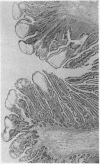
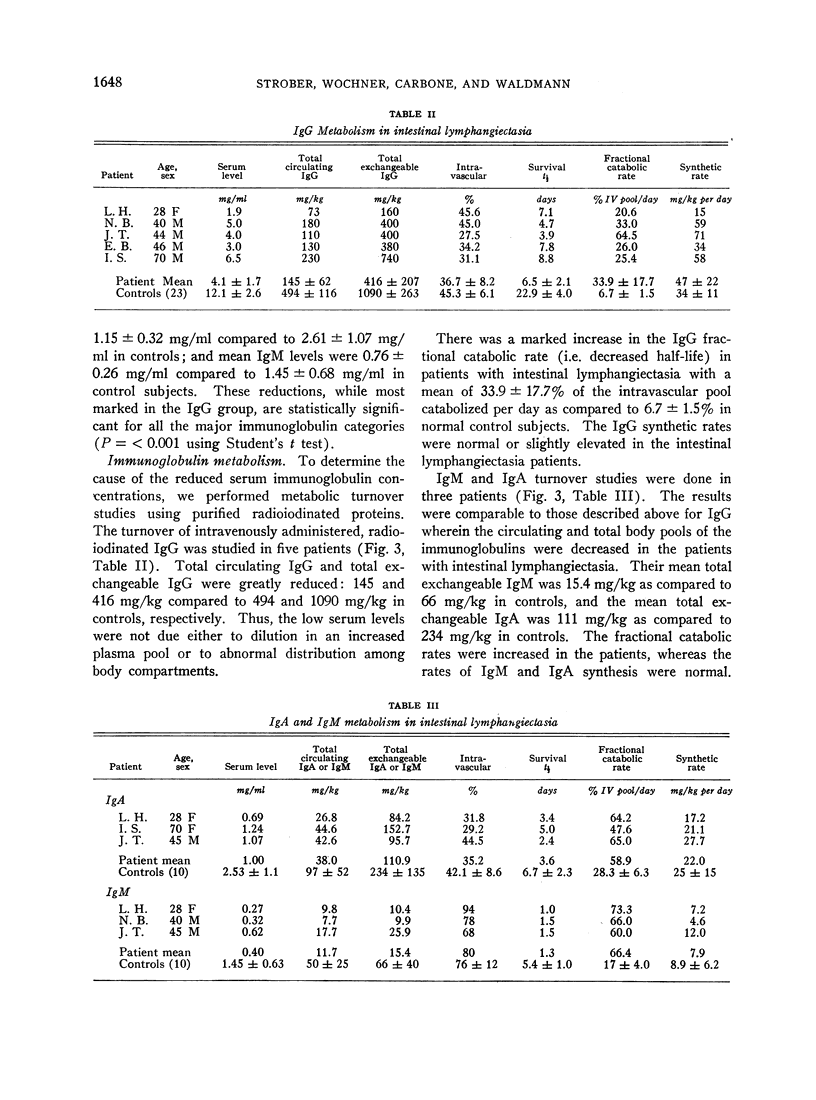
Abstract
Intestinal lymphangiectasia is a disease characterized by dilated intestinal lymphatics, protein-losing enteropathy, hypoalbuminemia, and edema. The immunologic status of 18 patients with intestinal lymphangiectasia was studied. Concentrations of IgG, IgA, and IgM were measured by immune precipitation and metabolism of these three immunoglobulins was studied using purified radioiodinated proteins. The serum concentration and total body pool of each immunoglobin were greatly reduced. The fraction of the intravascular protein pool catabolized per day was increased to 34% for IgG, 59% for IgA, and 66% for IgM; these are in contrast with control values of 7%, 28%, and 17%, respectively. Synthetic rates of the immunoglobulins were normal or slightly increased.
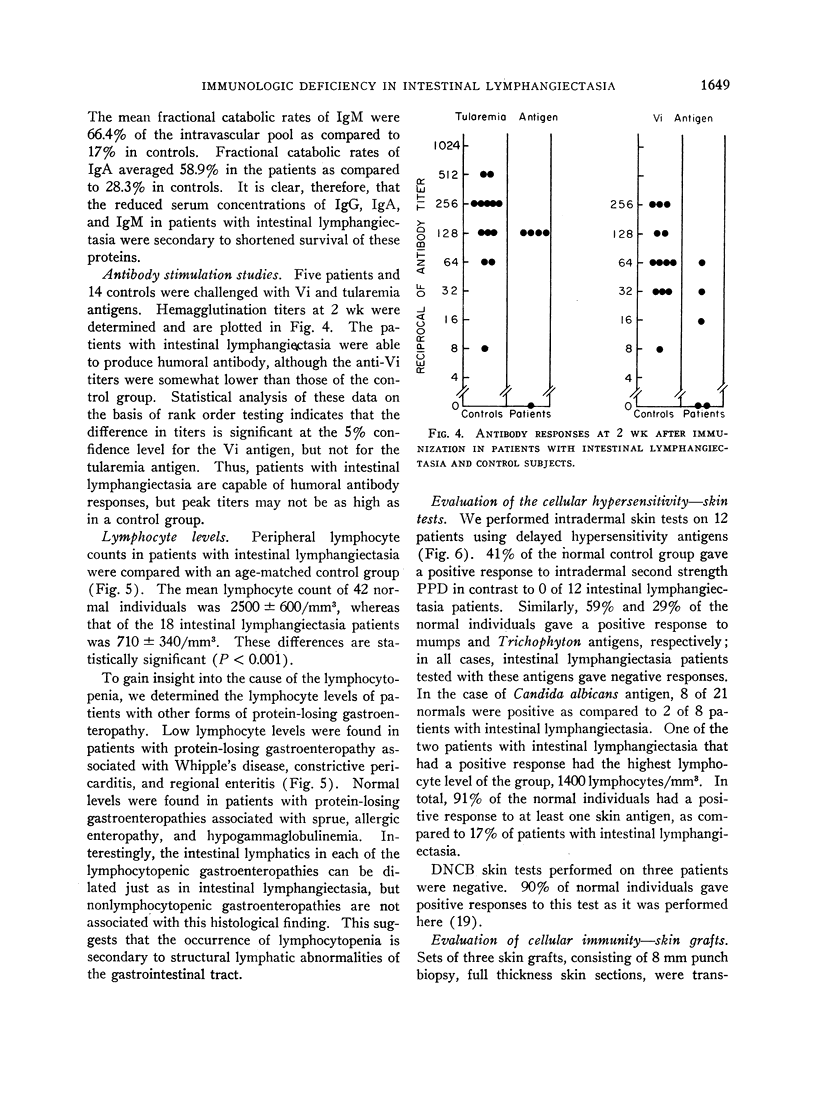
Primary circulating antibody response was tested in five patients with Vi and tularemia antigens. Titers elicited in patients with the Vi antigen were significantly lower than those seen in a control group, whereas no difference was seen between patient and control responses to the tularemia antigen.
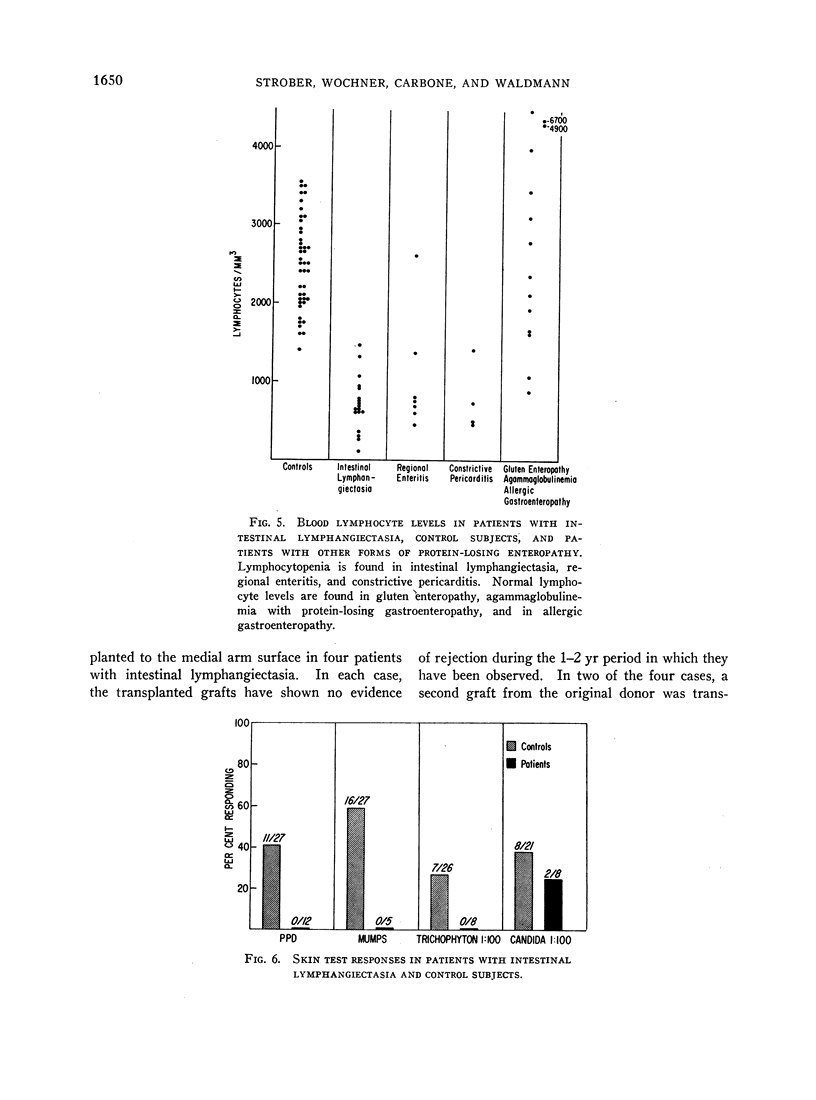
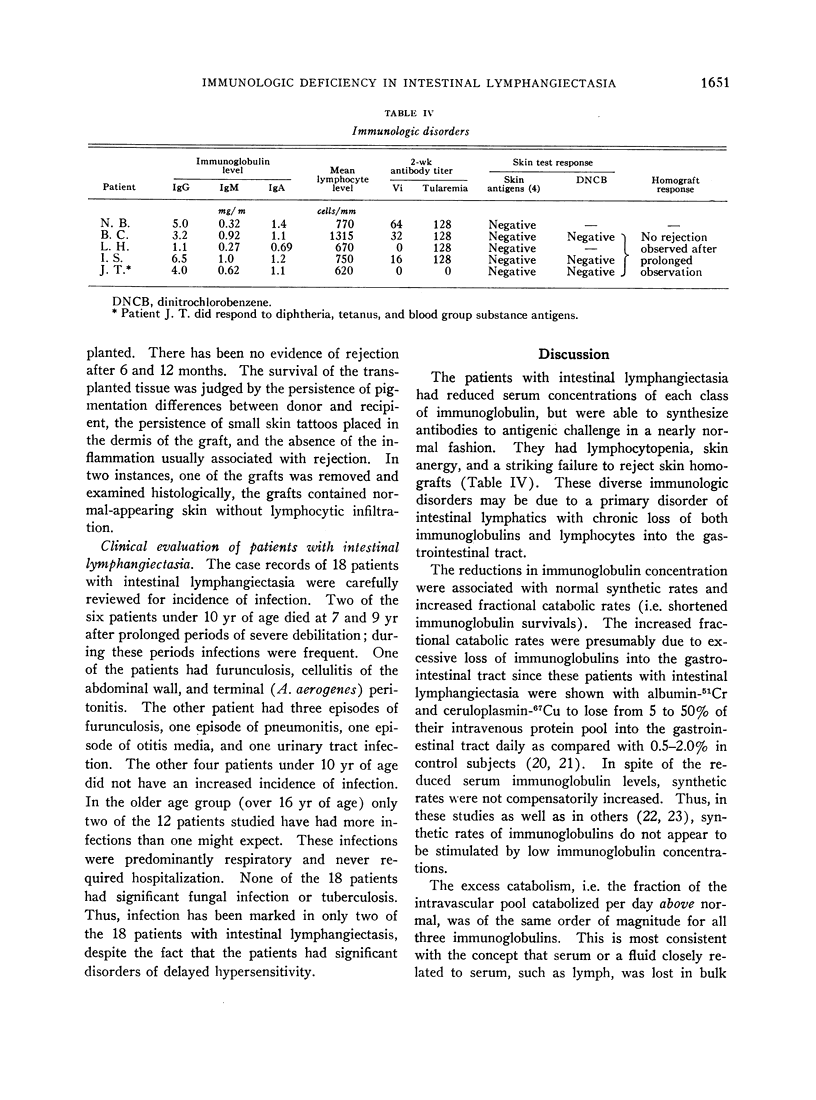
Lymphocytopenia was noted in patients with intestinal lymphangiectasia. The mean circulating lymphocyte count was 710 ± 340/mm3 in contrast to 2500 ± 600/mm3 in controls. Cellular hypersensitivity was studied with skin tests and skin grafts. 91% of normal individuals reacted to at least one of the four skin test antigens: purified protein derivative, mumps, Trichophyton, and Candida albicans; in contrast, only 17% of patients with intestinal lymphangiectasia had a positive reaction. Each of three patients tested with dinitrochlorobenzene had a negative reaction. Finally, all four patients who received skin homografts have retained these grafts for at least 12 months. The immunological disorders in patients with intestinal lymphangiectasia appear to result from loss of immunoglobulins and lymphocytes into the gastrointestinal tract secondary to disorders of lymphatic channels. Lymphocyte depletion then leads to skin anergy and impaired homograft rejection.
Full text
PDF
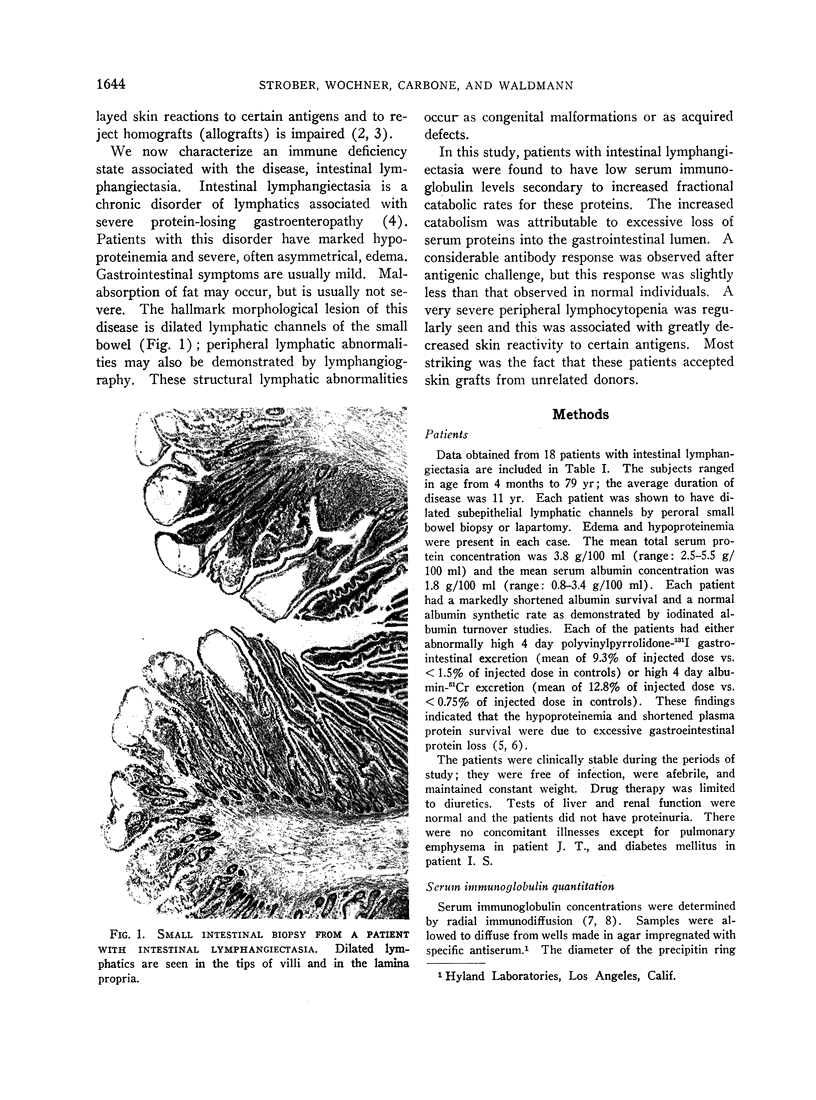
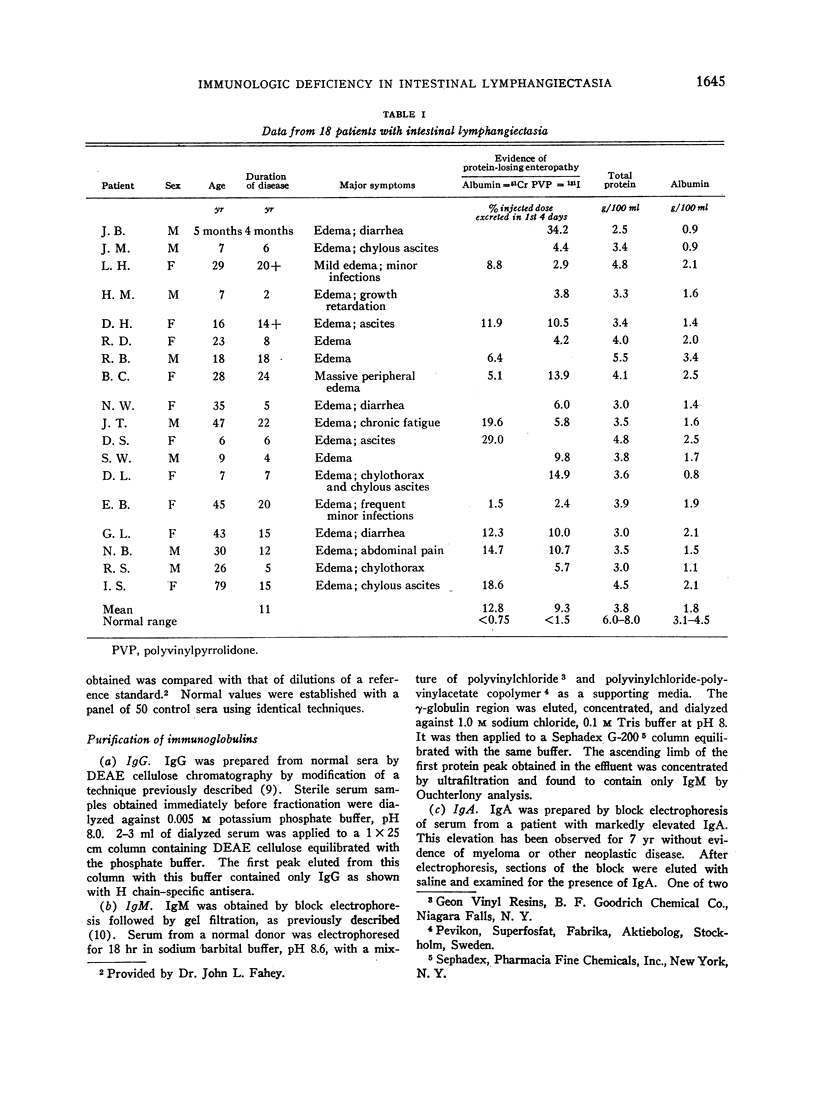

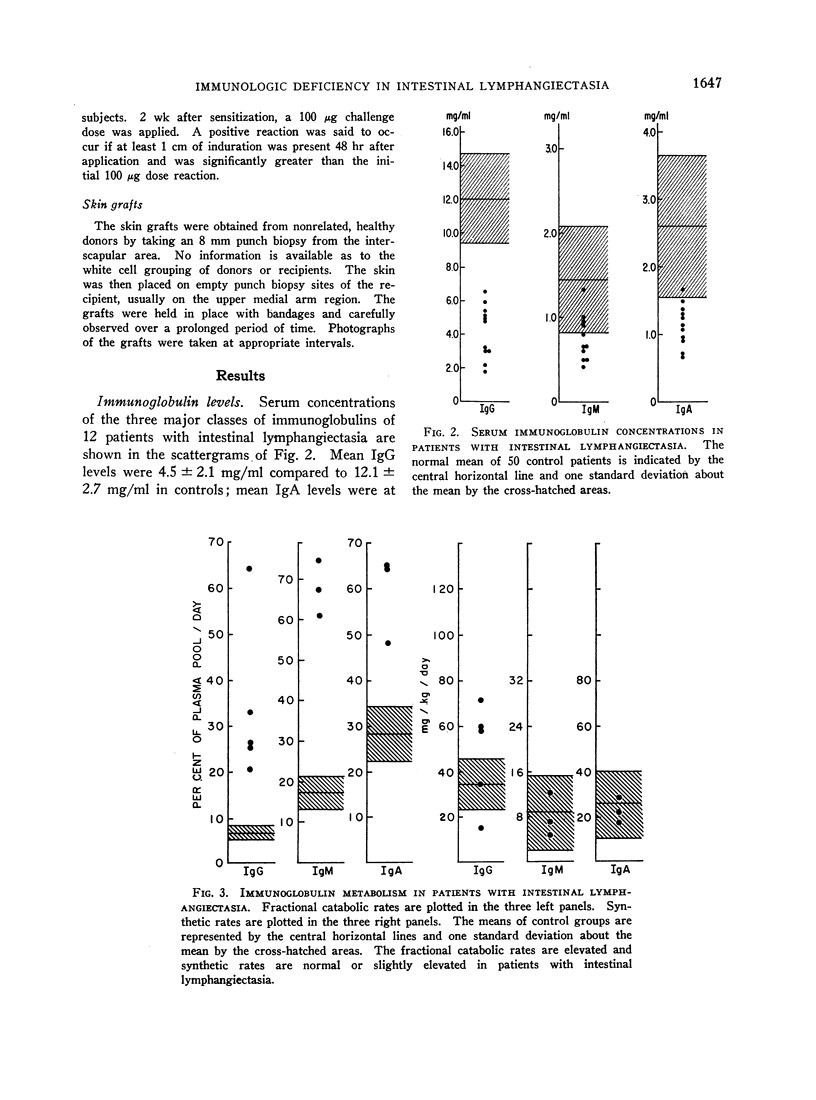





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AISENBERG A. C., LESKOWITZ S. Antibody formation in Hodgkin's disease. N Engl J Med. 1963 Jun 6;268:1269–1272. doi: 10.1056/NEJM196306062682304. [DOI] [PubMed] [Google Scholar]
- BARTH W. F., WOCHNER R. D., WALDMANN T. A., FAHEY J. L. METABOLISM OF HUMAN GAMMA MACROGLOBULINS. J Clin Invest. 1964 Jun;43:1036–1048. doi: 10.1172/JCI104987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERSON S. A., YALOW R. S., SCHREIBER S. S., POST J. Tracer experiments with I131 labeled human serum albumin: distribution and degradation studies. J Clin Invest. 1953 Aug;32(8):746–768. doi: 10.1172/JCI102789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILLINGHAM R. E., BRENT L. Further attempts to transfer transplantation immunity by means of serum. Br J Exp Pathol. 1956 Dec;37(6):566–569. [PMC free article] [PubMed] [Google Scholar]
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Quantitative studies on tissue transplantation immunity. II. The origin, strength and duration of actively and adoptively acquired immunity. Proc R Soc Lond B Biol Sci. 1954 Dec 15;143(910):58–80. doi: 10.1098/rspb.1954.0054. [DOI] [PubMed] [Google Scholar]
- BILLINGHAM R. E., SILVERS W. K., WILSON D. B. FURTHER STUDIES ON ADOPTIVE TRANSFER OF SENSITIVITY TO SKIN HOMOGRAFTS. J Exp Med. 1963 Sep 1;118:397–420. doi: 10.1084/jem.118.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON J. D., WALDMANN T. A., GOODMAN D. S., GORDON R. S., Jr Protein-losing gastroenteropathy in congestive heart-failure. Lancet. 1961 Apr 29;1(7183):899–902. doi: 10.1016/s0140-6736(61)91768-8. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., MCKELVEY E. M. QUANTITATIVE DETERMINATION OF SERUM IMMUNOGLOBULINS IN ANTIBODY-AGAR PLATES. J Immunol. 1965 Jan;94:84–90. [PubMed] [Google Scholar]
- FAHEY J. L., McCOY P. F., GOULIAN M. Chromatography of serum proteins in normal and pathologic sera: the distribution of protein-bound carbohydrate and cholesterol, siderophilin, thyroxin-binding protein, B12-binding protein, alkaline and acid phosphatases, radio-iodinated albumin and myeloma proteins. J Clin Invest. 1958 Feb;37(2):272–284. doi: 10.1172/JCI103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y., Hasegawa T., Watson C. G., Brooks J. R. The role of lymphoid cells in homograft rejection: circulating and cellular aspects. Transplantation. 1966 Nov;4(6):668–677. doi: 10.1097/00007890-196611000-00002. [DOI] [PubMed] [Google Scholar]
- GOOD R. A., KELLY W. D., ROTSTEIN J., VARCO R. L. Immunological deficiency diseases. Agammaglobulinemia, hypogammaglobulinemia, Hodgkin's disease and sarcoidosis. Prog Allergy. 1962;6:187–319. [PubMed] [Google Scholar]
- GORDON R. S., Jr Exudative enteropathy: abnormal permeability of the gastrointestinal tract demonstrable with labelled polyvinylpyrrolidone. Lancet. 1959 Feb 14;1(7068):325–326. doi: 10.1016/s0140-6736(59)90309-5. [DOI] [PubMed] [Google Scholar]
- HITZIG W. H., BIRO Z., BOSCH H., HUSER H. J. Agammaglobulinämie und Alymphocytose mit Schwund des lymphatischen Gewebes. Helv Paediatr Acta. 1958 Dec;13(6):551–585. [PubMed] [Google Scholar]
- HOLT P. R. DIETARY TREATMENT OF PROTEIN LOSS IN INTESTINAL LYMPHANGIECTASIA. THE EFFECT OF ELIMINATING DIETARY LONG CHAIN TRIGLYCERIDES ON ALBUMIN METABOLISM IN THIS CONDITION. Pediatrics. 1964 Nov;34:629–635. [PubMed] [Google Scholar]
- HUIZENGA K. A., WOLLAEGER E. E., GREEN P. A., McKENZIE B. F. Serum globulin deficiencies in non-tropical sprue, with report of two cases of acquired agammaglobulinemia. Am J Med. 1961 Oct;31:572–580. doi: 10.1016/0002-9343(61)90141-3. [DOI] [PubMed] [Google Scholar]
- JEFFRIES G. H., CHAPMAN A., SLEISENGER M. H. LOW-FAT DIET IN INTESTINAL LYMPHANGIECTASIA. ITS EFFECT ON ALBUMIN METABOLISM. N Engl J Med. 1964 Apr 9;270:761–766. doi: 10.1056/NEJM196404092701503. [DOI] [PubMed] [Google Scholar]
- JOACHIM G. R., CAMERON J. S., SCHWARTZ M., BECKER E. L. SELECTIVITY OF PROTEIN EXCRETION IN PATIENTS WITH THE NEPHROTIC SYNDROME. J Clin Invest. 1964 Dec;43:2332–2346. doi: 10.1172/JCI105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KADULL P. J., REAMES H. R., CORIELL L. L., FOSHAY L. Studies on tularemia. V. Immunization of man. J Immunol. 1950 Oct;65(4):425–435. [PubMed] [Google Scholar]
- KELLY W. D., LAMB D. L., VARCO R. L., GOOD R. A. An investigation of Hodgkin's disease with respect to the problem of homotransplantation. Ann N Y Acad Sci. 1960 May 31;87:187–202. doi: 10.1111/j.1749-6632.1960.tb23192.x. [DOI] [PubMed] [Google Scholar]
- LANDY M., LAMB E. Estimation of Vi antibody employing erythrocytes treated with purified Vi antigen. Proc Soc Exp Biol Med. 1953 Apr;82(4):593–598. doi: 10.3181/00379727-82-20188. [DOI] [PubMed] [Google Scholar]
- Laster L., Waldmann T. A., Fenster L. F., Singleton J. W. Albumin metabolism in patients with Whipple's disease. J Clin Invest. 1966 May;45(5):637–644. doi: 10.1172/JCI105379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGREGOR D. D., GOWANS J. L. SURVIVAL OF HOMOGRAFTS OF SKIN IN RATS DEPLETED OF LYMPHOCYTES BY CHRONIC DRAINAGE FROM THE THORACIC DUCT. Lancet. 1964 Mar 21;1(7334):629–632. doi: 10.1016/s0140-6736(64)91451-5. [DOI] [PubMed] [Google Scholar]
- MISTILIS S. P., SKYRING A. P., STEPHEN D. D. INTESTINAL LYMPHANGIECTASIA MECHANISM OF ENTERIC LOSS OF PLASMA-PROTEIN AND FAT. Lancet. 1965 Jan 9;1(7376):77–79. doi: 10.1016/s0140-6736(65)91657-0. [DOI] [PubMed] [Google Scholar]
- MITCHISON N. A. Studies on the immunological response to foreign tumor transplants in the mouse. I. The role of lymph node cells in conferring immunity by adoptive transfer. J Exp Med. 1955 Aug 1;102(2):157–177. doi: 10.1084/jem.102.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MTTCHISON N. A., DUBE O. L. Studies on the immunological response to foreign tumor transplants in the mouse. II. The relation between hemagglutinating antibody and graft resistance in the normal mouse and mice pretreated with tissue preparations. J Exp Med. 1955 Aug 1;102(2):179–197. doi: 10.1084/jem.102.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Wood M. L., Russell P. S. Adult Thymectomy: Effect on Recovery from Immunologic Depression in Mice. Science. 1965 Jul 23;149(3682):432–435. doi: 10.1126/science.149.3682.432. [DOI] [PubMed] [Google Scholar]
- NAJARIAN J. S., FELDMAN J. D. Passive transfer of transplantation immunity. I. Tritiated lymphoid cells. II. Lymphoid cells in millipore chambers. J Exp Med. 1962 May 1;115:1083–1093. doi: 10.1084/jem.115.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEZELOF C., JAMMET M. L., LORTHOLARY P., LABRUNE B., LAMY M. L'HYPOPLASIE HEREDITAIRE DU THYMUS: SA PLACE ET SA RESPONSABILITE DANS UNE OBSERVATION D'APLASIE LYMPHOCYTAIRE, NORMOPLASMOCYTAIRE ET NORMOGLOBULINEMIQUE DU NOURRISSON. Arch Fr Pediatr. 1964 Oct;21:897–920. [PubMed] [Google Scholar]
- PEARSON J. D., VEALL N., VETTER H. A practical method for plasma albumin turnover studies. Strahlentherapie. 1958;107(SONDERBD):290–297. [PubMed] [Google Scholar]
- PETERSEN V. P., HASTRUP J. Protein-losing enteropathy in constrictive pericarditis. Acta Med Scand. 1963 Apr;173:401–410. doi: 10.1111/j.0954-6820.1963.tb17423.x. [DOI] [PubMed] [Google Scholar]
- ROSEN F. S., GITLIN D., JANEWAY C. A. Alymphocytosis, agammaglobulinaemia, homografts, and delayed hypersen sitivity: study of a case. Lancet. 1962 Aug 25;2(7252):380–381. doi: 10.1016/s0140-6736(62)90232-5. [DOI] [PubMed] [Google Scholar]
- RUBIN C. E., BRANDBORG L. L., PHELPS P. C., TAYLOR H. C., Jr Studies of celiac disease. I. The apparent identical and specific nature of the duodenal and proximal jejunal lesion in celiac disease and idiopathic sprue. Gastroenterology. 1960 Jan;38:28–49. [PubMed] [Google Scholar]
- STOELINGA G. B., van MUNSTER J., SLOOFF J. P. Chylous effusions into the intestine in a patient with protein-losing gastroenteropathy. Pediatrics. 1963 Jun;31:1011–1018. [PubMed] [Google Scholar]
- WAKSMAN B. H., ARBOUYS S., ARNASON B. G. The use of specific "lymphocyte" antisera to inhibit hypersensitive reactions of the "delayed" type. J Exp Med. 1961 Dec 1;114:997–1022. doi: 10.1084/jem.114.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALDMANN T. A. Gastrointestinal protein loss demonstrated by Cr-51-labelled albumin. Lancet. 1961 Jul 15;2(7194):121–123. doi: 10.1016/s0140-6736(61)92646-0. [DOI] [PubMed] [Google Scholar]
- WALDMANN T. A., SCHWAB P. J. IGG (7 S GAMMA GLOBULIN) METABOLISM IN HYPOGAMMAGLOBULINEMIA: STUDIES IN PATIENTS WITH DEFECTIVE GAMMA GLOBULIN SYNTHESIS, GASTROINTESTINAL PROTEIN LOSS, OR BOTH. J Clin Invest. 1965 Sep;44:1523–1533. doi: 10.1172/JCI105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEAVER J. M., ALGIRE G. H., PREHN R. T. The growth of cells in vivo in diffusion chambers. II. The role of cells in the destruction of homografts in mice. J Natl Cancer Inst. 1955 Jun;15(6):1737–1767. [PubMed] [Google Scholar]
- WEBSTER M. E., LANDY M., FREEMAN M. E. Studies on Vi antigen. II. Purification of Vi antigen from Escherichia coli 5395/38. J Immunol. 1952 Aug;69(2):135–142. [PubMed] [Google Scholar]
- WEBSTER M. E., SAGIN J. F., ANDERSON P. R., BREESE S. S., FREEMAN M. E., LANDY M. Studies on Vi antigen. IV. Physicochemical characterization of Vi antigens isolated from V form Enterobacteriaceae. J Immunol. 1954 Jul;73(1):16–22. [PubMed] [Google Scholar]
- WESSLEN T. Passive transfer of tuberculin hypersensitivity by viable lymphocytes from the thoracic duct. Acta Tuberc Scand. 1952;26(1-2):38–53. [PubMed] [Google Scholar]
- Waldmann T. A., Morell A. G., Wochner R. D., Strober W., Sternlieb I. Measurement of gastrointestinal protein loss using ceruloplasmin labeled with copper. J Clin Invest. 1967 Jan;46(1):10–20. doi: 10.1172/JCI105502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A. Protein-losing enteropathy. Gastroenterology. 1966 Mar;50(3):422–443. [PubMed] [Google Scholar]
- Wochner R. D., Drews G., Strober W., Waldmann T. A. Accelerated breakdown of immunoglobulin G (IgG) in myotonic dystrophy: a hereditary error of immunoglobulin catabolism. J Clin Invest. 1966 Mar;45(3):321–329. doi: 10.1172/JCI105346. [DOI] [PMC free article] [PubMed] [Google Scholar]