed in plants expressing the GUS construct. PCR2-GFP–derived fluorescence was observed in epidermal cells and the stele of the root elongation zone (Figure 5E), while in the root hair zone of fully developed roots, PCR2 was localized predominantly in the epidermis (Figure 5F). Epidermal localization in this zone is further visualized in Figures 5G and 5H. Figure 5G shows PCR2 in plants grown under high Zn conditions. GFP-dependent fluorescence is very strong and also present in root hairs. The GFP signal was much weaker in plants grown under zinc-limiting conditions, and no green fluorescence could be observed in root hairs (Figure 5H). Since the intensity of the laser was similar for the two conditions, it has to be postulated that the protein level is strongly decreased under zinc deficiency. To verify this observation, we performed an immunoblot and could confirm that the protein levels under zinc deficiency were much weaker than those observed in the presence of 0.2 mM Zn (Figure 5I). To see whether the differences in protein levels directly correlated with the transcript levels, we performed an RT-PCR analysis. A strong downregulation of the PCR2 transcript levels could be observed in shoots of Zn-deficient plants, while in roots, downregulation was less pronounced (see Supplemental Figure 7 online). ZIP4 was strongly induced in roots, demonstrating that the plants indeed suffered from zinc deficiency. Interestingly, exposure to an excess of zinc, which resulted in the downregulation of ZIP4, did not significantly alter PCR2 transcript levels. These results indicate that PCR2 protein levels are partially regulated at the posttranscriptional level.
Microarray data (http://www.bar.utoronto.ca/efp) of gene expression patterns in roots should reflect the localization/expression of PCR2, since PCR1 is not expressed in this tissue. These data were in agreement with our localization results; PCR2 is strongly expressed in the vascular parenchyma in the young maturation zone of the roots (Brady et al., 2007), but in stage 3 roots (the zone where root hairs are fully elongated; ~0.45 to 2 mm from the root tip), it is expressed preferentially in the root epidermis, at a low level in the cortex, and expression in the stele is again high (Birnbaum et al., 2003). These expression data suggest that, in the very young parts of roots, PCR2 is preferentially involved in lateral transport of zinc and loading of zinc into the xylem and that in older parts, the major function of PCR2 is to extrude zinc, thereby preventing heavy metal toxicity.
To test this hypothesis, we analyzed how zinc distribution is altered when PCR2 is either present or absent from the elongation zone of roots under zinc-deficient or -sufficient conditions. Using the zinpyr-1 indicator for zinc in wild-type, pcr2, and complementation lines, we established that, under zinc-deficient conditions, young roots of wild-type plants exhibited the strongest zinc-dependent fluorescence signal in the vascular bundle (Figure 6A). By contrast, the strongest fluorescence signal was observed in the epidermal cells of the pcr2 mutant (Figure 6B). As observed for the other phenotypes, complementation of the pcr2 mutant restored the wild-type distribution pattern of zinc (Figure 6C). Quantification of the fluorescence levels in each layer of cells in the elongation zone of roots revealed that the epidermis of pcr2 roots exhibited a more than twofold higher fluorescence than wild-type and complemented plants, while the fluorescence in the stele of pcr2 roots was decreased to 50% that of the fluorescence in wild-type and complemented plants (Figure 6D). This indicates that, in pcr2 plants, <50% of the absorbed zinc was transferred to the vascular bundle and translocated to the shoot, and the major portion of the remaining zinc accumulated in the root epidermal cell layer. If this interpretation is correct, in addition to extruding Zn from the epidermal layer, PCR2 is also involved in radial transport of zinc from the epidermis to the cortex of roots. In zinc-sufficient half-strength Murashige and Skoog (MS) medium (Figures 6E and 6F), the root stele contained high levels of zinc both in the wild type and the pcr2 mutant, but the epidermal cells of only the pcr2 mutant, and not the wild type, exhibited a high level of zinc. This result indicates that the mutant is compromised in the export of zinc from the epidermal layer under zinc-sufficient conditions (Figure 6F), as it is under zinc-deficient conditions (Figure 6B).
Figure 6.
Zn Distribution in Roots of the Wild Type and pcr2 Plants Visualized by Zinpyr.
(A) to (C) Plants were grown under zinc-deficient conditions (half-strength MS with 10 nM ZnSO4), and their Zn distribution was visualized using the zinpyr-1 fluorescent dye.
(A) Control wild-type (Ws) plants.
(B) pcr2 knockout mutants.
(C) PCR2pro:PCR2-V5 (ap2:PCR2) complemented line.
(D) Fluorescence intensity shown in (A) to (C) was quantified. Values correspond to the mean ± se (n = 50) of the pixel intensity in the epidermis (ep), cortex (co), endodermis (en), and stele (st) layers of 10 roots (*P < 0.05; **P < 0.01 compared with the wild type by Student's t test).
(E) and (F) Zinpyr-1 fluorescence in the wild-type and pcr2 mutants grown under zinc-sufficient conditions (normal half-strength MS and 15 μM ZnSO4). Plants were grown in half-strength MS agar medium for 7 d and treated with 20 μM zinpyr-1 for 3 h. Bars = 10 μm.
Yeast Experiments Support That PCR2 Is a Zinc Transporter
The results described above strongly suggest that PCR2 is involved in zinc export from epidermal and vascular cells. To test whether PCR2 is indeed a zinc transporter, we cloned PCR2 into a yeast expression vector and expressed it in two different yeast strains that are sensitive to either low (ZHY3) or high (CM104) concentrations of zinc. Under zinc-deficient conditions, growth of the ZHY3 yeast strain is inhibited due to the deletion of the two zinc importers, Zrt1 and Zrt2 (Zhao and Eide, 1996). When grown under zinc-deficient conditions, growth of ZHY3 yeast heterologously expressing PCR2 was strongly reduced compared with that of ZHY3 yeast transformed with the empty vector (Figure 7A). A similar reduction in growth was observed for PCR2-expressing ZHY3 yeast cells in liquid culture containing limiting amounts of Zn (Figure 7B). By contrast, when Zn was present in excess, growth of the high zinc-sensitive yeast strain, CM104, which lacks the vacuolar Zrc1 and Cot1 transporters that confer zinc resistance (MacDiarmid et al., 2000), was restored by the expression of PCR2 (Figure 7C). To understand why yeast cells expressing PCR2 exhibit a zinc-resistance phenotype, we determined the zinc concentrations in CM104 yeast cells transformed with the empty vector or with PCR2. As shown in Figure 7D, the Zn concentrations of PCR2-expressing yeast were 30 to 40% less than those transformed with empty vector controls, suggesting that PCR2 removed zinc from the cell.
Since the heterologous expression of PCR2 in yeast confers tolerance to excess Zn and decreases the concentration of zinc in yeast cells, we speculate that PCR2 acts directly as an exporter. However, the small size of PCR2, which contains only two membrane-spanning α-helices, makes it unlikely that a single PCR2 protein can form an active transporter. Therefore, we examined whether PCR2 could itself constitute a functional transporter by forming multimer complexes to generate multiple transmembrane domains, which are a prerequisite for pore formation in the membrane. Several cases have been described in which homooligomers of membrane proteins containing only two membrane-spanning domains form functional channels (Bryan and Aguilar-Bryan, 1999; Voelker et al., 2006; Gazzarrini et al., 2009). Furthermore, the copper transporters are also small membrane proteins and contain only three membrane-spanning helices (Peña et al., 2000; Lee et al., 2002). Therefore, we tested whether PCR2 can form homooligomers. When proteins were extracted from yeast cells overexpressing PCR2 containing a V5 tag and a His tag (NCT-AtPCR2), and analyzed by SDS-PAGE, a major band could be observed at 19 kD, corresponding to the molecular mass of PCR2 containing the two tags, and a less pronounced band was observed at ~37 kD [Figure 8A, first lane at ethylene glycol bis(succinimidylsuccinate) (EGS) = 0]. When yeast membranes were treated with increasing concentrations of the cross-linking agent prior to protein solubilization, the band corresponding to the monomer gradually disappeared, and at low concentrations of the cross-linker (1 and 3 mM), a band corresponding to the dimer was more pronounced than in the absence of the cross-linker (Figure 8A, 2nd and 3rd lanes). Concomitantly, at 3 mM EGS, apparently large multimers were produced that only barely enter into the separation gel (Figure 8A, 3rd lane). This experiment shows that increasing the concentrations of the cross-linker EGS resulted first in a putative dimer, and at higher concentrations, in a more prominent high molecular mass band, which migrated at 160 kD or even higher, and cross-reacted with the V5 antibodies. This result indicates that PCR2 can either form multimers or can cross-react with one or several other partners. To determine which of these occurs and to exclude the possibility that a cross-linking agent produces artifacts, we performed experiments in the absence of the cross-linker. In the first experiment, we transformed yeast cells with a construct encoding PCR2 fused to a V5 tag at the C terminus (CT-PCR2). Microsomal proteins were purified from transformed yeast cells using a lysis buffer in the absence of DTT, separated by SDS-PAGE, and immunoblotted with anti-V5 antibody. In addition to detecting the monomer band at 17 kD, bands of 34, 51, and 68 kD, corresponding to the putative dimer, trimer, and tetramer, were observed. This suggests that PCR2 can form homooligomers; however, we cannot exclude the possibility that PCR2 interacts with a protein of a similar or larger size. To exclude this possibility, we used the NCT-PCR2 construct. Yeast membranes were extracted as described above, and similar results were obtained as for the CT-AtPCR2 construct, except that the bands migrated at a slightly higher molecular mass due to the additional tag introduced at the N terminus. Furthermore, we coexpressed CT-PCR2 and NCT-PCR2. Immunoblot analysis using membranes from yeast expressing the two constructs revealed bands corresponding to the single proteins (17 and 19 kD) as well as the corresponding multimers. In addition, bands corresponding to a hybrid of the two constructs were also observed at 36 kD (arrow in Figure 8B). These results strongly support the hypothesis that PCR2 can form homooligomers.
Figure 8.
Oligomerization of PCR2.
(A) Membrane proteins isolated from ZHY3 (zrt1 zrt2) yeast cells transformed with a V5-tagged pYES2 NT/C vector containing Arabidopsis PCR2 (NCT-PCR2). Cross-linking was performed using 0, 1, 3, and 5 mM EGS, and the PCR2 protein was detected by immunoblotting with an anti-V5 antibody. In addition to the monomer being detected at 19 kD (single dot), a band corresponding to a putative dimer was detected at 37 kD (double dots). Higher EGS concentrations resulted in high molecular mass complexes.
(B) Membrane proteins of yeast cells expressing the vector containing PCR2 with a V5 tag at the C terminus (CT-PCR2), the vector carrying a V5 tag at the C terminus and a His tag at the N terminus (NCT-PCR2), or both (CT-PCR2 and NCT-PCR2) at the same time (last lane). Cells were grown in SG-ura-leu medium to induce gene expression. Subsequently, proteins were extracted using a buffer with (+DTT) or without DTT (−DTT), separated by SDS electrophoresis without prior treatment with a cross-linker, and detected by immunoblotting using V5 antibodies. The arrow in the last lane indicates a putative dimer (36 kD) consisting of one molecule of NCT-PCR2 (19 kD) and one molecule of CT-PCR2 (17 kD). Dots indicate the oligomers containing NCT-AtPCR2, while asterisks indicate oligomers containing CT-PCR2.
PCR2 Functions Independently from HMA2 and HMA4
Among the different zinc transporters characterized in Arabidopsis, the P-type ATPases HMA4 and HMA2 play a central role in the export of zinc from cells and also in the translocation of zinc from the root to the shoot (Eren and Argüello, 2004; Hussain et al., 2004; Hanikenne et al., 2008). In A. thaliana and A. halleri, HMA4 is the predominant zinc transporter. However, in A. thaliana, both HMA2 and HMA4 have to be knocked out in order for plants to manifest a strong Zn phenotype. If PCR2 is a membrane protein that delivers Zn to HMA4 and/or HMA2, double and triple knockout plants would be expected to resemble either of the two single knockout plants or the hma2 hma4 double knockout under zinc-deficient conditions. To address this question, as a first step, we created a pcr2 hma4 double knockout and compared the phenotypes of the two single knockouts with those of the double knockout. Under conditions of zinc deficiency, the pcr2 single knockout had smaller leaves, a lower fresh weight, and lower chlorophyll concentrations than did the hma4 mutant. Importantly, an additive effect of zinc deficiency was observed for the pcr2 hma4 double knockout (Figures 9B, 9D, and 9E), indicating that the two membrane proteins have independent roles in transferring zinc to the shoot. Under conditions of excess zinc, no difference was observed between the wild-type and hma4 plants; however, the growth of pcr2 was strongly inhibited (Figure 9C). When grown in the presence of 130 μM zinc, the pcr2 mutant produced less than half the fresh weight of wild-type or hma4 knockout plants grown under the same conditions and contained a lower chlorophyll concentration than did the wild type or hma4 (Figures 9D and 9E). The pcr2 hma4 double knockout plants showed even more severe growth retardation and chlorosis than did either of the single knockout plants under Zn excess conditions (Figures 9C to 9E). This may be due to an increase in zinc concentration in the roots, since the absence of both hma4 and pcr2 might further increase the concentration of zinc in the root and lead to more pronounced toxicity symptoms.
Figure 9.
pcr2 hma4 Double and pcr2 hma2 hma4 Triple Knockout Mutants Are More Severely Affected Than Are the Corresponding Single or Double Knockout Plants.
(A) to (C) Wild-type, pcr2, hma4, and pcr2 hma4 plants grown for 3 weeks on half-strength MS agar plate (A), an agar plate of half-strength MS without zinc (B), or half-strength MS agar plate supplemented with 130 μM ZnSO4 (C).
(D) and (E) Fresh weight (FW; [D]) and chlorophyll concentration (E) of the plants shown in (A) to (C). Note that the effects of zinc deficiency and zinc toxicity are additive for the double knockout mutants. Values are means (±se) of 10 plants. (*P < 0.05; **P < 0.01 compared with the wild type by Student’s t test). One typical experiment out of three is shown. The two other experiments showed a similar pattern.
(F) and (G) The phenotype (F) and genotype (G) of the pcr2 hma2 hma4 (pcr2 hma2,4) triple knockout mutant.
(F) hma2 hma4 double mutant and pcr2 hma2 hma4 triple mutant plants were grown on soil for 2 weeks (left) and then supplemented with 2 mM ZnSO4 solution for 3 weeks (right).
(G) Genomic DNA PCR to confirm the T-DNA insertions in HMA2, HMA4, and PCR2 in pcr2 hma2 hma4 triple knockout plants. Gene-specific primers for HMA2, HMA4, and PCR2 were HMA2F and HMA2R, HMA4F and HMA4R, PCR2F and PCR2R, respectively. T-DNA–specific primer used was LB. Primer sequences are described in Methods. Note that PCR2F and PCR2R (R) primers, which amplified PCR2 gene in the hma2 hma4 double knockout mutant (left), could not amplify it in pcr2 hma2 hma4 triple knockout mutant (right).
To get further proof of an independent action between PCR2 and the two HMAs in zinc translocation to the shoot, we created a triple mutant pcr2 hma2 hma4. In the absence of Zn, the hma2 hma4 double knockout and the hma2 hma4 pcr2 triple mutant were both strongly impaired in growth (Figure 9F, left). It was previously reported that the hma2 hma4 double knockout requires additional zinc in the medium to grow properly (Hussain et al., 2004). Therefore, after growing the plants for 2 weeks on soil, we supplemented the soil with zinc by watering the plants with 2 mM Zn solution. Under this condition, the hma2 hma4 double knockout rapidly recovered. By contrast, growth of the triple mutant remained impaired and plants became chlorotic (Figure 9F, right). These plants only occasionally produced some seeds. This observation further indicates that PCR2 functions independently from HMA2 and HMA4.
DISCUSSION
Arabidopsis PCRs constitute a small gene family of 12 members that differ mainly in the length of the N-terminal domain (http://aramemnon.botanik.uni-koeln.de/; Supplemental Data Set 1 online). They can be subdivided into three clades: the first clade includes only PCR10 and the second clade consists of seven members, and none of these genes has been characterized so far. The third clade contains PCR1, PCR2, PCR3, and PCR11 (see Supplemental Figure 9 online). No microarray data are available for PCR3. PCR1 is strongly expressed in leaves but not in roots (see Supplemental Figure 1 online), PCR2 is expressed in leaves and roots (Figure 1), and At1g68610 is exclusively expressed in pollen (http://aramemnon.botanik.uni-koeln.de/). Hence, redundancy in function between PCR1, PCR2, and At1g68610 at the root level can be excluded.
In a previous article, we showed that heterologous expression of PCR1 and PCR2 in yeast restores cadmium tolerance in the cadmium-sensitive yeast strain ycf1 (Song et al., 2004). Since transporters of essential heavy metals are often not very specific for their substrates (Guerinot, 2000; Rogers et al., 2000; Hou et al., 2001; Blaudez et al., 2003; Hall and Williams, 2003), we measured Ca, Fe, Mg, Mn, and Zn concentrations in pcr2 plants and in their corresponding isogenic wild-type plants grown on half-strength MS media. Roots of pcr2 plants contained twice as much Zn and 1.5-fold more Fe than the corresponding wild type. These results prompted us to investigate in more detail the role of PCR2 in zinc transport and allocation.
A surprising result was that the PCR2 deletion mutant was more sensitive to high as well as to low zinc concentrations than was the corresponding wild-type plant. Wild-type plants expressing additional copies of PCR2 exhibited an increased resistance to heavy metals and grew better under zinc-limiting conditions than did the corresponding wild-type plants. Furthermore, the concentrations of zinc in roots of these plants were lower than those of the wild type. This initially appeared to be a contradictory observation; however, expression analysis of PCR2 revealed that the cell-specific expression of PCR2 could be responsible for its role under conditions of limiting or excess amounts of zinc (Figure 5). The PCR2 protein appears to fulfill two independent functions: it is involved in the loading of Zn into the xylem and in the detoxification of excess zinc at the epidermal cells.
Loading of zinc to the xylem has been attributed to the activities of two other transporters, the P-type ATPases HMA2 and HMA4 (Hussain et al., 2004; Verret et al., 2004). In A. halleri, three copies of the HMA4 ortholog, Ah-HMA4, are driven by strong promoters and play a central role in translocating zinc to the shoot, thus allowing the hyperaccumulation of zinc in the shoot (Hanikenne et al., 2008). Using the zinc-fluorophore zinpyr-1, it has been shown that, in contrast with the corresponding wild-type plants, the A. thaliana hma2 hma4 double knockout mutant and Ah-HMA4 RNA interference lines exhibit a strong fluorescence at the pericycle and endodermis, indicating that these plants cannot load Zn into the xylem parenchyma cells and the xylem (Sinclair et al., 2007; Hanikenne et al., 2008). The zinpyr-1 fluorescence pattern observed for pcr2 mutant was different from that of the hma2 hma4 knockout or the Ah-HMA4 RNA interference lines: it was strongest in epidermal cells under zinc-deficient conditions, whereas it was uniformly distributed in all tissues of the root under zinc-sufficient conditions. This fluorescence pattern was also very different from that observed from the wild-type plants, which was strongest in the stele, regardless of the zinc concentrations in the medium, as was previously reported by Sinclair et al. (2007). Thus, our results suggest that the pcr2 mutant is strongly impaired in zinc translocation from the epidermis to the xylem parenchyma under zinc-deficient conditions. In a recent publication, Wong and Cobbett (2009) presented evidence that HMA2 and HMA4 are the major factors involved in transferring cadmium from the root to the shoot. As shown in Supplemental Figure 6 online, a slight reduction in cadmium transfer can also be observed in the pcr2 mutant. However, the reduction is much less pronounced than that reported for the hma4 single or the hma2 hma4 double mutant. The fact that pcr2 plants exposed to cadmium accumulate much more of this toxic heavy metal in the roots and are much more cadmium sensitive is therefore likely due to the decreased excretion at the root level rather than to the decreased transfer of this heavy metal to the shoot. A recent analysis in rice of factors responsible for cadmium translocation revealed a quantitative trait locus that explains 16% of the phenotypic variation. The gene responsible for this trait has not been mapped so far, but the authors excluded the possibility that it encodes a heavy metal P-type ATPase (Ueno et al., 2009). It will be interesting to learn whether the gene encodes a transporter of heavy metal complexing agents, such as nicotinamine or citrate, a PCR-like protein, or a still unknown factor involved in cadmium translocation.
Although PCR2 is involved in long-distance transport of Zn, similarly as described for HMA2 and HMA4, its function is not likely to overlap completely with that of the HMAs because the sites of expression of HMA2, HMA4, and PCR2 are different. This is underlined by distinct expression patterns, while HMA2 and HMA4 are localized in the pericycle as well as in the xylem parenchyma, our data and published microarray data suggest that PCR2 is localized in the epidermis and xylem parenchyma. Furthermore, expression of these genes may also differ at the different stages of root development. Therefore, additional localization studies employing HMA2, HMA4, and PCR2 tagged with different markers are required to elucidate the respective roles of these transporters in zinc transport and allocation.
PCR2 performs different functions when zinc is present in sufficient or excessive amounts. The pcr2 mutant did not seem to have a problem in transferring zinc to the xylem under these conditions. Instead, it seemed to experience problems in excretion of toxic amounts of zinc from the epidermal cells (Figure 6F), as indicated by the unusually high zinpyr fluorescence in the epidermal cells. The relatively high concentration of zinc in the stele of pcr2 mutants may indicate that a second transporter with a lower transport capacity is present in this tissue. Alternatively, zinc might move also by diffusion through the apoplastic space to the stele, as suggested by White et al. (2002) and Baxter et al. (2009). The fact that high zinc toxicity is more pronounced in pcr2 plants than in hma4 plants may be due to its additional expression in the epidermal cells. Under conditions of excess zinc, hma2 and hma4 mutants may be able to reduce the root zinc concentration due to the presence of PCR2 in the epidermal cells, whereas pcr2 cells cannot remove excess zinc either by translocation or by excretion to the rhizosphere.
Our results showing that zinpyr fluorescence is localized in the epidermis of the pcr2 mutant under conditions of zinc deficiency (Figure 6B) strongly suggest that a considerable part of zinc is delivered to the apoplast before being delivered to the stele. To allocate zinc into the shoot tissue or to extrude the excess zinc from the plant, a polar localization was expected. Surprisingly, PCR2 was not polarly localized in epidermal cells, as was reported for BORON TRANPORTER4 of Arabidopsis, which is responsible for extrusion of boron under conditions of excess boron (Miwa et al., 2007). Under zinc-limiting conditions, however, our data showing that the PCR2 protein level is strongly reduced (Figures 5H and 5I) suggest that transport of zinc through PCR2 is very limited. Additionally a polar PCR2-dependent transport could be achieved by differential modulation of the oligomeric PCR2 state at the plasma membrane through an as yet unknown mechanism.
Under zinc excess conditions, our results (Figures 2C, 4B, and 9C) strongly suggest that PCR2 is part of the detoxification pathway. So far only vacuolar transporters have been described to be implicated in zinc detoxification. In root epidermal cells, MTP3 (Arrivault et al., 2006), which is strongly upregulated by excess of zinc, is probably the most important vacuolar zinc transporter. Furthermore, it is likely that ZIF1, which is expressed only in the stele under low zinc conditions but also in the root epidermis under Zn excess (Haydon and Cobbett, 2007), participates in vacuolar Zn sequestration, although the form of zinc transported by ZIF1 remains unclear. MTP1, another vacuolar zinc transporter (Kobae et al., 2004; Kawachi et al., 2009), which does not respond to zinc excess, and HMA3 (Morel et al., 2009) are also important for zinc detoxification, although probably only to a limited extent in root epidermal cells (see Supplemental Figure 8 online).
The structure of PCR2, predicted by ConPred_v2 and TmPred, contains only two transmembrane domains and as many as nine Cys residues in the transmembrane domain (see Supplemental Figure 2B online; http://aramemnon.botanik.uni-koeln.de/). This structure raises the question of whether PCRs can act as transporters or whether they are chaperone-like membrane proteins that deliver heavy metals to the corresponding transporters. We propose that PCRs act as transporters based on three lines of experimental evidence. First, PCR2 functions as a zinc exporter when expressed heterologously in yeast (Figure 7). So far, no zinc exporters at the plasma membrane have been identified in yeast, and genes in yeast conferring zinc tolerance are the tonoplast-associated transporters Zrc1 and Cot1. Neither is there evidence for a zinc-transporting P-type ATPase in the yeast genome, excluding the possibility that PCR2 interacts with a Zn efflux transporter in yeast at the plasma membrane. Expression of PCR2 in a low-Zn-sensitive yeast mutant, which has an impaired growth at low zinc levels, inhibited cell growth, as would be expected for a Zn efflux transporter, but not for a chaperone-like membrane protein. Expression of PCR2 in a yeast mutant sensitive to excess Zn enhanced cell growth and decreased Zn content in the cell, suggesting a role for PCR2 in removing excess heavy metals. While the increased zinc tolerance of yeast cells growing on agar plates could also be explained by the excretion of a chelator, such an explanation is unlikely for yeasts growing in liquid culture, since, under these conditions, excreted chelators would be too diluted to efficiently form complexes with the added heavy metal. Excretion of toxic ions has been described for Na+, where the SOS system reduces the internal concentrations of sodium (Shi et al., 2002) or boron (Miwa et al., 2007). A similar mechanism has also been proposed for A. thaliana PLEIOTROPIC DRUG RESISTANCE PROTEIN8 (PDR8), where cadmium efflux is slowest in knockout plants and highest in plants overexpressing PDR8 (Kim et al., 2007). The second line of evidence for the role of PCR2 as a transporter is that the phenotypes of pcr2, hma2, and hma4 double and triple knockouts were additive (Figure 9), which strongly suggests that the three transporters function independently. Finally, experiments expressing PCR2 in yeast indicate that this protein can form homooligomers (Figure 8). The fact that multimers of PCR2 bearing different tags can be observed makes it unlikely that PCR2 interacts with yeast proteins of similar sizes. Homo- and heterooligomerization of membrane proteins is often required to form functional units in the case of, for example, K+ channels (Heginbotham et al.,1997; Shamotienko et al., 1997). This is also the case for proteins containing only two α-helices, like PCR2. In mammals, the potassium channel of the sulfonylurea receptor is formed by four subunits of Kirs, which are surrounded by four ABC-type proteins that function as channel regulators (Aguilar-Bryan and Bryan, 1999). Also, the very small Chlorella virus ATCV-1 encodes a functional potassium channel (Gazzarrini et al., 2009). In Arabidopsis, the functional channel Ca2+ ACTIVATED OUTWARD RECTIFYING K+ CHANNEL3 is also formed by four peptides, each containing only two membrane-spanning domains (Voelker et al., 2006).
As mentioned above, PCRs form gene families in Arabidopsis and rice. Our study suggests that these genes encode functional transporters and probably act as secondary active exporters. It will be interesting to investigate which substrates are transported by other members of this family and what their role is in other organs, such as pollen. Furthermore, structural studies have to be envisaged to learn more about their mode of function.
METHODS
Yeast Strains and Growth Conditions
The DY1457 (MATα ade6 can1 his3 leu2 trp1 ura3), ZHY3 (MAT ade6 can1 his3 leu2 trp1 ura3 zrt1::LEU2 zrt2::HIS3) (Zhao and Eide, 1996), and CM104 (MATα ade6 can1 his3 leu2 trp1 ura3 zrc1Δ::HIS3 cot1Δ::URA3) (MacDiarmid et al., 2000) strains were used. Yeast strains were grown in YPD and transformed either with the empty vectors pYES2NT/C (Invitrogen) or pYEPGAL, a modified pYEPlac 181 vector (Gietz and Sugino, 1988) driven by the GAL4 promoter, or with PCR2 containing vectors pYES2NT/C-AtPCR2 (Song et al., 2004) or pYEPGAL-AtPCR2, a construct generated by the insertion of PCR2-V5 into the pYEPGAL vector. The transformed yeast strains were cultured on synthetic dextrose (SD) or synthetic galactose (SG) medium containing 1% raffinose supplemented with the required auxotrophic amino acids (amino acids without uracil for the pYES2NT/C vector, amino acids without Leu for the pYEPGAL vector, and amino acids without uracil and Leu for cotransformation with pYES2NT/C and pYEPGAL vectors). Zinc-limited half-strength SG agar plates contained 0.2 mM EDTA and 0.2 mM ZnCl2. For all experiments, yeast strains were precultured on liquid SD medium to OD600 = 2 at A600, washed twice with distilled water, and normalized against cell number.
In Vitro Cross-Linking Experiments and Immunoblot Analysis
For in vitro cross-linking experiments, total proteins were extracted from pYES2NT/C-PCR2 transgenic DY1457 yeast cells using an extraction buffer (50 mM HEPES-KOH, pH 7.4, 5 mM MgCl2, 1 mM EDTA, 10 mM DTT, 0.7 μg/mL pepstatin A, 5 μg/mL aprotinin, 20 μg/mL leupeptin, and 0.5 mM phenylmethylsulfonyl fluoride). Membrane proteins were sedimented by centrifugation at 100,000g for 1 h at 4°C and resuspended in a buffer containing 100 mM Tris-HCl, pH 7.5, and 150 mM NaCl. Ten micrograms of membrane proteins were incubated in ice for 20 min with increasing concentrations of EGS. Cross-linked proteins were mixed with sample buffer (60 mM Tris-HCl, pH 6.8, 25% [v/v] glycerol, 2% [w/v] SDS, and 0.0125% [w/v] bromophenol blue) and separated by SDS-PAGE. After electrophoresis, the proteins were electroblotted onto nitrocellulose membranes. The membranes were blocked in 1× TBST (0.1% [v/v] Tween 20 in 1× TBS) with 7.5% nonfat dry milk for 1 h at room temperature, washed twice in 1× TBST for 5 min each, and then incubated for 1.5 h at room temperature with the anti-mouse anti-V5 antibody (Invitrogen). After three washes of 15 min each in 1× TBST, the membrane was incubated for 1 h with sheep anti-mouse IgG conjugated to horseradish peroxidase (Invitrogen) and subsequently washed three times with 1× TBST. Chemiluminescence was detected using the ECL reagent according to the manufacturer’s instructions (Amersham Pharmacia Biotech), and the signals were developed using an x-ray film.
To test oligomerization of PCR2, the PCR2 constructs bearing tags at the N and C termini (pYES2NT/C-PCR2, called NCT-PCR2) and C terminus alone (pYEPGAL-PCR2 called CT-PCR2) were either double transformed into ZHY3 yeast cells or transformed with the corresponding empty vector. Yeast cells were grown in ura-synthetic galactose (SG −ura), −leu synthetic galactose (SG −leu), or –ura −leu synthetic galactose (SG −ura −leu) and collected at OD600 = 2. Total proteins were extracted using a lysis buffer (100 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 100 μg/mL phenylmethylsulfonyl fluoride, 1 μg/mL leupeptin, and 10 μg/mL pepstatin A). Microsomal proteins were pelleted by centrifugation at 100,000g for 1 h at 4°C and solubilized in the sample buffer described above, but with some samples containing 200 mM DTT.
Plant Growth Conditions
Arabidopsis thaliana (ecotypes Col and Ws) seeds sterilized with 1% sodium hypochloride were grown vertically on half-strength MS medium (Duchefa Biochemie; basal salts mixture) containing 0.8% (w/v) phytoagar (Duchefa Biochemie) and 1.5% (w/v) sucrose in a controlled environment with continuous light at 22°C. For experiments conducted under metal deficiency conditions, when not mentioned otherwise, ZnSO4 was added at a concentration of 10 nM to the MS media, which did not contain vitamins. For metal excess conditions, CdCl2, CuSO45H2O, FeCl36H2O, MgSO47H2O, MnSO42H2O, and ZnSO47H2O were supplemented into half-strength MS medium at the concentrations indicated in the figures. To determine the zinc concentration in zinc-deficient conditions, plants were grown on hydroponically in the following nutrient solution: 1.25 mM KNO3, 1.50 mM Ca(NO3)2, 0.75 mM MgSO47H2O, and 0.50 mM KH2PO4; with the micronutrients, 50 μM KCl, 50 μM H3BO3, 10 μM MnSO42H2O, 1.5 μM CuSO45H2O, 0.075 μM Na2MoO42H2O, and 72 μM Fe-EDTA. To analyze Zn sensitivity of plants, chlorophyll contents were measured from leaves of each plant using spectrophotometer as described previously (Oh et al., 1997)
We performed plant growth experiments in three laboratories. The response observed was always to the same direction, although some quantitative differences were observed, as revealed by comparing Figures 2 and 4.
Isolation of T-DNA Insertional Mutants
T-DNA insertional mutants from the University of Wisconsin Arabidopsis Knockout Facility were examined for the presence of T-DNA insertions in PCR1 or PCR2, as described by Krysan et al. (1999) (http://www.biotech.wisc.edu/NewServicesAndResearch/Arabidopsis/). PCRs were performed using the AtPcrR primer, which is common to the 3′ untranslated region sequences of PCR1 and PCR2, and a T-DNA–specific primer (JL-202, 5′-CATTTTATAATAACGCTGCGGACATCTAC-3′). Only one pcr2 T-DNA insertion lines could be isolated. The gene-specific PCR primer sets used for screening were as follows: AtPcrF, 5′-ACGCAATATTTAGTAAATGGTGATGGCTACTTTC-3′, and AtPcrR, 5′-CTTAGCGGGTCATGCCGCCTTGGAAGGCTG-3′. The homozygous plants were determined by genomic PCR and RT-PCR with gene-specific primers PCR2F, 5′-CTCTCTCTCTTCATGGAAGCTCAACACC-3′, and PCR2R, 5′-TTGTTCAGATCTCAACAAGCATATTAACAACAC-3′. The PCR products were sequenced and flanking regions were determined. A pcr1 T-DNA insertion line (salk_106043) was ordered from the SALK T-DNA insertion collection (http://signal.salk.edu/cgi-bin/tdnaexpress). The pcr1 homozygous plants were obtained by PCR screening and confirmed by RT-PCR using AtPcr1-F, 5′-TATTTTTCTCTCCATGGAAGCTCAACTTC-3′, and AtPcr1-R, 5′-AGAAGATTACACAACACATACACACACG-3′.
To create a pcr2 hma4 double knockout mutant, the pcr2 mutant was crossed to the hma4 mutant (Verret et al., 2004). F1 heterozygote plants were selected by BASTA selection, and F2 homozygote plants were identified by BASTA selection and RT-PCR. For RT-PCR of HMA4, the AtHMA4F (5′-GGCGTTACAAAACAAAGAAGAAGAG-3′) and AtHMA4R (5′-CAATGGCAACGCAAGCTGATACT-3′) primers were used. To generate a pcr2 hma2 hma4 triple knockout mutant, the pcr2 mutant was crossed to the hma2 hma4 mutant (Hussain et al., 2004). The F3 homozygote plants were selected by genomic PCR. For genomic PCR of PCR2, HMA2, and HMA4, PCR2F, PCR2R, HMA2F (5′-ATCCTGATATCAATCTGTTTTGTGGC-3′), HMA2R (5′-TGGATGGCTTGACTTGCTCTCAGTG-3′), HMA4F (5′-AGAAGAGAAAAAGAAAGTGAAGAAGTTGC-3′), HMA4R (5′-TTAATATTGACCTTTAGCTCCTAGG-3′), and T-DNA–specific JL-202 primer (LB) were used.
Assays of PCR1 and PCR2 Expression and Their Promoter Activity in Arabidopsis
To analyze the PCR1 and PCR2 expression levels in different tissues and under different metal conditions, total RNA was prepared using a phenol/chloroform method (Song et al., 2004) and RNeasy mini kit (Qiagen), and RT-PCRs were performed using PCR2-F, PCR2-R, AtPcr1-F, and AtPcr1-R at 57°C annealing temperature. The control RT-PCR to confirm the zinc-deficient or excessive conditions was performed by measuring the transcription level of ZIP4 using ZIP4-F (5′-GCTGCTGGTAGTGAAGAGAT-3′) and ZIP4-R (5′-ATCAGCTGCGATGAGGTCCA-3′) primers. TUBULIN and ACTIN genes were used for loading controls for RT-PCR, and RT-PCR was performed with TUB1 (5′-CTCACAGTCCCGGAGCTGACAC-3′), TUB2 (5′-GCTTCAGTGAACTCCATCTCGT-3′) or ACTIN1 (5′-CATCAGGAAGGACTTGTACGG-3′) and ACTIN2 (5′-GATGGACCTGACTCGTCATAC-3′) primers.
For construction of PCR2pro:GUS, 3.5 kb of the promoter (−3047) and open reading frame region (+477) of PCR2 was amplified using genomic DNA from A. thaliana (Ws) as template and the primers PCR2pF (5′-GGTCGTTCTATTCGTCATTCAACG-3′) and PCR2pR (5′-CATGAGTATATACATGCACAACC-3′). The PCR product was digested with BglII and Ecl136II and inserted into the BamHI and SmaI sites of the pBI101.2 binary vector (Clontech). To construct the PCR1pro:GUS construct, 1.2 kb of the PCR1 promoter region was amplified using genomic DNA from A. thaliana (Col-0) and the primers AtPcr1pF (5′-GACAGAGTGACTGTGAGTGATGG-3′) and AtPcr1pR (5′-CTTGAGCGGATCCGGAGAGAAAAATAAAGAG-3′). The PCR product was digested with HindIII and BamHI and inserted into the HindIII and BamHI sites of the pBI121 (Clontech) binary vector. The constructs were introduced into the Agrobacterium tumefaciens EHA105 strain, which was then used to transform A. thaliana (Col-0) by the dipping method (Clough and Bent, 1988). Kanamycin-selected T2 transformants were then assayed for GUS activity according to established methods (Gallagher, 1992).
Construction of the PCR2pro:PCR2-GFP and PCR2pro:PCR2-V5 Vectors for Arabidopsis Transformation
The pBI101-PCR2pro:PCR2-GFP and PCR2pro:PCR2-V5 constructs were made using the pBI101-PCR2pro:PCR2 and pYES2NT/C-PCR2-GFP and pYES2NT/C-PCR2 vectors. The pBI101-PCR2pro:PCR2 vector was constructed with the PCR2pro:PCR2 fragment. The PCR2pro:PCR2 fragment (−3047 to + 865) was synthesized from wild-type DNA using specific primers for PCR2 (AP2pro-F, 5′-AGATCTTGATAGACTTAATAACAAGTTGTGGGCG-3′; AP2pro-R, 5′-TGAATTACGTAGATATCAAGGCCTGCGGGTCATGCCGCCTTGGAAGAC-3′). The fragment was digested with BglII and EcoRV and then inserted into the BamHI and SacI sites of the pBI101 vector (Clontech). To make the pBI101-PCR2pro:PCR2-GFP and PCR2pro:PCR2-V5 constructs, the PCR2-GFP and PCR2-V5 fragments were isolated using restriction enzymes (BamHI/PmeI) from the pYES2NT/C-PCR2-GFP and pYES2NT/C-PCR2 vectors and inserted in frame into the BamHI and StuI sites of the pBI101-PCR2pro:PCR2 vector. pYES2NT/C-PCR2-GFP was prepared by insertion of PCR2 into YES2NT/C carrying GFP tag. The constructs were introduced into the A. tumefaciens EHA105 strain, which was then used to transform A. thaliana by the dipping method (Clough and Bent, 1988). The T3 transgenic plants were grown on half-strength MS-agar medium for 1 week and treated with propidium iodide or FM4-64 to stain the cell wall or various membranes, respectively. The roots of the plants were used for fluorescence analysis.
Measurement of Metal Contents
Metal contents in plants and yeast were measured using an atomic absorption spectrometer (Shimadzu Co.). The plants were harvested, briefly washed using cold water, and dried at 60°C for 2 d. Yeast cells were cultured in SD-ura medium to an OD600 of 3 and then pelleted by centrifugation. The cells were diluted to an OD of 0.1 and grown for 2 d at 30°C on SG-ura medium containing 50 μM ZnSO4, 50 μM MnSO4, 50 μM MgCl2, and 50 μM Fe(NO3)3 9H2O. Subsequently, the cells were harvested and washed twice with ice-cold water for 2 min. All samples were digested with 65% HNO3 for 6 to 15 h at 200°C.
To investigate metal translocation from the root to the shoot, Arabidopsis seedlings were cultured in Zn-deficient conditions (half-strength MS plate containing 10 nM ZnSO47H2O) at 16-h light for 10 d, placed on a plastic net, and incubated in one-tenth MS supplemented with 10 mM MES-KOH, pH 5.6, containing 10 μM ZnCl2 and 6.7 Bq of 65ZnCl2 for 10 h at room temperature. The seedlings were then washed using one-tenth MS containing 10 mM MES-KOH, pH 5.6, and 1 mM CaCl2 for only 10 min to avoid further translocation. Shoots and roots were separated and weighed. Radioactivity from absorbed zinc was measured using a γ-ray counter (Perkin-Elmer). The fact that plants had higher Zn concentrations than the medium suggest (e.g., Figure 3) that the washing procedures used were efficient and that, if at all, only small quantities of medium that do not affect the overall picture remained attached to the roots.
Protein Immunoblot and Sucrose Density Gradients
Immunoblots and sucrose density gradients were performed as described (Geisler et al., 2000). Microsomal membranes were isolated from Arabidopsis seedlings grown in half-strength MS liquid medium containing 1% sucrose for 10 d. Vesicles were resuspended in STED10 buffer solution (10% [w/v] sucrose, 100 mM Tris-HCl, pH 7.5, 1 mM EGTA, and 1 mM DTT) and loaded onto a 10 to 50% (w/v) continuous sucrose gradient made with the same buffer. Gradients were centrifuged at 100,000g for 15 h, and 0.6-mL fractions were collected. Aliquots of equal volume were blotted on nitrocellulose and probed with anti-V5 (Invitrogen), anti-AHA2 (Palmgren et al., 1991), anti-PIP1 (Kammerloher et al., 1994), anti-VM23 (Maeshima, 1992), anti-V-ATPase (2E7; Ward et al., 1992), and anti-BIP (tobacco-BIP, gift from A. Vitale). A refractometer was used to measure sucrose concentration.
Zinpyr-1 Imaging and Quantification of Fluorescence
Seven-day-old plants grown on zinc-sufficient or zinc-deficient half-strength MS plates were treated with PBS solution containing 20 μM zinpyr-1 at room temperature in darkness for 3 h. The seedlings were washed with PBS solution, immersed in propidium iodide for 5 min, and rinsed again. The green and red fluorescence of root samples was observed with a confocal laser scanning microscope (TCS SP2 Leica) using excitation at 490 nm and emission at 530 nm. Green fluorescence intensity was measured from images of the root cells using Adobe Photoshop 7.0.
Accession Numbers
Sequence data for the genes studied in this article can be found in the Arabidopsis Genome Initiative database under the following accession numbers: PCR1, At1G14880; PCR2, At1g14870; HMA2, At4g30110; HMA4, At2g19110; and PCR11, At1g68610. The relevant Arabidopsis Genome Initiative identifiers are included on the phylogenetic analysis shown in Supplemental Figure 9 online.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Expression Analysis of PCR1.
Supplemental Figure 2. The Sites of T-DNA Insertions in the pcr1 and pcr2 Knockout Plants Used and the Secondary Structure of PCR2 Predicted by Conpred II.
Supplemental Figure 3. Phenotype Analysis and Metal Contents of pcr2 and Ws Plants Shown in Figures 2 and 3.
Supplemental Figure 4. pcr1 Knockout Plants Do Not Exhibit a Heavy Metal–Related Phenotype.
Supplemental Figure 5. pcr2 Knockout Plants Grown on Clay and Subjected to Zinc Deficiency Show a Similar Phenotype as Plants Grown on Agar Plates.
Supplemental Figure 6. pcr2 Knockout Plants Retain Heavy Metals in the Root.
Supplemental Figure 7. Transcript Levels of PCR1, PCR2, and ZIP4 in Response to Zinc-Deficient and Zinc Excess Conditions.
Supplemental Figure 8. Model of Zinc Transport and Detoxification in Root Epidermis Cells.
Supplemental Figure 9. Molecular Phylogenetic Analyses of the PCR Family in Arabidopsis.
Supplemental Table 1. Metal Levels of Wild-Type and pcr2 Knockout Plants Grown on Normal Half-Strength MS-Agar Medium.
Supplemental Data Set 1. Multiple Sequence Alignment of PCR Family Members.
Supplementary Material
Acknowledgments
This work was supported by the European Union through the Sixth Framework Program for Research and Technological Development (Contract FOOD-CT-2006-016253 to E.M.), the Global Research Program of the Ministry of Science and Technology of Korea (K20607000006-08A0500-00610 to E.M. and Y.L.), and the Environmental Biotechnology National Core Research Center of Korea R15-2003-012-02003-0 to Y.L.). We acknowledge Dr. David Eide for providing the yeast strains CM104 and ZHY3. We thank Prof. Hans Lambers and Dr. Stefan Hörtensteiner for critical reading of the manuscript and Aekyung Han and Jong Soon Kim for plant maintenance.
References
- Aguilar-Bryan L., Bryan J. (1999). Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr. Rev. 20: 101–135 [DOI] [PubMed] [Google Scholar]
- Arrivault S., Senger T., Krämer U. (2006). The Arabidopsis metal tolerance protein MTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. Plant J. 46: 861–879 [DOI] [PubMed] [Google Scholar]
- Baxter I., Hosmani P.S., Rus A., Lahner B., Borevitz J.O., Muthukumar B., Mickelbart M.V., Schreiber L., Franke R.B., Salt D.E. (2009). Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet. 5: e1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum K., Shasha D.E., Wang J.Y., Jung J.W., Lambert G.M., Galbraith D.W., Benfey P.N. (2003). A gene expression map of the Arabidopsis root. Science 302: 1956–1960 [DOI] [PubMed] [Google Scholar]
- Blaudez D., Kohler A., Martin F., Sanders D., Chalot M. (2003). Poplar metal tolerance protein 1 confers zinc tolerance and is an oligomeric vacuolar zinc transporter with an essential leucine zipper motif. Plant Cell 15: 2911–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady S.M., Orlando D.A., Lee J.Y., Wang J.Y., Koch J., Dinneny J.R., Mace D., Ohler U., Benfey P.N. (2007). A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318: 801–806 [DOI] [PubMed] [Google Scholar]
- Broadley M.R., White P.J., Hammond J.P., Zelko I., Lux A. (2007). Zinc in plants. New Phytol. 173: 677–702 [DOI] [PubMed] [Google Scholar]
- Bryan J., Aguilar-Bryan L. (1999). Sulfonylurea receptors: ABC transporters that regulate ATP-sensitive K(+) channels. Biochim. Biophys. Acta 1461: 285–303 [DOI] [PubMed] [Google Scholar]
- Clemens S. (2001). Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212: 475–486 [DOI] [PubMed] [Google Scholar]
- Clemens S. (2006). Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88: 1707–1719 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1988). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Eide D., Broderiu M., Fett J., Guerinot M.L. (1996). A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl. Acad. Sci. USA 93: 5624–5628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren E., Argüello J.M. (2004). Arabidopsis HMA2, a divalent heavy metal-transporting PIB-type ATPase, is involved in cytoplasmic Zn homeostasis. Plant Physiol. 136: 3712–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S.R. (1992). GUS Protocols: Using the GUS Gene as a Reporter of Gene Expression. (San Diego, CA: Academic Press; ). [Google Scholar]
- Gazzarrini S., Kang M., Abenavoli A., Romani G., Olivari C., Gaslini D., Ferrara G., van Etten J.L., Kreim M., Kast S.M., Thiel G., Moroni A. (2009). Chlorella virus ATCV-1 encodes a functional potassium channel of 82 amino acids. Biochem. J. 420: 295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M., Frangne N., Gomès E., Martinoia E., Palmgren M.G. (2000). The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 124: 1814–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R.D., Sugino A. (1988). New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74: 527–534 [DOI] [PubMed] [Google Scholar]
- Gustin J.L., Loureiro M.E., Kim D., Na G., Tikhonova M., Salt D.E. (2009). MTP1-dependent Zn sequestration into shoot vacuoles suggests. Dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J. 57: 1116–1127 [DOI] [PubMed] [Google Scholar]
- Guerinot M.L. (2000). The ZIP family of metal transporters. Biochim. Biophys. Acta 1465: 190–198 [DOI] [PubMed] [Google Scholar]
- Hall J.L., Williams L.E. (2003). Transition metal transporters in plants. J. Exp. Bot. 54: 2601–2613 [DOI] [PubMed] [Google Scholar]
- Hanikenne M., Talke I.N., Haydon M.J., Lanz C., Nolte A., Motte P., Kroymann J., Weigel D., Kramer U. (2008). Evolution of metal hyperaccumulation. required cis-regulatory changes and triplication of HMA4. Nature 453: 391–395 [DOI] [PubMed] [Google Scholar]
- Haydon M.J., Cobbett C.S. (2007). A novel major facilitator superfamily protein at the tonoplast influences zinc tolerance and accumulation in Arabidopsis. Plant Physiol. 143: 1705–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L., Odessey E., Miller C. (1997). Tetrameric stoichiometry of a prokaryotic K+ channel. Biochemistry 36: 10335–10342 [DOI] [PubMed] [Google Scholar]
- Henriques R., Jasik J., Klein M., Martinoia E., Feller U., Schell J., Pais M.S., Koncz C. (2002). Knock-out of Arabidopsis metal transporter gene IRT1 results in iron deficiency accompanied by cell differentiation defects. Plant Mol. Biol. 50: 587–597 [DOI] [PubMed] [Google Scholar]
- Hershfinkel M. (2006). Zn2+, a dynamic signaling molecule. In Molecular Biology of Metal Homeostasis and Detoxification from Microbes to Man, Tamás M.J., Martinoia E., (Berlin: Springer; ), pp. 131–154 [Google Scholar]
- Hou Z.J., Narindrasorasak S., Bhushan B., Sarkar B., Mitra B. (2001). Functional analysis of chimeric proteins of the Wilson Cu(I)-ATPase (ATP7B) and ZntA, a Pb(II)/Zn(II)/Cd(II)-ATPase from Escherichia coli. J. Biol. Chem. 276: 40858–40863 [DOI] [PubMed] [Google Scholar]
- Hussain D., Haydon M.J., Wang Y., Wong E., Sherson S.M., Young J., Camakaris J., Harper J.F., Cobbett C.S. (2004). P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell 16: 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerloher W., Fischer U., Piechottka G.P., Schaffner A.R. (1994). Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J. 6: 187–199 [DOI] [PubMed] [Google Scholar]
- Kawachi M., Kobae Y., Mori H., Tomoioka R., Lee Y., Maeshima M. (2009). A mutant strain Arabidopsis thaliana lacks vacuolar membrane zinc transporter MTP1 revealed the latent tolerance to excessive zinc. Plant Cell Physiol. 50: 1156–1170 [DOI] [PubMed] [Google Scholar]
- Kim D.Y., Bovet L., Maeshima M., Martinoia E., Lee Y. (2007). The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 50: 207–218 [DOI] [PubMed] [Google Scholar]
- Kobae Y., Uemura T., Sato M.H., Ohnishi M., Mimura T., Maeshima M. (2004). Zinc transporter of Arabidopsis thaliana AtMTP1 is localized to vacuolar membranes and implicated in zinc homeostasis. Plant Cell Physiol. 45: 1749–1758 [DOI] [PubMed] [Google Scholar]
- Krämer U. (2005). MTP1 mops up excess zinc in Arabidopsis cells. Trends Plant Sci. 10: 313–315 [DOI] [PubMed] [Google Scholar]
- Krämer U., Clemens S. (2006). Functions and homeostasis of zinc, copper, and nickel in plants. In Molecular Biology of Metal Homeostasis and Detoxification from Microbes to Man, Tamás M.J., Martinoia E., (Berlin: Springer; ), pp. 214–272 [Google Scholar]
- Krämer U., Talke I.N., Hanikenne M. (2007). Transition metal transport. FEBS Lett. 581: 2263–2272 [DOI] [PubMed] [Google Scholar]
- Krysan P.J., Young J.K., Sussman M.R. (1999). T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 11: 2283–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Peña M.M., Nose Y., Thiele D.J. (2002). Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem. 277: 4380–4387 [DOI] [PubMed] [Google Scholar]
- MacDiarmid C.W., Gaither L.A., Eide D. (2000). Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J. 19: 2845–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima M. (1992). Characterization of the major integral protein of vacuolar membrane. Plant Physiol. 98: 1248–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. (1995). Mineral Nutrition of Higher Plants. (San Diego, CA: Academic Press; ). [Google Scholar]
- Miwa K., Takano J., Omori H., Seki M., Shinozaki K., Fujiwara T. (2007). Plants tolerant of high boron levels. Science 318: 1417. [DOI] [PubMed] [Google Scholar]
- Morel M., Crouzet J., Gravot A., Auroy P., Leonhardt N., Vavasseur A., Richaud P. (2009). AtHMA3, a P-1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol. 149: 894–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.A., Park J.H., Lee G.I., Paek K.H., Park S.K., Nam H.G. (1997). Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. Plant J. 12: 527–535 [DOI] [PubMed] [Google Scholar]
- Palmer C.M., Guerinot M.L. (2009). Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat. Chem. Biol. 5: 333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren M.G., Sommarin M., Serrano R., Larsson C. (1991). Identification of an autoinhibitory domain in the C-terminal region of the plant plasma membrane H+-ATPase. J. Biol. Chem. 266: 20470–20475 [PubMed] [Google Scholar]
- Palmgren M.G., Clemens S., Williams L.E., Krämer U., Borg S., Schjørring J.K., Sanders D. (2008). Zinc biofortification of cereals: Problems and solutions. Trends Plant Sci. 13: 464–473 [DOI] [PubMed] [Google Scholar]
- Peña M.M., Puig S., Thiele D.J. (2000). Characterization of the Saccharomyces cerevisiae high affinity copper transporter Ctr3. J. Biol. Chem. 275: 33244–33251 [DOI] [PubMed] [Google Scholar]
- Pence N.S., Larsen P.B., Ebbs S.D., Letham D.L.D., Lasat M.M., Garvin D.F., Eide D., Kochian V. (2000). The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc. Natl. Acad. Sci. USA 97: 4956–4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers E.E., Eide D.J., Guerinot M.L. (2000). Altered selectivity in an Arabidopsis metal transporter. Proc. Natl. Acad. Sci. USA 97: 12356–12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutzendubel A., Polle A. (2002). Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 53: 1351–1365 [PubMed] [Google Scholar]
- Shamotienko O.G., Parcej D.N., Dolly J.O. (1997). Subunit combinations defined for K+ channel Kv1 subtypes in synaptic membranes from bovine brain. Biochemistry 36: 8195–8201 [DOI] [PubMed] [Google Scholar]
- Sinclair S.A., Sherson S.M., Jarvis R., Camakaris J., Cobbett C.S. (2007). The use of the zinc-fluorophore, Zinpyr-1, in the study of zinc homeostasis in Arabidopsis roots. New Phytol. 174: 139–145 [DOI] [PubMed] [Google Scholar]
- Shi H., Quintero F.J., Pardo J.M., Zhu J.K. (2002). The putative plasma membrane Na(+)/H(+) antiporter SOS1 controls long-distance Na(+) transport in plants. Plant Cell 14: 465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W.Y., Martinoia E., Lee J., Kim D., Kim D.Y., Vogt E., Shim D., Choi K.S., Hwang I., Lee Y. (2004). A novel family of cys-rich membrane proteins mediates cadmium resistance in Arabidopsis. Plant Physiol. 135: 1027–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Terada Y., Nakai I., Nakanishi H., Yoshimura E., Mori S., Nishizawa N.K. (2003). Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell 15: 1263–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno D., Kono I., Yokosho K., Ando T., Yano M., Ma J.F. (2009). A major quantitative trait locus controlling cadmium translocation in rice (Oryza sativa). New Phytol. 182: 644–653 [DOI] [PubMed] [Google Scholar]
- Verret F., Gravot A., Auroy P., Leonhardt N., David P., Nussaume L., Vavasseur A., Richaud P. (2004). Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett. 576: 306–312 [DOI] [PubMed] [Google Scholar]
- Voelker C., Schmidt D., Mueller-Roeber B., Czempinski K. (2006). Members of the Arabidopsis AtTPK/KCO family form homomeric vacuolar channels in planta. Plant J. 48: 296–306 [DOI] [PubMed] [Google Scholar]
- Ward J.M., Reinders A., Hsu H.T., Sze H. (1992). Dissociation and reassembly of the vacuolar H+-ATPase complex from oat roots. Plant Physiol. 99: 161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P.J., Whiting S.N., Baker A.J.M., Broadley M.R. (2002). Does zinc move apoplastically to the xylem in roots of Thlaspi caerulescens? New Phytol. 153: 201–207 [Google Scholar]
- Wintz H., Fox T., Wu Y.Y., Feng V., Chen W., Chang H.S., Zhu T., Vulpe C. (2003). Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J. Biol. Chem. 278: 47644–47653 [DOI] [PubMed] [Google Scholar]
- Wong C.K., Cobbett C.S. (2009). HMA P-type ATPases are the major mechanism for root-to-shoot Cd translocation in Arabidopsis thaliana. New Phytol. 181: 71–78 [DOI] [PubMed] [Google Scholar]
- Zhao H., Eide D. (1996). The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J. Biol. Chem. 271: 23203–23210 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.