Abstract
Objectives
To determine the frequency, accrual, attribution and outcome of neuropsychiatric (NP) events and impact on quality of life over 3 years in a large inception cohort of SLE patients.
Methods
The study was conducted by the Systemic Lupus International Collaborating Clinics. Patients were enrolled within 15 months of SLE diagnosis. NP events were identified using the ACR case definitions and decision rules were derived to determine the proportion of NP disease attributable to SLE. The outcome of NP events was recorded and patient perceived impact determined by the SF-36.
Results
There were 1206 patients (89.6% female) with a mean (±SD) age of 34.5±13.2 years. The mean disease duration at enrollment was 5.4±4.2 months. Over a mean follow-up of 1.9±1.2 years 486/1206 (40.3%) patients had ≥1 NP events which were attributed to SLE in 13.0%–23.6% of patients using two a priori decision rules. The frequency of individual NP events varied from 47.1% (headache) to 0% (myasthenia gravis). The outcome was significantly better for those NP events attributed to SLE especially if they occurred within 1.5 years of the diagnosis of SLE. Patients with NP events, regardless of attribution, had significantly lower summary scores for both mental and physical health over the study.
Conclusions
NP events in SLE patients are variable in frequency, most commonly present early in the disease course and adversely impact patients’ quality of life over time. Events attributed to non-SLE causes are more common than those due to SLE, although the latter have a more favourable outcome.
Keywords: Lupus, Neuropsychiatric, Prospective, Inception cohort
The frequency of neuropsychiatric (NP) disease in systemic lupus erythematosus (SLE) varies between 37%–95% (1–5). Differences in the definition and ascertainment of NP manifestations, lack of consistency in the attribution of NP events and inclusion of subtle NP disease of uncertain clinical significance contribute to this variability. The fluctuating course of many NP manifestations emphasizes the need to evaluate their impact over time.
An international, multi-center, prospective, inception cohort study of NP events in SLE patients was undertaken using uniform definitions, diagnostic criteria determination of attribution. We previously reported on NP events at enrollment (6) including short-term outcome over a mean of 3.7 months. Here we report the clinical characteristics, outcome and impact on health-related quality of life (HRQOL) in an expanded cohort of patients evaluated over three years and up to 4 annual assessments with a mean follow-up of 23 months.
Patients and Methods
Research network
The study was performed by the Systemic Lupus International Collaborating Clinics (SLICC) (7) between October 1999 and February 2008. The study was approved by the Capital Health Research Ethics Board, Halifax, Nova Scotia, Canada and by the institutional research ethics boards of participating centers..
Patients
Patients fulfilled the American College of Rheumatology (ACR) classification criteria for SLE (8) and provided written informed consent. The date of diagnosis was when ≥4 ACR criteria were first recognized and enrollment occurred up to 15 months following the diagnosis. Patients were reviewed at enrollment and annually (±6 months) thereafter when new NP events since the previous visit and the status of old events were recorded. Other data included age, gender, ethnicity, education, medication use, SLE Disease Activity Index (SLEDAI) (9) and SLICC/ACR damage index (SDI) (10). HRQOL was measured by the SF-36 (11). Laboratory data included a complete blood count, serum creatinine, urinalysis, anti-DNA, C3 and C4.
Neuropsychiatric (NP) events
All NP events were characterized using the ACR case definitions and were diagnosed by clinical evaluation supported with appropriate investigations as per the ACR glossary (12) (See appendix #1). The exception was cognitive impairment for which the diagnosis was made by formal neuropsychological testing in only 9/43 (21%) of cases.
Outcome of NP events
A physician generated 7-point Likert scale compared the change in NP status between the onset of the event and time of study assessment (1=patient demise, 2=much worse, 3=worse, 4=no change, 5=improved, 6=much improved, 7=resolved).The time to resolution was the interval between the onset of the event and the date of resolution; if the NP event had not resolved the time was censored to onset of the event and the date of the final assessment. Analyses of both time to resolution and Likert outcome scores were undertaken. A patient generated mental (MCS) and physical (PCS) component summary score of the SF-36 (11) determined the impact of NP events on HRQOL.
Statistical analysis
NP events were attributed to SLE or non-SLE causes (See appendix #1). and categorized into central/peripheral and diffuse/focal nervous system manifestations as described (6, 12) (See appendix #2). SLICC centers were grouped into geographic locations (Canada, U.S.A./Mexico, Europe, Asia). For some analyses patients were categorized at each assessment as NP positive with (a) diffuse/central events only, (b) focal/peripheral events only, (c) both events, and (d) a NP negative group.
Chi-square and t-tests examined differences in demographics and NP status at enrollment between patients with missing data and patients who completed the study. Explanatory variables for time-to-case resolution for NP events were examined using Cox regression (adjusting for correlation of events in the same patient).
Likert outcome scores of NP events were analyzed using multi-level ordinal logistic regression, with odds ratios linked to the probability of higher, more favourable, scores, and accounting for correlation of multiple scores over time for the same event and multiple events for the same patient. SF-36 analyses utilized linear regression and generalized estimating equations with a first-order autoregressive correlation structure to allow for correlation between multiple SF-36 measurements for the same patient.
Results
Patients
1206 patients were recruited in 24 centers. Patients were predominantly female (89.6%) and Caucasian with a mean±SD age of 34.5±13.2 years (Table 1).
Table 1.
Demographic and clinical manifestations of SLE patients at enrollment visit
| Number of Patients | 1206 | |
| Gender, n, (%) | Female | 1080 (89.6) |
| Male | 126 (10.5) | |
| Age (years) (mean ± SD) at enrollment | 34.5 ± 13.2 | |
| Ethnicity, % | Caucasian | 47.4 |
| Hispanic | 16.5 | |
| Asian | 16.5 | |
| Black | 15.6 | |
| Other | 3.9 | |
| Region, % | Canada | 22.2 |
| US, Mexico | 41.4 | |
| Europe | 25.1 | |
| Asia | 11.3 | |
| Single/Married/Other, % | 46.6/40.8/12.6 | |
| Post secondary education, % | 62.1 | |
| Disease duration (months) (mean ± SD) | 5.4 ± 4.2 | |
| Number of ACR criteria (mean ± SD) | 4.5 ±1.01 | |
| Cumulative ACR manifestations, n, % | ||
| Malar rash | 501 (41.5) | |
| Discoid rash | 174 (14.4) | |
| Photosensitivity | 434 (35.9) | |
| Oral/nasopharyngeal ulcers | 539 (44.7) | |
| Serositis | 336 (27.8) | |
| Arthritis | 886 (73.5) | |
| Renal disorder | 346 (28.7) | |
| Neurological disorder | 71 (5.9) | |
| Hematologic disorder | 742 (61.5) | |
| Immunologic disorder | 923 (76.5) | |
| Antinuclear antibody | 1158 (96.0) | |
| SLEDAI score (mean ± SD) | 5.4 ± 5.5 | |
| SLICC/ACR score (mean ± SD) | 0.3 ± 0.8 | |
| Medications, % | ||
| Corticosteroids | 835 (69.2) | |
| Antimalarials | 744 (61.7) | |
| Immunosuppressants | 471 (39.1) | |
| Aspirin | 170 (14.1) | |
| Antidepressants | 105 (8.7) | |
| Anticonvulsants | 48 (3.9) | |
| Warfarin | 59 (4.9) | |
| Antipsychotics | 7 (0.6) | |
The mean disease duration was 5.4±4.2 months in an unselected patient population with moderate disease activity. The mean follow-up for NP events (the onset of NP events to the last assessment) was 1.9±1.2 years. No follow-up was available in 191/1206 (15.8%) patients and the assessment for the last anticipated date plus 6 months was unavailable in 353/1206 (29.3%). These patients were more likely to be younger (p<0.008), Hispanic or Black (p<0.0001), had less education (p<0.006) and higher SLEDAI scores (6.1±6.3 vs. 5.2±5.2; p<0.023). They were also less likely to have NP disease, attributed to SLE or non-SLE causes, at the enrollment assessment (p<0.023). There were 18/1206 (1.5%) deaths and in 4/18 (22.2%) the primary cause was attributed to NP events (intracranial hemorrhage (2), stroke (1), seizures (1)).
Frequency and attribution of NP events
486 of 1206 (40.3%) patients had at least one NP event during the study. 210 (17.4%) had two or more events. The 486 patients had 843 events encompassing 18/19 NP syndromes (Table 2).
Table 2.
The number of NP events by attribution over the period of study
| Event types | SLE NP events (model A) | SLE NP events (model B) | Non-SLE NP events | Total NP events | ||||
|---|---|---|---|---|---|---|---|---|
| # | % | # | % | # | % | # | % | |
| Headache | 0 | 0.00 | 0 | 0.0 | 397 | 67.9 | 397 | 47.1 |
| Mood Disorders | 18 | 12.1 | 47 | 18.2 | 92 | 15.7 | 139 | 16.5 |
| Seizures and Seizure Disorders | 39 | 26.2 | 54 | 20.9 | 9 | 1.5 | 63 | 7.5 |
| Cognitive Dysfunction | 8 | 5.4 | 22 | 8.5 | 21 | 3.6 | 43 | 5.1 |
| Anxiety Disorder | 0 | 0.0 | 0 | 0.0 | 42 | 7.2 | 42 | 4.9 |
| Cerebrovascular Disease | 18 | 12.1 | 40 | 15.5 | 0 | 0.0 | 40 | 4.7 |
| Acute Confusional State | 11 | 7.4 | 17 | 6.6 | 5 | 0.9 | 22 | 2.6 |
| Polyneuropathy | 8 | 5.4 | 10 | 3.9 | 10 | 1.7 | 20 | 2.4 |
| Mononeuropathy | 10 | 6.7 | 18 | 6.9 | 0 | 0.0 | 18 | 2.1 |
| Neuropathy, Cranial | 11 | 7.4 | 11 | 4.3 | 4 | 0.7 | 15 | 1.8 |
| Psychosis | 8 | 5.4 | 13 | 5.0 | 1 | 0.2 | 14 | 1.7 |
| Myelopathy | 5 | 3.4 | 10 | 3.9 | 0 | 0.0 | 10 | 1.2 |
| Movement Disorder | 4 | 2.7 | 5 | 1.9 | 1 | 0.2 | 6 | 0.7 |
| Aseptic Meningitis | 4 | 2.7 | 4 | 1.6 | 2 | 0.3 | 6 | 0.7 |
| Demyelinating Syndrome | 1 | 0.7 | 3 | 1.2 | 0 | 0.0 | 3 | 0.4 |
| Acute Inflammatory | 2 | 1.3 | 2 | 0.8 | 0 | 0.0 | 2 | 0.2 |
| Autonomic Disorder | 2 | 1.3 | 2 | 0.8 | 0 | 0.0 | 2 | 0.2 |
| Plexopathy | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 1 | 0.1 |
| Myasthenia Gravis | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total (%) NP Events | 149 | (17.7) | 258 | (30.6) | 585 | (69.4) | 843 | (100) |
The most frequent events were headache (migraine (49%), tension (38%), intractable 9%, cluster (3%), pseudotumour cerebri (1%),), mood disorders, seizures, cognitive dysfunction, anxiety disorder, cerebrovascular disease, acute confusional state, polyneuropathy and mononeuropathy. The remaining 10 NP syndromes had a prevalence of less than 2%; myasthenia gravis did not occur in any patient.
NP events attributed to SLE varied from 17.7% (model A) to 30.6% (model B) (Table 2). Of the 843 NP events 785 (93.1%) affected the central nervous system and 58 (6.9%) involved the peripheral nervous system. Diffuse and focal events were 666 (79%) and 177 (21%), respectively. The most frequent NP events attributed to SLE were seizures, mood disorders, cerebrovascular disease and acute confusional states.
Onset and accrual of NP events
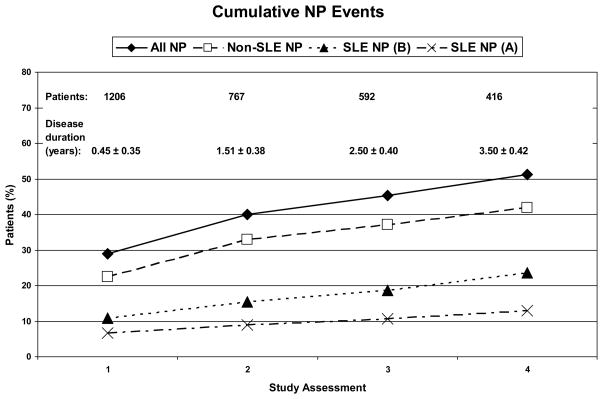
NP events were most frequent at the enrollment visit and the cumulative frequency of both SLE and non-SLE NP events increased over time (Figure 1). Of patients with follow-up to the final study assessment, 51.2% had at least one NP event. The proportion of patients with NP events attributed to SLE varied between 13.0% (model A) and 23.6% (model B). The proportion of patients with both SLE and non-SLE attributed NP events was 7.9% (model A) and 14.2% (model B).
Figure 1.
The cumulative frequency of patients with NP events at enrollment and at subsequent study assessments. The percentage of patients with NP events is shown at each time point for all NP events regardless of attribution (all NP), NP events attributed to non-SLE causes (non-SLE NP), NP events attributed to SLE as per attribution model B (SLE NP (B)) and NP events attributed to SLE as per attribution model A (SLE NP (A)).
Outcome of NP events
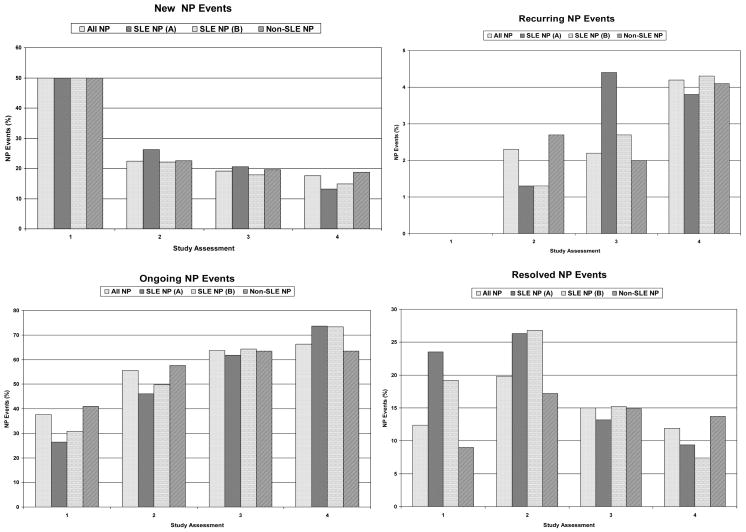
There was no difference in the attribution frequency of new, recurring or ongoing NP events (Figure 2). However, the rate of resolution of NP events attributed to SLE was higher than events due to non-SLE causes (model A: 55.0%vs.38.2%, hazard ratio (HR): 1.62, 95% CI=[1.24,2.11], p<0.001; model B: 51.9%vs.36.4%, HR: 1.53, 95% CI=[1.22,1.92], p<0.001). There was a higher resolution of focal vs. diffuse NP events (52.5%vs.38.1%, HR: 1.55, 95% CI=[1.21,1.98], p<0.001) but no difference between the resolution of central vs. peripheral NP events (41.4%vs.37.9%, HR: 1.23, 95% CI=[0.79,1.93], p=0.358).
Figure 2.
The frequency of NP events at enrollment and at subsequent study assessments characterized as new, recurring or ongoing from a previous assessment. At each assessment the status of the NP events into resolved or unresolved is shown. Summary data is shown for NP events regardless of attribution (all NP events), NP events attributed to SLE as per attribution model A (SLE NP (A)), NP events attributed to SLE as per attribution model B (SLE NP (B)) and NP events attributed to non-SLE causes (non-SLE NP).
To look for an interaction between disease duration and the effect of attribution on NP event resolution, disease duration at the occurrence of events was dichotomized at 1.5 years. For model A the estimated SLE attribution effect on resolution of events within 1.5 years of the diagnosis of SLE was larger than the effect for events occurring ≥1.5 years following the diagnosis (HR: 1.77vs. 0.89) (interaction coefficient=−0.69, 95%CI=[−1.52, 0.15], p=0.107). For model B, the same analysis led to a hazard ratio comparison of: 1.66vs.0.86 (interaction coefficient=−0.66, 95%CI=[−1.39,0.07], p=0.076).
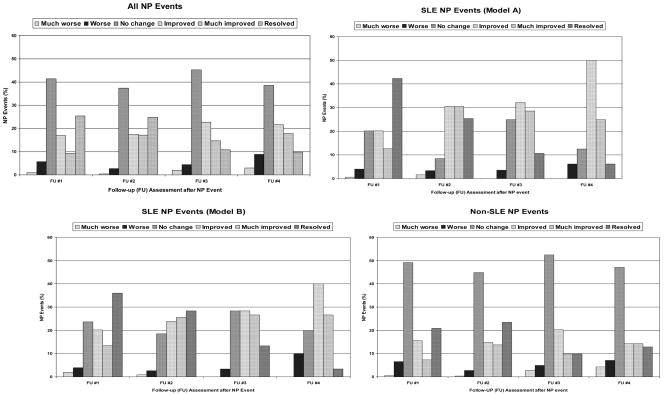
Favorable Likert outcome scores for NP events were more frequent for those attributed to SLE (model A or model B), particularly at the first two study assessments (Figure 3). Controlling for the duration of follow-up, multivariate ordinal regression analysis confirmed a significant positive association between favorable outcome scores and SLE NP events (model B) (odds ratio (OR): 1.51, 95%CI=[1.05, 2.21], p=0.028), focal NP events (OR: 1.83, 95%CI=[1.28, 2.64], p=0.001), US/Mexico (OR: 1.32, 95%CI=[0.90, 1.93]), European (OR: 1.66, 95%CI=[1.12,2.46]), and Asian (OR: 2.79, 95%CI=[1.41,5.50]) sites (p=0.007), and negative associations with older age at SLE diagnosis (OR: 0.69, 95%CI=[0.59,0.81]), p<0.001), longer disease duration at event onset (OR: 0.79, 95%CI=[0.69,0.90]), p=0.001) and higher SLEDAI scores computed without NP variables (OR: 0.95, 95%CI=[0.93, 0.98]), p=0.002), all of which were included in the models as continuous variables. The interaction between disease duration at event onset and attribution of an NP event was again only marginally significant (p = 0.095).
Figure 3.
The outcome of NP events over the duration of the study. Events are clustered into all NP events regardless of attribution (all NP events), NP events attributed to SLE as per attribution model A (SLE NP events (model A)), NP events attributed to SLE as per attribution model B (SLE NP events (model B)) and NP events attributed to non-SLE causes (non-SLE NP events). Within each panel the outcome of the events is scored as much worse, worse, no change, improved, much improved and resolved at assessments 1 through 4 compared to the onset of the event.
NP events and HRQOL
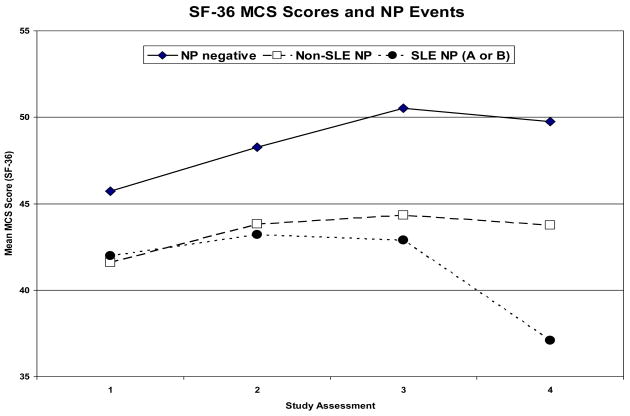
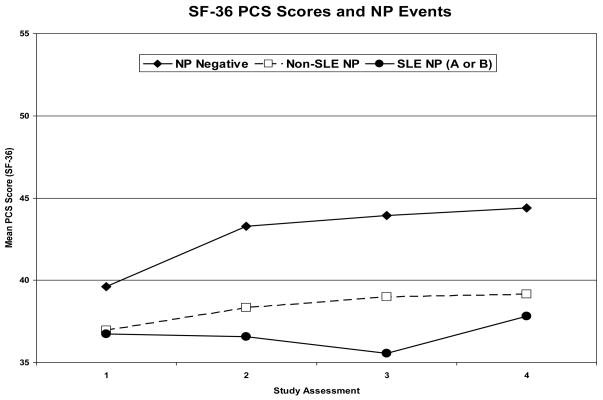
In a multivariate regression analysis there were significantly lower MCS scores in patients with NP events regardless of attribution compared to those without events (estimate: −9.7, 95%CI=[−12.7,−6.7]; p<0.001) (Figure 4). Controlling for gender, age at SLE diagnosis, disease duration at each visit, a summary multivariate analysis also demonstrated associations between lower MCS scores and diffuse NP events (e.g. patients with diffuse vs. focal NP events only: estimate=−5.0, 95%CI=[−9.2,−0.8]; global p = 0.041), higher SLEDAI scores (estimate: −0.15, 95%CI=[−0.30,−0.01], p=0.041) and higher SDI scores, both computed without NP variables (e.g., SDI >3 vs. <=3: estimate=−5.7, 95%CI=[−10.4,−0.9]; global p=0.039). Similarly the group means for the PCS scores were significantly lower in patients with NP events (estimate: −3.3, 95%CI=[−4.5,−2.1], p<0.001) regardless of attribution (Figure 4). In a summary multivariate analysis controlling for ethnicity, age at SLE diagnosis and disease duration at each visit, other significant associations with lower PCS scores were with study sites (e.g., US/Mexico vs. rest: estimate: −5.3, 95%CI=[−7.2, −3.4]; global p<0.001), female gender (estimate: −2.3, 95%CI=[−4.3,−0.3], p=0.024), lack of college education (estimate: −2.7, 95%CI=[−4.1,−1.3], p<0.001), higher SLEDAI scores (estimate: −0.35, 95%CI=[−0.48,−0.22], p<0.001) and higher SDI scores (e.g., SDI >3 vs. <=3: estimate: −5.2, 95%CI=[−8.9,−1.5]; global p<0.001) computed without NP variables.
Figure 4.
Mean MCS and PCS of the SF-36 over the period of study in patients with no NP events (NP negative), NP events attributed to non-SLE causes (non-SLE NP) and NP events attributed to SLE as per attribution model A or B (SLE NP A or B) at each of the study assessments. (See appendix #3).
Discussion
We have established a large, SLE disease inception cohort for the systematic evaluation of NP events in a long-term prospective study. A unique feature of the study is inclusion of all NP events regardless of the etiology, so that differences in the outcome and impact of NP events due to SLE and other causes could be compared. Attribution of NP events was determined using predefined decision rules which have previously provided a positive correlation between SLE NP events and pathogenic autoantibodies (13). Over the study 40.3% of patients had at least one NP event and 17.4% had multiple events. However, patients with NP events attributed to SLE varied from 13.0% to 23.6%, depending upon the stringency of the attribution rules. Likewise, only 17.7% to 30.6% of all NP events were attributed to SLE. Finally, many of the 19 syndromes occurred in less than 2% of patients, indicating that they are relatively infrequent, at least in the first 3 years of the disease.
The outcome of NP events in SLE patients, particularly those attributed directly to SLE, has been informed by clinical trials (14–19), retrospective and prospective observational cohorts and case series (20) (21) with inconsistent results. In the current study the most favorable outcomes occurred with NP events attributed to SLE compared to non-SLE causes and with focal NP compared to diffuse NP events. Furthermore, the outcome was best in SLE attributed events when they occurred early in the disease course, suggesting that the attribution and time of onset of NP events predict outcome. As for rheumatoid arthritis (22, 23) this may indicate a therapeutic window of opportunity when pathogenetic mediators are amenable to immunosuppressive and anti-inflammatory therapies. The current study confirms and expands the findings of previous cross-sectional studies reporting that NP events, regardless of attribution, are associated with a significant reduction in patient self-report HRQOL (3, 24, 25). Thus, in addition to lower group means for MCS and PCS scores of the SF-36 in patients with NP events compared to those without NP events at enrollment, the same group differences persist over the ensuing three years. Our results also emphasize the importance of assessing the impact of all NP manifestations as studies confined to specific subsets of NP disease such as cognitive dysfunction have not found a negative effect on HRQOL (26, 27).
There are potential limitations to our study. First, the frequency of patients with unavailable data (29%) or no follow-up (15.8%) by the final study assessment is high compared to some longitudinal lupus cohorts with rates as low as 11% (28, 29). However these cohorts were more homogenous and followed at single centers. In contrast, a 29% lost to follow-up over 3.5 years was reported in a large, multi-ethnic, multi-center cohort with very similar predictors as in our study (30). Second, restricting NP syndromes to the 19 ACR case definitions (12) could have excluded some NP presentations. However, none of the 1206 patients had an NP event which could not be captured within the ACR definitions. Finally, formal neuropsychological assessments were not performed on all patients and neuroimaging studies were only done if clinically indicated. Although additional abnormalities would likely have been detected by both techniques, our protocol was intended to reflect clinical practice and to avoid the inclusion of subtle NP disease with limited clinical significance (26, 31–36).
In summary our findings indicate a high cumulative frequency of NP events and a negative impact on HRQOL, even though the majority of NP events are not attributable to SLE. Those events attributed to SLE and focal NP events have a better outcome. Future studies will examine the long-term course and impact of nervous system disease and search for biomarkers and pathogenic mechanisms of NP events in this unique cohort of SLE patients.
Supplementary Material
Acknowledgments
Financial support:
J.G. Hanly (Canadian Institutes of Health Research grant MOP-57752, Capital Health Research Fund), M.B. Urowitz (Canadian Institutes of Health Research grant MOP-49529, Lupus Foundation of Ontario, Ontario Lupus Association, Lupus UK, Lupus Foundation of America, Lupus Alliance Western New York, Conn Smythe Foundation, Tolfo Family (Toronto), Li Su (MRC(UK) grant U.1052.00.009), S.C. Bae (Korea Healthcare technology R & D project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A080588), C. Gordon (Lupus UK, arthritis research campaign, Wellcome Trust Clinical Research Facility in Birmingham, UK), A. Clarke (Fonds de la recherche en sante de Quebec National Scholar, Singer Family Fund for Lupus Research), S. Bernatsky (Canadian Institutes of Health Research Junior Investigator Award; Fonds de la recherche en santé du Québéc Jeune Chercheure; Canadian Arthritis Network Scholar Award; McGill University Health Centre Research Institute), G.S Alarcón (University of Alabama at Birmingham, grant P60AR48095), D.D. Gladman (Canadian Institutes of Health Research), P.R. Fortin (Distinguished Senior Research Investigator of the Arthritis Society and Arthritis Centre of Excellence), M. Petri (Hopkins Lupus Cohort grant AR 43727, Johns Hopkins University General Clinical Research Center grant MO1 RR00052), S Manzi (National Institutes of Health research grants R01 AR46588, K24 AR002213 and M01 RR000056, R. Ramsey-Goldman (National Institutes of Health research grants M01-RR00048; K24 AR02318; P60 AR 48098), O. Nived (Swedish Medical Research council grant 13489), G. Sturfelt (Swedish Medical Research council grant 13489), V. Farewell (MRC(UK) grant U.1052.00.009).
We are grateful for the generous donation of our patients’ time and the dedication of all the research coordinators and research assistants in the SLICC network to the completion of this work. In addition the contribution of Dr Hwee-Cheng Chong at the University of Birmingham, Birmingham, UK is greatly appreciated.
References
- 1.Ainiala H, Hietaharju A, Loukkola J, Peltola J, Korpela M, Metsanoja R, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum. 2001;45(5):419–23. doi: 10.1002/1529-0131(200110)45:5<419::aid-art360>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Brey RL, Holliday SL, Saklad AR, Navarrete MG, Hermosillo-Romo D, Stallworth CL, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002;58(8):1214–20. doi: 10.1212/wnl.58.8.1214. [DOI] [PubMed] [Google Scholar]
- 3.Hanly JG, McCurdy G, Fougere L, Douglas JA, Thompson K. Neuropsychiatric events in systemic lupus erythematosus: attribution and clinical significance. J Rheumatol. 2004;31(11):2156–62. [PubMed] [Google Scholar]
- 4.Sanna G, Bertolaccini ML, Cuadrado MJ, Laing H, Mathieu A, Hughes GR. Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J Rheumatol. 2003;30(5):985–92. [PubMed] [Google Scholar]
- 5.Sibbitt WL, Jr, Brandt JR, Johnson CR, Maldonado ME, Patel SR, Ford CC, et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol. 2002;29(7):1536–42. [PubMed] [Google Scholar]
- 6.Hanly JG, Urowitz MB, Su L, Sanchez-Guerrero J, Bae SC, Gordon C, et al. Short-term outcome of neuropsychiatric events in systemic lupus erythematosus upon enrollment into an international inception cohort study. Arthritis Rheum. 2008;59(5):721–9. doi: 10.1002/art.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isenberg D, Ramsey-Goldman R. Systemic Lupus International Collaborating Group--onwards and upwards? Lupus. 2006;15(9):606–7. doi: 10.1177/0961203306071868. [DOI] [PubMed] [Google Scholar]
- 8.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 9.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 10.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–9. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 11.Thumboo J, Fong KY, Ng TP, Leong KH, Feng PH, Thio ST, et al. Validation of the MOS SF-36 for quality of life assessment of patients with systemic lupus erythematosus in Singapore. J Rheumatol. 1999;26(1):97–102. [PubMed] [Google Scholar]
- 12.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 13.Hanly JG, Urowitz MB, Siannis F, Farewell V, Gordon C, Bae SC, et al. Autoantibodies and neuropsychiatric events at the time of systemic lupus erythematosus diagnosis: results from an international inception cohort study. Arthritis Rheum. 2008;58(3):843–53. doi: 10.1002/art.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karlson EW, Liang MH, Eaton H, Huang J, Fitzgerald L, Rogers MP, et al. A randomized clinical trial of a psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis Rheum. 2004;50(6):1832–41. doi: 10.1002/art.20279. [DOI] [PubMed] [Google Scholar]
- 15.Mok CC, Lau CS, Wong RW. Treatment of lupus psychosis with oral cyclophosphamide followed by azathioprine maintenance: an open-label study. Am J Med. 2003;115(1):59–62. doi: 10.1016/s0002-9343(03)00135-9. [DOI] [PubMed] [Google Scholar]
- 16.Barile-Fabris L, Ariza-Andraca R, Olguin-Ortega L, Jara LJ, Fraga-Mouret A, Miranda-Limon JM, et al. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann Rheum Dis. 2005;64(4):620–5. doi: 10.1136/ard.2004.025528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCune WJ, Golbus J, Zeldes W, Bohlke P, Dunne R, Fox DA. Clinical and immunologic effects of monthly administration of intravenous cyclophosphamide in severe systemic lupus erythematosus. N Engl J Med. 1988;318(22):1423–31. doi: 10.1056/NEJM198806023182203. [DOI] [PubMed] [Google Scholar]
- 18.Tokunaga M, Saito K, Kawabata D, Imura Y, Fujii T, Nakayamada S, et al. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann Rheum Dis. 2007;66(4):470–5. doi: 10.1136/ard.2006.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denburg SD, Carbotte RM, Denburg JA. Corticosteroids and neuropsychological functioning in patients with systemic lupus erythematosus. Arthritis Rheum. 1994;37(9):1311–20. doi: 10.1002/art.1780370907. [DOI] [PubMed] [Google Scholar]
- 20.Karassa FB, Ioannidis JP, Boki KA, Touloumi G, Argyropoulou MI, Strigaris KA, et al. Predictors of clinical outcome and radiologic progression in patients with neuropsychiatric manifestations of systemic lupus erythematosus [In Process Citation] Am J Med. 2000;109(8):628–34. doi: 10.1016/s0002-9343(00)00603-3. [DOI] [PubMed] [Google Scholar]
- 21.Jonsen A, Bengtsson AA, Nived O, Ryberg B, Sturfelt G. Outcome of neuropsychiatric systemic lupus erythematosus within a defined Swedish population: increased morbidity but low mortality. Rheumatology (Oxford) 2002;41(11):1308–12. doi: 10.1093/rheumatology/41.11.1308. [DOI] [PubMed] [Google Scholar]
- 22.Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum. 2003;48(7):1771–4. doi: 10.1002/art.11156. [DOI] [PubMed] [Google Scholar]
- 23.Cush JJ. Early rheumatoid arthritis -- is there a window of opportunity? J Rheumatol Suppl. 2007;80:1–7. [PubMed] [Google Scholar]
- 24.Tam LS, Wong A, Mok VC, Zhu YE, Kwok LW, Li TK, et al. The relationship between neuropsychiatric, clinical, and laboratory variables and quality of life of Chinese patients with systemic lupus erythematosus. J Rheumatol. 2008;35(6):1038–45. [PubMed] [Google Scholar]
- 25.Hanly JG, Urowitz MB, Sanchez-Guerrero J, Bae SC, Gordon C, Wallace DJ, et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. 2007;56(1):265–73. doi: 10.1002/art.22305. [DOI] [PubMed] [Google Scholar]
- 26.Hanly JG, Cassell K, Fisk JD. Cognitive function in systemic lupus erythematosus: results of a 5-year prospective study. Arthritis Rheum. 1997;40(8):1542–3. doi: 10.1002/art.1780400825. [DOI] [PubMed] [Google Scholar]
- 27.Hanly JG, Fisk JD, Sherwood G, Eastwood B. Clinical course of cognitive dysfunction in systemic lupus erythematosus. J Rheumatol. 1994;21(10):1825–31. [PubMed] [Google Scholar]
- 28.Gladman DD, Koh DR, Urowitz MB, Farewell VT. Lost-to-follow-up study in systemic lupus erythematosus (SLE) Lupus. 2000;9(5):363–7. doi: 10.1191/096120300678828325. [DOI] [PubMed] [Google Scholar]
- 29.Moss KE, Ioannou Y, Sultan SM, Haq I, Isenberg DA. Outcome of a cohort of 300 patients with systemic lupus erythematosus attending a dedicated clinic for over two decades. Ann Rheum Dis. 2002;61(5):409–13. doi: 10.1136/ard.61.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertoli AM, Fernandez M, Calvo-Alen J, Vila LM, Sanchez ML, Reveille JD, et al. Systemic lupus erythematosus in a multiethnic U.S. cohort (LUMINA) XXXI: factors associated with patients being lost to follow-up. Lupus. 2006;15(1):19–25. doi: 10.1191/0961203306lu2257oa. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Crespo MR, Blanco FJ, Ramos A, Ciruelo E, Mateo I, Lopez Pino MA, et al. Magnetic resonance imaging of the brain in systemic lupus erythematosus. Br J Rheumatol. 1995;34(11):1055–60. doi: 10.1093/rheumatology/34.11.1055. [DOI] [PubMed] [Google Scholar]
- 32.Kozora E, West SG, Kotzin BL, Julian L, Porter S, Bigler E. Magnetic resonance imaging abnormalities and cognitive deficits in systemic lupus erythematosus patients without overt central nervous system disease. Arthritis Rheum. 1998;41(1):41–7. doi: 10.1002/1529-0131(199801)41:1<41::AID-ART6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Waterloo K, Omdal R, Husby G, Mellgren SI. Neuropsychological function in systemic lupus erythematosus: a five-year longitudinal study. Rheumatology (Oxford) 2002;41(4):411–5. doi: 10.1093/rheumatology/41.4.411. [DOI] [PubMed] [Google Scholar]
- 34.Hay EM, Huddy A, Black D, Mbaya P, Tomenson B, Bernstein RM, et al. A prospective study of psychiatric disorder and cognitive function in systemic lupus erythematosus. Ann Rheum Dis. 1994;53(5):298–303. doi: 10.1136/ard.53.5.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlomagno S, Migliaresi S, Ambrosone L, Sannino M, Sanges G, Di Iorio G. Cognitive impairment in systemic lupus erythematosus: a follow-up study. J Neurol. 2000;247(4):273–9. doi: 10.1007/s004150050583. [DOI] [PubMed] [Google Scholar]
- 36.Ginsburg KS, Wright EA, Larson MG, Fossel AH, Albert M, Schur PH, et al. A controlled study of the prevalence of cognitive dysfunction in randomly selected patients with systemic lupus erythematosus. Arthritis Rheum. 1992;35(7):776–82. doi: 10.1002/art.1780350711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.