Abstract
Objective:
Sleep disruption is common in widow(er)s. The objective of this study was to characterize the sleep of Spousally Bereaved (SB) seniors (60y+) studied within 4–19 months of being widowed.
Method:
Subjective (PSQI, 2-weeks diary) and objective (2-weeks actigraphy) baseline sleep measures were obtained in 47 (38f, 9m) Spousally Bereaved (SB) seniors, 33 (25f, 8m) Good Sleeper Controls (GSC), and 47 (38f, 9m) Older Adults with Insomnia (OAI); each group with the same mean age (72y). OAI subjects passed formal diagnostic criteria for primary or co-morbid insomnia. GSC subjects had no diagnosis of insomnia. At baseline (pre-treatment), all subjects completed 2 weeks of detailed sleep diary and wrist actigraphy, and completed the Pittsburgh Sleep Quality Index (PSQI) among other measures.
Results:
Significant group effects appeared in PSQI (GSC: 2.4, SB: 6.7, OAI: 10.5; Effect Sizes [ES]>1) and diary measures. In diary measures, for Total Sleep Time, Sleep Efficiency and Wake After Sleep Onset, SB were better than OAI and worse than GSC (0.47<ES<1.19). For Sleep Latency, SB were worse than GSC (ES=0.57), but similar to OAI. However, actigraphy results indicated no significant SB vs. GSC, or SB vs. OAI, differences in any of the sleep measures considered.
Conclusion:
The sleep disruption of bereaved seniors appears to be intermediate between GSC and OAI, as reported either retrospectively using the PSQI, or prospectively using a sleep diary. Only in diary sleep latency, were SB and OAI values similar. This pattern was not, however, observed when parallel objective actigraphic measures were considered
Keywords: insomnia, bereavement, widow, old, sleep, actigraph
INTRODUCTION
Losing a partner to death is a ubiquitous part of the human condition, especially in later life. More than 800,000 older Americans, for example, newly become widow(er)s every year (1). Apart from the severe emotional strain of the loss of a loved one, there are profound changes in lifestyle and status, often accompanied by reductions in financial security, perceived personal safety, and freedom of action. All of these facets of the situation are likely to lead to sleep disruption, and it is thus not surprising that late life Spousal Bereavement (SB) has been shown to be associated with significant sleep impairment (2).
As shown in a series of studies of bereaved seniors conducted by Reynolds and colleagues in the 1990s (2); although sleep disruption in SB is particularly prevalent in the depressed bereaved, even bereaved persons who fail to meet a formal diagnosis of depression have measurable sleep impairment. When a group of spousally bereaved seniors with sub-syndromal symptoms of depression were compared with an age and gender matched control group, all of the bereaved subjects endorsed poor sleep quality as indicated by a score > 5 on the Pittsburgh Sleep Quality Index (PSQI),(3) compared to only four of the 14 control subjects (4). Moreover, the level of sleep disruption (as measured by the PSQI) appeared to increase with grief severity (5). A recent Japanese epidemiological study using the PSQI with a sample of 2,800 adults (6) has indicated that being widowed (female) increased the probability of reporting Difficulty in Maintaining Sleep (DMS) with an odds ratio of 1.65, and of hypnotic use with an odds ratio of 2.12. A Swedish study (7) using a different instrument and a sample of 509 widows whose husbands had died of cancer three years prior (as compared to women whose husbands were still alive), indicated a Relative Risk (RR) of 1.95 with regard to sleep disturbances (RR CI = 1.5 − 3.4).
Accepted conceptualizations of insomnia have changed over recent years. In the United States, the 2005 NIH State-of-the-Science Conference Statement of the Manifestations and Management of Chronic Insomnia in Adults (8) has recommended the use of the term “co-morbid insomnia” (rather than “secondary” insomnia) to more accurately reflect “the limited understanding of mechanistic pathways in chronic insomnia [which] precludes drawing firm conclusions about the nature of these associations or the direction of causality” between insomnia and concurrent medical or psychiatric conditions. In fact, the treatment of insomnia is often associated with significant improvements in other symptoms of distress including depression,(9) anxiety disorders (including PTSD(10)), alcohol use disorder (11), and medical conditions including chronic pain, chronic medical conditions, and cancer (12). The consensus statement also highlighted the need for more research into the effectiveness of available insomnia treatments for co-morbid insomnia. As a response to this need, the present study seeks to elucidate how the sleep of bereaved seniors might differ from that of Good Sleeper Controls (GSC) of similar age, and from that of Older Adults with Insomnia (OAI) using PSQI, sleep diary and actigraphic measures.
METHOD
Spousally Bereaved (SB) Subjects
SB subjects for the present study were 47 widow(er)s (38f, 9m, age: 60y − 90y, mean 72.3y, sd 7.0y) who were recruited by advertisement, oral presentations and word of mouth. All subjects were required to either have a Pittsburgh Sleep Quality Index (PSQI) score of 5 or greater, or a laboratory PSG sleep efficiency ≤90%, at a screening night with oximetry (see below), although no potential subjects applying to be enrolled in the study were excluded by these criteria. All subjects were spousally bereaved and were studied in the laboratory between 4 mos. and 19 mos. after the loss event (mean 9.1 mos., s.d. 3.4 mos.). The longer post-loss intervals resulted mostly from the need to stabilize medical conditions in some subjects before they could be studied in the laboratory in accordance with subject protection rules. For such subjects bereavement remained an issue for them because they still sought the treatment provided by the protocol. The lower bound of post-loss interval resulted from us not wanting to start the protocol in the immediate post-loss interval when too much was happening in the new widow(er)'s life.
Spousal bereavement was defined as the loss of a co-habiting life partner of the same generation, whether or not the two people were formally married (most were). Following the baseline assessments to be described in this article, subjects took part in a study involving a potentially therapeutic intervention (see below) as well as assessments of sleep, physical health, mental health, and functioning. Intervention sessions were provided at no cost to the subject, and subjects were compensated for their time and provided with free transportation.
Subjects were required to be free of clinical unstable medical or psychiatric conditions that warranted immediate intervention (i.e., they were clinically stable and on a fixed treatment regimen), but otherwise exclusion criteria allowed for fairly inclusive recruitment. In particular, subjects were included even if they coded for Major Depressive Disorder (MDD); all subjects were given a Structured Clinical Interview for DSM-IV (SCID). Potential subjects who were on anti-depressant medication were not recruited. Occasional hypnotic use (either over-the-counter or prescribed) at the time of recruitment was permitted, but few subjects (<6%) reported such use, and subjects were asked to refrain from such use during the study.
For those who passed the screening processes, the intervention consisted of either Social Rhythm Therapy (SRT), which is designed to induce an active and regular lifestyle, or Emotion-Focused Therapy (EFT), which is designed to explore the triggers of grief. Each therapy lasted 6 mos. and involved ten one-on-one sessions. All of the data to be described here were, however, collected before any therapy had been given. The only contact with the therapist had been a “meet and greet” session at which the subject was introduced to the diary instruments and a baseline Hamilton Depression Rating Scale (HDRS) administered in a structured clinical interview (in addition to several questionnaire measures). This session took place several weeks before the diary and actigraphy data to be reported here were collected.
Older Adults with Insomnia (OAI) Subjects
OAI subjects were recruited for a randomized clinical trial of Brief Behavioral Treatment for Insomnia (BBTI) [PI D.J. Buysse]. Inclusion criteria regarding mental and physical health were very similar to those applied to the SB group, but those self-described as widow(er)s were not included in the OAI sample reported here. The data used here were collected before any treatment (BBTI or an informational control) had been given. Subjects were recruited both from physicians' offices (17%), and by advertising and other sources (83%), and were financially compensated for their time. Preliminary results from this study are reported elsewhere (13,14). OAI subjects were required to meet the general criteria for insomnia disorder in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV)(15) and the International Classification of Sleep Disorders, 2nd Edition (16). Specifically, these criteria include a sleep complaint lasting for at least one month; adequate opportunity and circumstances for sleep; and evidence of significant distress or daytime impairment. In order to enhance the generality and clinical relevance of the study, the exclusion criteria of DSM-IV for medical or psychiatric disorders were not applied. Therefore, many of the OAI subjects would be considered to have had “comorbid insomnia.” Initial assessment and diagnosis were assisted by the use of a screening sleep diary, locally-developed sleep, medical history, and medication surveys, and sleep and psychiatric symptom questionnaires. A sample of 47 OAI subjects were selected (for the present report) from a total of 80, based only on age and gender matching.
Good Sleeper Control (GSC) Subjects
GSC subjects were recruited as control subjects for the BBTI trial (PI D.J. Buysse) for which the OAI subjects were recruited (see above). The inclusion criteria were identical to those for the OAI, except that no diagnosis of insomnia was present. After excluding subjects who self-reported as being widowed, there were 33 GSC subjects.
All three groups had approximately the same gender ratio (GSC: 25f, 8m; SB: 38f, 9m; OAI 38f, 9m) and mean (± s.d.) age (GSC: 71.8y ±7.4; SB: 72.3y±7.0; OAI: 72.4y ±7.1), all subjects were within the 60y − 90y age range. The protocol complied with the University of Pittsburgh Biomedical Institutional Review Board (IRB). Full informed consent was obtained.
Procedure
Following recruitment and informed consent, all subjects completed a battery of questionnaires and received clinical interviews. The present analysis will focus upon the overall PSQI score for each subject. The PSQI was administered in the standard way, requiring responses pertaining to the previous month. Operational problems led to 1 of the 127 subjects' PSQI data not being included. Each subject was also given a 2-week Pittsburgh Sleep Diary (17) which was co-incident with the wearing of a wrist actigraph (Actiwatch □, Minimitter division of Respironics, Inc. Bend, Oregon) for 2-weeks, on the non-dominant arm, recording in one-minute epochs.
The PghSD comprises a diary with evening and morning pages. The present analysis focused on the morning pages, in which subjects were asked to note the time that they went to bed, first attempted sleep, fell asleep and arose in the morning (rise-time). Subjects were also asked to estimate the number of awakenings during the night and the total duration of wakefulness after sleep onset. Ratings of sleep quality, mood and alertness upon awakening where also made using visual analogue scales. From the PghSD we gleaned diary-based measures of Sleep Efficiency (SE), Sleep Latency (SL), Wake After Sleep Onset (WASO), and Total Sleep Time (TST), as well as habitual bedtimes and rise-times, across the 14 nights. Fourteen-night averages of the subjects’ reported SL, WASO and Time in Bed (TIB) were calculated. TST was defined as TIB minus (SL + WASO). SE was defined as 100 times TST divided by TIB. Problems with subject misunderstandings led to 4 of the 127 subjects' diary data not being included. Similar measures were derived from the concurrent actigraph record which was downloaded onto a computer from the wrist actigraph unit. Following quality control procedures, we applied the Actiware© 5.0 software (purchased with the device from the manufacturer) in conjunction with bedtimes and wake-times that were imported from our database constructed from the concurrent entries from the PghSD. A sensitivity level of medium was set for the software. In order to provide parallel measures to sleep diary, we examined TST, SE, WASO and SL selected from the 52 endpoints produced by the Actiware© software, averaging them over the 14 nights. Problems with device malfunction and/or subject misunderstandings led to 8 of the 127 subjects' actigraphy data not being included.
Before study, SB and OAI subjects completed an apnea screening night with PSG and oximetry at their habitual bedtime and rise-time and were required to have an Apnea-Hypopnea Index (AHI) of <30 (SB) or <20 (OAI), and a Periodic Limb movements with arousal (PLMA) index of <20. This screening night was given in our sleep laboratory for SB subjects and at the subject's home (using a Compumedics Siesta□ system) for OAI subjects, but were always at the subject's own habitual bedtime and rise-time. Fairly high AHI criteria were employed because of the desire to be inclusive and representative of older adults who often have higher average AHI scores than the young.
Immediately following the diary and actigraphy collection to be reported here, SB subjects went on to complete a 36h laboratory study with measurement of circadian rhythms and two nights of polysomnography (reported earlier 18)); OAI subjects completed three consecutive nights of polysomnography at home (reported elsewhere (13,14)). Polysomnography data will not be reported here.
Statistical Analysis
For each variable, a simple between-subjects ANOVA was performed to determine whether the 3 groups differed significantly. Pair-wise comparisons then compared SB vs. GSC, and SB vs. OAI for each variable that showed a significant main effect of group. A log transform was used for the SL ANOVA, though untransformed means for SL are reported.
RESULTS
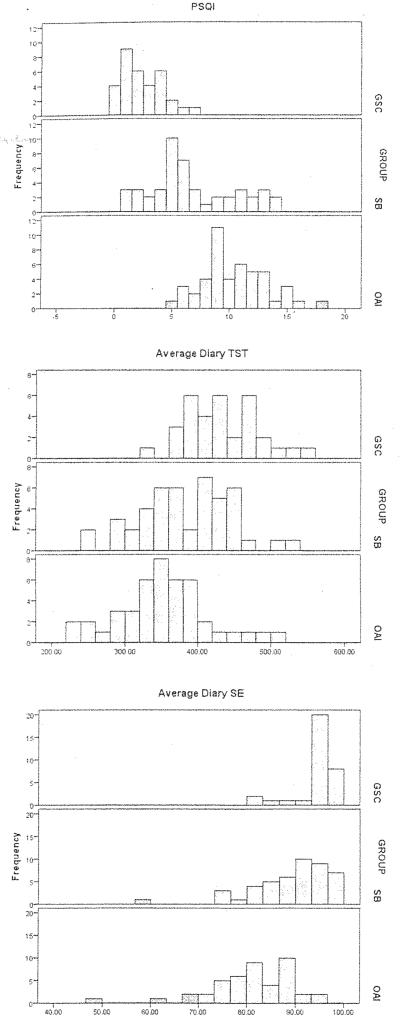
Results of the statistical analysis are given in Table 1. A selection of frequency distributions is presented in Figure 1. For the PSQI, SB scores were intermediate between GSC and OAI. In PSQI score, effect sizes were 1.41 for SB vs. GSC and 1.12 for SB vs. OAI. In diary measures also, SB sleep was intermediate between GSC and OAI. In Total Sleep Time (TST), Sleep Efficiency (SE) and Wake After Sleep Onset (WASO), SB reported significantly worse sleep than GSC, (effect sizes: 0.59 – 0.84), but significantly better sleep than OAI (effect sizes: 0.47 – 1.19). In SL, SB values were worse (longer) than GSC with an effect size of 0.57, but were no different from OAI.
Table 1.
See text for abbreviations. ↔ indicates a pair-wise comparison, with Y indicating Yes (p≤0.05), and N indicating No (p>0.05) with regard to statistical significance; the number in parenthesis after the Y is the effect size for that comparison. Units for TST, SL and WASO are minutes.
| Variable | GSC | SB | OAI | F | p | SB↔GSC | SB↔OAI |
|---|---|---|---|---|---|---|---|
| PSQI | 2.4 | 6.7 | 10.5 | 68.14 | <.001 | Y (1.41) | Y (1.12) |
| Diary TST | 433 | 385 | 355 | 16.44 | <.001 | Y (0.84) | Y (0.47) |
| Diary SE | 94.4% | 89.0% | 81.2% | 30.56 | <.001 | Y (0.79) | Y (0.94) |
| Diary SL | 13.0 | 25.7 | 34.0 | 10.18 | <.001 | Y (0.57) | N |
| Diary WASO | 12.2 | 21.3 | 50.9 | 16.44 | <.001 | Y (0.59) | Y (1.19) |
| Actig. TST | 399 | 379 | 381 | 1.96 | 0.15 | N | N |
| Actig. SE | 83.1% | 81.7% | 80.9% | 0.79 | 0.45 | N | N |
| Actig. SL | 13.0 | 17.0 | 17.4 | 1.48 | 0.23 | N | N |
| Actig. WASO | 50.1 | 50.6 | 55.6 | 0.62 | 0.54 | N | N |
Figure 1.
Frequency histograms for PSQI score (left panels), Diary Total Sleep Time (TST) (center panels) and Diary Sleep Efficiency (SE) (right panels); for Good Sleeper Controls (GSC) (top panels), Spousally Bereaved (SB) (middle panels), and Older Adults with Insomnia (OAI) (bottom panels). See text for details.
DISCUSSION
Sleep disturbance appears to be extremely common in spousally bereaved seniors (2,6). The magnitude of sleep disturbance endorsed by bereaved older adults, however, has not been directly compared to the nature and severity of sleep disturbances observed in older adults who meet formal diagnostic criteria for insomnia. In the present study, we sought to compare subjective sleep quality, and sleep disturbance logged retrospectively using the PSQI, and prospectively using sleep diaries and actigraphy, comparing SB with both GSC and OAI.
Self-report sleep measures differed among the three groups, with the SB group reporting significantly more problems than the GSC group, and fewer problems than the OAI group. As shown by Figure 1, and confirmed by the pair-wise comparisons reported in Table 1, SB values of PSQI, and sleep diary measures of TST and SE differed from those of the other two groups. Thus, in self-report measures, whether retrospective (PSQI), or prospective (diary), SB appeared to be intermediate between GSC and OAI. In diary SL, SB and OAI were very similar (and significantly worse than GSC), suggesting that the sleep disruption of SB may be particularly strong at the beginning of the night. Possible contributing factors for this are discussed below. We recognize that it is possible that the sleep of SB was worse than that of GSC simply because of inclusion criteria for the SB study, which required a PSQI score ?5 or a screening sleep efficiency (polysomnography with oximetry) ≤90. However, as noted above, no potential subjects volunteering for the study were excluded by these criteria. Also, the publicity material for subject recruitment did not emphasize sleep or its treatment. Thus, we feel fairly confident that the SB sample was broadly representative of widow(er)s seeking help. The only caveat would be that they were also prepared to participate in a 10 session, 6-month therapy program, with pre-and post-treatment 36h laboratory evaluations, which represented a fairly considerable commitment of time and effort. It is also possible that differences in SB versus OAI inclusion criteria regarding apnea (AHI<30 (SB) vs. AHI<20 (OAI)) may have minimized the difference between SB and OAI in WASO and SE measures. Examination of AHI scores for SB and OAI subjects confirmed that there was a significant difference (SB: 14.0, OAI: 7.6, p<0.001). Using a criterion of AHI ? 15, 19 of the 47 SB had OSA compared to 4 of the 47 OAI. No apnea criteria were imposed on GSC subjects, so the presence of an AHI exclusion criterion in SB (albeit with a high cut-off of 30) may have minimized the difference between SB and GSC. Overall, the effects of the AHI criteria differences would seem to be one of making the SE and WASO comparisons, if anything, more conservative. Although PSG sleep recordings were available for SB and OAI subjects, they were conducted in the laboratory for SB, whereas the OAI subjects were studied using at-home recording. Because of these differences we felt that there was not enough comparability for the PSG data to be reported. The 2-week diary and actigraphy data were, of course, collected at home in all three groups.
Spousal bereavement may be very potent in its impact on sleep in general, and sleep onset in particular, because of the salience of sleeping together in the spousal relationship. The bereaved senior may have spent several decades going to bed each night with the lost spouse, and because of this, bedtime routines and the sleeping environment of the widow(er) might be times in which the loss is felt particularly acutely. However, although reporting their sleep as worse than GSC, SB self-reported sleep was, in most measures, also significantly better than OAI. This may relate to group differences in the salience of the sleep disruption, which was merely one of a whole legion of challenges faced by the widow(er)s, but perhaps comprised the major single challenge in the lives of OAI subjects. It should also be noted that was a range of duration of bereavement (months since loss) in the SB group, and that there was an expected correlation between diary WASO and months since loss (rho= −0.380, p=0.01), with WASO decreasing as time since loss increased.
Given that bereavement has been shown to increase the risk of insomnia, depression, and poor physical health outcomes (19), the present observations raise the possibility that further attention to sleep disturbances in SB seniors may provide new prevention and therapeutic strategies to enhance mental and physical health outcomes in this population. Longitudinal studies are necessary to fully investigate the role of sub-threshold sleep disturbance in SB seniors as a potential mediation of health outcomes. The present findings also highlight the sleep-focused treatments for seniors may be an important component of healthy adjustment following bereavement.
Given the fairly strong effect sizes seen in group differences in the diary measures of TST, SE and WASO, (which ran at 0.59 – 0.84 for SB vs. GSC; and at 0.47 – 1.19 for SB vs. OAI), we were frankly surprised that equivalent differences were not apparent in the parallel measures obtained using the objective actigraphy record. As noted in the methods section, all three groups used identical actigraphy procedures, equipment and software, and the values of TST, SE, SL and WASO obtained using actigraphy appeared to be reasonable ones (Table 1). As noted by other investigators (20), actigraphic measures may sometimes be less reliable in older subjects, particularly in those with sleep disruption. Alternatively, it may be the case that objectively, there was indeed no difference between the three groups and that the differences were of perception. Further research is needed in order to determine our pattern of actigraphy findings.
The lack of coherence between objective and diary/self report measures seen in the present study does have relevance as to how insomnia is defined. Sleep disturbance and insomnia are generally considered to be increasing societal problems resulting in substantial distress, especially for older adults (21). Current formal diagnoses of insomnia now rely more upon perceptions of sleep quality, duration, and daytime functioning rather than upon strictly objective physiological measures (22, 23). Thus, the same objective levels of sleep disruption may be characterized as either insomnia or normal sleep, depending upon the perceptions of the patient. As this study shows, similar objective levels of insomnia may result in quite different subjective effects. The “slings and arrows of outrageous fortune” that so often beset older adults may themselves play an important role in determining their subjective level of insomnia. Moreover, the salience of that insomnia in the patient's life may itself affect the level of symptomatic distress that is reported.
In conclusion, the sleep disruption of bereaved seniors appears to be intermediate between that of GSC and that of OAI, as reported either retrospectively using the PSQI, or prospectively using a sleep diary. Only in diary sleep latency, were values for SB and OAI similar (with both worse than GSC). This pattern was not, however, observed when parallel actigraphic measures were considered.
REFERENCES
- 1.Murrell SA, Norris FH, Hutchins GL. Distribution and desirability of life events in older adults: Population and policy implications. J. Commun. Psychol. 1984;12:301–311. [Google Scholar]
- 2.Reynolds CF, Hock CC, Buysse DJ, Houck PR, Schlernitzauer M, Frank E, Mazumdar S, Kupfer DJ. Electroencephalographic sleep in spousal bereavement and bereavement-related depression of late life. Biol Psychiatry. 1992;31:69–82. doi: 10.1016/0006-3223(92)90007-m. [DOI] [PubMed] [Google Scholar]
- 3.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 4.Pasternak RE, Reynolds CF, Hoch CC, Buysse DJ, Schlernitzauer M, Machen M, Kupfer DJ. Sleep in spousally bereaved elders with subsyndromal depressive symptoms. Psychiatry Res. 1992;43:43–53. doi: 10.1016/0165-1781(92)90140-x. [DOI] [PubMed] [Google Scholar]
- 5.Brown LF, Reynolds CF, Monk TH, Prigerson HG, Dew MA, Houck PR, Mazumdar S, Buysse DJ, Hoch CC, Kupfer DJ. Social rhythm stability following late-life spousal bereavement: Associations with depression and sleep impairment. Psychiatry Res. 1996;62:161–169. doi: 10.1016/0165-1781(96)02914-9. [DOI] [PubMed] [Google Scholar]
- 6.Doi Y, Minowa M, Okawa M, Uchiyama M. Prevalence of sleep disturbance and hypnotic medication use in relation to sociodemographic factors in the general Japanese adult population. Journal of Epidemiology. 2000;10:79–86. doi: 10.2188/jea.10.79. [DOI] [PubMed] [Google Scholar]
- 7.Valdimarsdottir U, Helgason AR, Furst CJ, Adolfsson J, Steineck G. Long-term effects of widowhood after terminal cancer: a Swedish nationwide follow-up. Scand. J. Public Health. 2003;31:31–36. doi: 10.1080/14034940210165109. [DOI] [PubMed] [Google Scholar]
- 8.National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults; Sleep; June 13-15, 2005; 2005. pp. 1049–1057. [DOI] [PubMed] [Google Scholar]
- 9.Buysse DJ, McCall V, Roach J, Wessel T, Wilson P, Caron J, Roth T. Eszopiclone co-administered with fluoxetine for insomnia associated with Major Depressive Disorder (MDD): Sleep effects. Sleep. 2006;28(Abstract Supplement):A310–A311. [Google Scholar]
- 10.Germain A, Nielsen I. Impact of imagery rehearsal treatment on distressing dreams, psychological distress, and sleep parameters in nightmare patients. Behav Sleep Med. 2003;1:140–154. doi: 10.1207/S15402010BSM0103_2. [DOI] [PubMed] [Google Scholar]
- 11.Currie SR, Clark S, Hodgins DC, El Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction. 2004;99:1121–1132. doi: 10.1111/j.1360-0443.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev. 2005;25:559–592. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Buysse D, Germain A, Laurie B, Moul D, Franzen P, Miewald J, Reynolds C, Monk T. Effects of brief behavioral treatment for insomnia (BBTI) persist for six months in older adults. Sleep. 2007;30(Abstract Supplement):A233–A234. [Google Scholar]
- 14.Germain A, Moul DE, Franzen PL, Miewald JM, Reynolds CF, Monk TH, Buysse DJ. Effects of a brief behavioral treatment for late-life insomnia: Preliminary findings. Journal of Clinical Sleep Medicine. 2006;2:403–406. [PubMed] [Google Scholar]
- 15.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Fourth Edition American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 16.American Academy of Sleep Medicine The International Classification of Sleep Disorders, Second Edition (ICSD-2): Diagnostic and Coding Manual. (Second Edition) 2005 [Google Scholar]
- 17.Monk TH, Reynolds CF, Kupfer DJ, Buysse DJ, Coble PA, Hayes AJ, Machen MA, Petrie SR, Ritenour AM. The Pittsburgh Sleep Diary. J. Sleep Res. 1994;3:111–120. [PubMed] [Google Scholar]
- 18.Monk TH, Begley A, Billy BD, Fletcher ME, Germain A, Mazumdar S, Moul DE, Shear K, Thompson WK, Zarotney JR. Sleep and circadian rhythms in spousally bereaved seniors. Chronobiol. Int. 2008;25:83–98. doi: 10.1080/07420520801909320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendes de Leon CF, Kasl SV, Jacobs S. A prospective study of widowhood and changes in symptoms of depression in a community sample of the elderly. Psychol. Med. 1994;24:613–624. doi: 10.1017/s0033291700027768. [DOI] [PubMed] [Google Scholar]
- 20.Blackwell T, Redline S, Ancoli-Israel S, Schneider JL, Surovec S, Johnson NL, Cauley JA, Stone KL. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31:283–291. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Specchio LM, Prudenzano MP, de Tommaso M, Massimo M, Cuonzo F, Ambrosio R, Puca F. Insomnia, quality of life and psychopathological features. Brain Res. Bull. 2004;63:385–391. doi: 10.1016/j.brainresbull.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Buysse DJ. Insomnia State of the Science: An evolutionary, evidence-based assessment. Sleep. 2005;28:1029–1198. [PubMed] [Google Scholar]
- 23.Rybarczyh B, Stepanski E, Fogg L, et al. A Placebo-Controlled Test of Cognitive-Behavioral Therapy for Cognitive–Insomnia in Older Adults 2005. J. Consulting and Clinical Psychol. 73:1164–1174. doi: 10.1037/0022-006X.73.6.1164. [DOI] [PubMed] [Google Scholar]