Abstract
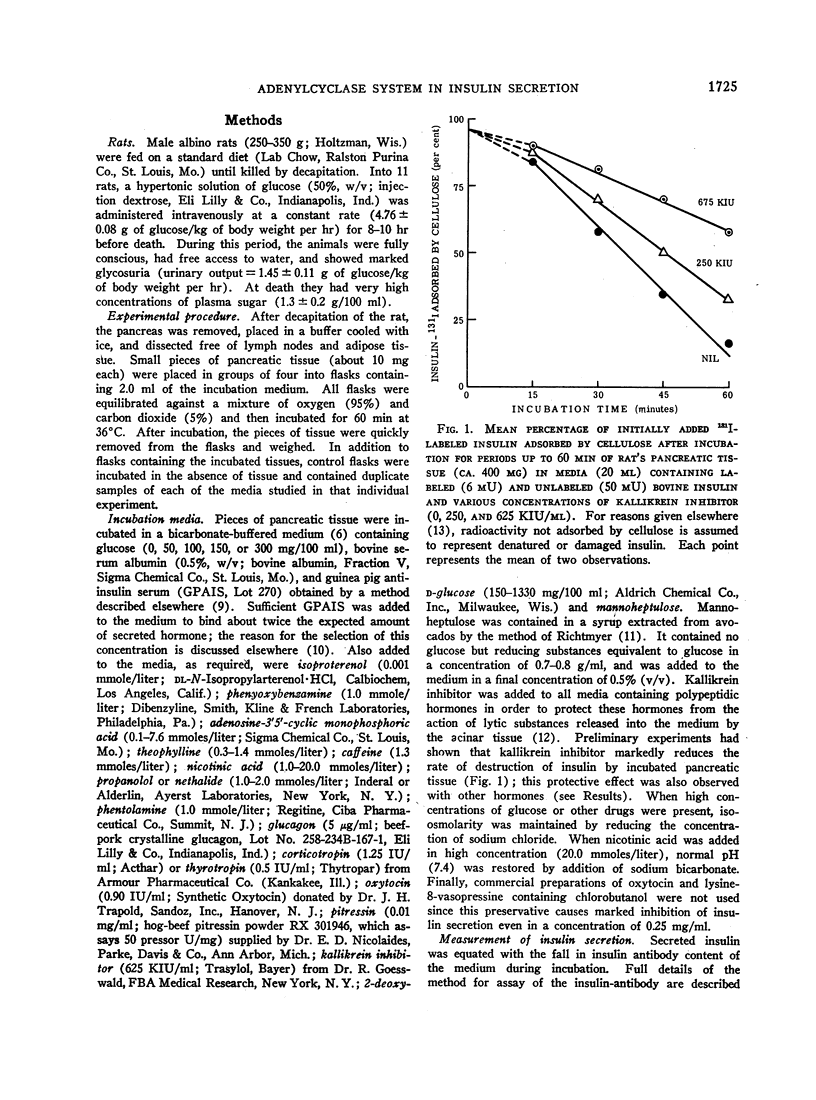
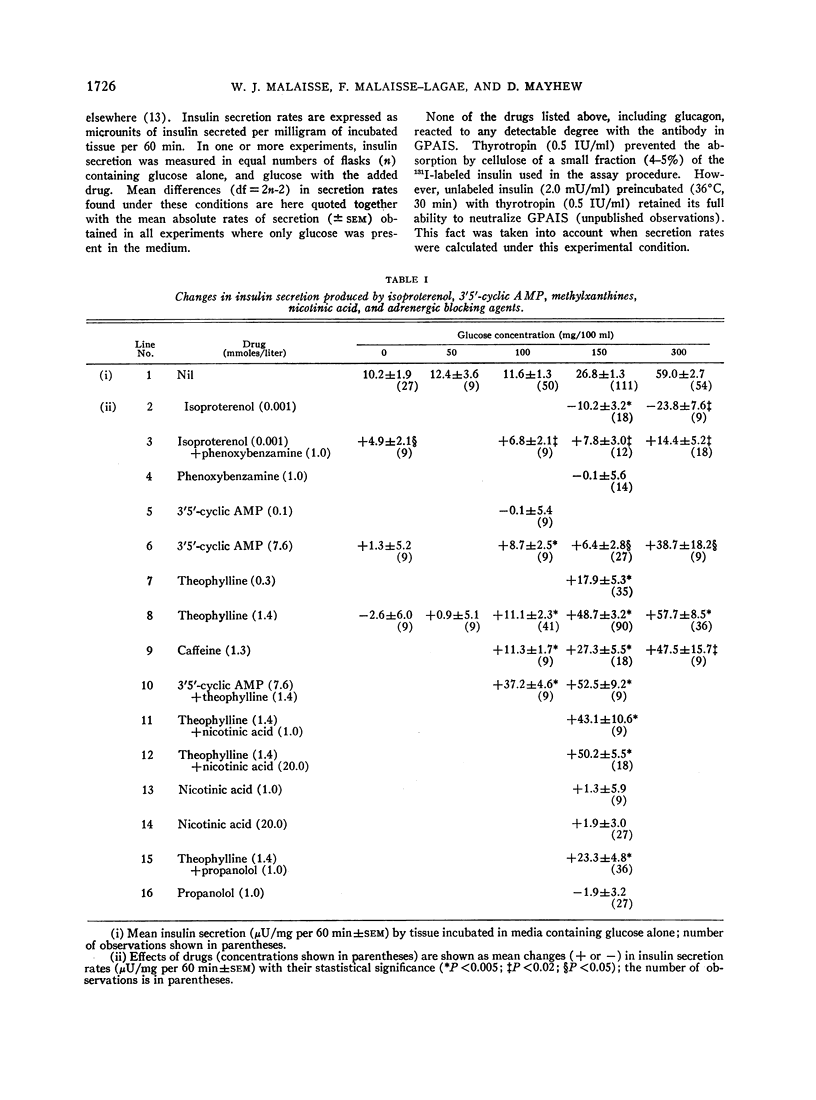
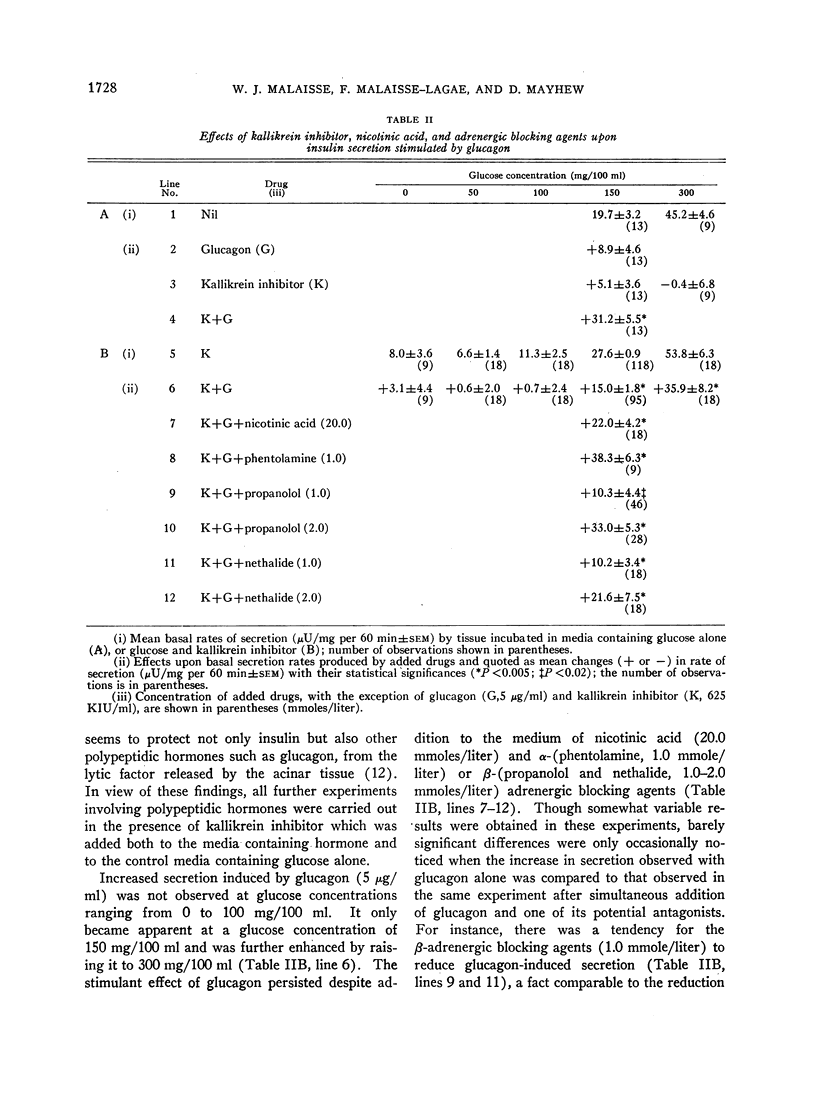
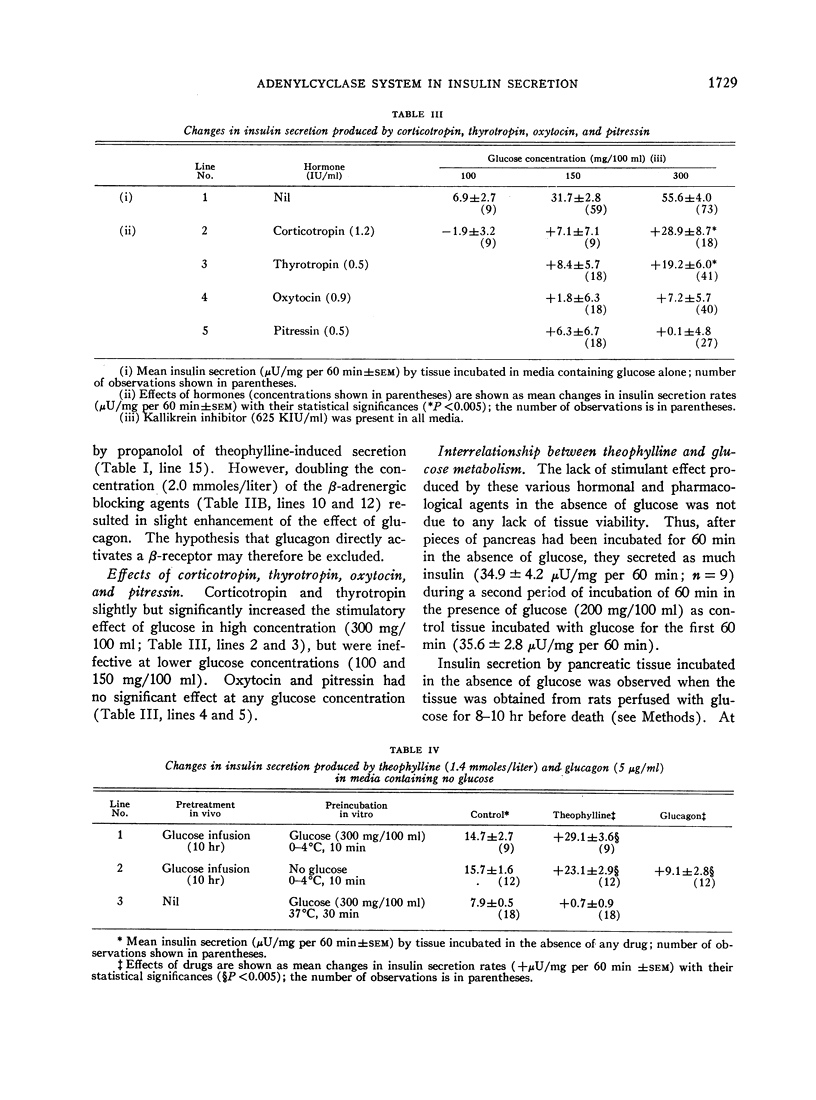
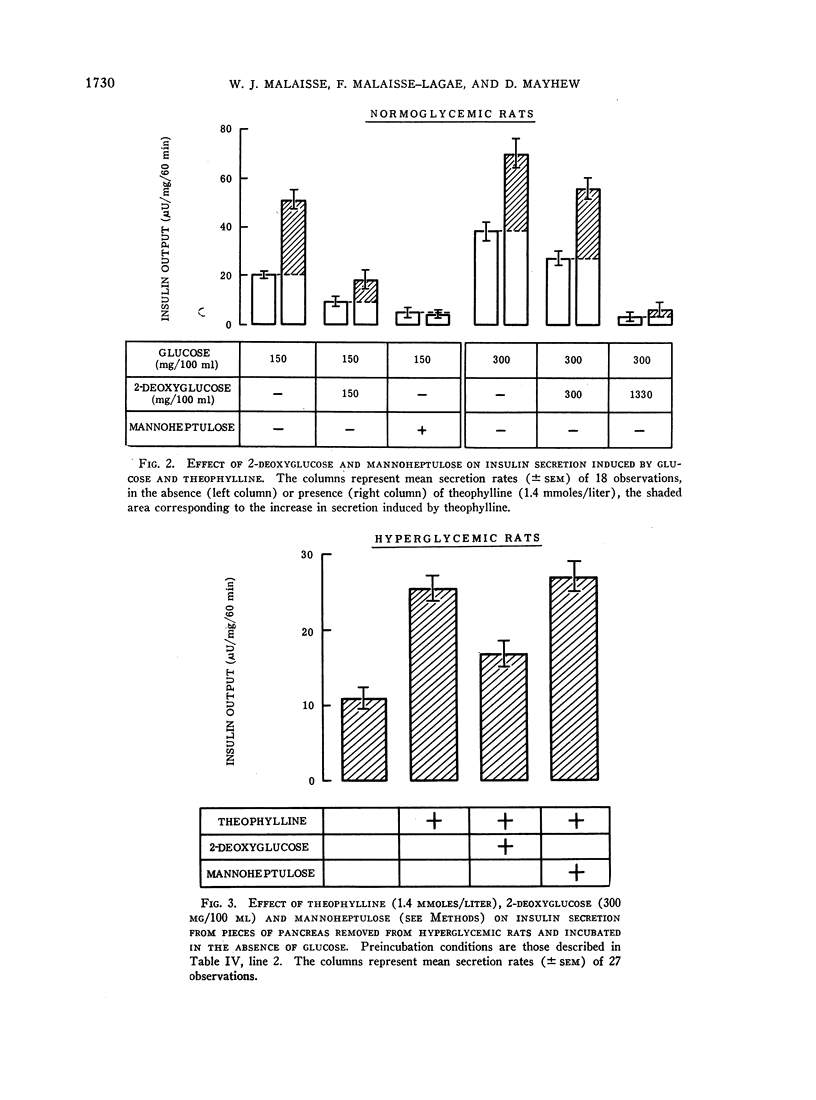
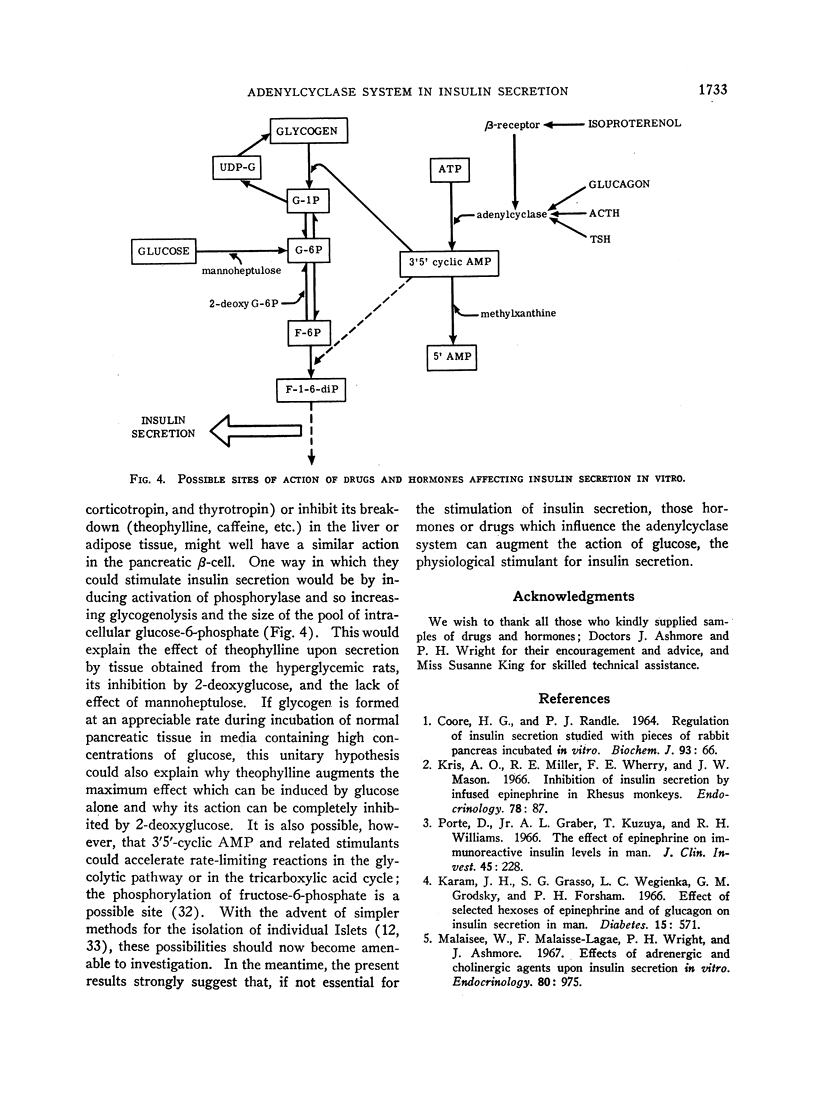
A possible role for adenylcyclase in insulin secretion was investigated. Isoproterenol, a predominantly β-adrenergic agent, when mixed with an α-adrenergic blocking agent (phenoxybenzamine), stimulated insulin secretion from pieces of the rat's pancreas in vitro. Theophylline, caffeine, 3′5′-cyclic AMP, glucagon, adrenocorticotropin (ACTH), and thyrotropin (TSH), all of which are thought to act through the adenylcyclase systems in the liver and adipose tissue, also stimulated insulin secretion in vitro; oxytocin and vasopressin, which do not stimulate lipolysis in adipose tissue, were inactive. In all cases, stimulation of insulin secretion could not be detected when glucose was absent or present in only low concentrations (less than 100 mg/100 ml) and was maximal at high levels of glucose (300 mg/100 ml). When pancreatic tissue was obtained from normoglycemic rats and contained no detectable glycogen in the Islets, the stimulant effects of glucose and of theophylline were reduced or abolished by mannoheptulose and 2-deoxyglucose. When tissue was derived from rats infused for 8-10 hr with glucose and contained glycogen, theophylline, even in the absence of glucose, stimulated secretion and this effect was reduced by 2-deoxyglucose but not by mannoheptulose. It is suggested that the β-cell contains an adenylcyclase system through which phosphorylase and possibly phosphofructokinase could be activated; and that insulin secretion could depend upon and be regulated by hormones and other substances which influence the rate at which glycolysis proceeds within the β-cell.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Balasse E., Rasio E., Conard V. Action insulino-mimétique de la vasopressine chez le chien. Arch Int Pharmacodyn Ther. 1966 Jun;161(2):392–397. [PubMed] [Google Scholar]
- Balasse E., Rasio E. Mécanisme d'action de l'ocytocine sur la concentration plasmatique des acides gras libres (A.G.L.) chez le chien. Arch Int Pharmacodyn Ther. 1965 Oct;157(2):356–359. [PubMed] [Google Scholar]
- Butcher R. W. Cyclic 3',5'-AMP and the lipolytic effects of hormones on adipose tissue. Pharmacol Rev. 1966 Mar;18(1):237–241. [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Inhibition of glucose phosphorylation by mannoheptulose. Biochem J. 1964 Apr;91(1):56–59. doi: 10.1042/bj0910056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964 Oct;93(1):66–78. doi: 10.1042/bj0930066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford P. M., Porte D., Jr, Wood F. C., Jr, Williams R. H. Effect of glucagon on serum insulin, plasma glucose and free fatty acids in man. Metabolism. 1966 Feb;15(2):114–122. doi: 10.1016/0026-0495(66)90032-1. [DOI] [PubMed] [Google Scholar]
- Devrim S., Recant L. Effect of glucagon on insulin release in vitro. Lancet. 1966 Dec 3;2(7475):1227–1228. doi: 10.1016/s0140-6736(66)92307-5. [DOI] [PubMed] [Google Scholar]
- Karam J. H., Grasso S. G., Wegienka L. C., Grodsky G. M., Forsham P. H. Effect of selected hexoses, of epinephrine and of glucagon on insulin secretion in man. Diabetes. 1966 Aug;15(8):571–578. doi: 10.2337/diab.15.8.571. [DOI] [PubMed] [Google Scholar]
- Kris A. O., Miller R. E., Wherry F. E., Mason J. W. Inhibition of insulin secretion by infused epinephrine in rhesus monkeys. Endocrinology. 1966 Jan;78(1):87–97. doi: 10.1210/endo-78-1-87. [DOI] [PubMed] [Google Scholar]
- LAZAROW A. FUNCTIONAL CHARACTERIZATION AND METABOLIC PATHWAYS OF THE PANCREATIC ISLET TISSUE. Recent Prog Horm Res. 1963;19:489–546. [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Lacy P. E., Wright P. H. Insulin secretion by isolated islets in presence of glucose, insulin and anti-insulin serum. Proc Soc Exp Biol Med. 1967 Feb;124(2):497–500. doi: 10.3181/00379727-124-31773. [DOI] [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., Wright P. H. A new method for the measurement in vitro of pancreatic insulin secretion. Endocrinology. 1967 Jan;80(1):99–108. doi: 10.1210/endo-80-1-99. [DOI] [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., Wright P. H., Ashmore J. Effects of adrenergic and cholinergic agents upon insulin secretion in vitro. Endocrinology. 1967 May;80(5):975–978. doi: 10.1210/endo-80-5-975. [DOI] [PubMed] [Google Scholar]
- Mansour T. E. Factors influencing activation of phosphofructokinase. Pharmacol Rev. 1966 Mar;18(1):173–179. [PubMed] [Google Scholar]
- Porte D., Jr, Graber A. L., Kuzuya T., Williams R. H. The effect of epinephrine on immunoreactive insulin levels in man. J Clin Invest. 1966 Feb;45(2):228–236. doi: 10.1172/JCI105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDMAN D. THE ADIPOKINETIC ACTION OF POLYPEPTIDE AND AMINE HORMONES UPON THE ADIPOSE TISSUE OF VARIOUS ANIMAL SPECIES. J Lipid Res. 1963 Apr;4:119–129. [PubMed] [Google Scholar]
- SAMOLS E., MARRI G., MARKS V. PROMOTION OF INSULIN SECRETION BY GLUCAGON. Lancet. 1965 Aug 28;2(7409):415–416. doi: 10.1016/s0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- Sutherland E. W., Robison G. A. The role of cyclic-3',5'-AMP in responses to catecholamines and other hormones. Pharmacol Rev. 1966 Mar;18(1):145–161. [PubMed] [Google Scholar]
- Turner D. S., McIntyre N. Stimulation by glucagon of insulin release from rabbit pancreas in vitro. Lancet. 1966 Feb 12;1(7433):351–352. doi: 10.1016/s0140-6736(66)91327-4. [DOI] [PubMed] [Google Scholar]
- WICK A. N., DRURY D. R., NAKADA H. I., WOLFE J. B. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957 Feb;224(2):963–969. [PubMed] [Google Scholar]
- Wright P. H., Malaisse W. J. A simple method for the assay of guinea pig anti-insulin serum. Diabetologia. 1966 Nov;2(3):178–188. doi: 10.1007/BF01222068. [DOI] [PubMed] [Google Scholar]
- Wright P. H., Malaisse W. J., Reynolds I. J. Assay of partially neutralized guinea pig anti-insulin serum. Endocrinology. 1967 Aug;81(2):226–234. doi: 10.1210/endo-81-2-226. [DOI] [PubMed] [Google Scholar]
- Wright P. H., Norman L. L. Some factors affecting insulin antibody production in guinea pigs. Diabetes. 1966 Sep;15(9):668–674. doi: 10.2337/diab.15.9.668. [DOI] [PubMed] [Google Scholar]



