Abstract
Background
Both apolipoprotein (Apo) C-III gene polymorphism and alcohol consumption have been associated with increased serum triglyceride (TG) levels, but their interactions on serum TG levels are not well known. The present study was undertaken to detect the interactions of the ApoC-III 3238C>G (rs5128) polymorphism and alcohol consumption on serum TG levels.
Methods
A total of 516 unrelated nondrinkers and 514 drinkers aged 15-89 were randomly selected from our previous stratified randomized cluster samples. Genotyping of the ApoC-III 3238C>G was performed by polymerase chain reaction and restriction fragment length polymorphism combined with gel electrophoresis, and then confirmed by direct sequencing. Interactions of the ApoC-III 3238C>G genotype and alcohol consumption was assessed by using a cross-product term between genotypes and the aforementioned factor.
Results
Serum total cholesterol (TC), TG, high-density lipoprotein cholesterol (HDL-C), ApoA-I and ApoB levels were higher in drinkers than in nondrinkers (P < 0.05-0.001). There was no significant difference in the genotypic and allelic frequencies between the two groups. Serum TG levels in nondrinkers were higher in CG genotype than in CC genotype (P < 0.01). Serum TC, TG, low-density lipoprotein cholesterol (LDL-C) and ApoB levels in drinkers were higher in GG genotype than in CC or CG genotype (P < 0.01 for all). Serum HDL-C levels in drinkers were higher in CG genotype than in CC genotype (P < 0.01). Serum TC, TG, HDL-C and ApoA-I levels in CC genotype, TC, HDL-C, ApoA-I levels and the ratio of ApoA-I to ApoB in CG genotype, and TC, TG, LDL-C, ApoA-I and ApoB levels in GG genotype were higher in drinkers than in nondrinkers (P < 0.05-0.01). But the ratio of ApoA-I to ApoB in GG genotype was lower in drinkers than in nondrinkers (P < 0.01). Multivariate logistic regression analysis showed that the levels of TC, TG and ApoB were correlated with genotype in nondrinkers (P < 0.05 for all). The levels of TC, LDL-C and ApoB were associated with genotype in drinkers (P < 0.01 for all). Serum lipid parameters were also correlated with age, sex, alcohol consumption, cigarette smoking, blood pressure, body weight, and body mass index in both groups.
Conclusions
This study suggests that the ApoC-III 3238CG heterozygotes benefited more from alcohol consumption than CC and GG homozygotes in increasing serum levels of HDL-C, ApoA-I, and the ratio of ApoA-I to ApoB, and lowering serum levels of TC and TG.
Introduction
Coronary artery disease (CAD) is the most common cause of death in industrialized countries with evidence that high plasma or serum triglyceride (TG) concentration is an independent risk factor [1-5]. It is well known that plasma TG concentration is modulated by both environmental and genetic factors [6]. Numerous studies have evaluated the influence of alcohol intake, an index of lifestyle, on plasma lipid and lipoprotein concentrations. Alcohol consumption can promote lipogenesis [7] and accordingly increase serum TG levels [8,9]. Alcohol in doses > 30 g/day in both sexes can augment the TG level. It has been found that the alcohol intake of 60 g/day increases the TG level by about 0.19 mg/dl per 1 gram of alcohol consumed [10].
Plasma apolipoprotein (Apo) C-III is a major component of TG-rich lipoproteins (chylomicrons and very low density lipoprotein) and a minor component of high density lipoprotein. The mature 79-amino-acid ApoC-III protein is synthesized predominantly in the liver but also to a lesser extent in the intestine. In vitro studies have indicated that ApoC-III is a noncompetitive inhibitor of lipoprotein lipase, thereby suggesting an important role in the catabolism of TG-rich lipoproteins [11]. Plasma ApoC-III concentrations were positively correlated with plasma TG levels, both in the normal population as well as in hypertriglyceridemic patients [12] or in transgenic animals [13]. ApoC-III gene has been mapped to chromosome 11q23.3 [14] and is flanked by the genes for ApoA-I and ApoA-IV in a 15-kb gene cluster [15]. Several polymorphic sites have been detected within and around the ApoC-III gene. The most extensively studied is the SstI polymorphism, due to a C→G substitution at nucleotide 3238, in the 3' untranslated region of the gene. Numerous studies have found an association between the presence of a polymorphic SstI site in the untranslated region of the ApoC-III gene with raised ApoC-III and TG concentrations [16-53] and with an increased risk of CAD [53-62]. However, little is known about the interactions of the ApoC-III gene polymorphism and alcohol consumption on serum lipid concentrations. Therefore, the aim of the present study was to determine the interactions of the ApoC-III 3238C>G (rs5128) polymorphism and alcohol consumption on serum lipid levels.
Materials and methods
Study subjects
A total of 1030 unrelated subjects who reside in 16 villages in Napo County, Guangxi Zhuang Autonomous Region, People's Republic of China were randomly selected from our previous stratified randomized cluster samples [63]. The age of the subjects ranged from 15 to 89 years, with an average age of 43.30 ± 17.69 years. There were 516 nondrinkers and 514 drinkers. All of the subjects were peasants. The study subjects were essentially healthy and had no evidence of diseases related to atherosclerosis, CAD and diabetes. None of them had been treated with β-adrenergic blocking agents and lipid-lowering drugs such as statins or fibrates. The present study was approved by the Ethics Committee of the First Affiliated Hospital, Guangxi Medical University. Informed consent was obtained from all subjects after they received a full explanation of the study.
Epidemiological survey
The survey was carried out using internationally standardized methods, following a common protocol. Information on demographics, socioeconomic status, and lifestyle was collected with standardized questionnaires. Smoking status was categorized into groups of cigarettes per day: <20 and ≥20. Alcohol consumption was categorized into groups of grams of alcohol per day: ≤25 and >25. The physical examination included blood pressure, body height, and body weight, and body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Sitting blood pressure was measured three times with use of a mercury sphygmomanometer after the subjects had a 5-minute rest, and the average of the three measurements was used in statistical analysis. Systolic blood pressure was determined by the first Korotkoff sound, and diastolic blood pressure by the fifth Korotkoff sound.
Biochemical analysis
Venous blood samples (8 ml) were drawn from a forearm vein of every subject after venous occlusion for a few seconds in a sitting position, after an overnight fast of 12 h and abstention from alcohol use for at least 12 h. A part of the sample (3 ml) was collected into glass tubes and allowed to clot at ambient temperature, and used to determine serum lipid levels, and another part of the sample (5 ml) was transferred into tubes with anticoagulate solution (4.80 g/L citric acid, 14.70 g/L glucose, and 13.20 g/L tri-sodium citrate) and used to extract deoxyribonucleic acid (DNA). Immediately following clotting serum was separated by centrifugation for 15 minutes at 3000 rpm. The levels of serum total cholesterol (TC), TG, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) in samples were determined by enzymatic methods with commercially available kits, Tcho-1, TG-LH (RANDOX Laboratories Ltd., Ardmore, Diamond Road, Crumlin Co. Antrim, United Kingdom, BT29 4QY), Cholestest N HDL, and Cholestest LDL (Daiichi Pure Chemicals Co., Ltd., Tokyo, Japan), respectively. Serum ApoA-I and ApoB levels were assessed by the immunoturbidimetric immunoassay using a commercial kit (RANDOX Laboratories Ltd.). All determinations were performed with an autoanalyzer (Type 7170A; Hitachi Ltd., Tokyo, Japan) in the Clinical Science Experiment Center of the First Affiliated Hospital, Guangxi Medical University.
DNA amplification and genotyping
Total genomic DNA was isolated from peripheral blood leukocytes using the phenol-chloroform method. The extracted DNA was stored at 4°C until analysis. Genotyping of the ApoC-III 3238C>G was performed by polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP) according to the previous reports [19]. The sequence of the forward and backward primers used was 5'-CACTAGCCCAGAGAGAGGAGTGCC-3' and 5'-CTGAGCCCAGCCGCACACTAA-3' (Sangon, Shanghai, China). Each reaction system of a total volume of 25 μL, comprised 0.2 μg of genomic DNA; 1.0 μL of each primer (10 pmol/μl); 2.5 μL of 10 × buffer solution; 1.5 μL of MgCl2 (25 mmol/L); 2.0 μL of dNTP (2.5 mmol/L); and 1.5 U of Taq polymerase (Takara). For the amplification, initial denaturation at 94°C for 5 minutes was followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 61°C for 30 s, and extension at 72°C for 45 s, with final extension at 72°C for 4 min. Each restriction enzyme reaction was performed with 8 μL of amplified DNA; 2 μL of 10 × buffer solution; and 0.2 U SstI restriction ezyme in a total volume of 25 μL digested at 64°C for 4 h. The digestive products were separated by electrophoresis on 2% sepharose gel for 60 min. The length of each digested DNA fragment was determined by comparing migration of a sample with that of standard DNA marker. Stained with ethidium bromide, the gel was visualized under ultraviolet light and photographed. Genotypes were scored by an experienced reader blinded to epidemiological and lipid results.
DNA sequencing
Six samples (CC, CG and GG genotypes in two, respectively) detected by the PCR-RFLP were also confirmed by direct sequencing. The PCR product was purified by low melting point gel electrophoresis and phenol extraction, and then the DNA sequence were analyzed by using an ABI Prism 3100 (Applied Biosyatems) in Shanghai Sangon Biological Engineering Technology & Services Co., Ltd., People's Republic of China.
Diagnostic criteria
The normal values of serum TC, TG, HDL-C, LDL-C, ApoA-I, and ApoB in our Clinical Science Experiment Center were 3.10-5.17, 0.56-1.70, 1.04-1.81, 1.70-3.37 mmol/L, 1.20-1.60, and 0.63-1.14 g/L; respectively [63]. Hypertension was diagnosed according to the criteria of 1999 The World Health Organization-International Society of Hypertension Guidelines for the management of hypertension [64]. The diagnostic criteria of overweight and obesity were according to the Cooperative Meta-analysis Group of China Obesity Task Force. Normal weight, overweight and obesity were defined as a BMI <24, 24-28, and >28 kg/m2, respectively [63,64].
Statistical analysis
Epidemiological data were recorded on a pre-designed form and managed with Excel software. The quantitative variables were presented as mean ± standard deviation (serum TG levels were presented as medians and interquartile ranges). The difference in general characteristics between nondrinkers and drinkers was tested by the Student's unpaired t test. The allele frequencies of the ApoC-III 3238C>G were determined by gene counting. A chi-square analysis was used to evaluate the allelic and genotypic frequencies that were calculated from the observed genotypic counts and to assess Hardy-Weinberg expectations. Interaction between the ApoC-III 3238C>G genotype and alcohol consumption was assessed by using a cross-product term between genotypes and the aforementioned factor. Statistical significance was evaluated with analysis of covariance (ANCOVA). The co-variables include sex, age, BMI, hypertension, and cigarette smoking. In order to evaluate the association of serum lipid parameters with several environmental factors and genotypes, unconditional logistic regression analysis was also performed in the combined population, nondrinkers, and drinkers; respectively. The statistical analyses were performed with the statistical software package SPSS 13.0 (SPSS Inc., Chicago, Illinois). A P value of less than 0.05 was considered statistically significant.
Results
General characteristics between nondrinkers and drinkers
Table 1 gives the general characteristics between the nondrinkers and drinkers. The ratio of male to female, the mean age, the levels of systolic blood pressure, diastolic blood pressure and pulse pressure, and the percentages of subjects who smoked cigarettes were higher in drinkers than in nondrinkers (P < 0.05-0.001). There was no significant difference in the BMI between the two groups (P > 0.05).
Table 1.
Comparison of the general characteristics and serum lipid levels between the nondrinkers and drinkers
| Parameter | Nondrinker (n = 516) |
Drinker (n = 514) |
t (χ2) | P |
|---|---|---|---|---|
| Male/female | 186/330 | 306/208 | 56.930 | 0.000 |
| Age (years) | 41.26 ± 19.53 | 45.35 ± 15.38 | -3.733 | 0.000 |
| Body mass index (kg/m2) | 21.66 ± 2.71 | 21.95 ± 2.39 | -1.821 | 0.069 |
| Systolic blood pressure (mmHg) | 120.03 ± 16.15 | 125.60 ± 15.63 | -5.624 | 0.000 |
| Diastolic blood pressure (mmHg) | 74.13 ± 9.82 | 77.79 ± 9.78 | -5.993 | 0.000 |
| Pulse pressure (mmHg) | 45.95 ± 11.89 | 47.83 ± 12.01 | -2.525 | 0.012 |
| Cigarette smoking [n(%)] | ||||
| Nonsmoker | 414(80.2) | 292(56.8) | ||
| <20 cigarettes/day | 62(12.0) | 106(20.6) | ||
| ≥20 cigarettes/day | 40(7.8) | 116(22.6) | 69.628 | 0.000 |
| Alcohol consumption [n(%)] | ||||
| Nondrinker | 516(100.0) | - | ||
| <25 g/day | - | 396(77.0) | ||
| ≥25 g/day | - | 118(23.0) | ||
| Total cholesterol (mmol/L) | 4.52 ± 0.99 | 4.65 ± 0.95 | -2.150 | 0.032 |
| Triglyceride (mmol/L)a | 0.97 ± 0.57 | 1.00 ± 0.61 | -2.488 | 0.013 |
| HDL-C (mmol/L) | 1.98 ± 0.45 | 2.14 ± 0.49 | -5.458 | 0.000 |
| LDL-C (mmol/L) | 2.40 ± 0.70 | 2.41 ± 0.70 | -0.229 | 0.819 |
| Apolipoprotein (Apo) A-I (g/L) | 1.40 ± 0.16 | 1.48 ± 0.13 | -8.805 | 0.000 |
| ApoB (g/L) | 0.89 ± 0.22 | 0.92 ± 0.20 | -2.290 | 0.022 |
| ApoA-I/ApoB | 1.68 ± 0.57 | 1.69 ± 0.46 | -0.310 | 0.757 |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. aThe value of TG was presented as median (interquartile range). The difference between the two ethnic groups was determined by the Wilcoxon-Mann-Whitney test.
Serum lipid levels between nondrinkers and drinkers
The levels of TC, TG, HDL-C, ApoA-I and ApoB were higher in drinkers than in nondrinkers (P < 0.05-0.001). There were no significant differences in the levels of LDL-C and the ratio of ApoA-I to ApoB between the two groups (P > 0.05 for each).
Results of electrophoresis and genotyping
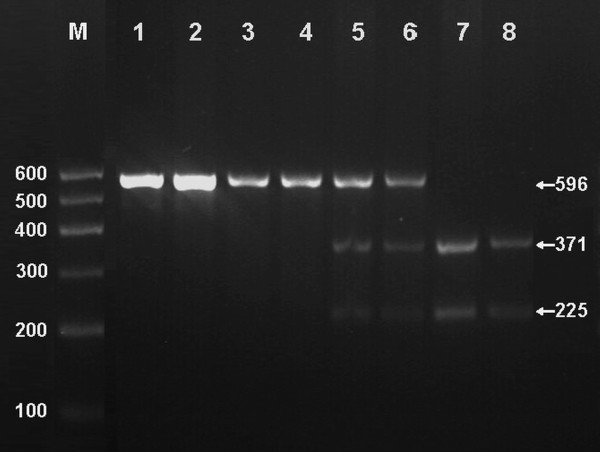
After the genomic DNA of the samples was amplified by PCR and imaged by 2% agarose gel electrophoresis, the purpose gene of 596 bp nucleotide sequences could be seen in the samples (Figure 1). The genotypes identified were named according to the presence or absence of the enzyme restriction sites, when a C to G transversion at nucleotide position 3238 of the ApoC-III gene. The presence of the cutting site indicates the 3238G allele, while its absence indicates the 3238C allele. Thus, the GG genotype is homozygote for the presence of the site (bands at 371 bp and 225 bp), CG genotype is heterozygote for the presence and absence of the site (bands at 596 bp, 371 bp and 225 bp), and CC genotype is homozygote for the absence of the site (band 596 bp; Figure 1). The genotype distribution was consistent with the Hardy-Weinberg equilibrium.
Figure 1.
Genotyping of PCR products of the samples. Lane M, 100 bp Marker Ladder; Lanes 1 and 2, the PCR products of the samples (596 bp); Lane 3 and 4, CC genotype (596 bp); Lanes 5 and 6, CG genotype (596 bp, 371 bp and 225 bp); and lanes 7 and 8, GG genotype (371 bp and 225 bp).
Results of sequencing
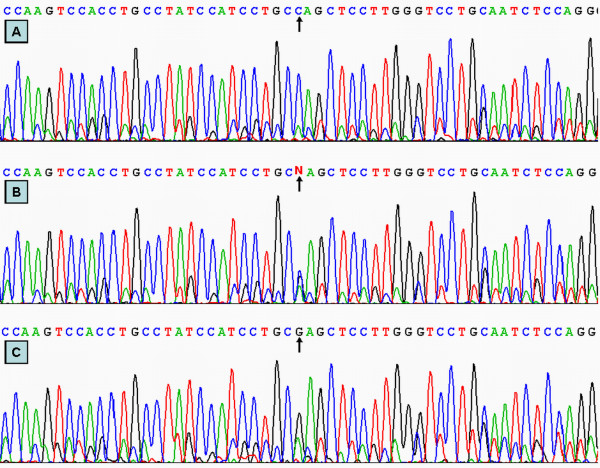
The results shown as CC, CG and GG genotypes by PCR-RFLP, CC, CG and GG genotypes were also confirmed by sequencing (Figure 2).
Figure 2.
A part of the nucleotide sequence of the ApoC-III 3238C>G. (A) CC genotype; (B) CG genotype; (C) GG genotype.
Genotypic and allelic frequencies
The genotypic and allelic frequencies of the ApoC-III 3238C>G are shown in Table 2. The frequencies of CC, CG and GG genotypes were 45.7%, 43.0% and 11.3% in nondrinkers, and 45.2%, 45.5% and 9.3% in drinkers (P > 0.05); respectively. The frequencies of C and G alleles were 67.2% and 32.8% in nondrinkers, and 67.9% and 32.1% in drinkers (P > 0.05); respectively.
Table 2.
Genotypic and allelic frequencies between the nondrinkers and drinkers [n (%)]
| Group | n | Genotype | Allele | |||
|---|---|---|---|---|---|---|
| CC | CG | GG | C | G | ||
| Nondrinker | 516 | 236(45.7) | 222(43.0) | 58(11.3) | 694(67.2) | 338(32.8) |
| Drinker | 514 | 232(45.2) | 234(45.5) | 48(9.3) | 698(67.9) | 330(32.1) |
| χ2 | - | 1.290 | 0.010 | |||
| P | - | 0.525 | 0.752 | |||
Genotypes and serum lipid levels
As shown in Table 3, the levels of TG in nondrinkers were higher in CG genotype than in CC genotype (P < 0.01), and the ratio of ApoA-I to ApoB in nondrinkers was higher in GG genotype than in CG genotype (P < 0.05).
Table 3.
Comparison of serum lipid levels among the genotypes and between the nondrinkers and drinkers
| Group | Genotype | n | TC (mmol/L) |
TG (mmol/L) |
HDL-C (mmol/L) |
LDL-C (mmol/L) |
ApoA-I (g/L) |
ApoB (g/L) |
ApoA-I/ ApoB |
|---|---|---|---|---|---|---|---|---|---|
| Nondrinker | CC | 236 | 4.49 ± 0.88 | 0.91 ± 0.57 | 1.97 ± 0.42 | 2.41 ± 0.69 | 1.40 ± 0.13 | 0.89 ± 0.21 | 1.70 ± 0.64 |
| CG | 222 | 4.53 ± 1.11 | 1.01 ± 0.59b | 1.98 ± 0.48 | 2.40 ± 0.68 | 1.40 ± 0.18 | 0.91 ± 0.22 | 1.62 ± 0.41 | |
| GG | 58 | 4.57 ± 1.03 | 0.93 ± 0.60 | 2.01 ± 0.43 | 2.40 ± 0.83 | 1.42 ± 0.12 | 0.86 ± 0.28 | 1.84 ± 0.75c | |
| F | - | - | 0.186 | 12.517 | 0.187 | 0.013 | 0.445 | 1.275 | 3.685 |
| P | - | - | 0.830 | 0.002 | 0.829 | 0.987 | 0.641 | 0.280 | 0.026 |
| Drinker | CC | 232 | 4.72 ± 0.90** | 0.97 ± 0.63** | 2.07 ± 0.45* | 2.38 ± 0.71 | 1.47 ± 0.14** | 0.91 ± 0.20 | 1.70 ± 0.46 |
| CG | 234 | 4.75 ± 0.86* | 0.95 ± 0.54 | 2.23 ± 0.53b** | 2.37 ± 0.66 | 1.49 ± 0.13** | 0.91 ± 0.20 | 1.73 ± 0.47** | |
| GG | 48 | 5.29 ± 0.91bd** | 1.28 ± 0.93bd** | 2.12 ± 0.49 | 2.77 ± 0.72bd* | 1.49 ± 0.14** | 1.06 ± 0.18bd** | 1.44 ± 0.30bd** | |
| F | - | - | 8.662 | 18.352 | 6.253 | 7.173 | 1.382 | 12.457 | 8.300 |
| P | - | - | 0.000 | 0.000 | 0.002 | 0.000 | 0.252 | 0.000 | 0.000 |
TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ApoA-I, apolipoprotein A-I; ApoB, apolipoprotein B. The value of TG was presented as median (interquartile range). The difference among the genotypes was determined by the Kruskal-Wallis test. aP < 0.05 and bP < 0.01 in comparison with CC genotype of the same subgroup;cP < 0.05 and dP < 0.01 in comparison with CG genotype of the same subgroup;*P < 0.05 and **P < 0.01 in comparison with the same subgroup of the nondrinkers.
The levels of TC, TG, LDL-C and ApoB in drinkers were higher in GG genotype than in CC or CG genotype (P < 0.01 for all). The levels of HDL-C in drinkers were higher in CG genotype than in CC genotype (P < 0.01). The ratio of ApoA-I to ApoB in drinkers was lower in GG genotype than in CC or CG genotype (P < 0.01 for each).
Serum TC, TG, HDL-C and ApoA-I levels in CC genotype, TC, HDL-C, ApoA-I levels and the ratio of ApoA-I to ApoB in CG genotype, and TC, TG, LDL-C, ApoA-I and ApoB levels in GG genotype were higher in drinkers than in nondrinkers (P < 0.05-0.01). But the ratio of ApoA-I to ApoB in GG genotype was lower in drinkers than in nondrinkers (P < 0.01).
Correlation between genotype and serum lipid parameters
Multivariate logistic regression analysis showed that the levels of TC, TG LDL-C and ApoA-I were correlated with genotype in the combined population (P < 0.05-0.01). The levels of TC, TG and ApoB were correlated with genotype in nondrinkers (P < 0.05 for all). The levels of TC, LDL-C and ApoB were associated with genotype in drinkers (P < 0.01 for all). Serum lipid parameters were also correlated with age, sex, alcohol consumption, cigarette smoking, blood pressure, body weight, and BMI in both nondrinkers and drinkers (Table 4).
Table 4.
Correlative factors for the serum lipid parameters between the nondrinkers and drinkers
| Lipid parameter | Risk factor | Odds ratio | χ2 | P | 95% CI |
|---|---|---|---|---|---|
| Nondrinkers plus drinkers | |||||
| TC | Age | 1.030 | 45.053 | 0.000 | 1.021-1.039 |
| Body mass index | 1.144 | 22.000 | 0.000 | 1.081-1.210 | |
| ApoC-III 3238C>G genotype | 1.324 | 6.542 | 0.011 | 1.068-1.642 | |
| TG | Body mass index | 1.223 | 30.735 | 0.000 | 1.139-1.314 |
| Pulse pressure | 1.018 | 5.255 | 0.022 | 1.003-1.034 | |
| Alcohol consumption | 1.744 | 14.249 | 0.000 | 1.307-2.329 | |
| Cigarette smoking | 0.614 | 4.056 | 0.044 | 0.382-0.987 | |
| ApoC-III 3238C>G genotype | 1.517 | 8.448 | 0.004 | 1.145-2.009 | |
| LDL-C | Age | 1.030 | 14.365 | 0.000 | 1.014-1.046 |
| Sex | 1.898 | 5.549 | 0.018 | 1.114-3.236 | |
| Body weight | 1.107 | 43.419 | 0.000 | 1.074-1.141 | |
| Alcohol consumption | 0.704 | 4.248 | 0.039 | 0.504-0.983 | |
| ApoC-III 3238C>G genotype | 1.709 | 9.600 | 0.002 | 1.218-2.400 | |
| ApoA-I | Age | 0.745 | 12.294 | 0.000 | 0.632-0.878 |
| Sex | 0.414 | 9.812 | 0.002 | 0.238-0.719 | |
| Pulse pressure | 0.974 | 4.009 | 0.045 | 0.949-0.999 | |
| Alcohol consumption | 0.327 | 12.749 | 0.000 | 0.177-0.604 | |
| ApoB | Age | 1.036 | 38.386 | 0.000 | 1.025-1.048 |
| Body weight | 1.086 | 45.102 | 0.000 | 1.060-1.113 | |
| Alcohol consumption | 3.154 | 7.351 | 0.007 | 1.375-7.234 | |
| ApoC-III 3238C>G genotype | 1.678 | 15.923 | 0.000 | 1.301-2.163 | |
| Sex | 1.678 | 3.923 | 0.048 | 1.004-2.219 | |
| Nondrinkers | |||||
| TC | Age | 1.142 | 13.511 | 0.000 | 1.064-1.225 |
| Body mass index | 1.175 | 14.633 | 0.000 | 1.082-1.277 | |
| ApoC-III 3238C>G genotype | 1.324 | 6.542 | 0.011 | 1.068-1.642 | |
| Cigarette smoking | 0.639 | 4.668 | 0.031 | 0.425-0.959 | |
| TG | Body mass index | 1.163 | 7.954 | 0.005 | 1.047-1.292 |
| Age | 1.314 | 9.279 | 0.002 | 1.102-1.566 | |
| Cigarette smoking | 0.280 | 5.302 | 0.021 | 0.095-0.827 | |
| ApoC-III 3238C>G genotype | 2.200 | 12.048 | 0.044 | 1.410-3.434 | |
| LDL-C | Age | 1.039 | 13.847 | 0.000 | 1.018-1.060 |
| Body weight | 1.131 | 34.897 | 0.000 | 1.086-1.178 | |
| Sex | 3.379 | 9.542 | 0.002 | 1.561-7.317 | |
| ApoA-I | Body weight | 1.061 | 4.339 | 0.037 | 1.004-1.122 |
| Body mass index | 0.671 | 14.044 | 0.000 | 0.545-0.827 | |
| Age | 0.733 | 11.783 | 0.001 | 0.614-0.875 | |
| Cigarette smoking | 1.667 | 4.219 | 0.040 | 1.024-2.713 | |
| ApoB | Age | 1.143 | 11.084 | 0.001 | 1.057-1.237 |
| Body weight | 1.084 | 25.440 | 0.000 | 1.051-1.119 | |
| ApoC-III 3238C>G genotype | 1.574 | 6.223 | 0.013 | 1.102-2.248 | |
| Drinkers | |||||
| TC | Age | 1.326 | 15.892 | 0.000 | 1.154-1.523 |
| Body weight | 1.052 | 12.961 | 0.000 | 1.023-1.082 | |
| ApoC-III 3238C>G genotype | 1.874 | 16.157 | 0.000 | 1.380-2.545 | |
| TG | Alcohol consumption | 0.674 | 10.599 | 0.001 | 0.532-0.855 |
| Body mass index | 1.270 | 23.776 | 0.000 | 1.154-1.399 | |
| LDL-C | Height | 1.063 | 7.372 | 0.007 | 1.017-1.112 |
| ApoC-III 3238C>G genotype | 2.319 | 10.577 | 0.001 | 1.397-3.849 | |
| ApoA-I | Body weight | 1.078 | 6.384 | 0.037 | 1.017-1.143 |
| Systolic blood pressure | 0.942 | 7.787 | 0.005 | 0.903-0.982 | |
| ApoB | Age | 1.040 | 17.141 | 0.000 | 1.021-1.060 |
| Body weight | 1.084 | 22.706 | 0.000 | 1.049-1.121 | |
| Pulse pressure | 0.974 | 5.365 | 0.021 | 0.952-0.996 | |
| Alcohol consumption | 0.360 | 13.214 | 0.000 | 0.207-0.624 | |
| ApoC-III 3238C>G genotype | 1.863 | 11.039 | 0.001 | 1.291-2.689 |
TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; ApoA-I, apolipoprotein A-I; ApoB, apolipoprotein B; CI, confidence interval
Discussion
The results of the present study show that the levels of TC, TG, HDL-C, ApoA-I and ApoB were higher in drinkers than in nondrinkers. There was no significant difference in the levels of LDL-C and the ratio of ApoA-I to ApoB between the two groups. These findings are consistent with those of several previous studies. A moderate intake of alcohol is associated with protection against CAD, probably due in part to a dose-dependent increase in HDL-C [65,66]. According to Rimma et al. [67], a daily dose of 30 g alcohol results in an average HDL level rise of 3.99 mg/dl, and an ApoA-I level rise of 8.82 mg/dl. Alcohol also causes an increase of TG lipase activity and a decrease of the HDL removal from the circulation [68]. A decrease in LDL-C with increased alcohol intake has also been reported in some studies, but this effect is less consistent and probably depends on the combination of one or more unmeasured factors [68].
The present study shows that there was no significant difference in the allelic and genotypic frequencies of the ApoC-III 3238C>G between the nondrinkers and drinkers. The frequency of G allele was 32.8% in nondrinkers, and 32.1% in drinkers, which is quite similar to the results in Taiwanese (0.30-0.43) [46,69], Japanese (0.25-0.48) [70], and Indians (0.36) [27], but is higher than those reported for Caucasians in whom the G allele frequency was 0.00-0.11 [27,55]. These results suggest that there exists significant racial variation of allele frequencies in this locus.
The relationship between the ApoC-III 3238C>G polymorphism and plasma or serum lipid levels in humans has been evaluated in a large number of studies. However, previous findings on the association of this polymorphism with the changes in plasma lipid levels are inconsistent [71-73]. Previous cohort studies, as well as case-control and familial studies have shown significant association between the rare allele of the polymorphic SstI site (3238G) and higher plasma TG levels [16-53] and CAD [53-62]. This association has been reported in studies carried out with Caucasians, Chinese, Mayans, Japanese (living in Japan or living in Southern Brazil), Koreans, Arabs, and Asian Indians [27,49,55,69,70]. However, several reports failed to find a significant genetic effect on TG concentrations [71-73]. In a previous work, Kee and coworkers found no association between variability at the SstI ApoC-III gene site (in the 3%-noncoding region) and lipid, lipoproteins and complex lipoprotein particles in a sample of men from northern France [72]. They thought that the SstI polymorphism is not major contributors to the risk of dyslipidemia in the population of northern France. Results from the current study are consistent with many studies cited above which reported associations of ApoC-III gene polymorphism with altered lipid metabolism. The levels of TG in nondrinkers were higher in CG genotype than in CC genotype, and the ratio of ApoA-I to ApoB in nondrinkers was higher in GG genotype than in CG genotype. The levels of TC, TG, LDL-C and ApoB in drinkers were higher in GG genotype than in CC or CG genotype. The levels of HDL-C in drinkers were higher in CG genotype than in CC genotype. The ratio of ApoA-I to ApoB in drinkers was lower in GG genotype than in CC or CG genotype.
The interactions of the ApoC-III 3238C>G polymorphism and alcohol consumption on serum lipid levels are not well known. In the present study, we showed that serum TC, TG, HDL-C and ApoA-I levels in CC genotype, TC, HDL-C, ApoA-I levels and the ratio of ApoA-I to ApoB in CG genotype, and TC, TG, LDL-C, ApoA-I and ApoB levels in GG genotype were higher in drinkers than in nondrinkers. But the ratio of ApoA-I to ApoB in GG genotype was lower in drinkers than in nondrinkers. The levels of TG were correlated with genotype in nondrinkers, whereas the levels of TG were positively associated with alcohol consumption in drinkers. These findings suggest that the ApoC-III 3238CG heterozygotes benefited more from alcohol consumption than CC and GG homozygotes in increasing serum levels of HDL-C, ApoA-I, and the ratio of ApoA-I to ApoB, and lowering serum levels of TC and TG. The effect of different kinds of wine on the lipid profiles is not well known. In a previous study, Ruidavets et al. [74] found that wine was positively associated with HDL-C. Beer was positively associated with HDL-C in men and with TGs in men and women. When taking drinking patterns into account, wine drinkers had higher HDL-C levels than non-wine drinkers. In another study, Choudhury et al. [75] also showed serum TGs levels were significantly lower in those who drank beer. Thus, we hypothesize that the interactions between the ApoC-III 3238C>G polymorphism and different kinds of alcoholic beverage on serum lipid levels may be different.
Conclusion
The results of the present study show that there was no significant difference in genotypic and allelic frequencies of the ApoC-III 3238C>G polymorphism between the nondrinkers and drinkers. But the interactions of the ApoC-III 3238C>G polymorphism and alcohol consumption on serum lipid levels are different among the three genotypes. The ApoC-III 3238CG heterozygotes benefited more from alcohol consumption than CC and GG homozygotes in increasing serum levels of HDL-C, ApoA-I, and the ratio of ApoA-I to ApoB, and lowering serum levels of TC and TG.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
YR and LY conceived the study, participated in the design, carried out the epidemiological survey, collected the samples, performed the statistical analyses, and drafted the manuscript; LM, LK, LX, ZL and LW carried out the biochemical analysis; WJ, YD and LW carried out the epidemiological survey, collected the samples, and helped to carry out the genotyping. All authors read and approved the final manuscript.
Contributor Information
Yin Ruixing, Email: yinruixing@yahoo.com.cn.
Li Yiyang, Email: liyiyang721@yahoo.com.cn.
Li Meng, Email: yimongxi@yahoo.com.cn.
Li Kela, Email: likela@126.com.
Long Xingjiang, Email: 511052795@qq.com.
Zhang Lin, Email: bugemiulin@sina.com.
Liu Wanying, Email: liuwanying1224@yahoo.com.cn.
Wu Jinzhen, Email: yinrx2003@yahoo.com.cn.
Yang Dezhai, Email: ydz678@163.com.
Lin Weixiong, Email: lin78018@yahoo.com.cn.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 30360038).
References
- Austin MA. Plasma triglyceride as a risk factor for coronary heart disease. The epidemiologic evidence and beyond. Am J Epidemiol. 1989;129:249–59. doi: 10.1093/oxfordjournals.aje.a115130. [DOI] [PubMed] [Google Scholar]
- Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–9. doi: 10.1097/00043798-199604000-00014. [DOI] [PubMed] [Google Scholar]
- Talmud PJ, Hawe E, Miller GJ, Humphries SE. Nonfasting apolipoprotein B and triglyceride levels as a useful predictor of coronary heart disease risk in middle-aged UK men. Arterioscler Thromb Vasc Biol. 2002;22:1918–23. doi: 10.1161/01.ATV.0000035521.22199.C7. [DOI] [PubMed] [Google Scholar]
- Arai H, Yamamoto A, Matsuzawa Y, Saito Y, Yamada N, Oikawa S, Mabuchi H, Teramoto T, Sasaki J, Nakaya N, Itakura H, Ishikawa Y, Ouchi Y, Horibe H. Serum lipid survey and its recent trend in the general Japanese population in 2000. J Atheroscler Thromb. 2005;12:98–106. doi: 10.5551/jat.12.98. [DOI] [PubMed] [Google Scholar]
- Satoh H, Nishino T, Tomita K, Tsutsui H. Fasting triglyceride is a significant risk factor for coronary artery disease in middle-aged Japanese men: Results from a 10-year cohort study. Circ J. 2006;70:227–31. doi: 10.1253/circj.70.227. [DOI] [PubMed] [Google Scholar]
- Wu LL. Review of risk factors for cardiovascular diseases. Ann Clin Lab Sci. 1999;29:127–33. [PubMed] [Google Scholar]
- You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1–G6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- Castelli WP, Doyle JT, Gordon T, Hames CG, Hjortland MC, Hulley SB, Kagan A, Zukel WJ. Alcohol and blood lipids. The cooperative lipoprotein phenotyping study. Lancet. 1977;2:153–5. doi: 10.1016/S0140-6736(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Ruixing Y, Shangling P, Hong C, Hanjun Y, Hai W, Yuming C, Jinzhen W, Feng H, Meng L, Muyan L. Diet, alcohol consumption, and serum lipid levels of the middle-aged and elderly in the Guangxi Bai Ku Yao and Han populations. Alcohol. 2008;42:219–29. doi: 10.1016/j.alcohol.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A prospective study of trigliceryde level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882–8. doi: 10.1001/jama.276.11.882. [DOI] [PubMed] [Google Scholar]
- Wang C, McConathy WJ, Kloer HU, Alaupovic P. Modulation of lipoprotein lipase activity by apolipoproteins. J Clin Invest. 1985;75:384–90. doi: 10.1172/JCI111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le NA, Gibson JC, Ginsberg HN. Independent regulation of plasma apolipoprotein C-II and C-III concentrations in very low density and high density lipoproteins: implications for the regulation of the catabolism of these lipoproteins. J Lipid Res. 1988;29:669–77. [PubMed] [Google Scholar]
- Ito Y, Azrolan N, O'Connell A, Walsh A, Breslow JL. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 1990;249:790–3. doi: 10.1126/science.2167514. [DOI] [PubMed] [Google Scholar]
- Bruns GA, Karanthasis SK, Breslow JL. Human apolipoprotein AI-CIII gene complex is located on chromosome 11. Arteriosclerosis. 1984;4:97–102. doi: 10.1161/01.atv.4.2.97. [DOI] [PubMed] [Google Scholar]
- Talmud PJ, Humphries SE. Apolipoprotein C-III gene variation and dyslipidaemia. Curr Opin Lipidol. 1997;8:154–8. doi: 10.1097/00041433-199706000-00005. [DOI] [PubMed] [Google Scholar]
- Hegele RA, Connelly PW, Hanley AJ, Sun F, Harris SB, Zinman B. Common genomic variation in the APOC3 promoter associated with variation in plasma lipoproteins. Arterioscler Thromb Vasc Biol. 1997;17:2753–8. doi: 10.1161/01.atv.17.11.2753. [DOI] [PubMed] [Google Scholar]
- Ordovas JM, Civeira F, Genest J Jr, Craig S, Robbins AH, Meade T, Pocovi M, Frossard PM, Masharani U, Wilson PW. Restriction fragment length polymorphisms of the apolipoprotein A-I, C-III, A-IV gene locus. Relationships with lipids, apolipoproteins, and premature coronary artery disease. Atherosclerosis. 1991;87:75–86. doi: 10.1016/0021-9150(91)90234-T. [DOI] [PubMed] [Google Scholar]
- Rees A, Stocks J, Paul H, Ohuchi Y, Galton D. Haplotypes identified by DNA polymorphisms at the apolipoprotein A-1 and C-III loci and hypertriglyceridaemia. A study in a Japanese population. Hum Genet. 1986;72:168–71. doi: 10.1007/BF00283939. [DOI] [PubMed] [Google Scholar]
- Hong SH, Park WH, Lee CC, Song JH, Kim JQ. Association between genetic variations of apo AI-CIII-AIV cluster gene and hypertriglyceridemic subjects. Clin Chem. 1997;43:13–7. [PubMed] [Google Scholar]
- Ferrell RE, Kamboh MI, Majumder PP, Valdez R, Weiss KM. Genetic studies of human apolipoproteins. XIII. Quantitative polymorphism of apolipoprotein C-III in the Mayans of the Yucatan Peninsula. Hum Hered. 1990;40:127–35. doi: 10.1159/000153919. [DOI] [PubMed] [Google Scholar]
- Dallongeville J, Meirhaeghe A, Cottel D, Fruchart JC, Amouyel P, Helbecque N. Gender related association between genetic variations of APOC-III gene and lipid and lipoprotein variables in northern France. Atherosclerosis. 2000;150:149–57. doi: 10.1016/S0021-9150(99)00362-7. [DOI] [PubMed] [Google Scholar]
- Wu JH, Kao JT, Wen MS, Lo SK. DNA polymorphisms at the apolipoprotein A1-CIII loci in Taiwanese: correlation of plasma APOCIII with triglyceride level and body mass index. J Formos Med Assoc. 2000;99:367–74. [PubMed] [Google Scholar]
- Waterworth DM, Talmud PJ, Bujac SR, Fisher RM, Miller GJ, Humphries SE. Contribution of apolipoprotein C-III gene variants to determination of triglyceride levels and interaction with smoking in middle-aged men. Arterioscler Thromb Vasc Biol. 2000;20:2663–9. doi: 10.1161/01.atv.20.12.2663. [DOI] [PubMed] [Google Scholar]
- Waterworth DM, Talmud PJ, Humphries SE, Wicks PD, Sagnella GA, Strazzullo P, Alberti KG, Cook DG, Cappuccio FP. Variable effects of the APOC3-482C > T variant on insulin, glucose and triglyceride concentrations in different ethnic groups. Diabetologia. 2001;44:245–8. doi: 10.1007/s001250051607. [DOI] [PubMed] [Google Scholar]
- Buzza M, Fripp Y, Mitchell RJ. Apolipoprotein AI and CIII gene polymorphisms and their association with lipid levels in Italian, Greek and Anglo-Irish populations of Australia. Ann Hum Biol. 2001;28:481–90. doi: 10.1080/03014460010019777. [DOI] [PubMed] [Google Scholar]
- Corella D, Guillén M, Sáiz C, Portolés O, Sabater A, Folch J, Ordovas JM. Associations of LPL and APOC3 gene polymorphisms on plasma lipids in a Mediterranean population: interaction with tobacco smoking and the APOE locus. J Lipid Res. 2002;43:416–27. [PubMed] [Google Scholar]
- Chhabra S, Narang R, Krishnan LR, Vasisht S, Agarwal DP, Srivastava LM, Manchanda SC, Das N. Apolipoprotein C3 SstI polymorphism and triglyceride levels in Asian Indians. BMC Genet. 2002;3:9. doi: 10.1186/1471-2156-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo E, González-Lamuño D, Ruiz JC, Fernández-Fresnedo G, Isla D, González-Cotorruelo J, Zubimendi JA, De Francisco AL, García-Fuentes M, Arias M. Apolipoprotein C-III and E polymorphisms and cardiovascular syndrome, hyperlipidemia, and insulin resistance in renal transplantation. Am J Transplant. 2002;2:343–8. doi: 10.1034/j.1600-6143.2002.20409.x. [DOI] [PubMed] [Google Scholar]
- Minihane AM, Finnegan YE, Talmud P, Leigh-Firbank EC, Williams CM. Influence of the APOC3 -2854T>G polymorphism on plasma lipid levels: effect of age and gender. Biochim Biophys Acta. 2002;1583:311–4. doi: 10.1016/s1388-1981(02)00264-0. [DOI] [PubMed] [Google Scholar]
- Humphries SE, Berglund L, Isasi CR, Otvos JD, Kaluski D, Deckelbaum RJ, Shea S, Talmud PJ. Loci for CETP, LPL, LIPC, and APOC3 affect plasma lipoprotein size and sub-population distribution in Hispanic and non-Hispanic white subjects: the Columbia University BioMarkers Study. Nutr Metab Cardiovasc Dis. 2002;12:163–72. [PubMed] [Google Scholar]
- Couillard C, Vohl MC, Engert JC, Lemieux I, Houde A, Almeras N, Prud'homme D, Nadeau A, Despres JP, Bergeron J. Effect of apoC-III gene polymorphisms on the lipoprotein-lipid profile of viscerally obese men. J Lipid Res. 2003;44:986–93. doi: 10.1194/jlr.M300043-JLR200. [DOI] [PubMed] [Google Scholar]
- Espino-Montoro A, Barrios-Artillo M, López-Chozas JM, Cayuela A, Stiefel P, Villar J. Influence of polymorphism (RFLP-sstI) at the apolipoprotein C-III gene locus on the lipoprotein metabolism and insulin resistance in essential hypertensive patients. Interaction between gender and genetic polymorphism. Nutr Metab Cardiovasc Dis. 2003;13:194–201. doi: 10.1016/S0939-4753(03)80011-X. [DOI] [PubMed] [Google Scholar]
- Surguchov AP, Page GP, Smith L, Patsch W, Boerwinkle E. Polymorphic markers in apolipoprotein C-III gene flanking regions and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 1996;16:941–7. doi: 10.1161/01.atv.16.8.941. [DOI] [PubMed] [Google Scholar]
- Li GP, Wang JY, Yan SK, Chen BS, Xue H, Wu G. Genetic effect of two polymorphisms in the apolipoprotein A5 gene and apolipoprotein C3 gene on serum lipids and lipoproteins levels in a Chinese population. Clin Genet. 2004;65:470–6. doi: 10.1111/j.1399-0004.2004.00251.x. [DOI] [PubMed] [Google Scholar]
- Garenc C, Aubert S, Laroche J, Girouard J, Vohl MC, Bergeron J, Rousseau F, Julien P. Population prevalence of APOE, APOC3 and PPAR-alpha mutations associated to hypertriglyceridemia in French Canadians. J Hum Genet. 2004;49:691–700. doi: 10.1007/s10038-004-0208-6. [DOI] [PubMed] [Google Scholar]
- Guettier JM, Georgopoulos A, Tsai MY, Radha V, Shanthirani S, Deepa R, Gross M, Rao G, Mohan V. Polymorphisms in the fatty acid-binding protein 2 and apolipoprotein C-III genes are associated with the metabolic syndrome and dyslipidemia in a South Indian population. J Clin Endocrinol Metab. 2005;90:1705–11. doi: 10.1210/jc.2004-1338. [DOI] [PubMed] [Google Scholar]
- Garenc C, Couillard C, Laflamme N, Cadelis F, Gagné C, Couture P, Julien P, Bergeron J. Effect of the APOC3 Sst I SNP on fasting triglyceride levels in men heterozygous for the LPL P207L deficiency. Eur J Hum Genet. 2005;13:1159–65. doi: 10.1038/sj.ejhg.5201469. [DOI] [PubMed] [Google Scholar]
- Ruiz-Narváez EA, Yang Y, Nakanishi Y, Kirchdorfer J, Campos H. APOC3/A5 haplotypes, lipid levels, and risk of myocardial infarction in the Central Valley of Costa Rica. J Lipid Res. 2005;46:2605–13. doi: 10.1194/jlr.M500040-JLR200. [DOI] [PubMed] [Google Scholar]
- Herron KL, Lofgren IE, Adiconis X, Ordovas JM, Fernandez ML. Associations between plasma lipid parameters and APOC3 and APOA4 genotypes in a healthy population are independent of dietary cholesterol intake. Atherosclerosis. 2006;184:113–20. doi: 10.1016/j.atherosclerosis.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Huang MC, Wang TN, Liu YL, Pa TH, Tu HP, Huang YC, Chang WT, Ko YC. Effect of SstI polymorphism of the apolipoprotein CIII gene and environmental factors on risks of hypertriglyceridemia in Taiwan aborigines. Circ J. 2006;70:1030–6. doi: 10.1253/circj.70.1030. [DOI] [PubMed] [Google Scholar]
- Hallman DM, Srinivasan SR, Chen W, Boerwinkle E, Berenson GS. Longitudinal analysis of haplotypes and polymorphisms of the APOA5 and APOC3 genes associated with variation in serum triglyceride levels: the Bogalusa Heart Study. Metabolism. 2006;55:1574–81. doi: 10.1016/j.metabol.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Dorfmeister B, Cooper JA, Stephens JW, Ireland H, Hurel SJ, Humphries SE, Talmud PJ. The effect of APOA5 and APOC3 variants on lipid parameters in European Whites, Indian Asians and Afro-Caribbeans with type 2 diabetes. Biochim Biophys Acta. 2007;1772:355–63. doi: 10.1016/j.bbadis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Fiegenbaum M, de Andrade FM, Hutz MH. Association between plasma lipid parameters and APOC3 genotypes in Brazilian subjects: effect of gender, smoking and APOE genotypes. Clin Chim Acta. 2007;380:175–81. doi: 10.1016/j.cca.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Miller M, Rhyne J, Chen H, Beach V, Ericson R, Luthra K, Dwivedi M, Misra A. APOC3 promoter polymorphisms C-482T and T-455C are associated with the metabolic syndrome. Arch Med Res. 2007;38:444–51. doi: 10.1016/j.arcmed.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollex RL, Ban MR, Young TK, Bjerregaard P, Anand SS, Yusuf S, Zinman B, Harris SB, Hanley AJ, Connelly PW, Huff MW, Hegele RA. Association between the -455T>C promoter polymorphism of the APOC3 gene and the metabolic syndrome in a multi-ethnic sample. BMC Med Genet. 2007;8:80. doi: 10.1186/1471-2350-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien KL, Fang WH, Wen HC, Lin HP, Lin YL, Lin SW, Wu JH, Kao JT. APOA1/C3/A5 haplotype and risk of hypertriglyceridemia in Taiwanese. Clin Chim Acta. 2008;390:56–62. doi: 10.1016/j.cca.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Bonnet E, Bernard J, Fauvel J, Massip P, Ruidavets JB, Perret B. Association of APOC3 polymorphisms with both dyslipidemia and lipoatrophy in HAART-receiving patients. AIDS Res Hum Retroviruses. 2008;24:169–71. doi: 10.1089/aid.2007.0076. [DOI] [PubMed] [Google Scholar]
- Lahiry P, Ban MR, Pollex RL, Feldman RD, Sawyez CG, Huff MW, Young TK, Bjerregaard P, Hegele RA. Common variants APOC3, APOA5, APOE and PON1 are associated with variation in plasma lipoprotein traits in Greenlanders. Int J Circumpolar Health. 2007;66:390–400. doi: 10.3402/ijch.v66i5.18311. [DOI] [PubMed] [Google Scholar]
- Parzianello L, Oliveira G, Coelho JC. Apolipoprotein CIII polymorphism and triglyceride levels of a Japanese population living in Southern Brazil. Braz J Med Biol Res. 2008;41:462–7. doi: 10.1590/S0100-879X2008005000022. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Osaka M, Tani M, Azuma H, Sacks FM, Shimokado K, Yoshida M. Apolipoprotein CIII links hyperlipidemia with vascular endothelial cell dysfunction. Circulation. 2008;118:731–42. doi: 10.1161/CIRCULATIONAHA.108.784785. [DOI] [PubMed] [Google Scholar]
- Ruiz JR, Labayen I, Ortega FB, Moreno LA, González-Lamuño D, Martí A, Nova E, Fuentes MG, Redondo-Figuero C, Martínez JA, Sjöström M, Castillo MJ. AVENA Study Group. Birth weight and blood lipid levels in Spanish adolescents: influence of selected APOE, APOC3 and PPARgamma2 gene polymorphisms. The AVENA Study. BMC Med Genet. 2008;9:98. doi: 10.1186/1471-2350-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiyang L, Ruixing Y, Meng L, Kela L, Xingjiang L, Lin Z, Wanying L, Shangling P, Dezhai Y, Weixiong L. Apolipoprotein C-III gene polymorphism and several environmental factors with serum lipid levels in the Guangxi Hei Yi Zhuang and Han populations. J Investig Med. 2010;58:777–85. doi: 10.231/JIM.0b013e3181e5e146. [DOI] [PubMed] [Google Scholar]
- Tsai MY, Ordovas JM. APOC3 mutation, serum triglyceride concentrations, and coronary heart disease. Clin Chem. 2009;55:1274–6. doi: 10.1373/clinchem.2009.124669. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Liu Y, Liu BW, Fan P, Cavanna J, Galton DJ. Common genetic variants of lipoprotein lipase and apolipoproteins AI-CIII that relate to coronary artery disease: a study in Chinese and European subjects. Mol Genet Metab. 1998;64:177–83. doi: 10.1006/mgme.1998.2712. [DOI] [PubMed] [Google Scholar]
- Russo GT, Meigs JB, Cupples LA, Demissie S, Otvos JD, Wilson PW, Lahoz C, Cucinotta D, Couture P, Mallory T, Schaefer EJ, Ordovas JM. Association of the Sst-I polymorphism at the APOC3 gene locus with variations in lipid levels, lipoprotein subclass profiles and coronary heart disease risk: the Framingham offspring study. Atherosclerosis. 2001;158:173–81. doi: 10.1016/S0021-9150(01)00409-9. [DOI] [PubMed] [Google Scholar]
- Olivieri O, Stranieri C, Bassi A, Zaia B, Girelli D, Pizzolo F, Trabetti E, Cheng S, Grow MA, Pignatti PF, Corrocher R. ApoC-III gene polymorphisms and risk of coronary artery disease. J Lipid Res. 2002;43:1450–7. doi: 10.1194/jlr.M200145-JLR200. [DOI] [PubMed] [Google Scholar]
- Baroni MG, Berni A, Romeo S, Arca M, Tesorio T, Sorropago G, Di Mario U, Galton DJ. Genetic study of common variants at the Apo E, Apo AI, Apo CIII, Apo B, lipoprotein lipase (LPL) and hepatic lipase (LIPC) genes and coronary artery disease (CAD): variation in LIPC gene associates with clinical outcomes in patients with established CAD. BMC Med Genet. 2003;4:8. doi: 10.1186/1471-2350-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra S, Narang R, Lakshmy R, Vasisht S, Agarwal DP, Srivastava LM, Manchanda SC, Das N. Apolipoprotein C3 SstI polymorphism in the risk assessment of CAD. Mol Cell Biochem. 2004;259:59–66. doi: 10.1023/B:MCBI.0000021345.31556.c9. [DOI] [PubMed] [Google Scholar]
- Payseur BA, Clark AG, Hixson J, Boerwinkle E, Sing CF. Contrasting multi-site genotypic distributions among discordant quantitative phenotypes: the APOA1/C3/A4/A5 gene cluster and cardiovascular disease risk factors. Genet Epidemiol. 2006;30:508–18. doi: 10.1002/gepi.20163. [DOI] [PubMed] [Google Scholar]
- Muendlein A, Saely CH, Marte T, Schmid F, Koch L, Rein P, Langer P, Aczel S, Drexel H. Synergistic effects of the apolipoprotein E epsilon3/epsilon2/epsilon4, the cholesteryl ester transfer protein TaqIB, and the apolipoprotein C3 -482 C>T polymorphisms on their association with coronary artery disease. Atherosclerosis. 2008;199:179–86. doi: 10.1016/j.atherosclerosis.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Ruiz-Narváez EA, Sacks FM, Campos H. Abdominal obesity and hyperglycemia mask the effect of a common APOC3 haplotype on the risk of myocardial infarction. Am J Clin Nutr. 2008;87:1932–8. doi: 10.1093/ajcn/87.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker J, Perumal G, Rao VS, Khadrinarasimhiah NB, John S, Hebbagodi S, Mukherjee M, Kakkar VV. Genetic studies on the APOA1-C3-A5 gene cluster in Asian Indians with premature coronary artery disease. Lipids Health Dis. 2008;7:33. doi: 10.1186/1476-511X-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruixing Y, Yuming C, Shangling P, Fengping H, Tangwei L, Dezhai Y, Jinzhen W, Limei Y, Weixiong L, Rongshan L, Jiandong H. Effects of demographic, dietary and other lifestyle factors on the prevalence of hyperlipidemia in Guangxi Hei Yi Zhuang and Han populations. Eur J Cardiovasc Prev Rehabil. 2006;13:977–84. doi: 10.1097/01.hjr.0000239476.79428.25. [DOI] [PubMed] [Google Scholar]
- Ruixing Y, Limei Y, Yuming C, Dezhai Y, Weixiong L, Muyan L, Fengping H, Jinzhen W, Guangqing Y, Zhenbiao N. Prevalence, awareness, treatment, control and risk factors of hypertension in the Guangxi Hei Yi Zhuang and Han populations. Hypertens Res. 2006;29:423–32. doi: 10.1291/hypres.29.423. [DOI] [PubMed] [Google Scholar]
- De Oliveira E, Silva ER, Foster D, McGee Harper M, Seidman CE, Smith JD, Breslow JL, Brinton EA. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation. 2000;102:2347–52. doi: 10.1161/01.cir.102.19.2347. [DOI] [PubMed] [Google Scholar]
- Agarwal DP. Cardioprotective effects of light-moderate consumption of alcohol: a review of putative mechanisms. Alcohol Alcohol. 2002;37:409–15. doi: 10.1093/alcalc/37.5.409. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–8. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen MJ, Kesaniemi YA. Effects of alcohol lipoproteins in relation to coronary heart disease. Curr Opin Lipidol. 1995;6:243–50. doi: 10.1097/00041433-199508000-00009. [DOI] [PubMed] [Google Scholar]
- Ko YL, Ko YS, Wu SM, Teng MS, Chen FR, Hsu TS, Chiang CW, Lee YS. Interaction between obesity and genetic polymorphisms in the apolipoprotein CIII gene and lipoprotein lipase gene on the risk of hypertriglyceridemia in Chinese. Hum Genet. 1997;100:327–33. doi: 10.1007/s004390050511. [DOI] [PubMed] [Google Scholar]
- Bai H, Saku K, Liu R, Imamura M, Arakawa K. Association between coronary heart disease and the apolipoprotein A-I/C-III/A-IV complex in a Japanese population. Hum Genet. 1995;95:102–4. doi: 10.1007/BF00225084. [DOI] [PubMed] [Google Scholar]
- Marcil M, Boucher B, Gagné E, Davignon J, Hayden M, Genest J Jr. Lack of association of the apolipoprotein A-I-C-III-A-IV gene XmnI and SstI polymorphisms and of the lipoprotein lipase gene mutations in familial combined hyperlipoproteinemia in French Canadian subjects. J Lipid Res. 1996;37:309–19. [PubMed] [Google Scholar]
- Kee F, Amouyel P, Fumeron F, Arveiler D, Cambou JP, Evans A, Cambien F, Fruchart JC, Ducimetière P, Dallongeville J. Lack of association between genetic variations of apo A-I-C-III-A-IV gene cluster and myocardial infarction in a sample of European male: ECTIM study. Atherosclerosis. 1999;145:187–95. doi: 10.1016/S0021-9150(99)00066-0. [DOI] [PubMed] [Google Scholar]
- Thu NN, Mai TT, Ohmori R, Kuroki M, Chuyen NV, Hung NT, Kawakami M, Kondo K. Plasma triglyceride and HDL-cholesterol concentrations in Vietnamese girls are affected by lipoprotein lipase, but not apolipoprotein CIII polymorphism. J Nutr. 2006;136:1488–92. doi: 10.1093/jn/136.6.1488. [DOI] [PubMed] [Google Scholar]
- Ruidavets JB, Ducimetière P, Arveiler D, Amouyel P, Bingham A, Wagner A, Cottel D, Perret B, Ferrières J. Types of alcoholic beverages and blood lipids in a French population. J Epidemiol Community Health. 2002;56:24–8. doi: 10.1136/jech.56.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury SR, Ueshima H, Kita Y, Kobayashi KM, Okayama A, Yamakawa M, Hirao Y, Ishikawa M, Miyoshi Y. Alcohol intake and serum lipids in a Japanese population. Int J Epidemiol. 1994;23:940–7. doi: 10.1093/ije/23.5.940. [DOI] [PubMed] [Google Scholar]