Abstract
The age-induced decline in the body's ability to fight disease is exacerbated by obesity and metabolic disease. Using a mouse model of diet-induced obesity, the combined challenge of a high-fat diet and age on liver morphology and biochemistry was characterized, while evaluating the potential of 15 min per day of high frequency (90 Hz), extremely low-magnitude (0.2 G) mechanical signals (LMMS) to suppress lipid accumulation in the liver. Following a 36-week protocol (animals 43 weeks of age), suppression of hepatomegaly and steatosis was reflected by a 29% lower liver mass in LMMS animals as compared with controls. Average triglyceride content was 101.7 ± 19.4 μg mg−1 tissue in the livers of high-fat diet control (HFD) animals, whereas HFD + LMMS animals realized a 27% reduction to 73.8 ± 22.8 μg mg−1 tissue. In HFD + LMMS animals, liver free fatty acids were also reduced to 0.026 ± 0.009 μEq mg−1 tissue from 0.035 ± 0.005 μEq mg−1 tissue in HFD. Moderate to severe micro- and macrovesicular steatosis in HFD was contrasted to a 49% reduction in area covered by the vacuoles of at least 15 μm2 in size in HFD + LMMS animals. These data provide preliminary evidence of the ability of LMMS to attenuate the progression of fatty liver disease, most likely achieved indirectly by suppressing adipogenesis and thus the total adipose burden through life, thereby reducing a downstream challenge to liver morphology and function.
Keywords: diet-induced obesity, aging, liver steatosis, mechanical loading, triglycerides, free fatty acids
Nonalcoholic fatty liver disease (NAFLD) is a common disorder associated with excess adiposity, occurring in 10–15% of normal-weight individuals but afflicting up to 80% of the obese.1 Evidence indicates that this disease is the hepatic component of the obesity-induced metabolic syndrome, with gradual weight loss recognized as the principal therapeutic strategy to slow its progression. An increase in NAFLD has paralleled the rising incidence of obesity, with statistics from 1986 to 1988 showing that 25.5% of hospitalizations with a discharge diagnosis of NAFLD had a concurrent diagnosis of obesity, compared with 43.3% over the period of 2004–2006.
Recent reports indicate that low-magnitude mechanical signals (LMMS) suppress adiposity2 and diet-induced obesity (DIO)3 by inhibiting adipogenesis in a young adult mouse. LMMS is a high-frequency (30–90 Hz), extremely low-magnitude mechanical signal, with the 12 μ displacement of the oscillating platform barely perceptible to human touch. After 6 weeks, LMMS increased the number of bone marrow-derived stem cells, and after 12 weeks a marked reduction in visceral and total body adiposity was measured. It was hypothesized that organs compromised by lipid accumulation would therefore be protected by these mechanical signals. In this study, using a high-fat diet mouse model of DIO, we investigate the ability of LMMS to protect against several indices of NAFLD.
At 7 weeks of age, single-housed C57BL/6J male mice were given free access to a high-fat diet (HFD, 45% kcal fat, 58V8 Research Diet, Richmond, IN, USA). Sixteen mice were randomized into either HFD + LMMS (5 days per week of 15 min per day of a 90Hz, 0.2G mechanical signal, where 1G = earth's gravitational field) or age-matched sham handled controls (HFD). Eight additional animals were retained on a regular chow diet to serve as an age-matched regular diet (RD) control. The LMMS signal was delivered by placing mice on a gently oscillating platform (Marodyne Medical, Lakeland, FL, USA), a load challenge which generated <5 microstrain in the tibia,4 a deformation several orders of magnitude below the peak strains experienced during strenuous activity.5 Animal mass and food consumption were measured weekly. Whole-body in vivo micro-computed tomography scans were used to establish fat, lean and bone volume of the torso at 12, 24 and 36 weeks of LMMS (VivaCT, Scanco Medical, Bruttisellen, Switzerland).6
Livers of LMMS and control mice were harvested at 36 weeks (43 weeks of age). Total triglycerides (TGs) and nonesterified free fatty acids (NEFAs) were measured on total lipid extracts using enzymatic kits (TG Kit, Sigma, Saint Louis, MO, USA; NEFA C, Wako Chemicals, Richmond, VA, USA). Primary enzyme-linked immunosorbent assay data are expressed as concentrations (per mg tissue), and estimated total content changes were calculated by multiplication of liver mass to concentration per individual animal.
In a blinded manner, full field, hematoxylin and eosin-stained histological sections of each liver were qualitatively reviewed by a pathologist (KRS). Further, four random, nonoverlapping images were acquired from each section, and automated quantification of vesicle size and area fraction established for each digitized field (ImageJ, NIH, Bethesda, MD, USA). The percentage of the filed covered by vesicles >15 μm2 was then determined.
All data are shown as mean ± s.d., with two tailed t-tests used throughout (significance at 5%). One animal from each group was euthanized shortly before the final scan due to dermatitis. One LMMS mouse expired during the last scan due to anesthesia overdose, but the scan data were included. Tissue samples were not harvested for these three animals. Sample size for all ex vivo tissue analyses: n = 7 for HFD and n = 6 for HFD + LMMS.
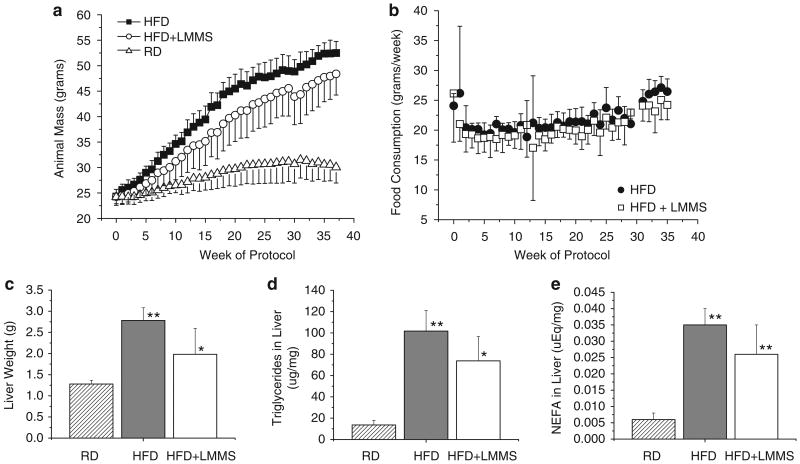
Weekly measures of animal mass showed that HFD + LMMS animals did not gain as much weight as HFD on the 45% fat diet (Figure 1a), but both groups experienced significant DIO compared with age-matched RD animals. At 36 weeks (43 weeks of age), HFD + LMMS mice (48.4 ± 4.2 g) were 7.8% lighter than HFD (52.5 ± 2.3 g, P = 0.07). Both groups weighed significantly more (> 50%) than the age-matched, RD controls (30.6 ± 3.1 g; P < 0.001). Although weekly high-fat food consumption of HFD tended to be higher than HFD + LMMS (median difference of 5.5% of control over LMMS), the difference was significant at only 3 of the 36 time points (Figure 1b). The sum of weekly averages showed a 5.7% increase in total food consumption of HFD over HFD + LMMS mice (P = 0.02).
Figure 1.
Average animal weight (a) for the high-fat control (HFD) and low-magnitude mechanical signals (HFD + LMMS) mice, as compared with an age-matched regular chow-diet control group over the 36-week study period (n = 8 per group; body mass of high-fat diet (HFD) + LMMS is lower than HFD at weeks 8–10 and 15–25 at P < 0.05; HFD is greater than regular diet (RD) from week 2 onward, and HFD + LMMS is greater than RD from week 6 onward). Food consumption between HFD and HFD + LMMS mice (b) is significantly different (P < 0.05) at only three time points (weeks 20–22). LMMS provided significant protection against the hepatomegaly (liver enlargement) often associated with long-term HFD (c), with a 28.7% decrease in average liver weight (P < 0.05 for HFD + LMMS to HFD and RD; HFD to RD is P < 0.001). The reduction in liver size was partially attributed to suppressed lipid accumulation and macrovesicular formation in LMMS animals. The concentration (per mg of tissue) of triglycerides (TGs) (d; HFD + LMMS to HFD significant at P < 0.05, with both significantly different to RD at P < 0.001) and nonesterified free fatty acids (NEFAs) (e; HFD + LMMS nsd to HFD, both significantly different to RD at P < 0.001) were lower by 27 and 25%, respectively, in the LMMS mice as compared with HFD. Multiplying (per animal) liver mass and (per animal) concentrations of TG and free fatty acid (FFA), indicated that LMMS mice had 46.2% (P = 0.013) lower total TG and 42% (P = 0.02) lower NEFA burden in the whole liver compared with diet-induced obesity (DIO) CON. Although LMMS were only 7.8% lighter than HFD at 36 weeks, and both groups were > 50% higher in body mass as compared with RD controls, the consequences of a HFD on the liver seems modulated in the LMMS mice. Nsd, non significant difference. *P < 0.05, **P < 0.001.
Measured at 12 weeks (19 weeks of age), adipose development in the mouse torso was suppressed by 20.4% in the HFD + LMMS animals (P = 0.05, Figure 2a). At the same time point, HFD + LMMS animals exhibited a 14.1% increase in total bone volume fraction (P = 0.03, Figure 2b). Trends for decreased fat and increased bone volume in LMMS animals continued at the 24 and 36 weeks time points, but did not achieve statistical significance. From the 36-week in vivo computed tomography scans, marked enlargement of liver volume was visible in HFD compared with LMMS (Figure 2c).
Figure 2.
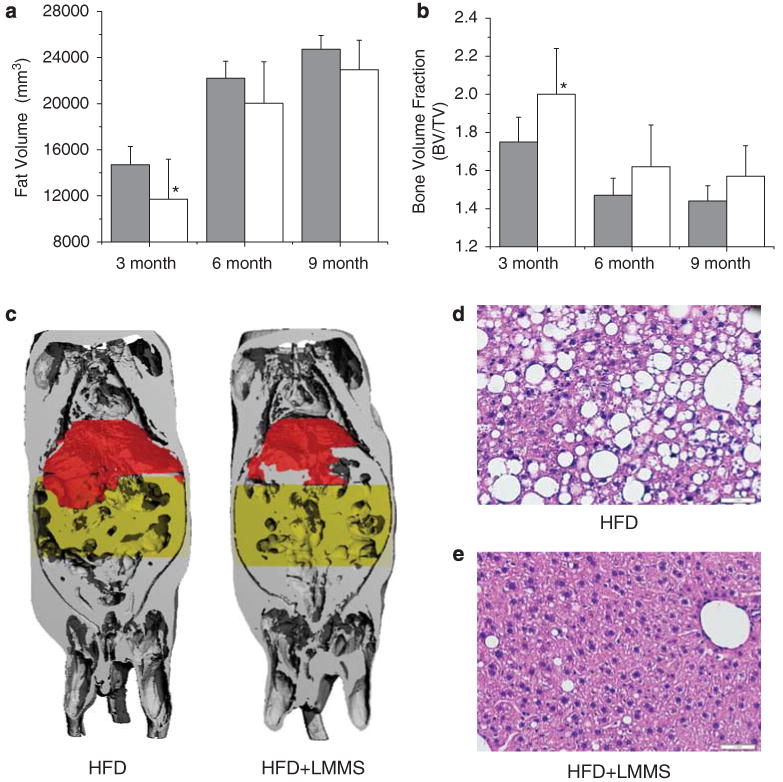
In vivo micro-computed tomography scans at 3, 6 and 9 months of low-magnitude mechanical signals (LMMS) were analyzed for total adipose and bone volume for each animal. After 3 months (12 weeks), adipose tissue was suppressed by 20.4% (P = 0.05) in the LMMS mice (a), paralleled by a 14.1% (P = 0.03) increase in bone volume of the whole body (b), differences that persisted as trends at 6 and 9 months. Coronal-view reconstruction of a 9-month whole-body scan of a diet-induced obesity (HFD) mouse (c-left) shows the total (gray) and abdominal (yellow) adiposity of the animal. Also shown is the relative size of the liver (red), significantly larger in the control (HFD)—as compared with HFD + LMMS (c-right)—mouse. The differences of liver morphology and biochemical indices of fatty liver disease were also evident in histological examination of the liver. CON livers (d) contained large lipid deposits (macrovesicles) within and between the hepatocytes, whereas livers from LMMS mice (e) had a more normal-appearing morphology, with smaller and fewer lipid vacuoles. Scale is 1mm. *P < 0.05.
Post-mortem weights of harvested livers (Figure 1c) showed that LMMS livers were 28.8% lighter than controls (2.78 ± 0.30g vs 1.98 ± 0.61g; P = 0.02), although the livers in both the HFD and HFD + LMMS groups were markedly enlarged compared with age-matched RD animals (1.28 ± 0.87 g). Total liver TGs were 27.4% (P = 0.04) lower in the LMMS mice as compared with HFD (Figure 1d), whereas NEFA concentration in the liver was 24.7% lower in the LMMS animals (P = 0.08; Figure 1e). Multiplying the reduction in liver weight by the suppression in TG concentration, the total amount of TG in the liver indicated that LMMS animals were 46.2% lower than HFD (P = 0.01), and total NEFA in LMMS livers was 42.1% lower (P = 0.02).
Examined by a pathologist blinded to group assignments, hematoxylin and eosin- stained histological sections showed both large (macrovesicles) and small lipid vacuoles (micro-vesicles) in hepatocytes throughout the HFD livers, with marked micro- and macrovesicular steatosis involving ∼85% of the hepatic parenchyma (Figure 2d). There was focal-minimal portal triaditis in both HFD and HFD + LMMS liver sections, but no evidence of acute or chronic lobular hepatitis. Automated analysis of digitized histological images indicated that 49% less area of the sections from LMMS mice (Figure 2e) was comprised of lipid vacuoles of at least 15μm2 as compared with HFD (19.7 ± 4.6% vs 9.5 ± 6.4%; P = 0.01).
The interrelated conditions of metabolic syndrome share underlying mediators, mechanisms and pathways, which over time compromise the function of major organ systems.7 Deteriorating organ function is magnified by an age-related decline of the organism's ability to resist and repair tissue damage. The study reported here, using a C57BL/6J model of DIO, confirms an interaction between a HFD endured over an extended period to potentiate the development of at least one pathological condition: fatty liver disease. Liver mass of a healthy male C57BL/6J mice fed a regular chow diet over 36 weeks was 1.3 ± 0.09g, essentially half that measured here in the control HFD animals. Gross enlargements in the liver closely correlate with a number of pathologies, with NAFLD generally being the most prevalent, as evidenced by the pervasive accumulation of lipid in and around the hepatocytes.
The biosynthesis of TGs most often occurs in adipocytes, with occurrence in other cell types typically linked to various disease states eventually inducing apoptosis and lipotoxicity,8 whereas an overall increase in TG and NEFA levels will inevitably compromise liver function.9 The data presented here indicate that several indices of fatty liver disease can be suppressed by LMMS, including protection against liver enlargement, reductions in TG and NEFA, and the suppression of macrovesicle invasion, all reflecting improved physiological function of the liver. Although the protective mechanism has not yet been determined, we propose that the observed benefit is to an extent an aggregate of a reduced total body adipose burden from early in life, achieved in part by the mechanical biasing of stem cells away from adipogenesis,10 ultimately translating into improved metabolic function in the older animal. A significant reduction in food consumption of LMMS animals was only observed in 3 of the 36 stages of the study, after the animal mass differences had already been established and while perhaps contributing to the reduced steatosis, may also indicate a decrease in the metabolic demand of a leaner animal. The contribution of decreased food consumption to the liver pathology will need to be addressed by detailed pair-feeding experiments, and are a limitation of this study.
Exercise can decrease the risk of metabolic disease and suppress the complications of type 2 diabetes mellitus not only by reducing the adipose burden, but by suppressing key adipokines and inflammatory factors,11 and aging-associated insulin resistance.12 Reductions in TG for lifestyle interventions such as exercise vary widely, but are typically less than the ∼25% reductions observed in this study,13 and clinical reductions in free fatty acid are even more difficult to achieve through weight loss and lifestyle intervention.14 Although the direct effect of LMMS treatment on insulin resistance has not been determined for this long-term study, it has been shown that 12 weeks of LMMS improved glucose tolerance of animals on an RD.2 What is surprising here, however, is that mechanical signals so small and brief could influence liver morphology and function, through a pathway independent of the metabolic expenditure of calories. These studies lend some insight into the complexity of the interaction between diet and aging, and may, in part, explain why exercise is so often indicated for the treatment of NAFLD even if the specific molecular mechanisms responsible for this benefit have not been identified.
Translating this finding to the clinic, it is important to recognize that even brief (< 1 min) exposure to high magnitude (> 1G) vibration can damage a range of physiologic systems, including the liver, as evidenced by a marked increase in liver toxicity and enlargement.15 Exposure to the magnitude of mechanical signals investigated here (< 0.4 G) is considered safe by NIOSH and ISO for up to 4h each day.16 These data also suggest that mechanical factors need not be large to be influential, even in the clinic,17 and could still be delivered to help maintain the viability of tissues and function of organs. Indeed, it is entirely possible that a fraction of the many undisputed benefits of exercise could be derived from the direct mechanical stimulation of cells, rather than necessarily upregulating the metabolism of the entire organism.
Acknowledgments
We thank S Lublinsky for help with animal imaging. This work was supported by the National Institutes of Health Grant AR 43498.
Footnotes
Conflict of interest:
YKL, JEP, SJ and CTR have submitted a series of patents to the US Patent and Trademark Office regarding the method and application of the technology. CTR is the scientific founder of Marodyne Medical, LLC and both he and the company may benefit from the results of this research. The remaining authors declared no conflict of interest.
References
- 1.Duvnjak M, Lerotic I, Barsic N, Tomasić V, Virović Jukić L, Velagić V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol. 2007;13:4539–4550. doi: 10.3748/wjg.v13.i34.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci USA. 2007;104:17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24:50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40:1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Rubin CT, Lanyon LE. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J Theor Biol. 1984;107:321–327. doi: 10.1016/s0022-5193(84)80031-4. [DOI] [PubMed] [Google Scholar]
- 6.Luu YK, Lublinsky S, Ozcivici E, Capilla E, Pessin JE, Rubin CT, et al. In vivo quantification of subcutaneous and visceral adiposity by micro-computed tomography in a small animal model. Med Eng Phys. 2009;31:34–41. doi: 10.1016/j.medengphy.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonseca-Alaniz MH, Takada J, Alonso-Vale MI, Lima FB. Adipose tissue as an endocrine organ: from theory to practice. J Pediatr (Rio J) 2007;83:S192–S203. doi: 10.2223/JPED.1709. [DOI] [PubMed] [Google Scholar]
- 9.Kashyap SR, Diab DL, Baker AR, Yerian L, Bajaj H, Gray-McGuire C, et al. Triglyceride levels and not adipokine concentrations are closely related to severity of nonalcoholic fatty liver disease in an obesity surgery cohort. Obesity (Silver Spring) 2009;17:1696–1701. doi: 10.1038/oby.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable β-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank LL, Sorensen BE, Yasui Y, Tworoger SS, Schwartz RS, Ulrich CM, et al. Effects of exercise on metabolic risk variables in overweight postmenopausal women: a randomized clinical trial. Obes Res. 2005;13:615–625. doi: 10.1038/oby.2005.66. [DOI] [PubMed] [Google Scholar]
- 12.Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med. 2000;30:327–346. doi: 10.2165/00007256-200030050-00002. [DOI] [PubMed] [Google Scholar]
- 13.Kris-Etherton PM, Taylor DS, Smiciklas-Wright H, Mitchell DC, Bekhuis TC, Olson BH, et al. High-soluble-fiber foods in conjunction with a telephone-based, personalized behavior change support service result in favorable changes in lipids and lifestyles after 7 weeks. J Am Diet Assoc. 2002;102:503–510. doi: 10.1016/s0002-8223(02)90116-1. [DOI] [PubMed] [Google Scholar]
- 14.Mensink M, Blaak EE, Wagenmakers AJ, Saris WH. Lifestyle intervention and fatty acid metabolism in glucose-intolerant subjects. Obes Res. 2005;13:1354–1362. doi: 10.1038/oby.2005.164. [DOI] [PubMed] [Google Scholar]
- 15.Griffin JJ. Handbook of Human Vibration. Academic Press; London: 2001. [Google Scholar]
- 16.International Standards Organization. Evaluation of Human Exposure to Whole-Body Vibration. ISO 2631/1. ISO; Geneva: 1985. [Google Scholar]
- 17.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculo-skeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–1474. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]