Abstract
In higher eukaryotes, translation of some mRNAs occurs by internal initiation. It is not known, however, whether this mechanism is used to initiate the translation of any yeast mRNAs. In this report, we identify naturally occurring nucleotide sequences that function as internal ribosome entry sites (IRESes) within the 5′ leader sequences of Saccharomyces cerevisiae YAP1 and p150 mRNAs. When tested in the 5′ untranslated regions of monocistronic reporter genes, both leader sequences enhanced translation efficiency in vegetatively growing yeast cells. Moreover, when tested in the intercistronic region of dicistronic mRNAs, both sequences were shown to contain IRESes that functioned in living cells. The activity of the p150 leader was much greater than that of the YAP1 leader. The second cistron was not expressed in control dicistronic constructs that lacked these sequences or contained the 5′ leader sequence of the CLN3 mRNA in the intercistronic region. Further analyses of the p150 IRES revealed that it contained several nonoverlapping segments that were able independently to mediate internal initiation. These results suggested a modular composition for the p150 IRES that resembled the composition of IRESes contained within some cellular mRNAs of higher eukaryotes. Both YAP1 and p150 leaders contain several complementary sequence matches to yeast 18S rRNA. The findings are discussed in terms of our understanding of internal initiation in higher eukaryotes.
Translation initiation in eukaryotes involves recruitment of the translation machinery by mRNA. This can occur at the 5′ cap-structure or at a segment contained within the mRNA, generally referred to as an internal ribosome entry site (IRES) (for reviews see ref. 1). Internal initiation is cap independent and enables some mRNAs to be efficiently translated in the presence of primary or higher-order RNA structures that might otherwise inhibit linear scanning from the cap to the initiation codon. Internal initiation also appears to be used by some mRNAs when cap-dependent translation is reduced, for example, during poliovirus infection, at the G2/M phase of the cell cycle, or under conditions of hypoxia (2–5). Although IRESes have been identified within some cellular and viral mRNAs of higher eukaryotes, it remained unclear if IRESes were also used by mRNAs of lower eukaryotes (6). Two well-characterized mammalian IRESes, from encephalomyocarditis virus and poliovirus, did not function when tested in living yeast (7, 8), but there have been conflicting reports concerning the ability of the poliovirus IRES to function in these lysates (8, 9). The notion that Saccharomyces cerevisiae has the capacity to internally initiate translation received support from a study that showed that the leader sequences from hepatitis C virus and coxsackievirus B1 mRNAs were able to confer cap-independent translation in yeast cell-free lysates (10). This same study showed that the 5′ leader sequences from yeast TFIID and HAP4 mRNAs also mediated internal initiation in yeast lysates. It was not, however, reported whether these sequences functioned similarly in vivo. More recently, Paz et al. (11) fortuitously identified a segment of the Escherichia coli lacI gene that functioned as an IRES in living yeast when the cells were starved.
In the present study, two naturally occurring nucleotide sequences that function as IRESes in vegetatively growing yeast were identified in the 5′ leader sequences of S. cerevisiae YAP1 and p150 mRNAs. We selected these 5′ leaders for analysis because they are unusually long for yeast leader sequences (12) and they contain upstream AUGs, two features that may inhibit initiation by a scanning mechanism and that have been found in other 5′ leaders that contain IRESes (13–15). The 5′ leader of CLN3 mRNA encoding cyclin 3 protein served as a control for these studies. The translation of this mRNA appeared to be cap dependent in that cyclin 3 protein was poorly expressed in yeast mutants with reduced cap-binding activity of translation initiation factor eIF4E (16).
The YAP1 mRNA encodes an AP1-like transcription factor that appears to provide yeast with resistance to a variety of compounds (17, 18). In 1994, Iizuka et al. (10) showed that in yeast lysates, translation of a monocistronic mRNA containing the YAP1 leader sequence was enhanced when the mRNA was capped, but that a cap analogue failed to inhibit its translation. Thus, translation mediated by the YAP1 leader appeared to involve both cap-dependent and -independent components. In the present study, the YAP1 leader sequence was tested in the 5′ untranslated region (UTR) of a monocistronic reporter construct and was shown to enhance the translation efficiency of this mRNA in living cells. Moreover, when tested in the intercistronic region of a dicistronic mRNA, this sequence functioned as an IRES.
We also identified and characterized a second, more powerful IRES in the transcribed leader sequence of p150 mRNA. This mRNA, transcribed from gene TIF4631, encodes the yeast homologue of mammalian translation initiation factor eIF4G. Primer extension identified two p150 mRNAs that differ in the length of their leader sequences (19). The longer leader sequence, which contains 508 nucleotides, is the focus of the present study. It contains 11 upstream ORFs, none of which overlap the main eIF4G ORF. We found that the p150 leader sequence functioned as a translational enhancer; the translation mediated by this leader appeared to be cap independent. We surmised that translation from this leader sequence might involve internal initiation and we confirmed this by using a dicistronic analysis. Studies using deletions of the p150 5′ leader sequence showed that the IRES in this mRNA lacks distinct boundaries. Moreover, nonoverlapping fragments with IRES activity were identified, indicating a modular composition resembling that of some other cellular IRESes (e.g., ref. 20).
Materials and Methods
Plasmid Construction.
The plasmid pMyr (Stratagene) was used as backbone for both dicistronic and monocistronic constructs. An adaptor containing restriction sites HindIII, PstI, NheI, EcoRI, NcoI, and XbaI was introduced into the pMyr vector immediately downstream of the GAL1 promoter, by using HindIII and XbaI as cloning sites. The PstI and XbaI sites were used as cloning sites for a fragment from the RPh dicistronic reporter vector (20, 21). The resulting construct, pMyr-RP, encodes a dicistronic mRNA designated RP that contains Renilla (sea pansy) and Photinus (firefly) luciferase proteins as the first and second cistrons, respectively. Note that these cloning steps resulted in a 5′ UTR that differs slightly from that in the RP mRNA used previously (20, 21). The iso-1-cytochrome c (CYC1) terminator sequence contained within pMyr-1 vector provides signals for termination of transcription and polyadenylation.
The p150, YAP1, and CLN3 leader sequences were PCR amplified by using yeast genomic DNA as a template. These leader sequences were cloned into the intercistronic region of the pMyr-RP vector by using EcoRI and NcoI restriction sites that were introduced at the 5′ and 3′ ends of the leader sequences to generate constructs designated as pMyr-p150/RP, pMyr-YAP1/RP, and pMyr-CLN3/RP. A hairpin structure with a predicted stability of −50 kcal/mol (21) was introduced into the 5′ UTR of the dicistronic constructs to generate pMyr-p150/RPh, pMyr-YAP1/RPh, and pMyr-CLN3/RPh. Deletions and fragments of the p150 leader were generated by PCR amplification of the p150 sequence, again by using EcoRI and NcoI as cloning sites. Monocistronic constructs containing the Photinus luciferase gene were generated in the modified pMyr vector. The Photinus luciferase gene was obtained from the pGL3-control vector (Promega) as an NcoI/XbaI fragment and cloned by using these same sites to generate construct pMyr/P. The leader sequences from YAP1, p150, and CLN3 mRNAs, as well as the hairpin structure were cloned into the pMyr/P vector by using the same restriction sites used for the dicistronic constructs. Constructs containing the chloramphenicol acetyltransferase (CAT) gene were cloned into the pGAD10 vector (CLONTECH). The pGAD10 vector was digested with HindIII and an adaptor containing restriction sites HindIII, PstI, NheI, EcoRI, NcoI, and XbaI was introduced into this site, which is immediately downstream of the alcohol dehydrogenase (ADH) promoter. The CAT gene was obtained from the pCAT3 vector (Promega) and cloned into the modified pGAD10 vector by using NcoI and XbaI restriction sites. The p150 leader sequence was introduced into this vector as an EcoRI/NcoI fragment to generate the construct designated p150/CAT. The hairpin structure described above was introduced 5′ of this leader sequence to generate the construct designated p150/CATh.
Analysis of Reporter Gene Activity.
The yeast strain EGY48 (MATα, his3, trp1, ura3, LexAop(X6)-LEU2; CLONTECH) was used throughout the study. Yeast strains harboring the pMyr-based plasmids were grown overnight in 4 ml of synthetic-defined medium without uracil and containing glucose. The following morning, cells were harvested, washed with 4 ml of H2O, and grown for 3 h in 4 ml of synthetic defined medium without uracil with the addition of 2% galactose and 1% raffinose. Cells harboring the pGAD10-based constructs did not require induction and were cultured in 4 ml of synthetic defined/uracil/glucose medium overnight. Cells were lysed with 1× lysis buffer (diluted freshly from 5× stock; Promega) in tubes with glass beads. Tubes were vortexed twice for 30 s and recovered in a microcentrifuge (Eppendorf) spun at top speed for 3 min at 4°C. The supernatant was recovered and 20 μl of the lysate was used to assay luciferase activities by using the dual reporter assay system (Promega). CAT activity was measured by using n-butyl-CoA according to Technical Bulletin No. 84 (Promega).
Northern Analysis.
RNA was isolated from 4 ml of cell-culture samples. Cells were pelleted, washed with H2O, and resuspended in 400 μl of TES buffer (100 mM Tris⋅HCl, pH 7.5/10 mM EDTA/0.5% SDS). RNA was extracted by using preheated phenol (65°C); the mixture was vortexed for 1 min and incubated at 65°C for 1 h. Samples were put on ice for 5 min and then centrifuged at 15,000 rpm for 5 min and the top aqueous phase was collected and reextracted with phenol once and chloroform once. RNA was precipitated with isopropyl alcohol, and the precipitate was washed with 70% ethanol, dried, and dissolved in H2O. RNA samples were separated by gel electrophoresis using 1% formaldehyde/agarose gels and transferred to Nytran SuperCharge nylon membrane (Schleicher & Schuell). The blots were probed with full-length firefly luciferase RNA antisense probe that was labeled with 32P.
Results
Sequences Contained Within the YAP1 Leader Sequence Increase Translation Efficiency.
To determine the effect on translation of the YAP1 leader, this 164-nt sequence was tested in the 5′ UTR of a firefly (Photinus) luciferase reporter mRNA (YAP1/P; Fig. 1A). Cells were transformed with constructs expressing the parent Photinus (−/P) mRNA or the YAP1/P mRNA. Cells were also transformed with a construct containing the 364-nt 5′ leader of the CLN3 mRNA (CLN3/P) as a spacer control. The transcription of these monocistronic mRNAs was under control of the GAL1 promoter; mRNA expression was induced with galactose, cells were lysed after 3 h, and luciferase activities determined and normalized to Photinus luciferase mRNA levels (Fig. 1B). The results showed that the translation efficiency of the YAP1/P mRNA was ≈6-fold greater than that of either the control −/P or CLN3/P mRNAs. To investigate the possibility that the translation mediated by the YAP1 transcribed leader sequence has a cap-independent component, it was tested in a dicistronic mRNA for its ability to mediate internal initiation.
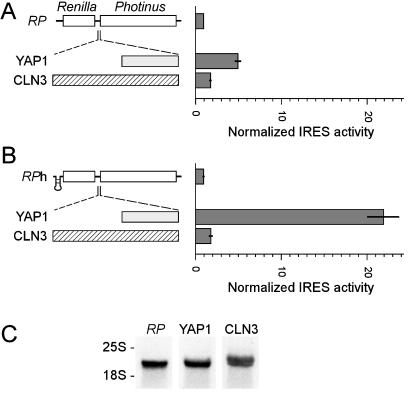
Figure 1.
Analysis of S. cerevisiae YAP1 leader sequence in the 5′ UTR of Photinus luciferase monocistronic mRNA. (A) Schematic representations of the monocistronic mRNAs −/P, YAP1/P, and CLN3/P are indicated. Yeast were transformed with the pMyr constructs that expressed these mRNAs, monocistronic mRNA expression was induced by galactose, and Photinus luciferase activities were determined 3 h after induction. Photinus luciferase activities were divided by the Photinus luciferase mRNA levels (shown in B). In this figure and those that follow, the ordinate for the histograms is formed by the constructs indicated immediately to the left. (B) Northern blot hybridizations of total RNA purified from cells transformed with the constructs indicated, and probed with a RNA probe complementary to the Photinus luciferase gene. The position of the 18S rRNA is indicated. Photinus luciferase mRNA levels were quantified by using a PhosphorImager. Similar results were obtained in three independent experiments.
YAP1 Leader Sequence Contains an IRES.
The leader sequence of YAP1 mRNA was placed in the intercistronic region of a dual luciferase dicistronic mRNA (Fig. 2A) to determine if it contained an IRES. In these mRNAs, the upstream cistron encodes Renilla (sea pansy) luciferase and the downstream cistron encodes Photinus luciferase (20, 21). Cells were transformed with constructs encoding the parent RP mRNA, or with constructs containing the YAP1 or CLN3 leaders in the intercistronic region of the RP mRNA. The results indicated that the YAP1 leader sequence enhanced the translation of the downstream Photinus luciferase cistron approximately 5-fold relative to that of the RP mRNA. In contrast, the CLN3 leader appeared to have almost no effect on the expression of the second cistron relative to that of the RP mRNA.
Figure 2.
Analysis of S. cerevisiae YAP1 leader sequence in the intercistronic region of a dicistronic reporter mRNA with Renilla and Photinus luciferase-coding sequences as cistrons. (A) Schematic representations of the RP, YAP1/RP, and CLN3/RP dicistronic mRNAs are indicated. Yeast were transformed with pMyr constructs that expressed these mRNAs. IRES activities were determined 3 h after galactose induction of dicistronic mRNA induction and are represented as ratios of Photinus luciferase activity to Renilla luciferase activity. Values represent activities that have been normalized to those of the RP vector. Horizontal bars indicate SEM. (B) Dicistronic constructs with a hairpin structure at the 5′ end of the mRNA. These experiments were performed as in A; however, the IRES activities were normalized to those of the parent RPh construct. (C) Northern blot of total RNA purified from cells transformed with the dicistronic constructs indicated, and probed as in Fig. 1. The positions of the 25S and 18S rRNAs are indicated.
In the dicistronic constructs, hairpin structures were then inserted upstream of the Renilla luciferase gene to block scanning and thereby reduce the translation of this gene. These hairpin structures blocked Renilla luciferase expression by more than 90%. Their use showed that the YAP1 leader permitted translation of the Photinus luciferase gene even when translation of the Renilla luciferase gene was blocked (Fig. 2B). This suggests that the YAP1 leader did not increase expression of the second cistron by reinitiation or leaky scanning.
To exclude the possibility that enhanced expression of the downstream cistron was from shorter monocistronic mRNAs that might be generated by mechanisms including RNA fragmentation or unusual splicing events, RNA was isolated from transformed cells and analyzed on Northern blots using a probe to the downstream Photinus luciferase gene. The results indicated that the dicistronic mRNAs were intact (Fig. 2C) and the translation of the second cistron could not be accounted for by initiation via shorter transcripts.
p150 Leader Sequence Contains a Potent Translational Enhancer.
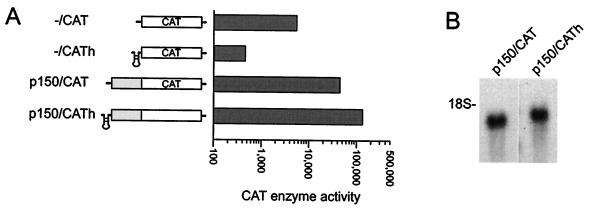
The 5′ leader of the mRNA encoding the p150 protein was determined by primer extension analysis to contain 508 nucleotides (19). This sequence contains 11 ORFs and it does not appear to contain or be part of an intron (22), consistent with the observation that only 4% of yeast genes contain introns (23, 24), 90% of which encode ribosomal proteins (25). The presence of upstream ORFs in the p150 leader might be expected to inhibit translation by a scanning mechanism. As with YAP1, this sequence was tested in the 5′ UTR of a monocistronic reporter mRNA. The results indicated that constructs containing this sequence enhanced the translation efficiency of the reporter gene up to 10-fold. However, the analysis was complicated by the appearance of a second band, ≈1 kb, which may be a partial degradation product of the luciferase mRNA. This RNA was too short to encode a functional Photinus luciferase protein (26, 27). The p150 leader was then tested in the 5′ UTR of a different reporter gene (CAT) to further evaluate whether it was functioning as a translational enhancer. The results with the CAT reporter were similar to those obtained with the Photinus luciferase reporter gene (activities are represented on a logarithmic scale in Fig. 3A). The p150 leader sequence enhanced the translation efficiency of the CAT reporter gene 9-fold.
Figure 3.
Analysis of S. cerevisiae p150-transcribed leader sequence in the 5′ UTR of CAT monocistronic mRNA. (A) Schematic representations of the monocistronic mRNAs are indicated, without (−/CAT, p150/CAT) or with (−/CATh, p150/CATh) a hairpin structure at the 5′ end of the mRNA. Yeast were transformed with the pGAD10 constructs that overexpressed these mRNAs. CAT activities were determined, divided by CAT mRNA levels, and represented on a logarithmic scale. (B) Northern blot hybridizations of total RNA purified from cells transformed with the constructs indicated and probed with an RNA probe complementary to the CAT gene. The position of the 18S rRNA is indicated. Similar results were obtained in two independent experiments.
To determine whether any translation mediated by the p150 5′ leader was cap independent, a hairpin structure was inserted at the 5′ end of this construct (Fig. 3A). Although this hairpin structure inhibited translation of a control CAT mRNA by >90%, the translation mediated by the p150 leader sequence was not inhibited, but rather was enhanced by approximately 3-fold. The CAT mRNA levels appeared to be unaffected (Fig. 3B). These results indicated that the translation mediated by this leader sequence was cap independent.
Identification of a Potent Yeast IRES in p150 mRNA.
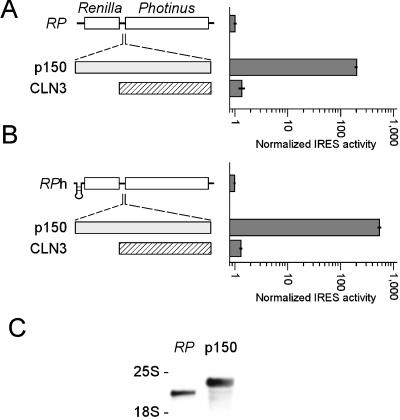
To determine if the translation was truly cap independent, the p150 leader was tested in the intercistronic region of the dual luciferase RP dicistronic mRNA. In this location, the p150 leader functioned as a potent IRES (Fig. 4A). It enhanced the translation of the downstream Photinus luciferase cistron ≈200-fold relative to that of the RP parent vector. This increase in Photinus luciferase activity in the p150/RP mRNA results in Photinus luciferase protein levels that are approximately twice those of Renilla protein levels.
Figure 4.
Dicistronic analysis of S. cerevisiae p150 leader sequence. (A) Schematic representations of the RP, p150/RP, and CLN/RP dicistronic mRNAs are indicated. Experiments were performed and presented as in Fig. 2, with the exception that activities are represented on a logarithmic scale. Horizontal bars indicate SEM. (B) Dicistronic constructs with a hairpin structure at the 5′ end of the mRNA. (C) Northern blot of total RNA purified from cells transformed with the dicistronic constructs indicated and probed as in Fig. 1. The positions of the 25S and 18S rRNAs are indicated.
Blocking the translation of the upstream Renilla luciferase gene with a hairpin structure resulted in an even greater enhancement of the Photinus-to-Renilla luciferase ratio, indicating that the translation facilitated by this sequence was not dependent on the translation of the upstream Renilla luciferase cistron (Fig. 4B). As with the findings with YAP1, the enhanced expression of the downstream cistron did not appear to require RNA fragmentation or unusual splicing events as determined by Northern analysis (Fig. 4C).
The p150 IRES Lacks Distinct Boundaries and Shorter Fragments Have IRES Activity.
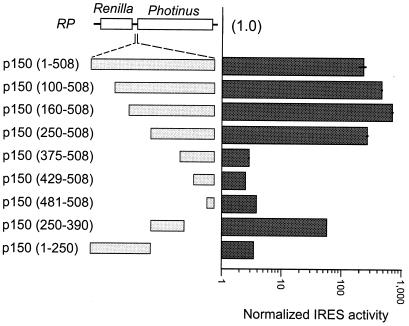
The p150 leader sequence was sequentially deleted from the 5′ end and fragmented into shorter segments, each of which was tested for IRES activity (Fig. 5). The results indicated that most of the activity was associated with nucleotides 160–508; however, all fragments had some level of IRES activity. Deletion of nucleotides 1–100 and 100–160 increased translation by internal initiation, raising the possibility that these 160 nucleotides contain sequences that inhibit IRES activity. The leader sequence in construct p150 () corresponds to that of a shorter leader sequence that occurs naturally (19). This shorter leader sequence has a level of IRES activity that is similar to that of the 508-nt leader.
Figure 5.
Deletion and fragment analysis of the p150 IRES in S. cerevisiae cells. Schematic representations of the dicistronic constructs used in this analysis are indicated. Constructs are based on the RP vector; inserts include the full p150 leader sequence [p150 ()] in addition to 5′ deletions and fragments of this sequence. The construct designations indicate, in parentheses, the exact nucleotide sequences present in each construct. Cells were induced and IRES activities are represented as in Fig. 4. Values represent activities that have been normalized to those of the RP construct. Horizontal bars indicate SEM.
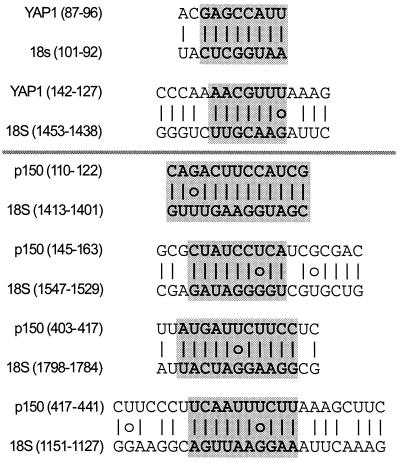
Identification of Complementary Sequence Matches to 18S rRNA Within the YAP1 and p150 Leader Sequences.
In earlier studies, we noted that many eukaryotic mRNAs contain short complementary sequence matches to 18S rRNA, raising the possibility that ribosome recruitment at some cellular IRESes might occur by base pairing between mRNA and 18S rRNA (20, 28–30). Comparison of the YAP1 and p150 leader sequences to yeast 18S rRNA identified two and four complementary sequence matches, respectively (Fig. 6). These matches contain stretches of up to 10 nucleotides of perfect complementarity. In addition, two of the matches are part of more extensive complementary matches of up to 25 nucleotides with 84% complementarity. The complementary matches at nucleotides 110–122 and at nucleotides 145–163 of the p150 IRES are correlated with a 60-nt segment of the IRES that appears to inhibit IRES activity [in Fig. 5, compare the activities of constructs p150 () to p150 ()]. In contrast, two other complementary matches of the p150 IRES at nucleotides 403–417 and 447–441 do not appear to have an obvious effect on IRES activity [in Fig. 5, compare p150 () to p150 ()].
Figure 6.
Complementary sequence matches between YAP1 or p150 leader sequences and 18S rRNA. Vertical lines indicate base pairing and open circles represent GU base pairing. The longest uninterrupted stretches of complementarity for each match are indicated by the shaded nucleotides.
Discussion
Although several studies have suggested that the yeast translation machinery may be capable of mediating internal initiation (10, 11), the present study provides unequivocal evidence that yeast IRESes contained within YAP1 and p150 leader sequences can actually function in vegetatively growing cells. In addition, numerous segments with complementarity to yeast 18S rRNA were identified within both leader sequences. We previously noted that many other mRNAs and cellular IRESes contain similar features and also showed that complementary sequence matches to 18S rRNA could function as cis-acting sequences that affect translation (20, 29, 30). In the case of the 9-nt IRES-module characterized from the transcribed leader of the mRNA that encodes the Gtx homeodomain, this segment is 100% complementary to 18S rRNA. Recruitment of ribosomes at this site appeared to involve base pairing to 18S rRNA within 40S ribosomal subunits (unpublished data). These observations raise the possibilities that recruitment of ribosomes at some cellular IRESes, including the yeast YAP1 and p150 IRESes, may occur directly by means of base pairing to rRNA and that this mechanism might be consistent with the modular nature of these cellular IRESes.
The leader sequence of the YAP1 mRNA contains an IRES that appears to contribute to the efficient translation of this mRNA. In other studies, it has been shown that sequence features of this leader could affect both translation and mRNA stability (17, 31). One of these features, a short upstream ORF, did not inhibit translation of the main ORF, even though it was recognized by a large fraction of the scanning ribosomes (17). Inasmuch as upstream ORFs generally inhibit the translation of downstream cistrons (32), these results indicated that reinitiation and leaky scanning were also involved in the efficient translation of the YAP1 mRNA (reviewed in ref. 33).
p150, the second IRES characterized in this study, was particularly active. To determine if the full IRES had discrete boundaries, the leader sequence was sequentially deleted from the 5′ end. Although most of the IRES activity was localized to nucleotides 160–508, the 5′ IRES boundary was not distinct. Moreover, several nonoverlapping segments functioned independently, suggesting that this IRES has a modular composition. In our previous studies of the IRES contained within the mRNA that encodes the Gtx homeodomain protein, the apparent modularity was pursued to identify a 9-nt segment that functioned independently as an IRES-module (20).
The notion that short nucleotide sequences can recruit the translation machinery is not consistent with the proposal that higher-order RNA conformations are uniformly important for the activity of some cellular IRESes (34). Indeed, the results obtained from deletion and fragment analyses of IRESes contained within other mammalian and insect cellular mRNAs indicate that many of these IRESes may also be modular (21, 35–38). The modular composition of cellular IRESes contrasts with those found in viruses. For example, in picornaviruses, the IRESes comprise several hundred nucleotides and contain RNA conformations that appear to be highly conserved and that are important for activity (see ref. 39).
How widely internal initiation is used by yeast or higher eukaryotic mRNAs is presently unknown. The identification of numerous insect and mammalian IRESes may reflect a more extensive use of this mechanism in higher eukaryotes, or it may reflect incidental bias that has resulted in the evaluation of many more mRNAs from insects and mammals than from yeast. Some mammalian IRESes do not function in living yeast (7, 8). In the case of poliovirus, the inactivity of its IRES in S. cerevisiae reflects a specific blockage that occurs via a short inhibitory RNA (reviewed in ref. 40). The inactivity of some mammalian IRESes in yeast may also reflect trans-factor requirements that are not provided by yeast cells or differences related to the ability of a sequence to bind a component of the translation machinery that is not identical to that in yeast. For example, p150 is the yeast homologue of mammalian translation initiation factor eIF4G (19), but the two are not functionally interchangeable (41).
In higher eukaryotes, IRESes are used by some mRNAs during the G2/M phase of the cell cycle (3, 4) and under conditions that reduce cap-dependent translation, as seen, for example, during different types of stress (e.g., ref. 5). In yeast, internal initiation may also be used to facilitate the translation of essential genes under similar conditions, including the condition of nutritional deficiency (11). It may be significant that IRESes were identified within the YAP1 and p150 leader sequences given that overexpression of YAP1 confers general resistance to many compounds (reviewed in refs. 17 and 18). In addition, expression of p150 when cap-dependent translation is reduced may contribute to the translation of other mRNAs under these conditions.
The identification of yeast IRESes that function in vegetatively growing cells suggests that yeast and higher eukaryotes may use similar mechanisms to initiate translation. The analysis of these mechanisms should be facilitated in yeast; many strains of yeast exist with mutations in genes involved in translation. The ability to easily manipulate this organism genetically may also enable the identification of specific factors involved in internal initiation and should enable us to critically test the hypothesis that base pairing between certain IRES sequences and 18S rRNA is important for recruitment of ribosomes at these sites. In addition to these scientific interests, the identification of yeast IRESes that function as translational enhancers in monocistronic mRNAs may also have applications for bioengineering.
Acknowledgments
We thank Matthew Busse for excellent technical assistance and Dr. Joseph Gally for critical reading of the manuscript. This work was supported by grants from the National Science Foundation (MCB9982574 to V.P.M), the U.S. Public Health Service (NS39837 to G.M.E), and the G. Harold and Leila Y. Mathers Charitable Foundation (to G.M.E.).
Abbreviations
- IRES
internal ribosome entry site
- UTR
untranslated region
- CAT
chloramphenicol acetyltransferase
References
- 1.Gray N K, Hentze M W. Mol Biol Rep. 1994;19:195–200. doi: 10.1007/BF00986961. [DOI] [PubMed] [Google Scholar]
- 2.Kwon H, Pelletier N, DeLuca C, Genin P, Cisternas S, Lin R, Wainberg M A, Hiscott J. J Biol Chem. 1998;273:7431–7440. doi: 10.1074/jbc.273.13.7431. [DOI] [PubMed] [Google Scholar]
- 3.Cornelis S, Bruynooghe Y, Denecker G, Van Huffel S, Tinton S, Beyaert R. Mol Cell. 2000;5:597–605. doi: 10.1016/s1097-2765(00)80239-7. [DOI] [PubMed] [Google Scholar]
- 4.Pyronnet S, Pradayrol L, Sonenberg N. Mol Cell. 2000;5:607–616. doi: 10.1016/s1097-2765(00)80240-3. [DOI] [PubMed] [Google Scholar]
- 5.Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Mol Cell Biol. 1998;18:3112–3119. doi: 10.1128/mcb.18.6.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy J E G. Microbiol Mol Biol Rev. 1998;62:1492–1553. doi: 10.1128/mmbr.62.4.1492-1553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evstafieva A G, Beletsky A V, Borovjagin A V, Bogdanov A A. FEBS Lett. 1993;335:273–276. doi: 10.1016/0014-5793(93)80745-g. [DOI] [PubMed] [Google Scholar]
- 8.Coward P, Dasgupta A. J Virol. 1992;66:286–295. doi: 10.1128/jvi.66.1.286-295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altmann M, Blum S, Pelletier J, Sonenberg N, Wilson T M, Trachsel H. Biochim Biophys Acta. 1990;1050:155–159. doi: 10.1016/0167-4781(90)90158-x. [DOI] [PubMed] [Google Scholar]
- 10.Iizuka N, Najita L, Franzusoff A, Sarnow P. Mol Cell Biol. 1994;14:7322–7330. doi: 10.1128/mcb.14.11.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paz I, Abramovitz L, Choder M. J Biol Chem. 1999;274:21741–21745. doi: 10.1074/jbc.274.31.21741. [DOI] [PubMed] [Google Scholar]
- 12.Cigan A M, Donahue T F. Gene. 1987;59:1–18. doi: 10.1016/0378-1119(87)90261-7. [DOI] [PubMed] [Google Scholar]
- 13.Akiri G, Nahari D, Finkelstein Y, Le S Y, Elroy-Stein O, Levi B Z. Oncogene. 1998;17:227–236. doi: 10.1038/sj.onc.1202019. [DOI] [PubMed] [Google Scholar]
- 14.Oh S-K, Scott M P, Sarnow P. Genes Dev. 1992;6:1643–1653. doi: 10.1101/gad.6.9.1643. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein J, Sella O, Le S-Y, Elroy-Stein O. J Biol Chem. 1997;272:9356–9362. doi: 10.1074/jbc.272.14.9356. [DOI] [PubMed] [Google Scholar]
- 16.Danaie P, Altmann M, Hall M N, Trachsel H, Helliwell S B. Biochem J. 1999;340:135–141. [PMC free article] [PubMed] [Google Scholar]
- 17.Vilela C, Linz B, Rodrigues-Pousada C, McCarthy J E. Nucleic Acids Res. 1998;26:1150–1159. doi: 10.1093/nar/26.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer B E, Wolfger H, Kuchler K. Biochim Biophys Acta. 1999;1461:217–236. doi: 10.1016/s0005-2736(99)00160-1. [DOI] [PubMed] [Google Scholar]
- 19.Goyer C, Altmann M, Lee H S, Blanc A, Deshmukh M, Woolford J L, Jr, Trachsel H, Sonenberg N. Mol Cell Biol. 1993;13:4860–4874. doi: 10.1128/mcb.13.8.4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chappell S A, Edelman G M, Mauro V P. Proc Natl Acad Sci USA. 2000;97:1536–1541. doi: 10.1073/pnas.97.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoneley M, Paulin F E M, Le Quesne J P C, Chappell S A, Willis A E. Oncogene. 1998;16:423–428. doi: 10.1038/sj.onc.1201763. [DOI] [PubMed] [Google Scholar]
- 22.Costanzo M C, Hogan J D, Cusick M E, Davis B P, Fancher A M, Hodges P E, Kondu P, Lengieza C, Lew-Smith J E, Lingner C, et al. Nucleic Acids Res. 2000;28:73–76. doi: 10.1093/nar/28.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ares M, Jr, Grate L, Pauling M H. RNA. 1999;5:1138–1139. doi: 10.1017/s1355838299991379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis C A, Grate L, Spingola M, Ares M., Jr Nucleic Acids Res. 2000;28:1700–1706. doi: 10.1093/nar/28.8.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warner J R. Trends Biochem Sci. 1999;24:437–440. doi: 10.1016/s0968-0004(99)01460-7. [DOI] [PubMed] [Google Scholar]
- 26.Sala-Newby G, Kalsheker N, Campbell A K. Biochem Biophys Res Commun. 1990;172:477–482. doi: 10.1016/0006-291x(90)90697-l. [DOI] [PubMed] [Google Scholar]
- 27.Sung D, Kang H. Photochem Photobiol. 1998;68:749–753. doi: 10.1562/0031-8655(1998)068<0749:tntaas>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Mauro V P, Edelman G M. Proc Natl Acad Sci USA. 1997;94:422–427. doi: 10.1073/pnas.94.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tranque P, Hu M C-Y, Edelman G M, Mauro V P. Proc Natl Acad Sci USA. 1998;95:12238–12243. doi: 10.1073/pnas.95.21.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu M C-Y, Tranque P, Edelman G M, Mauro V P. Proc Natl Acad Sci USA. 1999;96:1339–1344. doi: 10.1073/pnas.96.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz-Echevarria M J, Peltz S W. Cell. 2000;101:741–751. doi: 10.1016/s0092-8674(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 32.Geballe A P, Sachs M S. In: Translational Control of Gene Expression. Sonenberg N, Hershey J W B, Mathews M B, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 2000. pp. 595–614. [Google Scholar]
- 33.Kozak M. Gene. 1999;234:187–208. doi: 10.1016/s0378-1119(99)00210-3. [DOI] [PubMed] [Google Scholar]
- 34.Le S-Y, Maizel J V., Jr Nucleic Acids Res. 1997;25:362–369. doi: 10.1093/nar/25.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Q, Sarnow P. Nucleic Acids Res. 1997;25:2800–2807. doi: 10.1093/nar/25.14.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sella O, Gerlitz G, Le S-Y, Elroy-Stein O. Mol Cell Biol. 1999;19:5429–5440. doi: 10.1128/mcb.19.8.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huez I, Creancier L, Audigier S, Gensac M-C, Prats A-C, Prats H. Mol Cell Biol. 1998;18:6178–6190. doi: 10.1128/mcb.18.11.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauring A, Overbaugh J. Mol Cell. 2000;6:939–945. doi: 10.1016/s1097-2765(05)00084-5. [DOI] [PubMed] [Google Scholar]
- 39.Le S Y, Siddiqui A, Maizel J V., Jr Virus Genes. 1996;12:135–147. doi: 10.1007/BF00572952. [DOI] [PubMed] [Google Scholar]
- 40.Das S, Ott M, Yamane A, Venkatesan A, Gupta S, Dasgupta A. Front Biosci. 1998;3:D1241–D1252. doi: 10.2741/a359. [DOI] [PubMed] [Google Scholar]
- 41.Barco A, Carrasco L. J Gen Virol. 1998;79:2651–2660. doi: 10.1099/0022-1317-79-11-2651. [DOI] [PubMed] [Google Scholar]