Abstract
Treatment failure and drug resistance create obstacles to long-term management of HIV-1 infection. Nearly 60% of infected persons fail their first highly active antiretroviral therapy (HAART) regimen, partially because of nonadherence, requiring a switch to a second regimen to prevent drug resistance. Among HIV-infected youth, a group with rising infection rates, treatment switch is often delayed; virologic and immunologic consequences of this delay are unknown. We conducted a retrospective, longitudinal study of drug resistance outcomes of initial HAART in U.S. youth enrolled between 1999–2001 in a multicenter, observational study and experiencing delayed switch in their first nonsuppressive treatment regimen for up to 3 years. HIV-1 genotyping was performed on plasma samples collected longitudinally, and changes in drug resistance mutations, CD4+ T cell numbers and viral replication capacity were assessed. Forty-four percent (n = 18) of youth in the parent study experiencing virologic nonsuppression were maintained on their initial HAART regimen for a median of 144 weeks. Drug resistance was detected in 61% (11/18) of subjects during the study. Subjects on non-nucleoside reverse transcriptase inhibitor (NNRTI) regimens developed more (8/10) drug resistance mutations than those on protease-inhibitor (PI) regimens (2/7) (p = 0.058). Subjects developing NNRTI-resistance (NNRTI-R), showed a trend toward lower CD4+ T cell gains (median: −6 cells/mm3 per year) than those without detectable NNRTI-R (median: +149 cells/mm3 per year) (p = 0.16). HIV-1–infected youth maintained on initial nonsuppressive NNRTI-based HAART regimens are more likely to develop drug-resistant viremia than with PI-based HAART. This finding may have implications for initial treatment regimens and transmission risk in HIV-infected youth, a group with rising infection rates.
Introduction
Fifty percent of newly diagnosed persons with HIV-1 infection worldwide are youth, with an estimated 10.3 million persons between the ages of 15 and 24 infected by 2005.1 In the United States, confidential name-based reporting from 33 states show that 14% (5132) of the 37,330 incident HIV/AIDS cases reported in 2005 were in persons between the ages of 13 and 25, and 40,000 were living with HIV-1.2 Successful treatment of HIV-1 infection in youth is particularly challenging because of the unique psychosocial issues of at-risk and infected youth.3–5
Antiretroviral treatment failure and subsequent drug resistance are major obstacles to the long-term management of HIV-1 infection in youth, and have implications for transmission and for the future of the HIV epidemic. HIV-1 infected youth receiving highly active antiretroviral treatment (HAART) are reported to have low adherence, with rates between 29% and 50% reported in HIV-1–infected youth initiating HAART.3,6 Their adherence rates are substantially lower than the 90%–95% adherence rates reported for successful long-term control of virus replication and the prevention of drug resistance.3,7 In a U.S. study, 59% of youth initiating HAART achieved clinically undetectable viral load levels (VL) at week 24 of therapy compared to 80%–90% in adult cohorts.4,8–11 Therefore, it is likely that a significant proportion of youth starting HAART will experience virologic failure and subsequent development of drug resistance. Furthermore, the effect of virologic failure and drug resistance may increase if the recommendation of maintaining the initial treatment regimen while improving adherence to therapy in preparation for treatment switch is selected.12
The major drug classes for initial therapy in infected persons include regimens that are either NNRTI- or PI-based. While the efficacy of initial HAART is similar with NNRTI- and PI-based regimens,4,8,12 the long-term persistence of NNRTI-resistance variants in plasma,13 coupled with the high likelihood of initial treatment failure in adolescents, may need to be considered when selecting the major drug class for initial therapy in infected youth. Moreover, the long-term persistence of drug-resistant viremia may have implications for transmission given the rising rates of infection in this group and the increasing overall rates of the transmission of drug-resistant HIV-1.14,15 Indeed, recent studies have shown that NNRTI-R variants are the dominant drug-resistant variants being transmitted in U.S. adult and pediatric cohorts.16–19 In a recent study of the prevalence of drug resistance in primary HIV-1 infection in youth, 18% had major class drug resistant primary infection. The majority (80%) had NNRTI-resistance, thereby excluding the use of NNRTI-based treatment regimens for these youth.14 The extent of drug resistance during first treatment failure and the potential adverse effects on CD4+ T cells in youth have not been studied and constitute an important gap in our understanding of how to best treat youth and other populations at risk for nonadherence to drug regimens. In this study, we examined, in the context of an observational U.S. study, the extent of delayed treatment switch, drug resistance, and immunologic effects of first HAART failure in youth.
Methods
Study population
This is a nested retrospective study conducted within Pediatric AIDS Clinical Trial Group (PACTG) 381. PACTG 381 is an open-label longitudinal, observational study in which youth ages 12–24 infected with HIV-1 through risk behaviors with limited to no antiretroviral exposure were starting HAART between 1999 and 2001 and followed for up to 3 years. Immunologic data and treatment outcomes, but not genotypic drug resistance outcomes on the cohort, have been previously published.4,8 The subjects in our study therefore consisted of individuals experiencing virologic failure in whom longitudinal virologic and immunologic data were already collected, and for whom stored plasma samples were available for drug resistance testing. Virologic failure here is defined as patients who had the first of two consecutive measurements of VL greater than 400 copies per milliliter after a period of viral load suppression of at least 0.7 log10 lower than pretreatment levels, and also control subjects are defined as those who had no virologic suppression after 12 weeks on HAART. The performance of this retrospective substudy on deidentified, stored plasma samples was approved by the Johns Hopkins Institutional Review Board.
Time points analyzed
Plasma samples were analyzed at the following time points: at baseline or prior to initiation of HAART (T0); time of virologic failure (T1); time after virologic failure (T2), which corresponds to samples obtained every 12–24 weeks after T1; and finally time of switch or treatment discontinuation (T3).
Laboratory methods
Amplification and sequencing of HIV-1 gag-pol and assessment of HIV-1 pol replication capacity. A region of HIV-1 gag-pol (HXB2 positions 2002 to 3495) was amplified from plasma using a modification of a previously published nested reverse transcriptase polymerase chain reaction (RT-PCR) method.20,21 Briefly, 140 to 1000 μL of plasma was pelleted before viral RNA isolation for RT-PCR amplification of a 1.5kb region of HIV-1 gag-pol. The PCR product was gel-purified and ligated into the gapped green fluorescent protein (GFP) tagged HIV-vector pNL4-3ΔE-GFP (supplied by R.F. Siliciano) for subsequent use in a replication capacity (RC) assay before being transformed into STBL-2 competent cells (Invitrogen, Carlsbad, CA).20 Plasmid DNA from individual clones were sequenced. Sequences were aligned in Bioedit® (www.mbio.ncsu.edu/BioEdit/bioedit.html) and cleaned for PCR-induced errors using CleanCollapse (available from S.C.R. at http://sray.med.som.jhmi.edu/SCRoftware/CleanCollapse) to minimize template resampling.22 Application of the Akaike Information Criterion as implemented in ModelTestVersion 3.7 identified the general time reversible model with correction for invariant sites and γ-distributed rate variation (GTR + I + Γ) as the best-fit phylogenetic model (parameters available on request). Phylogenetic analysis using a heuristic search algorithm (three random-addition sequences with tree-bisection-reconnection branch swapping) and bootstrap assessment (using the neighbor-joining algorithm with 1000 permutations) was performed using Nimble Tree Version 2.6 (available from S.C.R. at http://sray.med.som.jhmi.edu/SCRoftware), and PAUP* Version 4b10 (Sinauer Associates, Sunderland, MA). Trees were visualized using Mega Version 3.23 The sequences have been submitted to GenBank (Accession numbers: EU346204–EU346359). The sequences were analyzed for sites of known drug resistance mutations in reverse transcriptase and protease (http://hivdb.stanford.edu).
Subject-derived HIV-1 pol sequences amplified from plasma before and following the development of drug resistance mutations were analyzed for HIV-1 pol replication capacity (HIV-1 pol RC) using a previously published in-house assay that uses the laboratory strain NL43 as the reference sequence.20 For those subjects in whom multiple distinct drug resistant clones were identified at a given time point, the pol RC was represented as an average of the measurements obtained. In subjects without detectable drug resistance mutations in plasma, a clone with the consensus wild-type HIV-1 sequence per time point was selected for pol RC analysis. To decrease variability, all assays were performed using a single stock of reference wild-type NL43. Changes of more than 5% from pretreatment levels were considered qualitatively different.
Statistical analysis
Drug resistance mutations were identified and the subjects were subdivided into two groups based on the presence or absence of NNRTI-R mutations. The distinction made for analyses were as follows: NNRTI-R included any mutations conferring NNRTI resistance, and NNRTI-S class included lack of mutations at NNRTI-R sites. Statistical analyses were performed on the changes in viral load and in CD4+, CD8+ naïve, and memory T cells and activated CD8+ T cells from pretreatment levels. The viral load and immunologic data used for these analyses were performed in the parent trial and included analyses of CD4 62L/45RA, CD8 62L/45RA, CD4 45RO/45RA and CD8 38/DR as previously reported.4 To adjust for different follow-up times, slopes per year were calculated as the difference in each outcome between the pretreatment measure and the measurement closest to and before antiretroviral treatment discontinuation, divided by time on treatment. Wilcoxon signed rank tests were used to test for changes within groups. Between-group comparisons were done using Wilcoxon rank sum tests and Fisher's exact tests for continuous and categorial data, respectively. All calculations were done using the Statistical Analysis System (SAS; version 9.0; SAS Institute, Cary, NC).
Results
Subject characteristics
Table 1 summarizes the demographic, virologic, and immunologic characteristics and initial antiretroviral treatment regimens for the 18 subjects. These 18 subjects, cared for at eight U.S. PACTG 381 participating sites, represent nearly 44% (18/41) of the youth experiencing virologic nonsuppression on initial HAART who also had virologic data out to week 24. Eight of the 49 subjects with virologic failure did not have available virologic data through week 24 and were therefore not included in the study. However, among the 41 evaluable subjects with week 24 data, 23 (56%) patients met the virologic criteria for our study. Plasma samples were unavailable on 4, and in 1 subject plasma virus was not amplified.
Table 1.
Demographic, Treatment, Immunologic, and Virologic Characteristics at Study Entry
| NNRTI (n = 10) [Median (range)] | PI (n = 8) [Median (range)] | p value | |
|---|---|---|---|
| Age | 19.8 (18.0–21.5) | 20.1 (16.7–21.7) | 0.827c |
| Gender (M/F) | 4/6 | 6/2 | 0.188d |
| Race (Ba/Hb) | 9/1 | 3/5 | 0.043 |
| Antiretroviral treatment | 101.6 (40.5–212.0) | 46.0 (16.2–149.7) | 0.138c |
| Duration (weeks) | |||
| CD4 at start of HAART (cells/mm3) | 354 (10–600) | 235 (48–528) | 0.409c |
| CD4% at start of HAART | 19.0 (3.0–44.0) | 12.5 (5.0–34.0) | 0.514c |
| VL at start of HAART log10 HIV-1 RNA (copies/mL) | 4.9 (4.1–5.7) | 4.9 (4.0–5.5) | 0.965c |
B, Black.
H, Hispanic (regardless of race).
Wilcoxon rank sum test.
Fisher's exact test.
NNRTI, non-nucleoside reverse reverse transcriptase inhibitor; PI, protease inhibitor.
Ten (56%) of the 18 subjects were started on NNRTI- and 8 on PI-based HAART. The median viral load before HAART initiation was similar in both groups (4.9 log10 HIV-1 RNA copies per milliliter), and the median initial CD4+ T cell count was 354 and 235 cells/mm3 (p = 0.409) for the NNRTI- and PI-treated groups, respectively. The median duration of maintenance of the nonsuppressive regimen was 101.6 weeks (39 to 208 weeks) for the NNRTI-treated and 46 weeks (16 to 144 weeks) for the PI-treated group. This difference was not statistically significant (p = 0.138).
Genotypic drug resistance patterns in youth maintained on their first nonsuppressive HAART
Baseline Drug Resistance Patterns. The drug resistance mutations present at baseline and during treatment failure are summarized in Table 2. HIV-1 pol was successfully amplified at baseline from 17 of the 18 subjects and longitudinally on all 18. A median of 10 HIV-1 pol clones (range 4 to 18) were analyzed per time point on each subject, providing a total of 188 RT sequences and 232 protease sequences for assessing the extent of genotypic drug resistance in the cohort. Phylogenetic analysis confirmed patient specificity and the diversity of the viral variants examined (data not shown). This analysis showed temporal clustering of the sequences with sequences derived from the earlier visits clustering closer to the root of the tree, a pattern consistent with forward virus evolution as a result of nonsuppressive therapy (data not shown).
Table 2.
Antiretroviral Treatment Regimens and Drug Resistance Mutations Detected at Baseline and Sequentially During Treatment Failure
| Subject | Antiretroviral study regimen | Duration on failing regimen (weeks) | Drug resistance mutations at baseline (T0) (weeks) | Drug resistance mutations at (T1) (weeks) | Drug resistance mutations at (T2) (weeks) | Drug resistance mutations at (T3) (weeks) |
|---|---|---|---|---|---|---|
| Group 1: NNRTI-Treated | ||||||
| 1 | EFV/AZT/3TC | 98 | WT | K103N (12) | K103N, M184V (36) | K103N, M184V, P225H (84) |
| 2 | EFV/AZT/3TC | 152 | WT | K103N (12) | ns | K103N (156) |
| 3 | EFV/AZT/3TC | 141 | WT | WT (16) | WT (60) | K103N, V108I (144) |
| 4 | EFV/AZT/3TC | 48 | WT | WT (16) | ns | WT (48) |
| 5 | EFV/AZT/3TC | 119.1 | D67N, K219Q | D67N, K219Q (12) | D67N, V75I, Y115F, G190E (48) | D67N, K70R, V75I, Y115F, G190E, T215F (120) |
| 6 | EFV × 1 wk → NFV → LPV/r/AZT/3TC | 212.0 | WT | K103N (24) | K103N (48) | K103N (208) |
| 7 | EFV/AZT/3TC | 156 | WT | WT (12) | WT (36) | K103N, V106I (156) |
| 8 | EFV/AZT/3TC | 60 | WT | K103N, M184I/V, M230L (24) | K103N, M184V, M230L (36) | K103N (60) |
| 9 | EFV/AZT/3TC | 48.2 | WT | K103N, M184V (12) | ns | K103N, M184V (48) |
| 10 | EFV × 1 wk → NFV/AZT/3TC | 40.5 | WT | M184V (24) | ns | WT (39) |
| Group 2: PI-Treated | ||||||
| 11 | NFV/AZT/3TC | 16.2 | WT | WT (12) | WT (16) | |
| 12 | NFV/AZT/3TC | 84.3 | WT | M46L (12) | L90M, M184V (48) | L90M, M184V (84) |
| 13 | IDV → SQV/AZT/3TC | 60 | WT | Unable to amplify | Unable to amplify | WT (60) |
| 14 | NFV/AZT/3TC | 19.3 | L90M | ns | ns | L90M (16) |
| 15 | NFV/AZT → D4T/3TC | 149.7 | WT | WT (24) | D30N, M184V (60) | D30N, N88D, M184V (144) |
| 16 | NFV/DDI/D4T | 36 | WT | WT (12) | WT (24) | WT (36) |
| 17 | NFV/AZT/3TC | 148.9 | ns | WT (12) | WT (24) | WT (144) |
| 18 | NFV/AZT/3TC | 28.3 | WT | WT (12) | ns | WT (24) |
EFV, efavirenz; AZT, zidovudine; 3TC, lamivudine; NFV, nelfinavir; IDV, indinavir; SQV, saquinavir; D4T, stavudine; DDI, didanosine: WT, wild-type; ns, no sample.
Of the 17 subjects in whom HIV-1 pol was successfully amplified from pretreatment samples, 88% (15/17) had wild-type, drug-sensitive HIV-1. Drug-resistant virus was detected in two subjects. One patient (#5) had the thymidine analogue resistance mutations (TAMS) D67N and K219Q present at baseline and another (#14) the PI-drug resistance mutation L90M. None of the subjects had NNRTI-resistant virus detected at baseline.
Drug resistance patterns during nonsuppressive therapy among NNRTI- and PI-treated adolescents. Drug resistance mutations to the major class of drugs were more commonly detected in the NNRTI-treated subjects than those on PI-based HAART (p = 0.058), with NNRTI-resistance (NNRTI-R) developing in 8 of the 10 (80%) NNRTI-treated subjects. The K103N resistance mutation was detected first and was dominant in 7 of the 8 subjects (88%). This mutation persisted as the dominant replicating plasma HIV-1 variant for up to 208 weeks (median 144 weeks; range, 48–208 weeks; Table 2). Additionally, 50% of the subjects (4/8) maintained on nonsuppressive NNRTI-based therapy developed other NNRTI-R mutations over the study period. These mutations included V106I (patient #7), V108I (patient #3), P225H (patient #1), and M230L (patient #8) substitutions, which accumulated with the K103N mutation and subsequently became the dominant plasma variant (Table 2).
In contrast, in the PI-treated group, the one subject (patient #14) who had the L90M mutation present at baseline maintained viremia with the L90M variant throughout the study period without the development of additional mutations. Among the remaining 7 subjects in the PI-treated group, only 2 (29%) subjects developed drug resistance mutations. In the first subject (patient #15), the D30N mutation was detected at 60 weeks post-HAART and was not present at the 24-week time point. This mutation profile was later replaced with variants having the D30N and N88D mutations at week 144. In the second subject (#12), the PI-resistance mutation was not detected at week 12 at the time of virologic failure but was subsequently detected at week 48 and remained dominant in the plasma through week 84 of study. The remaining 5 subjects had no detectable plasma viremia with PI resistant variants for up to 144 weeks of study treatment.
Patterns of nucleoside reverse transcriptase inhibitor mutations. Thirty-three percent (6/18) of the youth developed the lamivudine-associated resistance mutation (M184V): four (#1, 8, 9, 10) in the NNRTI- and two (#12, 15) in the PI-treated group. This mutation was detected at a median of 24 weeks (range, 12–36) of nonsuppressive therapy and was present with the major class mutation in most subjects (5/6). TAMS were only detected in the one subject (#5) who had TAMS detected at baseline, and in this subject, additional TAMS developed over 120 weeks of study (Table 2).
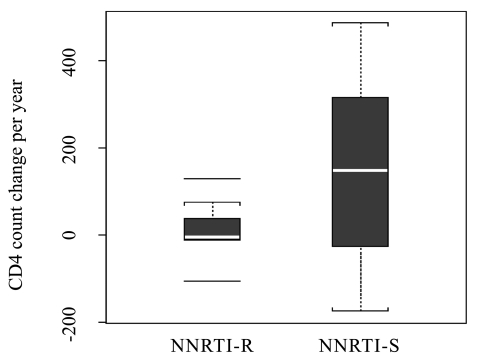
Drug resistance mutations and immunologic outcomes. In order to assess the association between the acquisition of NNRTI-R and virologic and immune outcomes, we compared rates of change in viral load, naïve and memory CD4+, CD8+ and CD8+/CD38+/HLA-DR+ T cell numbers between the 8 subjects who developed NNRTI-R mutations and the 10 subjects without NNRTI-R variants (NNRTI-S). Subjects with NNRTI-R had a trend towards lower total CD4+ T cell gains (median counts of −6 cells/mm3 per year) than subjects without NNRTI-R (median counts of +149 cells/mm3 per year), although this difference was not statistically significant (p = 0.161; Fig. 1). Similar trends were observed for the CD4+ T cell gains when analyzed by treatment class, with the median CD4+ T cell count of +148.5 cells/mm3 per year for the PI-treated and −6 cells/mm3 per year for the NNRTI-treated group (p = 0.101). Analysis of naive and memory CD4+ and CD8+ T cell subsets revealed no statistically significant differences in median slopes between those with and without NNRTI-R. Similarly, no differences in median slopes in CD8+-activated T cells were observed in NNRTI-R versus NNRTI-S subjects. Overall, there were no differences in the viral load slopes when analyzed by NNRTI-R or by treatment class with the overall change in log 10 HIV-1 RNA of −0.05 and −0.27 for NNRTI-R and NNRTI-S, respectively (p = 0.364).
FIG. 1.
CD4+ T cell slopes (cells/mm3 change per year) by presence or absence of non-nucleoside reverse transcriptase inhibitor (NNRTI) resistance.
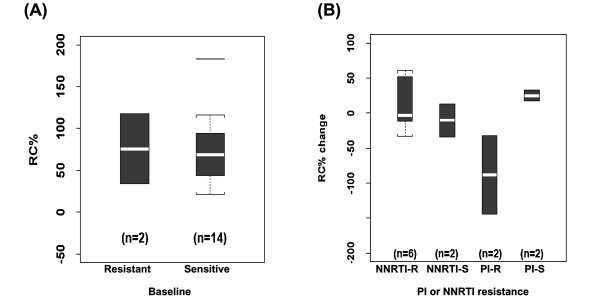
Analysis of changes in HIV-1 pol replication capacity before and during nonsuppressive HAART in HIV-1–infected youth. To assess the association between the acquisition of NNRTI-R and replication capacity (RC), we compared baseline HIV-1 pol RC with changes during ongoing virus replication on nonsuppressive HAART. Samples were analyzed on 16 of the 18 subjects at baseline, and longitudinally in 14 subjects. In four subjects (#8, #11, #13, and #17), not all of the HIV-1 pol clones were functional in the RC assay, precluding longitudinal analysis. For the 14 subjects with wild-type HIV-1 infection at baseline, the pol RC ranged from 21%–183%; median 69% (Fig. 2A). With the development of NNRTI-R, the changes in pol RC were variable ranging from −33% to +60% with a median change of −3.3% from baseline. However, with the acquisition of PI-R mutations, a decline in pol RC (median of −88%; range, −39% to −144%) from pretreatment levels was observed in both subjects (Fig. 2B).
FIG. 2.
Replication capacity at baseline for subjects infected with drug-resistant and wild-type HIV-1 (A) and change in pol RC as a function of initial treatment class and by the presence or absence of antiretroviral class resistance (B).
Discussion
In this study of HIV-1–infected youth experiencing initial HAART failure, we found that nearly 44% of subjects experiencing virologic failure were maintained on the initial non-suppressive HAART regimen for a median of 144 weeks. In assessing the drug resistance patterns that ensued in this patient cohort, we found that the development and accumulation of drug resistance mutations among NNRTI-treated youth were more likely than among PI-treated youth. Subjects with NNRTI-drug resistance also exhibited a trend, although not statistically significant, toward lower CD4+ T cell gains compared to those maintained on nonsuppressive PI-based HAART. In addition, we found that with the acquisition of NNRTI-R mutations, HIV-1 pol RC was more likely to remain at or above patient-derived wild-type levels while the acquisition of PI-resistant variants led to markedly lower HIV-1 pol RC levels. Our study finding of lower rates of drug resistance development in youth failing initial PI-based regimens as compared to youth failiing NNRTI-based regimens is similar to data obtained from homeless adults by Bangsberg et al.24 In homeless persons failing NNRTI- or PI-based HAART, PI-treated subjects had fewer detectable resistance mutations than NNRTI-treated persons. Also, 69% of the NNRTI-treated subjects had detectable NNRTI-R compared to only 23% with PI resistance mutations in the PI-treated.24
The unexpected overall lack of accumulation of TAMS or PI resistance mutations detectable in plasma in our study may be due to the decay of drug-resistant variants from plasma due to the loss of drug-selective pressure from nonadherence or may represent a slower pace of accumulation of non NNRTI-R mutations.25,26 With the restriction of our analysis to plasma samples, we cannot exclude that our study subjects acquired PI-resistance or variants with TAMS that were subsequently archived in cellular populations. Interestingly, this relative lack of TAMS in plasma during initial HAART failure has also recently been reported in Ugandan patients with virologic nonsuppression on NNRTI-based regimens.27 The notion of maintaining patients with virologic failure on a nonsuppressive regimen rather than discontinuing therapy is in part supported by studies in adults and children showing continued CD4+ T cell gains despite rebound viremia on PI-based regimens.28–30 In our study, we found that HIV-1–infected youth who had sustained NNRTI-R viremia during treatment failure tended to have lower CD4+ T cell gains than those harboring wild-type virus or drug resistance mutations to either NRTI or PI classes. This trend requires further study.
The limitations of this study include the small sample size restricting the ability to reach statistical significance, the lack of timed drug levels to assess drug exposure, and the lack of cellular samples to look for archived virus. Similarly, the direct effect of developing resistance mutations to NNRTIs or PIs cannot be distinguished from other effects induced by the drugs used for treatments such as the potential for PIs to inhibit apoptosis that might lead to less immunologic decline.31,32 Furthermore, the use of nelfinavir in most of the study subjects may limit generalizability of the findings to the newer PI-based HAART regimens that may be more forgiving with respect to the selection of drug resistance.33 Additionally, although we attempted to adjust for time on HAART by dividing by time on treatment, the difference in time on the treatment regimen, although not statistically different, may be a potential confounder.
Moreover, while in PACTG 381, youth experiencing virologic failure maintained high levels of CD8+ naïve T levels,8 we found no meaningful differences in the CD8+ T cell naïve slopes to suggest differences in thymic output as an explanation for the observed lower CD4+ T cell gains.34 Additionally, there were no differences in immune activation between the two groups of youth as a possible explanation for the observed CD4+ T cell trends.35
The rapid selection of NNRTI-R mutations has been reported in adults for whom NNRTI-based therapy failed36–39 and even in women and infants receiving single dose nevirapine for prevention of mother-to-child transmission.16,37,40,41 Similarly, the long-term persistence of plasma viremia with NNRTI-R variants has also been reported when NNRTI-R arises during treatment failure or is acquired during primary infection.13 However, the demonstration of these findings in HIV-1–infected U.S. youth has not previously been reported and not only adds to our knowledge, but has important implications for treatment and for the transmission of drug resistant HIV-1 in a population with increasing rates of infection including primary drug-resistant infection.15
In conclusion, this study of HIV-1 drug resistance in U.S. youth experiencing delayed treatment switch while on non-suppressive HAART showed that those failing initial PI-based HAART were less likely to maintain plasma viremia with variants resistant to the major drug class. In addition, those failing PI-based HAART were more likely to experience a non-statistically significant trend toward better CD4+ T cell gains than those failing initial NNRTI-based HAART. While strategies such as directly observed therapy and cell phone reminders have been shown to be effective in enhancing medication adherence among youth that will likely impact on drug resistance outcomes in youth receiving HAART, these strategies are not routinely incorporated into care of HIV-1 infected persons.42,43
Given the high rates of treatment failure in youth, the potential for delay in antiretroviral treatment switch, and the risk of transmission of drug-resistant variants in this population, additional knowledge of the rates and consequences of treatment failure for youth receiving NNRTI and PI-based initial HAART will be important for guiding initial and successive treatment strategies for this high risk group.
Acknowledgments
We thank Roxann Ashworth for her assistance editing the sequences.
This work was supported by funding from the National Institutes Health/National Institute of Allergy and Infectious Diseases R01AI 055312 (D. Persaud) and supported in part by the Pediatric AIDS Clinical Trials Group of the National Institutes of Allergy and Infectious Diseases and the Pediatric/Perinatal HIV Clinical Trials Network and Adolescent Trials Network of the National Institute of Child Health and Human Development
References
- 1.Preventing HIV/AIDS in young people: A systematic review of the evidence from developing countries. UNAIDS Inter-Agency Task Team On Young People. UNAIDS. 2007. www.who.int/child-adolescent-health/ [Dec 12;2007 ]. www.who.int/child-adolescent-health/
- 2.Centers for Disease Control Prevention HIV/AIDS Surveillance. Centers for Disease Control and Prevention. 2007. www.cdc.gov/ [Dec 12;2007 ]. www.cdc.gov/
- 3.Murphy DA. Wilson CM. Durako SJ. Muenz LR. Belzer M. Antiretroviral medication adherence among the REACH HIV-infected adolescent cohort in the USA. AIDS Care. 2001;13:27–40. doi: 10.1080/09540120020018161. [DOI] [PubMed] [Google Scholar]
- 4.Flynn PM. Rudy BJ. Douglas SD, et al. Virologic and immunologic outcomes after 24 weeks in HIV type 1-infected adolescents receiving highly active antiretroviral therapy. J Infect Dis. 2004;190:271–279. doi: 10.1086/421521. [DOI] [PubMed] [Google Scholar]
- 5.Futterman DC. HIV in adolescents and young adults: Half of all new infections in the United States. Top HIV Med. 2005;13:101–105. [PubMed] [Google Scholar]
- 6.Murphy DA. Belzer M. Durako SJ. Sarr M. Wilson CM. Muenz LR. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2005;159:764–770. doi: 10.1001/archpedi.159.8.764. [DOI] [PubMed] [Google Scholar]
- 7.Parienti JJ. Massari V. Descamps D et al. Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis. 2004;38:1311–1316. doi: 10.1086/383572. [DOI] [PubMed] [Google Scholar]
- 8.Rudy BJ. Lindsey JC. Flynn PM, et al. Immune reconstitution and predictors of virologic failure in adolescents infected through risk behaviors and initiating HAART: Week 60 results from the PACTG 381 cohort. AIDS Res Hum Retroviruses. 2006;22:213–221. doi: 10.1089/aid.2006.22.213. [DOI] [PubMed] [Google Scholar]
- 9.Pulido F. Arribas JR. Miro JM, et al. Clinical, virologic, and immunologic response to efavirenz-or protease inhibitor-based highly active antiretroviral therapy in a cohort of antiretroviral-naive patients with advanced HIV infection (EfaVIP 2 study) J Acquir Immune Defic Syndr. 2004;35:343–350. doi: 10.1097/00126334-200404010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Matthews GV. Sabin CA. Mandalia S, et al. Virological suppression at 6 months is related to choice of initial regimen in antiretroviral-naive patients: A cohort study. AIDS. 2002;16:53–61. doi: 10.1097/00002030-200201040-00008. [DOI] [PubMed] [Google Scholar]
- 11.Friedl AC. Ledergerber B. Flepp M, et al. Response to first protease inhibitor- and efavirenz-containing antiretroviral combination therapy. The Swiss HIV Cohort Study. AIDS. 2001;15:1793–1800. doi: 10.1097/00002030-200109280-00008. [DOI] [PubMed] [Google Scholar]
- 12.Department of Health Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents— Dec 1, 2007. www.hivatis.org. [Dec 12;2007 ]. www.hivatis.org
- 13.Smith DM. Wong JK. Shao H, et al. Long-term persistence of transmitted hiv drug resistance in male genital tract secretions: Implications for secondary transmission. J Infect Dis. 2007;196:356–360. doi: 10.1086/519164. [DOI] [PubMed] [Google Scholar]
- 14.Viani RM. Peralta L. Aldrovandi G, et al. Prevalence of primary HIV-1 drug resistance among recently infected adolescents: A multicenter adolescent medicine trials network for HIV/AIDS interventions study. J Infect Dis. 2006;194:1505–1509. doi: 10.1086/508749. [DOI] [PubMed] [Google Scholar]
- 15.Shet A. Berry L. Mohri H, et al. Tracking the prevalence of transmitted antiretroviral drug-resistant HIV-1: A decade of experience. J Acquir Immune Defic Syndr. 2006;41:439–446. doi: 10.1097/01.qai.0000219290.49152.6a. [DOI] [PubMed] [Google Scholar]
- 16.Persaud D. Palumbo P. Ziemniak C, et al. Early archiving and predominance of nonnucleoside reverse transcriptase inhibitor-resistant HIV-1 among recently infected infants born in the United States. J Infect Dis. 2007;195:1402–1410. doi: 10.1086/513871. [DOI] [PubMed] [Google Scholar]
- 17.Richman DD. Morton SC. Wrin T, et al. The prevalence of antiretroviral drug resistance in the United States. AIDS. 2004;18:1393–1401. doi: 10.1097/01.aids.0000131310.52526.c7. [DOI] [PubMed] [Google Scholar]
- 18.Descamps D. Chaix ML. Andre P, et al. French national sentinel survey of antiretroviral drug resistance in patients with HIV-1 primary infection and in antiretroviral-naive chronically infected patients in 2001–2002. J Acquir Immune Defic Syndr. 2005;38:545–552. doi: 10.1097/01.qai.0000155201.51232.2e. [DOI] [PubMed] [Google Scholar]
- 19.Little SJ. Holte S. Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347:385–394. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H. Zhou Y. Alcock C, et al. Novel single-cell-level phenotypic assay for residual drug susceptibility and reduced replication capacity of drug-resistant human immunodeficiency virus type 1. J Virol. 2004;78:1718–1729. doi: 10.1128/JVI.78.4.1718-1729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Persaud D. Siberry GK. Ahonkhai A, et al. Continued production of drug-sensitive human immunodeficiency virus type 1 in children on combination antiretroviral therapy who have undetectable viral loads. J Virol. 2004;78:968–979. doi: 10.1128/JVI.78.2.968-979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith DB. McAllister J. Casino C. Simmonds P. Virus ‘quasispecies': Making a mountain out of a molehill? J Gen Virol. 1997;78(Pt 7):1511–1519. doi: 10.1099/0022-1317-78-7-1511. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S. Tamura K. Jakobsen IB. Nei M. MEGA2: Molecular evolutionary genetics analysis software. Bioinformatics. 2001;17:1244–1245. doi: 10.1093/bioinformatics/17.12.1244. [DOI] [PubMed] [Google Scholar]
- 24.Bangsberg DR. Acosta EP. Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20:223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- 25.Gallego O. de MC. Perez-Elias MJ, et al. Drug resistance in patients experiencing early virological failure under a triple combination including indinavir. AIDS. 2001;15:1701–1706. doi: 10.1097/00002030-200109070-00014. [DOI] [PubMed] [Google Scholar]
- 26.Descamps D. Flandre P. Calvez V, et al. Mechanisms of virologic failure in previously untreated HIV-infected patients from a trial of induction-maintenance therapy. Trilege (Agence Nationale de Recherches sur le SIDA 072) Study Team) JAMA. 2000;283:205–211. doi: 10.1001/jama.283.2.205. [DOI] [PubMed] [Google Scholar]
- 27.Spacek LA. Shihab HM. Kamya MR, et al. Response to antiretroviral therapy in HIV-infected patients attending a public, urban clinic in Kampala, Uganda. Clin Infect Dis. 2006;42:252–259. doi: 10.1086/499044. [DOI] [PubMed] [Google Scholar]
- 28.Deeks SG. Grant RM. Sustained CD4 responses after virological failure of protease inhibitor-containing therapy. Antivir Ther. 1999;4(Suppl 3):7–11. [PubMed] [Google Scholar]
- 29.Perez EE. Rose SL. Peyser B, et al. Human immunodeficiency virus type 1 protease genotype predicts immune and viral responses to combination therapy with protease inhibitors (PIs) in PI-naive patients. J Infect Dis. 2001;183:579–588. doi: 10.1086/318538. [DOI] [PubMed] [Google Scholar]
- 30.Deeks SG. Wrin T. Liegler T, et al. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N Engl J Med. 2001;344:472–480. doi: 10.1056/NEJM200102153440702. [DOI] [PubMed] [Google Scholar]
- 31.Barreiro P. Soriano V. Casas E. Gonzalez-Lahoz J. Different degree of immune recovery using antiretroviral regimens with protease inhibitors or non-nucleosides. AIDS. 2002;16:245–249. doi: 10.1097/00002030-200201250-00014. [DOI] [PubMed] [Google Scholar]
- 32.Benito JM. Lopez M. Martin JC, et al. Differences in cellular activation and apoptosis in HIV-infected patients receiving protease inhibitors or nonnucleoside reverse transcriptase inhibitors. AIDS Res Hum Retroviruses. 2002;18:1379–1388. doi: 10.1089/088922202320935456. [DOI] [PubMed] [Google Scholar]
- 33.Walmsley S. Bernstein B. King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:2039–2046. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 34.Douglas SD. Rudy B. Muenz L, et al. T-lymphocyte subsets in HIV-infected and high-risk HIV-uninfected adolescents: Retention of naive T lymphocytes in HIV-infected adolescents. The Adolescent Medicine HIV/AIDS Research Network. Arch Pediatr Adolesc Med. 2000;154:375–380. doi: 10.1001/archpedi.154.4.375. [DOI] [PubMed] [Google Scholar]
- 35.Hunt PW. Martin JN. Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 36.Wirden M. Simon A. Schneider L, et al. Interruption of non-nucleoside reverse transcriptase inhibitor (NNRTI) therapy for 2 months has no effect on levels of human immunodeficiency virus type 1 in plasma of patients harboring viruses with mutations associated with resistance to NNRTIs. J Clin Microbiol. 2003;41:2713–2715. doi: 10.1128/JCM.41.6.2713-2715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson JB. Becker-Pergola G. Guay LA, et al. Identification of the K103N resistance mutation in Ugandan women receiving nevirapine to prevent HIV-1 vertical transmission. AIDS. 2000;14:F111–F115. doi: 10.1097/00002030-200007280-00001. [DOI] [PubMed] [Google Scholar]
- 38.Deeks SG. International perspectives on antiretroviral resistance. Nonnucleoside reverse transcriptase inhibitor resistance. J Acquir Immune Defic Syndr. 2001;26(Suppl 1):S25–S33. doi: 10.1097/00042560-200103011-00004. [DOI] [PubMed] [Google Scholar]
- 39.Palmer S. Boltz V. Maldarelli F, et al. Selection and persistence of non-nucleoside reverse transcriptase inhibitor-resistant HIV-1 in patients starting and stopping non-nucleoside therapy. AIDS. 2006;20:701–710. doi: 10.1097/01.aids.0000216370.69066.7f. [DOI] [PubMed] [Google Scholar]
- 40.Eshleman SH. Jackson JB. Nevirapine resistance after single dose prophylaxis. AIDS Rev. 2002;4:59–63. [PubMed] [Google Scholar]
- 41.Eshleman SH. Hoover DR. Chen S, et al. Resistance after single-dose nevirapine prophylaxis emerges in a high proportion of Malawian newborns. AIDS. 2005;19:2167–2169. doi: 10.1097/01.aids.0000194800.43799.94. [DOI] [PubMed] [Google Scholar]
- 42.Puccio JA. Belzer M. Olson J, et al. The use of cell phone reminder calls for assisting HIV-infected adolescents and young adults to adhere to highly active antiretroviral therapy: A pilot study. AIDS Patient Care STDs. 2006;20:438–444. doi: 10.1089/apc.2006.20.438. [DOI] [PubMed] [Google Scholar]
- 43.Parsons GN. Siberry GK. Parsons JK, et al. Multidisciplinary, inpatient directly observed therapy for HIV-1-infected children and adolescents failing HAART: A retrospective study. AIDS Patient Care STDs. 2006;20:275–284. doi: 10.1089/apc.2006.20.275. [DOI] [PubMed] [Google Scholar]