Abstract
Background
We adapted an event-related brain potential word repetition paradigm, sensitive to early Alzheimer’s disease (AD), for functional MRI (fMRI). We hypothesized that AD would be associated with reduced differential response to new/old congruous words.
Methods
Fifteen mild AD patients (mean age = 72.9) and 15 normal elderly underwent 1.5T fMRI during a semantic category decision task.
Results
We found robust between-groups differences in BOLD response to congruous words. In controls, the New > Old contrast demonstrated larger responses in much of the left-hemisphere (including putative P600 generators: parahippocampal, cingulate, fusiform, perirhinal, middle temporal (MTG) and inferior frontal gyri (IFG)); the Old > New contrast showed modest activation, mainly in right parietal and prefrontal cortex. By contrast, there were relatively few regions of significant New > Old responses in AD patients, mainly in the right-hemisphere, and their Old > New contrast did not demonstrate a right-hemisphere predominance. Across subjects, the spatial extent of New > Old responses in left medial temporal lobe (MTL) correlated with subsequent recall and recognition (r’s ≥ 0.60). In controls, the magnitude of New - Old response in left MTL, fusiform, IFG, MTG, superior temporal and cingulate gyrus correlated with subsequent cued recall and/or recognition (0.51 ≤ r’s ≤ 0.78).
Conclusions
A distributed network of mostly left-hemisphere structures, which are putative P600 generators, appears important for successful verbal encoding (with New > Old responses to congruous words in normal elderly). This network appears dysfunctional in mild AD patients, as reflected in decreased word repetition effects particularly in left association cortex, paralimbic and MTL structures.
Keywords: Memory, semantic, language, medial temporal lobe, cingulate
INTRODUCTION
Most prior fMRI studies of memory encoding in Alzheimer’s disease (AD) have found abnormal medial temporal lobe (MTL) activation, across paradigms using verbal (Remy, Mirrashed, Campbell, & Richter, 2005), picture (Machulda et al., 2003), and face-name associative (Sperling et al., 2003) encoding tasks. In contrast, using word repetition “priming” paradigms, some fMRI studies of verbal memory in AD have found neocortical regions with relatively normal New - Old word differences, e.g. selected portions of left inferior frontal gyrus (IFG) and middle frontal gyrus (MFG) (BA 45/47 and 44/46) (Lustig & Buckner, 2004). This pattern of results is as expected if the MTL is critical for the normal function of explicit/declarative memory circuitry, but the frontal neocortex is either mainly involved in implicit verbal memory processes (e.g. phonological or semantic priming) or its activation is insufficient for normal encoding and explicit learning to occur.
We have adapted an event-related brain potential (ERP) incidental learning paradigm (with cross-modal category-target word associations), shown by our prior ERP studies to be very sensitive to early AD (Olichney et al., 2002; Olichney et al., 2006; Olichney et al., 2008), even at the MCI stage, for functional MRI (fMRI). This paradigm normally produces robust incidental learning of the semantically congruous category-exemplar words, but not of the incongruous target words (Olichney et al., 2000). P600 word repetition effects (with larger P600s to New than Old congruous words) on this paradigm have correlated positively with superior verbal memory abilities. The P600 word repetition effect is either absent or reduced (amplitudes < 2.5 microvolts) in 83% of patients with chronic amnesia (Olichney et al., 2000), 81% of MCI patients who later convert to AD (Olichney et al., 2008), and 91% of patients with mild AD (Olichney et al., 2006). Invasive electrophysiological studies have identified many candidate P600 neural generators, including limbic (hippocampus, parahippocampal gyrus (PHG), paralimbic (cingulate, temporal pole) and association neocortical (fusiform, IFG, middle temporal gyrus (MTG), superior temporal gyrus (STG)) regions (Fernández et al., 1999; Guillem, Rougier & Claverie, 1999; Halgren et al., 1994). Functional imaging studies have shown many of these regions are important for memory retrieval and/or recognition processes (Kahn et al., 2005; Kirchhoff, Wagner, Maril, & Stern, 2000; Wagner et al., 1998a; Wagner, Desmond, Glover & Gabrieli, 1998b; Wagner, 1999). In addition, the association neocortical regions noted above have each been implicated as being involved in semantic processing (Bookheimer, 2002; Chao, Haxby & Martin, 1999).
Our prior ERP studies have shown that when incongruous words are repeated in this paradigm, there is no significant modulation of the P600, but instead a decrement of an earlier component, the N400, which likely reflects a diminished semantic processing load (Olichney et al 2000, Chwilla, Brown & Hagoort, 1995). The amplitude of this distinct word repetition effect has consistently not correlated with memory abilities or subsequent memory for the stimuli (Olichney et al 2000, 2002, and 2008). This effect has been interpreted as reflecting implicit memory processes, most likely semantic “priming” (Olichney et al 2000; Taylor & Olichney 2007).
The main study objective was to define the neuroanatomical structures which mediate the congruous repetition effect (related to learning efficiency and explicit memory) in normal elderly (NE) and how these are impacted by mild AD. We hypothesized, in line with our prior ERP studies, that AD would have diminished New > Old congruous word repetition effects on this fMRI paradigm. Regions of significant inter-group differences in BOLD response may provide potentially useful markers for early AD and memory failure. While the whole brain analyses of the incongruous word repetition effects are beyond the scope of this paper, we have included analyses of these effects within the medial temporal lobe in order to contrast these effects with those elicited by congruous words (which are much more robustly learned by normal elderly). The use of multi-modal stimuli and a semantic judgment task may be advantageous in producing activation of higher association cortex, a predilection site for AD pathology (Arnold, Hyman, Flory, Damasio, & van Hoesen, 1991). Also, because some recent fMRI studies have suggested that delays in the hemodynamic BOLD response may precede the loss or reduction of BOLD responses on cognitive activation paradigms in early-stage AD (Rombouts, Goekoop, Stam, Barkhof, & Scheltens, 2005a), we sought to characterize the time-course of the BOLD response to New and Repeated congruous words in the MTL and other regions of interest (ROIs) (i.e. putative P600 generators) of mild AD, compared to NE.
METHODS
Subjects
Fifteen patients with mild Probable AD (McKhann et al., 1984) (mean age: 72.9 years; education: 14.7 years; mean MMSE = 24.4, range 20-28) and 15 NE (mean age: 68.7 years; education: 15.5 years) were recruited from the Shiley-Marcos ADRC and the San Diego community. There were no significant intergroup differences in age, education or gender (Table 1). Subjects were all right-handed English native speakers. Exclusion criteria included history of other neurological (CNS) or psychiatric disorders; cardiac, respiratory, renal, or hepatic failure; and severe loss of hearing (e.g. use of hearing aid, difficulty hearing conversational speech) or vision (corrected distant visual acuity poorer than 20/50). All subjects gave informed consent prior to their participation. The research protocol was approved by the UCSD Human Research Protection Program and performed in accordance with the 1964 Declaration of Helsinki.
Table 1.
Demographic, behavioral and subsequent memory performance data (mean ± SD).
| Mild AD ( n = 15) |
Normal (n = 15) |
AD vs. Normal (p-value) |
||
|---|---|---|---|---|
| Demographics | Age | 72.9 ± 8.6 | 68.7 ± 12.1 | .28 |
| Education | 14.7 ± 2.3 | 15.5 ± 2.4 | .41 | |
| Gender | 10M,5F | 9M,6F | .71 | |
| Accuracy rate (%) | Congruous | 88.5 ± 8.0 | 96.6 ± 3.2 | .001* |
| Incongruous | 87.7 ± 12 | 98.8 ± 1.4 | .001* | |
| RT (ms) | Congruous-New | 1181 ± 266 | 1149 ± 287 | .75 |
| Congruous-Old | 986 ± 238 | 742 ± 221 | .007* | |
| Incongruous-New | 1499 ± 600 | 1243 ± 275 | .15 | |
| Incongruous-Old | 1273 ± 479 | 995 ± 268 | .06 | |
| RT Priming (New – Old, ms) |
Congruous | 195 ± 187a | 407 ± 171c | .003* |
| Incongruous | 226 ± 170b | 248 ± 147d | .71 | |
| Subsequent memory scores | Free Recall (Total count) | 0.5 ± 0.9 | 13.9 ± 5.3 | < .0001* |
| Cued Recall – Cong (%) | 17 ± 14 | 85 ± 11 | < .0001* | |
| Cued Recall – Incong (%) | 0.0 ± 0.0 | 1.7 ± 4.4 | .16 | |
| Recognition – Cong (%) | 39 ± 17 | 94 ± 6.7 | < .0001* | |
| Recognition – Incong (%) | 0.9 ± 3.3 | 63 ± 30 | < .0001* |
Legend: RT = Response time; Cong = Congruous; Incong = Incongrous
p< 0.05 (t-tests or chi-square);
Paired t-tests (within-group, 2-tailed):
Congruous New vs. Congruous Old in AD: t = 4.0, p = .001
Incongruous New vs. Incongruous Old in AD: t = 5.0, p = .0003
Congruous New vs. Congruous Old in Normal: t = 9.3, p < .0001
Incongruous New vs. Incongruous Old in Normal: t = 6.5, p < .0001
Materials and procedure
A set of 72 stimuli was constructed (144 trials total, including repetitions), each consisting of a unique short auditory category statement followed by a visual target word (noun), half of which were semantically congruous (e.g. “Part of the face – CHEEKS”) and half of which were incongruous (e.g. “A citrus fruit – PORT”).
Subjects were briefly trained on a semantic category decision task outside of the scanner until reliable performance was demonstrated. The task was to indicate whether a visual target word belonged to an auditorily stated category. Auditory category statements were presented via noise-attenuating headphones, and projected visual stimuli were viewed through a mirror (visual angle ~ 0.5°). Responses were made with a two-button mouse placed in the dominant/right hand and response time (RT) data recorded. On each trial, a fixation crosshair and an auditory category statement (total duration = 3 s, including inter-stimulus interval) were presented together, followed by a visual target word (duration =500 ms). Variable inter-trial intervals (5, 10, 12.5 and 15 s) were used (see Supplemental Fig. 1 for an illustration of single trial timing). Stimuli were presented in 6 runs of 24 trials (12 new and 12 repeated items; all repetitions occurred within runs), each run lasting 265 seconds (106 TRs). The lag between repetition of items was, on average, 93 s (range: 15-178 s). Runs 1, 3, and 5 consisted of 5/6 congruous items and 1/6 incongruous items. Runs 2, 4, and 6 consisted of 5/6 incongruous items and 1/6 congruous items. Therefore, across runs, 50% of trials were congruous and 50% were incongruous. Immediately following the MRI session, participants were given unanticipated tests of free recall, cued recall, and multiple-choice recognition, in that order (Olichney et al., in press). In the cued-recall task, participants were given a list of category statements and asked to fill in the associated target words seen earlier (regardless of congruity). The multiple-choice recognition task consisted of category statements, each with six possible completions (four congruous, two incongruous; chance performance = 16.7%). The cued-recall and multiple-choice questionnaires were weighted towards congruous trials (35 congruous and 8 incongruous items; maximum score = 43).
Imaging methods and analysis
Image acquisition
Imaging was performed on a 1.5 T Siemens MRI scanner. High-resolution (1×1×1 mm3) T1-weighted anatomical images of the entire brain were acquired (180 sagittal slices, 1 mm thickness, TR = 11.4 ms, TE = 4.4 ms, flip angle = 10°, FOV = 256 mm). This sequence provided high-resolution (1 mm × 1 mm × 1 mm) T1 – weighted images of the entire brain. BOLD response was assessed with T2*-weighted gradient-echo planar imaging (EPI) sequences (29 axial slices, 4 mm thickness, 4 × 4 mm in-plane resolution, TR=2.5 sec, TE=32 ms, flip angle=90°, FOV=256 mm). For each functional run, 106 repetitions were performed which resulted in time series fMRI data for the entire bilateral cerebral hemispheres, most of the cerebellum and brainstem.
Individual subject data analyses
The functional and structural MRI data processing and analyses were performed primarily with the AFNI software package (Cox, 1996), for details, see Olichney et al., in press. Functional image runs were analyzed in an event-related manner. Timepoints with large head movements not correctable or containing scanner artifacts were censored from the analyses (19.5% of timepoints in NE, 22.7% in AD; t=1.19 p=0.24). Small head movements were corrected for in each functional run, with AFNI program “3dVolreg”, which registers all brain volumes to a reference volume, chosen to minimize the total correction (Cox, http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dvolreg.html). The average linear displacement (x, y, z) and rotation (pitch, roll, yaw) were estimated at 0.16 mm and 0.15° relative to the reference volume in the non-censored time points. Multiple linear regression analysis with stick-function (square wave with duration=1 TR or 2.5 s) regressors was performed on the motion-corrected concatenated time series data, with BOLD signal intensity as the dependent variable, predicted by the independent effects of four experimental conditions (Congruous-New, Congruous-Old, Incongruous-New, Incongruous-Old) and by the residual motion estimates in six orthogonal planes (3 linear, 3 rotational). This analysis produced functional activation maps for all four conditions as well as for two contrasts (New vs. Old Congruous; New vs. Old Incongruous) for each timepoint. In this paper, we primarily report the results for the congruous trials (New and Old trials separately, and the New vs. Old contrast), focusing on the congruous word repetition effects and their relationship to behavior, especially declarative memory. Further analyses were conducted at 3-TR (7.5 s) and 4-TR (10 s) after the onset of the trial to capture the rising/peak (~4.5 s after visual target word onset) and falling (~7 s after target word onset) phases, respectively, of the BOLD response to the visual target word onset. The Hemodynamic Response Function (HRF) for each voxel by experimental condition was also estimated using stick-function references at timepoints 0- through 5-TR (0-12.5 s post-trial onset). HRFs shown here are averaged across subjects within a region-of-interest (ROI).
Group analyses
Individual subject maps were smoothed (isotropic Gaussian kernel, full-width half-maximum (FWHM) =4 mm) and spatially transformed into standardized anatomical coordinates (Talairach & Tournoux, 1988). Data were analyzed for the patient and normal groups separately. Statistical maps (t-tests) were generated, thresholded at p< 0.025 (one-tailed), to evaluate the neural response to each condition relative to baseline, and to evaluate the repetition effects (New vs. Old contrasts). For between group comparisons, t-tests were conducted which compared the beta estimates for New-Old words in each voxel between the AD and NE subjects. For the whole brain analyses, clusters of ≥ 12 adjacent voxels were considered significant (whole brain α < 0.05 with Monte Carlo Simulation; connectivity radius = 5.66 mm, 23,660 voxels in whole brain mask). For voxels active in both 3-TR and 4-TR cluster maps (yellow or purple voxels in Figs 1-3), the probability that this is due to chance alone is 0.4×10−7 corresponding to p=0.015 to find one such voxel in the entire brain.
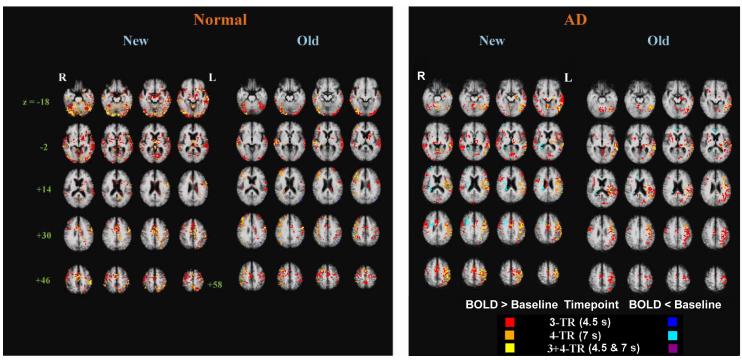
Fig.1.
Map of the BOLD response to Congruous-New and Congruous-Old words. Clusters of voxels with significant response relative to baseline (BOLD > Baseline, 12 or more adjacent voxels, with voxel-wise one-tailed p < 0.025) at 3- and 4-TR timepoints are superimposed on the group-averaged anatomical images of normal controls and AD patients, respectively (N = 15 per group). Axial slices are labeled with the corresponding z-coordinate from the atlas of Talairach & Tournoux (1988).
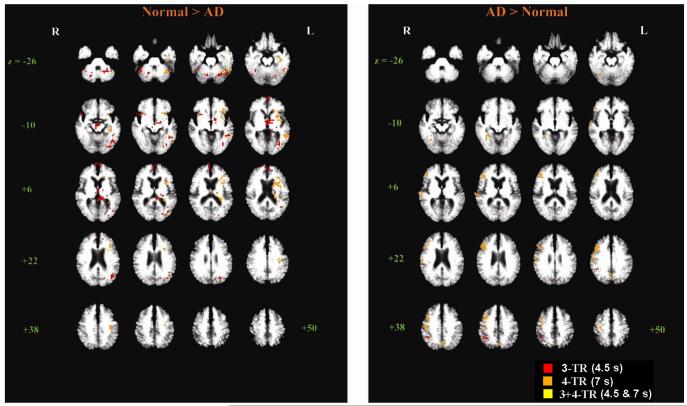
Fig.3.
Between-group differences of the New > Old congruous repetition effects. Clusters of voxels (12 or more adjacent voxels, with voxel-wise one-tailed p < 0.025) with significant Normal > AD and AD > Normal differences at 3- and 4-TR timepoints are superimposed on the group-averaged anatomical images of normal elderly controls. Axial slices are labeled with the corresponding z-coordinate.
To test for possible hemispheric differences in activation patterns, masks were constructed to define the left and right cerebral hemisphere for each subject. These masks included the entire cerebral hemisphere and ipsilateral diencephalon, but excluded the ventricles and midbrain (present on the lower axial slices). The AFNI program ‘3dROIstats’ was then used to calculate the average beta coefficient (BOLD response) for each subject in each hemisphere for each timepoint (3-TR and 4-TR) and repetition condition (New/Old). These data were submitted to a split-plot ANOVA with one between-subject factor (group) and three within-subject factors (hemisphere, latency, repetition).
To test the relationship between MTL activity and subsequent memory, the magnitude (New - Old BOLD response, averaged across 3- and 4-TR timepoints) and spatial extent (automated voxel counts of significant New > Old BOLD response across 3- and 4-TR timepoints) of repetition effects in left and right MTL were correlated with cued recall and multiple-choice recognition scores. MTL was defined as the region encompassing the entire PHG and hippocampus (per AFNI’s Talairach daemon) and resampled to 4-mm isotropic voxels. Analogous methods were also used to 1) examine the effect of repeating semantically incongruous words on the MTL BOLD response and 2) conduct exploratory analyses in several ROIs, which are putative P600 generators—i.e. fusiform, IFG, MTG, STG, anterior and posterior cingulate cortex (ACC and PCC), as defined by the AFNI Talairach daemon.
Group discrimination- logistic regression analyses
Forward step and backward step logistic regressions were performed (SPSS, version 18) in order to find the strongest fMRI predictors of group classification. First, a forward step regression (probability in = 0.05, probablility out = 0.10) was performed with 7 main fMRI measures (4 based on spatial extent and 3 based on magnitude of BOLD responses): New > Old voxel counts in the left MTL, and entire left hemisphere; Old > New voxel counts in the left MTL and entire right hemisphere; and New-Old change in magnitude of BOLD response in the left MTL, left hemisphere, and right hemisphere. Next, backwards step regressions were performed to control for potential effects of age, education and gender (probability out= 0.10).
RESULTS
Behavioral data
Behavioral results are summarized in Table 1. While the AD group performed the semantic decision task fairly well, their performance was poorer than NE (t’s ≥ 3.57, p’s < 0.005). To Congruous-New items, there was no significant group difference in RTs, while AD responded more slowly to Congruous-Old targets (t = 2.91, p < 0.01). Both groups showed repetition priming (i.e. faster RTs to Old targets) regardless of semantic congruity (t’s ≥ 4.0, p’s < 0.005; see Table 1 legend), but controls had significantly greater RT priming than AD for congruous items (t = 3.25, p < 0.005). On the post-scan memory tests, as expected, AD performed more poorly than NE (all p’s < 0.0001 except for cued recall of incongruous items, Table 1). In addition, there were large effects of semantic congruity on subsequent recall and recognition with greater memory for congruous than incongruous target words in both groups (Table 1).
Imaging results
To examine the neural response to congruous trials, t-test maps were created to contrast BOLD response for New and Old conditions relative to baseline. Widespread and robust BOLD responses were seen for Congruous-New trials in both NE and AD groups throughout much of the bilateral cerebral hemispheres at both 3- and 4-TR timepoints (4.5 s and 7.0 s after visual target word onset). In NE, the BOLD response to Congruous-Old trials was less widespread. The spatial extent of activation was greater at 3- than at 4-TR, but many regions showed responses at both timepoints (yellow voxels in Fig. 1). Importantly, NE demonstrated a so-called “HERA” (Hemispheric Encoding/Retrieval Asymmetry) pattern (Tulving, Kapur, Craik, Moscovitch, & Houle, 1994a), with more activation in the left hemisphere to New words (left > right: 165,312 > 34,158 mm3), for which encoding demands are higher, and right > left (57,408 > 44,352 mm3) activation, particularly in the pre-frontal cortex (Figure 1) to Old words, for which retrieval processes are expected to be greater. Also, more prolonged activation to New than Old words were observed in the left (compare orange voxels in Fig. 1), while more prolonged activation to Old than New words were seen in the right-hemisphere (see orange voxels in Fig. 1). In contrast, AD consistently showed a left > right pattern in response to both New and Old words (New: 101,056 > 34,752 mm3; Old: 71,168 > 13,760 mm3), and, unlike NE, they had almost no prolonged right-hemisphere activation to Old stimuli (see lack of orange or yellow voxels in right side of Fig. 1), and even had some regions of “deactivation” (BOLD < baseline) in right frontal and deep right hemisphere structures.
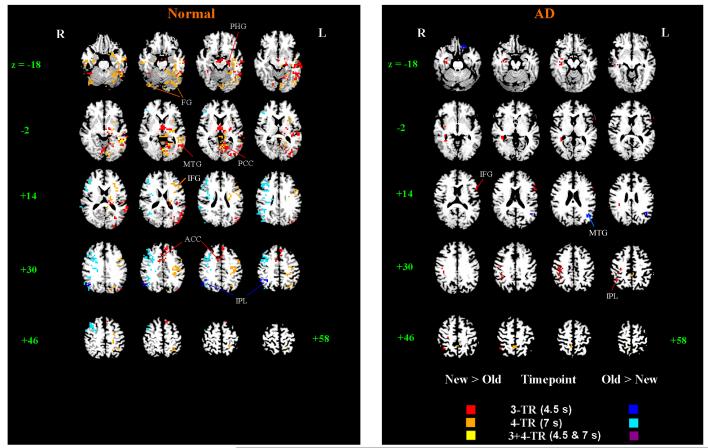
The congruous word repetition effect (i.e. New > Old BOLD response) at 3-TR revealed significant clusters mainly in the left hemisphere in NE: left anterior PHG, bilateral fusiform gyri (BA 37), left middle and superior temporal gyri (BA39, 21), bilateral anterior cingulate, left thalamus, lingual gyrus, and PCC (see red and yellow voxels in Fig. 2 and Table 2A for cluster report). Adding significant clusters at 4-TR (orange in Fig. 2) to the 3-TR map (red voxels), additional clusters became active in neighboring regions of the left PHG, fusiform (BA 37, 19), MTG (BA21, 22) and thalamus. Also, new clusters appeared in left IPL (BA 40), PCC, left primary motor and sensory cortex, left MFG and IFG (BA 9, 44, 45), and right middle and inferior temporal gyrus (BA 21, 20). In contrast, clusters of Old > New response appeared only in the right-hemisphere. At 3-TR, one cluster in the IPL (BA 40, 39) was active (dark blue voxels). At 4-TR, a very large cluster (10,048 mm3 ) of activation which included much of the inferior and middle frontal gyri (BA 9, 6, 46) was present. Other significant clusters were present in right parietal cortex, posterior middle temporal and mid-cingulate gyri (light blue in Fig. 2).
Fig.2.
Maps of the Congruous repetition effects on BOLD response. Clusters of voxels (12 or more adjacent voxels, with voxel-wise one-tailed p < 0.025) with significant New > Old (‘hot’ colors) and Old > New (‘cold’ colors) effects at 3- and 4-TR timepoints. To improve anatomical detail, functional maps are superimposed on the anatomical images of a representative normal elderly control (left) and AD (right) subject. PHG = parahippocampal gyrus, FG = fusiform gyrus, MTG = middle temporal gyrus, ACC = anterior cingulate cortex, PCC = posterior cingulate cortex, IFG = inferior frontal gyrus, IPL = inferior parietal lobule. Axial slices are labeled with the corresponding z-coordinate.
Table 2A.
Clusters with congruous word repetition effects, Normal Elderly (n = 15).
| New > Old 3TR (4.5 seconds after visual word) Center and Extent | |||||||
|---|---|---|---|---|---|---|---|
| Structure (Brodmann’s Area) | CM (x, y, z) |
Volume | Mean (SEM) |
Peak* | MI (x, y, z) |
t | p |
| L Thalamus, L antPHG(27), HC, Mammillary Body |
−8, −14, 1 | 4992 | 1.87(0.10) | R Mammillary Body | 2, −13, −8 | 5.03 | 9 × 10−5 |
| LMTG(37), L Fusiform(37), LITG(20) | −49, −52, −6 | 4160 | 1.28(0.04) | L Culmen | −42, −53, −20 | 3.36 | .002 |
| L LG(18,19), L Cuneus(18,30), L Post. Cingulate(30) |
−9, −64, 5 | 3968 | 1.41(0.05) | L Cuneus | −6, −61, 8 | 3.20 | .003 |
| LMTG(39), LSTG(39) | −51, −63, 21 | 2304 | 1.09(0.04) | LSTG | −62, −41, 16 | 3.66 | .001 |
| LMTG(21) | −59, −24, −5 | 2176 | 1.28(0.05) | LMTG(21) | −66, −33, 0 | 4.24 | 4.1 × 10−4 |
| LMOG(18,19) | −32, −79, 12 | 1600 | 1.08(0.05) | LMOG(18) | −30, −81, 4 | 3.01 | .005 |
| CG(32) | 0, 12, 39 | 1344 | 1.15(0.06) | LMFG(8) | −6, 19, 44 | 2.39 | .015 |
| LSFG(8), L medFG(8) | −10, 33, 43 | 1280 | 1.33(0.08) | LSFG | −10, 27, 48 | 3.90 | .001 |
| Transverse G(41), STG(41), L PostCG, | −49, −26, 13 | 1152 | 1.53(0.07) | L Transverse TG | −38, −29, 12 | 3.35 | .002 |
| R Fusiform(37) | 38, −48, −17 | 1024 | 1.49(0.09) | R Culmen | 42, −53, −20 | 3.26 | .003 |
| L PreCG(6) | −17, −18, 68 | 768 | 1.33(0.09) | L PreCG | −14, −21, 68 | 2.65 | .010 |
| New > Old 4TR (7 seconds after visual word) Center and Extent | |||||||
|---|---|---|---|---|---|---|---|
| Structure (Brodmann’s Area) | CM (x, y, z) |
Volume | Mean (SEM) |
Peak* | MI (x, y, z) |
t | p |
| L Fusiform(19,37), LPHG(36), L Culmen, L Declive |
−27, −61, −10 | 11776 | 1.25(0.03) | L Declive | −46, −53, −20 | 2.85 | .006 |
| PostCG(2,3), PreCG(4), IPL(40) | −36, −22, 40 | 3264 | 1.07(0.03) | LIPL | −38, −29, 40 | 3.26 | .003 |
| LPHG, L Uncus, LSTG(22) | −31, −2, −19 | 2624 | 1.42(0.08) | LPHG | −22, −1, −16 | 2.57 | .011 |
| Thalamus, L lentiform Nucleus | −11, −31, 5 | 2240 | 1.22(0.08) | L Thalamus | −6, −33, 4 | 2.70 | .008 |
| L Insula(13) | −33, 15, 15 | 1792 | 1.10(0.06) | L Insula | −30, 11, 12 | 5.07 | 8 × 10−5 |
| LIFG(44, 45), LMFG(9,6,8) | −49, 8, 29 | 1664 | 1.17(0.05) | LIFG/MFG | −46, 11, 28 | 3.26 | .003 |
| MTG(22) | −51, −44, 3 | 1408 | 1.15(0.06) | LMTG | −58, −37, 4 | 2.73 | .008 |
| RPHG(28), HC | 28, −17, −21 | 1024 | 1.33(0.10) | R Culmen | 22, −25, −20 | 2.48 | .013 |
| R Declive | 37, −77, −15 | 1024 | 1.74(0.15) | R Declive | 42, −77, −16 | 3.61 | .001 |
| RMTG(20), RITG(20) | 52, −19, −15 | 896 | 1.03(0.07) | R MTG | 50, −21, −12 | 2.47 | .013 |
| L Thalamus, L Insula | −24, −14, 21 | 896 | 0.97(0.06) | L Thalamus | −18, −17, 16 | 3.13 | .004 |
| LSPL(7), LIPL(40) | −32, −48, 52 | 832 | 1.10(0.06) | LIPL | −30, −41, 56 | 3.66 | .001 |
| LMTG(21), LITG(20) | −52, −13, −15 | 768 | 1.10(0.06) | LMTG | −50, −13, −16 | 3.47 | .002 |
| L Precuneus(19), L Cuneus(19), LIPL(40), LSPL(7) |
−27, −69, 37 | 768 | 1.00(0.08) | L Cuneus | −22, −73, 32 | 3.81 | .001 |
| Old > New 3TR (4.5 seconds after visual word) Center and Extent | |||||||
|---|---|---|---|---|---|---|---|
| Structure (Brodmann’s Area) | CM (x, y, z) |
Volume | Mean (SEM) |
Peak* | MI (x, y, z) |
t | p |
| RIPL (40,39) | 43,−57,38 | 1536 | 1.10 (0.05) | RAG, RIPL (39,40) | 46,−61,36 | 3.51 | .002 |
| Old > New 4TR (7 seconds after visual word) Center and Extent | |||||||
|---|---|---|---|---|---|---|---|
| Structure (Brodmann’s Area) | CM (x, y, z) |
Volume | Mean (SEM) |
Peak* | MI (x, y, z) |
t | p |
| RMFG (9,6,46), RIFG (46) | 37,16, 32 | 10048 | 1.14 (0.02) | RMFG (R46) | 42,31,16 | 3.18 | .003 |
| RSMG, RMTG (22) | 38,−47,30 | 1280 | 1.04 (1.42) | RSMG (39) | 38,−49,28 | 2.38 | .016 |
| RSMG, RIPL (40) | 56,−48,26 | 1024 | 0.84 (0.04) | RSMG (39) | 50,−53,28 | 2.80 | .007 |
| RCG, R Caudate | 22,−20,30 | 1024 | 1.06 (0.08) | RCG, R Caudate | 14,−17,28 | 4.15 | 5 × 10−4 |
| RAG, RMTG (19), R Precuneus | 40,−71,32 | 896 | 0.89 (0.06) | RMTG (39) | 42,−73,28 | 2.44 | .014 |
Legend: Clusters above list center first, then other surrounding structures with activations.
Peak is the brain region of Maximum Intensity (MI); t and p values are listed for the voxel with Maximum Intensity. AG = Angular Gyrus, ant = Anterior, CG = Cingulate Gyrus, G = Gyrus, HC = Hippocampus, IFG = Inferior Frontal Gyrus, IPL = Inferior Parietal Lobule, ITG = Inferior Temporal Gyrus, L = Left, , LG = Lingual Gyrus, medFG = Medial Frontal Gyrus, MFG = Middle Frontal Gyrus, MOG = Middle Occipital Gyrus, MTG = Middle Temporal Gyrus, PHG = Parahippocampal Gyrus, PostCG = Postcentral Gyrus, PreCG = Precentral Gyrus R = Right, SFG = Superior Frontal Gyrus, SMG = Supramarginal Gyrus, SPL = Superior Parietal Lobule, STG = Superior Temporal Gyrus.
Compared to controls, the AD group showed far fewer brain regions of New > Old repetition effect (right side of Fig. 2, see Table 2B for cluster report). At 3-TR, two of the larger clusters were in right parietal cortex, and included the IPL (BA40) (volumes = 1408 and 832 mm3, center coordinates = [37, −21, 39] and [38, −46, 44]). The largest cluster was in the right cerebellum (tuber and declive). Other small clusters were present in left IFG (BA 44, 45), right PHG and tail of caudate at 3-TR, and in right precuneus at 4-TR. Two clusters of significant Old > New BOLD response were present in the left hemisphere: inferior and medial frontal (BA 11, 47, 25) and MTG (BA 39, z= +18 to +26 in Fig. 2) at 3-TR; none were present at 4-TR.
Table 2B.
Clusters with congruous word repetition effects, Alzheimer’s disease (n = 15).
| New > Old 3TR (4.5 seconds after visual word) Center and Extent | |||||||
|---|---|---|---|---|---|---|---|
| Structure (Brodmann’s Area) | CM (x, y, z) |
Volume | Mean (SEM) |
Peak* | MI (x, y, z) |
t | p |
| R Tuber, R Declive | 39, −65, −28 | 1536 | 1.56(0.15) | R Tuber | 46, −61, −28 | 2.40 | .015 |
| R PostCG(2,3), RIPL(40), RPreCG(4) | 37, −21, 39 | 1408 | 1.42(0.08) | R PreCG | 42, −13, 40 | 3.11 | .004 |
| RPHG, R Insula | 34, −15, −10 | 1216 | 1.39(0.09) | R Insula | 38, −9, −4 | 2.82 | .007 |
| RIPL(40) | 38, −46, 44 | 832 | 1.87(0.19) | R IPL | 38, −49, 44 | 2.23 | .021 |
| R Caudate Tail | 34, −38, 4 | 768 | 1.46(0.11) | RCT | 34, −41, 4 | 4.08 | .001 |
| LIFG(44,45) | −54, 14, 17 | 768 | 1.46(0.13) | LIFG(45) | −54, 11, 20 | 3.89 | .001 |
| New > Old 4TR (7 seconds after visual word) Center and Extent | |||||||
|---|---|---|---|---|---|---|---|
| Structure (Brodmann’s Area) | CM (x, y, z) |
Volume | Mean (SEM) |
Peak* | MI (x, y, z) |
t | p |
| R Paracentral Lobule(5), R Precuneus | 2, −41, 51 | 960 | 2.03(0.15) | L Precuneus | −2, −45, 52 | 2.38 | .015 |
| Old > New 3TR (4.5 seconds after visual word) Center and Extent | |||||||
|---|---|---|---|---|---|---|---|
| Structure (Brodmann’s Area) | CM (x, y, z) |
Volume | Mean (SEM) |
Peak* | MI (x, y, z) |
t | p |
| LIFG (47), LMFG (11), LmedFG(25) | −21, 23,−19 | 832 | 1.80(0.12 ) | LIFG (47) | −26,19,−20 | 2.89 | 0.005 |
| LMTG (39) | −42,−59,24 | 768 | 1.37(0.07) | LSTG (39) | −42,−57,20 | 3.21 | 0.003 |
| 4TR (7 seconds after visual word) Center and Extent |
|---|
| No Clusters Found |
Legend: Clusters above list center first, then other surrounding structures with activations.
Peak is the brain region of Maximum Intensity (MI); t and p values are listed for the voxel with Maximum Intensity. CT = Caudate Tail, IFG = Inferior Frontal Gyrus, IPL = Inferior Parietal Lobule, L = Left, medFG = Medial Frontal Gyrus, MFG = Middle Frontal Gyrus, MTG = Middle Temporal Gyrus, PHG = Parahippocampal Gyrus, PostCG = Postcentral Gyrus, PreCG = Precentral Gyrus, R = Right.
The above deconvolution analyses were repeated with reference functions which included only trials with correct behavioral responses (i.e. 96.6% of the congruous trials for NE, and 88.5% of trials for AD). The results were virtually identical, except the AD group no longer had a significant cluster of New > Old BOLD response in the right cerebellum at shift 3TR (perhaps due to increased motor demands or indecision on the incorrect trials).
The split-plot ANOVA which tested for effects of group, hemisphere, repetition and latency shift on the magnitude of the BOLD response found a significant 3-way interaction of Group × Hemisphere × Repetition [F(1,28)= 7.96, p = 0.0087], due to the NE group showing New > Old responses in the left hemisphere and the AD group having similar magnitude New > Old effects in the right hemisphere. In addition, main effects of repetition [F(1,28)= 4.30, p = 0.047] and latency shift [F(1,28)= 22.3, p = 0.0001] were present, due to New words having larger BOLD responses than Old words and both groups having larger BOLD responses at shift 3TR (4.5 seconds after visual target onset) than shift 4TR (7 s post-target onset).
In summary, the normal controls displayed a HERA pattern in which the left hemisphere responded more during initial encoding (i.e. New > Old), but the right hemisphere responded greater to repeated stimuli (i.e. Old > New). The patient group failed to show this pattern, but instead had scant New > Old effects present mostly in the right hemisphere.
Between-group t-test maps comparing the New > Old repetition effect in controls vs. patients yielded many significant clusters (Fig. 3). In the contrast of “Normal > AD”, the cluster map at 3-TR included 9 significant clusters in the left hemisphere, including fusiform (BA 37), posterior MTG (BA 37, 39, 19), medial frontal gyrus (BA 10), and thalamus. Three right-hemisphere clusters in the IFG (BA 47), fusiform and cerebellum were also identified. At 4-TR, 9 left-hemisphere clusters, including PHG, uncus, fusiform (BA 37), insula, IFG (BA 47) and MTG (BA 21, 22), but no right-hemisphere clusters were present. As for the “AD > Normal” contrast, only 1 cluster was significant, in the right IPL (BA 40), at 3-TR (red voxels in Fig. 3). At 4-TR, 1 left-hemisphere cluster (left precuneus (BA7)) and 5 right-sided clusters were present, including the right PHG, middle frontal (BA 9, 10), precentral (BA 4), and superior temporal (BA 42, 22) gyri. Note that two of the AD > NE clusters are in “default mode” regions (right IPL, left precuneus), where New > Old responses would not be predicted to occur. See Table 3 for a complete list of all significant clusters with intergroup differences.
Table 3A.
NE > AD Clusters, Congruous New – Old Words.
| Structure (Brodmann’s area) | CM |
Volume | Mean (SE) | MI | t | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||||
|
3-TR (4.5 seconds after visual word),
Center and Extent |
||||||||||
| Thalamus, L Subthalamic nuc. | −4 | −9 | 0 | 2816 | 3.01(0.17) | 6 | −13 | −8 | 2.98 | .003 |
| L FG(37), L Culmen, L Declive | −32 | −57 | −19 | 2048 | 2.09(0.11) | −22 | −65 | −16 | 3.67 | 5 × 10−4 |
| medFG(10, 9)(R>L), R SFG | −1 | 59 | 13 | 1984 | 2.54(0.11) | −2 | 63 | 4 | 2.24 | .017 |
| L MTG(39), LSTG(22), L SG | −35 | −63 | 25 | 1344 | 1.85(0.10) | −42 | −65 | 24 | 2.18 | .018 |
| L ITG, L MTG(37) | −45 | −52 | −5 | 1216 | 1.86(0.08) | −42 | −61 | −8 | 3.89 | 2.8 × 10−4 |
| L OLG, L IOG(18, 19), L MOG, L FG | −32 | −71 | −3 | 1152 | 1.78(0.09) | −26 | −77 | −8 | 3.27 | .001 |
| Corpus Callosum(L>R), L Pulvinar, L PHG |
−8 | −33 | 9 | 1024 | 2.28(0.15) | −6 | −37 | 8 | 2.65 | .006 |
| L Thalamus, L Claustrum, L Caudate | −22 | −23 | 16 | 960 | 1.91(0.16) | −22 | −13 | 20 | 4.58 | 4.4 × 10−5 |
| R Cerebellum | 17 | −63 | −20 | 896 | 1.75(0.11) | 10 | −61 | −16 | 3.27 | .001 |
| R Culmen, R FG(37), R Declive, R Tuber, R ITG(20) |
38 | −49 | −19 | 832 | 2.28(0.17) | 42 | −53 | −20 | 3.49 | .001 |
| R IFG(47), R Insula(13), R STG(38, 22), R Claustrum |
40 | 16 | −4 | 768 | 2.15(0.10) | 46 | 15 | −4 | 2.74 | .005 |
| L MOG(19), L Cuneus, L MTG | −33 | −76 | 10 | 768 | 1.94(0.14) | −30 | −81 | 8 | 3.37 | .001 |
|
4TR (7 seconds after visual word),
Center and Extent |
||||||||||
| L Insula(13), L Claustrum, L IFG(45) | −31 | 13 | 15 | 4224 | 1.77(0.05) | −18 | −1 | 24 | 2.24 | .016 |
| L FG (37), L Declive, L Culmen, L ITG | −44 | −52 | −17 | 1344 | 2.20(0.16) | −50 | −53 | −16 | 2.67 | .006 |
| L Claustrum, L Insula (13), L IFG(47) | −29 | 22 | 0 | 1216 | 1.79(0.08) | −38 | 19 | 0 | 2.42 | .011 |
| L Insula (13), L Caudate | −27 | −34 | 16 | 1216 | 2.10(0.10) | −26 | −37 | 12 | 3.55 | 5 × 10−4 |
| L Uncus(28), L MTG(21), L STG, L PHG(38) |
−33 | −1 | −28 | 1024 | 2.55(0.27) | −30 | −1 | −24 | 2.46 | .010 |
| L PHG(36), L Hippocampus, L Caudate | −38 | −23 | −12 | 960 | 2.01(0.12) | −38 | −17 | −16 | 4.12 | 1.5 × 10−4 |
| L MTG(22, 21) | −53 | −47 | 2 | 960 | 2.02(0.14) | −54 | −45 | 4 | 3.31 | .015 |
| L PreCG(4), L PostCG(3) | −35 | −17 | 39 | 896 | 1.72(0.15) | −30 | −9 | 44 | 3.26 | .001 |
Legend: Clusters above list center first, then other surrounding structures with significant intensity differences. The t and p values are listed for the voxel with Maximum Intensity (MI). FG = Fusiform Gyrus, IFG = Inferior Frontal Gyrus, IOG = Inferior Occipital Gyrus, ITG = Inferior Temporal Gyrus, L = Left, medFG = Medial Frontal Gyrus, MOG = Middle Occipital Gyrus, MTG = Middle Temporal Gyrus, OLG = Occipital Lingual Gyrus, PHG = Parahippocampal Gyrus, PostCG = Postcentral Gyrus, PreCG = Precentral Gyrus, R = Right, SFG = Superior Frontal Gyrus, SG = Superior Gyrus, STG = Superior Temporal Gyrus.
BOLD responses in MTLs and correlations with memory
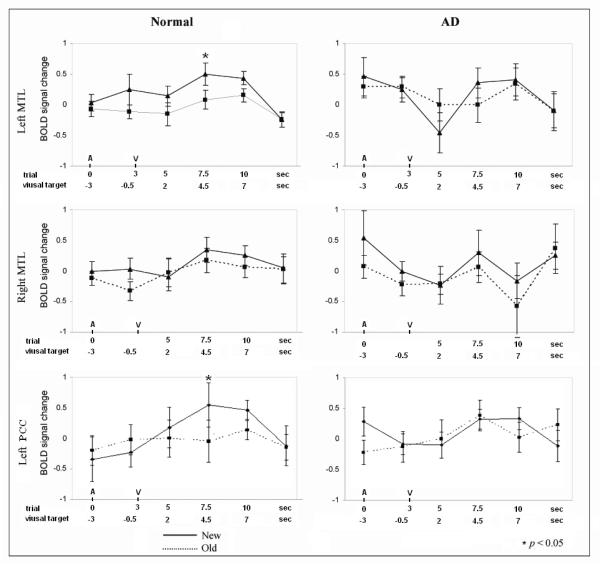
HRF was estimated within the left and right MTL ROIs (entire PHG and hippocampus per AFNI Daemon). The timecourse of the BOLD response in the left MTL of the NE showed a significant congruous repetition effect (New > Old) at 3-TR (t = 2.20, p = 0.045) and marginal effect at 4-TR (t = 1.87, p = 0.08), but no repetition effects in the right MTL (Fig. 4). In AD, no significant New > Old difference was found at 3- or 4-TR in either MTL (Fig. 4). Collapsing across 3- and 4-TR, NE showed greater spatial extent (i.e. voxel count) than AD in New > Old activation in the left MTL (means = 23.7 in NE, 14.1 in AD; t = 4.03, p < 0.0005). The extent was correlated with subsequent cued recall scores (r = 0.65, p <0.0005) across all subjects, but not within NE or AD (r’s ≤ 0.36). However, a “selectivity index” (Red - Blue voxels) for the direction of activation correlated with cued recall both within NE (r = 0.67, p = 0.006) and across all subjects (r = 0.59, p = 0.001). This measure also correlated with recognition memory across all subjects (r = 0.55, p = 0.002), with a non-significant trend (r = 0.44, p = 0.09) within NE. Similarly, in NE, Pearson correlations showed significant correlations between the magnitude of repetition effect (New – Old, BOLD response averaged across 3- and 4-TR timepoints) in the left MTL and subsequent cued recall (r = 0.61, p = 0.016), but not with recognition (r = 0.36, p = 0.19). Neither cued recall nor recognition correlated with the magnitude of right MTL activation (r’s ≤ 0.14). With regard to Old > New activation (blue voxels), the spatial extent in the right MTL did correlate with recall and recognition across all subjects (r’s ≥ 0.41, p’s ≤ 0.03) and with recognition within AD (r = 0.74, p = 0.003). Neither NE nor AD showed any correlations between activation magnitude (Old - New, BOLD response averaged across 3- and 4-TR timepoints) in the right MTL and subsequent memory (∣r’s∣ ≤ 0.25).
Fig.4.
Estimates of the hemodynamic response function (HRF) for Congruous-New and Congruous-Old words in left and right medial temporal lobes, and left posterior cingulate cortex (PCC). Timescale is shown both relative to trial/Auditory phrase onset (A, upper scale) and to Visual target word (V, lower scale).
Parallel analyses of the incongruous word repetition effects in the MTLs showed no significant intergroup differences in spatial extent of New > Old or Old > New activation in either the left or right MTL (all p’s > 0.17) for these difficult to learn stimuli. Furthermore, the spatial extent of these effects did not correlate significantly with subsequent memory scores within either group (e.g. r = 0.33, p =0.23 between right MTL New > Old words and cued recall for incongruous words, all other r’s < 0.33). The NE group had a greater extent of New > Old activation in the left MTL for congruous than incongruous items (23.7 vs. 16.1 voxels, t = 3.67, p= 0.003), while there was no significant effect of congruity on left MTL activation extent in AD (t= −1.87, p= 0.08).
Correlations of P600 generator ROIs with memory
Exploratory correlation analyses were conducted for the magnitude of the repetition effects in bilateral fusiform gyrus, IFG, cingulate cortex, MTG and STG. The analyses in left and right fusiform in NE revealed strong correlations between the differential BOLD response and subsequent cued recall and recognition (r’s ≥ 0.61, p’s ≤ 0.015). Significant correlations were also found in NE between the activation magnitude and subsequent recall and recognition in bilateral IFG (r’s ≥ 0.56), and between activation magnitude and recall in left MTG, STG, anterior and posterior cingulate (r’s ≥ 0.51, see bottom of Figure 4 for estimated hemodynamic response in left PCC). Across all subjects, both recall and recognition correlated with activation magnitude in left IFG (r’s ≥ 0.42), STG (r’s ≥ 0.48) and fusiform gyrus (r’s ≥ 0.43). The magnitude of New – Old BOLD response in the left PCC correlated with subsequent cued recall (r = 0.51, p = 0.05). No such correlations were found within the AD group (e.g. r’s ≤ 0.38, p’s > 0.17 between left IFG, FG, MTG or STG activation and subsequent recall or recognition) in these ROIs. This lack of correlations within the AD group may be due to floor effects, especially pronounced for free and cued recall (see Table 1). It should also be noted that many AD participants had absent (beta estimate ~ 0.0) or reversed (Old > New) repetition effects in these large ROIs. In summary, all of the left hemisphere putative P600 cortical regions’ congruous word repetition effect (New – Old) had at least one significant correlation with subsequent memory scores, either the main recall or recognition measure (FR, CR or MC). In contrast, only two right hemisphere ROIs showed significant correlations, namely the right fusiform and IFG.
To follow-up on the significant findings within the bilateral inferior frontal gyri, additional exploratory analyses were done in BA 44, 45 and 47. Across all subjects, New – Old BOLD response in both left and right BA 47 correlated with subsequent recall (r = 0.40, p = 0.03 on the left; r = 0.50, p = 0.006 in right BA 47) and recognition (r = 0.38, p = 0.04 on left, r = 0.45, p = 0.01 on right). In AD, New – Old BOLD response in right BA 45 correlated with total cued recall score (r = 0.59, p = 0.026).
Correlations between BOLD responses and semantic task performance (accuracy and priming)
Analyses testing for correlations between accuracy on the primary semantic judgment task and BOLD response in the cortical ROIs (putative P600 generators) found significant correlations with the magnitude of right IFG (r = 0.49, p = 0.007), left (r = 0.41, p = 0.029) and right (r = 0.59, p = 0.001) anterior cingulate (r’s > 0.41, p’s < 0.03) and left MTG (r = 0.41, p = 0.026) New – Old BOLD responses. In addition, an inverse correlation was present between New – Old BOLD response in the right STG and task accuracy (r = −0.37, p = 0.05). It should be noted that these correlations were not significant within the NE group, but appeared to be driven by significant correlations within AD (r’s ≥ 0.54, p’s ≤ 0.04 for all these ROIs except the right STG for which r = −0.48, p = 0.07). There was no significant correlation between the extent of MTL activation and task accuracy within either the NE (r = 0.13, p > 0.65) or AD (r = 0.12, p > 0.65) group.
Correlation analyses between the BOLD response in these same cortical ROIs with RT priming (response time to New – Old congruous trials) showed a significant correlation between the New- Old BOLD response of the left IFG and RT priming (r = 0.47, p = 0.009). IFG subregion analyses showed this correlation was significant only within left BA 47, and not within BA 44 or 46 (r’s ≤ 0.29, p’s ≥ 0.13). While similar magnitude correlations were present in the NE (r = 0.31, p = 0.29) and AD groups (r = 0.43, p = 0.11) when analyzed separately, these did not reach statistical significance within these smaller samples. None of the other ROIs showed significant correlations with RT priming.
Group discrimination
The forward step and backward step logistic regression models converged on a very highly significant model (chi-square = 29.2, df=3, p < 0.0001) in which lower left hemisphere New > Old voxel counts (B= −.023), lower left MTL New > Old voxel counts (B= −.242) and larger right hemisphere New – Old BOLD response (B= 2.71) were associated with increased likelihood of having AD (see Table 4). This model classified 93.3% of subjects correctly (14/15 in each group). Adding the demographic variables of age, sex and education did not produce any significant improvements to this model.
Table 4.
Logistic regression model to discriminate AD and NE groups.
| B | S.E. | Wald | Sig | Exp (B) | |
|---|---|---|---|---|---|
| L. Hemisphere New > Old voxels | −0.23 | 0.011 | 4.714 | 0.03 | .977 |
| L. MTL New > Old voxels | −0.242 | 0.135 | 3.212 | 0.073 | .785 |
| R. Hemisphere BOLD response (New-Old) | 2.709 | 1.332 | 4.133 | 0.042 | 15.008 |
| Constant | 16.798 | 6.854 | 6.007 | 0.014 | 1.973 × 107 |
Legend: Cutoff for group classification = 0.500 (1 = AD, 0 = NE). B = Slope. S.E. = Standard Error. Wald = Wald statistic. Exp (B) = Odds ratio.
DISCUSSION
During a semantic judgment task, normal elderly showed widespread New > Old BOLD response in the left hemisphere. Many of these clusters were in regions known to produce P600-like brain potentials (left PHG, cingulate, MTG, IFG). Converging evidence suggests that this circuit of interconnected P600 generators is particularly important for successful encoding and “memory binding” (Kahn et al., 2005; Kirchhoff et al., 2000; Wagner et al., 1998b; Wagner 1999). The magnitude and spatial extent of New > Old BOLD responses in several of these regions (left MTL/PHG, PCC, IFG, fusiform) correlated with subsequent recall and/or recognition.
The main abnormal findings in AD were: 1) MTL failure, with a severe loss of New > Old BOLD response in the left MTL, which is the more relevant hemisphere for learning verbal materials; 2) Widespread left hemisphere dysfunction, not an overall decrement of BOLD response in AD, but a selective loss of New > Old repetition effects in this hemisphere. Since these same effects correlated strongly with verbal declarative memory in normal elderly, it is likely that this loss of left hemisphere New > Old effects in AD may account for their dense verbal memory deficits; 3) Some evidence of right hemisphere dysfunction as well, with a loss of the Old > New effects seen in right parietal and prefrontal regions of the normal elderly. It should also be noted that a few right-hemispheric ROIs, (i.e. fusiform and BA 47 in the inferior frontal gyrus) likely related to semantic processing, also showed New > Old effects in the normal elderly and that these effects also correlated with memory for the experimental stimuli. Our logistic regression analyses achieved excellent separation (93.3% sensitivity and specificity) of the AD and normal elderly groups, using fMRI variables which quantify the main MTL and hemispheric responses noted above. This is in line with our prior ERP studies of mild AD with this paradigm (Olichney et al 2006), which also achieved excellent group discrimination (100% sensitivity, 82% specificity). This range of discriminability is consistent with a potentially useful biomarker for AD (The Ronald and Nancy Reagan Research Institute & NIA Working Group, 1998).
This fMRI word repetition paradigm involves attention, perceptual, conceptual and episodic memory processes and requires a motor response. As such, it produced widespread activation of the cerebral cortex (sensory, association, paralimbic, limbic and motor cortex), which was generally more pronounced for the novel stimuli (Figure 1). One disadvantage of using a complex cognitive task such as category judgment, along with cross-modal stimuli, is that it is difficult to isolate the specific cognitive processes performed by a given anatomical structure. However, when New vs. Old congruous words contrasts were made at timepoints chosen to model the BOLD response to the visual target words, a neural circuit of interconnected structures thought important for verbal memory emerged in the NE activation maps, as discussed in detail in Results above. As hypothesized, the magnitude of the BOLD response to New - Old congruous words in several ROIs (putative P600 generators) correlated with declarative memory (both recall and recognition) for the verbal stimuli. In contrast, the degree of activation (New vs. Old BOLD response) in the MTLs when semantically incongruous words were repeated did not correlate with any of our memory measures (neither with total recall or recognition scores for incongruous or all words).
In contrast to normal elderly, the AD group showed little evidence of New > Old BOLD responses in the left hemisphere and a generalized decrease in fMRI repetition effects. Our results resemble those of Golby et al. (2005), who found reduced activation, for novel vs. repeated visual scenes (color photographs), along the ventral visual stream, with most marked decrements in the MTL and fusiform regions. It should be noted that our results were not due to a general failure of cognitive activation, or an inadequate signal-to-noise ratio in AD, but rather a relatively selective decrement in New/Old effects. The spatial extent of left hemisphere activation was similar in AD and NE when collapsed across New and Old words, but as hypothesized, the AD group showed a selective loss of New > Old effects in this hemisphere. Old words elicited similar spatial extents of activation in both groups but with different hemispheric patterns (right > left in NE, left > right in AD), as will be discussed further below.
Relatively few prior fMRI studies of AD have used purely verbal stimuli (Lustig & Buckner, 2004) and we are not aware of any prior published studies which used cross-modal audio-visual stimuli to probe incidental learning in AD. The use of multi-modal stimuli with integrative tasks such as semantic judgment may be advantageous in producing activation of higher association cortex, which is a predilection site for AD pathology relative to primary sensory and motor areas (Arnold, Hyman, Flory, Damasio, & van Hoesen, 1991; Braak & Braak, 1991). Remy et al (2005), in a block design fMRI study of verbal encoding and recognition, found activation of the left hippocampus, fusiform, IPL and MFG in normal elderly but a complete lack of activation during encoding in AD (relative to a reading condition with more rapidly presented words). Lustig and Buickner (2004), using visually presented word lists and a semantic (living/nonliving) judgment, found relative sparing of New/Old word effects in the inferior and middle frontal gyrus of early-stage AD patients. The magnitude of the fMRI effects in left BA 45/47 showed moderate correlations with repetition-priming, and was interpreted as evidence of relatively preserved priming in AD. Our results resemble Lustig and Buckner (2004) in that both AD and normal elderly groups had significant New > Old BOLD responses which included portions of BA 44 and 45 in the inferior frontal gyrus (IFG). Similarly, we found that New > Old BOLD response in the left IFG was associated with greater RT priming (r=.47, p < 0.01) across our entire sample, which did not reach statistical significance within AD. We believe this is due to limited power to detect moderate correlations in smaller sample sizes. Within AD, the correlation coefficient was of similar strength in our study (r=.43, p = 0.11) and their prior report (r=.39, p = 0.07, with n= 24 AD participants). As a further refinement to Lustig and Buckner’s study, we found that RT priming on our task correlated significantly with the magnitude of New – Old BOLD responses in BA 47, but not BA 45. The former area has been shown to be concerned with semantic aspects of language processing, while the latter is thought primarily to be concerned with syntactic or phonemic processing (Bookheimer 2002, Daprato and Bookheimer 1999, Wagner et al 2000).
In the present study, we found different patterns of BOLD response across the two hemispheres in AD versus NE. The NE showed a “HERA” pattern (with initial encoding preferentially activating the left, and repeat presentations resulting in increased right-hemisphere responses), which may represent memory recognition processes. Some prior PET studies have suggested that right pre-frontal activity is more closely related to memory retrieval effort, rather than a reliable marker of retrieval success (Kapur et al 1995). Thus, the Old > New BOLD responses in many of our NE subjects may reflect greater retrieval effort, rather than success, as is supported by the lack of significant correlations between right frontal Old > New activation and memory performance. In fact, one region of the right anterior inferior frontal gyrus (BA 47) showed New > Old BOLD responses were associated with higher recall scores. This finding is more consistent with Cabeza’s proposed “HAROLD” (Hemisphere Asymmetry Reduction in Older Adults) model in which the right pre-frontal cortex is more likely to participate in verbal encoding processes in Older than Younger persons (Cabeza 2002). This age-related reduction is asymmetry is thought to have a compensatory function, which may help with declining frontal lobe function. One study limitation is that retrieval was not being systematically manipulated or demanded by our semantic encoding paradigm, but some investigators believe that this is not required for making meaningful applications or testing of the HERA model (Babiloni et al 2006, Habib et al 2003).
We acknowledge that not all New > Old responses on this paradigm are due to memory encoding and not all Old > New responses are due to memory retrieval processes. The complex nature of the task which involves attention, perceptual, conceptual and episodic memory processes along with motor preparation and response has been acknowledged above. We attempted to deal with this complexity by examining correlations with subsequent memory, RT priming and accuracy on the primary semantic task within selected ROIs, which mostly have established roles in episodic memory or semantic memory. Another possible interpretation of the increased right hemisphere response to Old words in normal older participants is that it may indicate resumption of ‘default mode’ activity, as much of this Old > New activity was in the right IPL, a region considered central to this network. The relationship of impaired default network function and memory impairment is currently an area of intensive investigation (Buckner et al., 2005; Celone et al., 2006; Greicius, Srivastava, Reiss, & Menon, 2004; Rombouts et al., 2005b) which may fundamentally advance our understanding of how attention and short-term memory interact. The precuneus and posterior cingulate are two of the earliest regions to show severe atrophy and amyloid deposition in AD (Buckner et al 2005). The connectivity of the PCC makes it a likely “crossroad” between limbic structures critical for memory and neocortical regions supporting exogenous attention (Vincent et al., 2006; Vogt, Finch, & Olson, 1992). In particular, its reciprocal connections with the PHG (Suzuki & Amaral, 1994) may be relevant to why both of these structures (in the left hemisphere) show New > Old effects during this incidental verbal learning paradigm. On our cross-modal incidental learning paradigm, NE showed significant activation in left PCC to New words which attenuated with word repetition while AD patients had only modest BOLD event-related responses in the PCC, similar for New and Old words. This adds to the growing literature showing abnormal cingulate function or responses in early AD (Del Sole et al., 2008; Greicius et al., 2004; Johnson et al., 2006; Lustig et al., 2003). Celone et al (2006), using independent component analysis, found that patients with AD or advanced MCI had the least task-related hippocampal activity and the least task-related ‘deactivation’ in left cingulate and bilateral parietal cortex during face-name encoding. Our cross-modal semantic task, in contrast, strongly suggests that left PCC can also be in relative synchrony with its ipsilateral MTL connections, as a sign of successful associative encoding.
These fMRI results agree well with our ERP studies wherein normal controls have shown large P600 repetition effects with a left-hemisphere bias, while mild AD patients have severely attenuated repetition effects with a right-central peak (Olichney et al., 2006), intriguingly near the two right parietal New > Old clusters found in AD. Pariente et al (2005) previously reported left IPL activation in mild AD (hyperactivation compared to healthy elderly) during the successful encoding of name-face associations. Their face encoding task also produced hyperactivation of the right parietal and frontal cortex, which they interpreted as reflecting compensating strategies for memory impairment. Hemispheric abnormalities were also reported in AD, with excessive right-sided activation during encoding and a left-sided emphasis during recognition, which could be interpreted as a “reversed HERA” pattern, although some investigators reserve this term for changes in prefrontal cortex activity only (Habib, Nyberg & Tulving, 2003). Our present study has a somewhat similar pattern of results in which AD patients showed a loss of the normal HERA effect with increased continued left-hemisphere activation to repeated words in conjunction with decreased right hemisphere responses. We interpret this pattern as reflecting ongoing deep semantic encoding, but possibly impaired recognition processes for the repeated words. For example, recognition memory (but not recall) in AD was indexed by the extent of Old > New BOLD response in the right medial temporal lobe. Exploratory subregion analyses of the IFG also found that larger New – Old BOLD response in right BA 45 was associated with higher recall (but not recognition) scores in our mild AD group, suggesting this region might help compensate for left-hemisphere dysfunction (Thompson et al, 2003) and the verbal and semantic memory encoding deficits which comprise an established cardinal feature of AD (Granholm & Butters, 1988; Martin, Brouwers, Cox & Fedio, 1985).
Our correlation analyses showed that New > Old BOLD responses in the left MTL and PCC correlated with subsequent recall within NE. In our participants, the strongest correlations of successful recall were the extent of left MTL activation (New > Old) and the magnitude of left STG New – Old activation, suggesting that the efficiency of semantic encoding, and dominant temporal lobe function, is well indexed by this paradigm. Another study limitation is that the use of a standardized template in Talairach space to measure the MTL and its BOLD responses. Greater MTL atrophy in the AD group may have led to a greater percentage of noisy voxels, and thus handicap our ability to find significant activation. On the other hand, the use of a standard uniform ROI of a fixed dimension does not bias the chance of finding false positive voxels (i.e. due to chance alone) between groups and between subjects. Manually corrected MTL voxel counts have also been done (with method described in Olichney et al, in press ePub) which are very highly correlated (r = 0.88-0.92 range across measures) with the automated counts and led to essentially the same results.
We also found significant correlations between memory performance and bilateral fusiform and IFG response, both putative P600 generators (Halgren et al., 1994). Both BA 47 and fusiform cortex have been shown to be sensitive to semantic processing (Bookheimer 2002, McCarthy et al 1995, Wagner et al. 2000). Thus, it seems likely that New > Old BOLD response in these areas is a sign of “deeper” more elaborative semantic encoding within our NE cohort. As noted above, bilateral IFG and BA 47 activation would be predicted by Cabeza’s HAROLD model, rather than unilateral left frontal activation, in our normal elderly. Prior fMRI studies of memory encoding have shown that greater left fusiform activity is associated with subsequent recall (Dickerson et al., 2007; Wagner et al., 1998a), and more bilateral fusiform activity is associated with subsequent recognition (Garoff, Slotnick, & Schacter, 2005). Interestingly, the magnitude of bilateral IFG New – Old activation did correlate with subsequent memory in NE, but not in AD (who had significant left IFG activation in BA 44 and 45). Previous literature has suggested the prefrontal regions have a compensatory role for medial temporal activity declines in encoding and recognition in older adults (Grady, McIntosh, & Craik, 2005; Gutchess et al., 2005). Outside of the IFG, the AD group showed a lack of any other significant New – Old clusters in their entire left hemisphere. Perhaps, New – Old effects need to be also present across several key left hemisphere regions (e.g. also MTL, FG, lateral temporal cortex) in order for effective memory “binding” with enduring associations to occur.
Regarding which brain regions appeared to predict success on the primary semantic judgment task, we found that the magnitude of New > Old activation in the bilateral anterior cingulate, right IFG and left MTG were all correlated with task accuracy. The ACC is closely related to conflict monitoring and decision making (Jones, Cho, Nystrom, Cohen & Braver, 2002) and therefore correlations with task accuracy might be predicted. We interpret the behavioral correlations with right IFG and left MTG activation as providing additional evidence that these regions participate in semantic processes and semantic judgments. However, independent replication and caution is advised before concluding that activation of these regions are necessarily a sign of high performance on semantic judgment tasks.
Similar to Rombouts and colleagues’ (2005a) fMRI study of face encoding, the intergroup fMRI differences were affected by the precise time period analyzed. Analyses designed to examine peak BOLD response (shift 3-TR) showed more pervasive differences than analyses of the sustained BOLD response (shift 4-TR). However, it should be kept in mind that apparent differences in the latency of a “significant” BOLD response can be confounded by differences in BOLD signal intensity (Henson et al 2002). Thus, some of the voxels activated at 4-TR (7 seconds post-visual target word), may have been smaller, rather than later, BOLD responses. Our use of deconvolution analyses with independent estimates of the BOLD response at consecutive time points, nonetheless, remains the generally accepted best method for estimating the shape of the hemodynamic response, while canonical “gamma” functions (Cohen, 1997) are generally preferred for estimating the amplitude of response (Birn, Cox & Bandettini, 2002). “Peak” (3-TR) BOLD responses showed only one cluster where the AD group had more New > Old responses, and this was in the right parietal cortex, a ‘default-mode’ region expected to normally deactivate during memory encoding. The model for “sustained” (4-TR) BOLD response showed clear differences in hemispheric response patterns: Nearly all the Normal > AD clusters were in the left-hemisphere (e.g. PHG, IFG), while nearly all AD > Normal clusters were on the right, including decrements in Old > New activation in right prefrontal regions thought important for memory recognition (e.g. BA 9, 46) (Tulving et al., 1994b) and stimulus familiarity (Henson, Rugg, Shallice, Josephs, & Dolan, 1999). The increase in right hemisphere New > Old BOLD response in AD appears to have been largely driven by an attenuation of BOLD response to the Old words, with some “deactivation” at the later timepoints, rather than a delay in the peak BOLD response to New words. Thus, our study, along with Rombouts et al’s (2005a) results, supports the notion that mild AD patients have abnormal brain dynamics when performing various memory encoding tasks. But, unlike Rombouts and colleagues, we did not find significant delays in the modeled peak BOLD response of our AD group, relative to NE.
In conclusion, our results suggest that a distributed left-hemisphere network of putative P600 generators (e.g. parahippocampal, inferior frontal and fusiform gyri, hippocampus, cingulate cortex), which normally has New > Old BOLD responses to congruous words, is important for successful verbal encoding. This network appears highly dysfunctional in mild AD who show decreased congruous word repetition effects, particularly in left association cortex, paralimbic, and MTL structures. In addition, the right ventrolateral prefrontal cortex (especially BA 47) and fusiform gyrus appeared to participate in successful verbal encoding in our normal elderly, perhaps by mediating elaborative semantic encoding. The differences in medial temporal lobe and cortical hemispheric response patterns allowed excellent group discrimination when the main fMRI measures were used in logistic regression models. Thus, this word repetition fMRI paradigm has the potential to be a clinically useful marker of early Alzheimer’s disease and also has provided some new insights into the spatio-temporal mechanisms which underlie their verbal encoding deficits.
Supplementary Material
Supplemental E-Figure 1. Timing of sample single trial (Event-related fMRI data acquisition). Each trial beings with the presentation of a fixation crosshair (“+”) and an auditory category statement (total duration of audio file = 3 s, including brief inter-stimulus interval), then a visual target word is presented for 500 ms, followed by a blank screen for a variable inter-trial interval of 2.5, 5, 7.5 or 10 s to produce trials of variable duration (7.5, 10, 12.5 or 15 s in total). Since the target word is presented 3 seconds following trial onset, the 3- and 4-TR latencies capture the rising/peak (~4.5 s) and falling (~7 s) phases, respectively, of the expected BOLD response to the target word (based on Cohen’s gamma-function (Cohen, 1997)).
Table 3B.
AD > NE Clusters, Congruous New – Old Words.
| Structure (Brodmann’s area) | CM | Volume | Mean (SE) | MI |
t | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||||
|
3-TR (4.5 seconds after visual word),
Center and Extent |
||||||||||
| R IPL(40), R SPL(7) | 40 | −47 | 43 | 960 | 2.64(0.20) | 38 | −49 | 44 | 2.73 | .005 |
|
4TR (7 seconds after visual word),
Center and Extent |
||||||||||
| R MFG(9, 46), R IFG | 41 | 20 | 29 | 5760 | 2.28(0.06) | 42 | 11 | 36 | 2.96 | .003 |
| PreCG(4), R PostCG(3, 2) | 46 | −17 | 38 | 2240 | 2.11(0.08) | 46 | −13 | 40 | 2.66 | .006 |
| R Cerebellum | 28 | −65 | −39 | 1024 | 1.51(0.09) | 26 | −69 | −36 | 2.28 | .015 |
| R LG(19), R FG(37) | 32 | −52 | −5 | 1024 | 1.95(0.18) | 30 | −45 | −4 | 2.10 | .022 |
| L Precuneus(7) | −2 | −63 | 40 | 832 | 2.74(0.21) | −2 | −65 | 44 | 2.05 | .024 |
| R STG(42, 22), R TTG(41) | 60 | −20 | 9 | 768 | 2.63(0.20) | 58 | −25 | 8 | 3.10 | .002 |
Legend: Clusters above list center first, then other surrounding structures with significant intensity differences. The t and p values are listed for the voxel with Maximum Intensity (MI). FG = Fusiform Gyrus, IFG = Inferior Frontal Gyrus, IPL = Inferior Parietal Lobule, L = Left, LG = Lingual Gyrus, MFG = Middle Frontal Gyrus, PHG = Parahippocampal Gyrus, PostCG = Postcentral Gyrus, PreCG = Precentral Gyrus, R = Right, = Superior Parietal Lobule, STG = Superior Temporal Gyrus, TTG = Transverse Temporal Gyrus.
ACKNOWLEDGMENTS
Special thanks to the Center for Mind and Brain, Laboratory of Cognitive Imaging (LOCI), VA San Diego Healthcare System, the UC Davis Alzheimer’s Disease Center (ADC), and the Shiley-Marcos Alzheimer’s Disease Research Center (ADRC). We would like to thank Gregory Brown, Terry Jernigan, Eric Wong, Simion Kreimer, Shaunna Morris, and the late Leon Thal for advising the adaptation of this paradigm for fMRI. We thank Jeremy Smith and Alexander Bressler for technical support, and Lannah Lua and Lillian Chi for manuscript preparation.
FUNDING Supported by NIH grants #R01-AG18442, R01-AG08313, P30-AG10129 & P50-AG05131 and the California Department of Health’s Alzheimer’s Disease Program.
Footnotes
Disclosure: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John M. Olichney, Department of Neurology, University of California, Davis, Davis, CA, USA.
Jason R. Taylor, Medical Research Council, Cognition and Brain Sciences Unit, Cambridge, UK.
Shiaohui Chan, Department of English, National Taiwan Normal University, Taipei, Taiwan.
Jin-Chen Yang, Department of Neurology, University of California, Davis, Davis, CA, USA.
Andrew Stringfellow, University of California, San Diego, CA, USA.
Dieter G. Hillert, Department of Neurosciences, University of California, San Diego, CA, USA.
Amanda L. Simmons, Dept. of Psychology, California State University, Sacramento, CA, USA.
David P. Salmon, Department of Neurosciences, University of California, San Diego, CA, USA.
Vicente Iragui-Madoz, Department of Neurosciences, University of California, San Diego, CA, USA.
Marta Kutas, Department of Cognitive Science, University of California, San Diego, CA, USA.
REFERENCES
- Arnold SE, Hyman BT, Flory J, Damasio AR, van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cerebral Cortex. 1991;1(1):103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Vecchio F, Cappa S, Pasqualetti P, Rossi S, Miniussi C, et al. Functional frontoparietal connectivity during encoding and retrieval processes follows HERA model. A high-resolution study. Brain Research Bulletin. 2006;68(4):203–212. doi: 10.1016/j.brainresbull.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Detection versus estimation in event-related fMRI: choosing the optimal stimulus timing. Neuroimage. 2002;15(1):252–264. doi: 10.1006/nimg.2001.0964. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annual Review of Neuroscience. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: An independent component analysis. Journal of Neuroscience. 2006;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nature Neuroscience. 1999;2(10):913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Chwilla DJ, Brown CM, Hagoort P. The N400 as a function of the level of processing. Psychophysiology. 1995;32(3):274–285. doi: 10.1111/j.1469-8986.1995.tb02956.x. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Del Sole A, Clerici F, Chiti A, Lecchi M, Mariani C, Maggiore L, Mosconi L, Lucignani G. Individual cerebral metabolic deficits in Alzheimer’s disease and amnestic mild cognitive impairment: An FDG PET study. European Journal of Nuclear Medicine and Molecular Imaging. 2008;35(7):1357–1366. doi: 10.1007/s00259-008-0773-6. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Bookheimer SY. Form and content: dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24(2):427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Miller SL, Greve DN, Dale AM, Albert MS, Schacter DL, Sperling RA. Prefrontal-hippocampal-fusiform activity during encoding predicts intraindividual differences in free recall ability: an event-related functional-anatomic MRI study. Hippocampus. 2007;17(11):1060–1070. doi: 10.1002/hipo.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellgiebel A, Schermuly I, Gerhard A, Keller I, Albrecht J, Weibrich C, Muller MJ, Stoeter P. Functional relevant loss of long association fibre tracts integrity in early Alzheimer’s disease. Neuropsychologia. 2008;46(6):1698–1706. doi: 10.1016/j.neuropsychologia.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Fernández G, Effern A, Grunwald T, Pezer N, Lehnertz K, Dümpelmann M, Roost DV, Elger CE. Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science. 1999;285(5433):15082–1585. doi: 10.1126/science.285.5433.1582. [DOI] [PubMed] [Google Scholar]
- Garoff RJ, Slotnick SD, Schacter DL. The neural origins of specific and general memory: The role of the fusiform cortex. Neuropsychologia. 2005;43(6):847–859. doi: 10.1016/j.neuropsychologia.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43(10):1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Granholm E, Butters N. Associative encoding and retrieval in Alzheimer’s and Huntington’s disease. Brain and Cognition. 1988;7(3):335–347. doi: 10.1016/0278-2626(88)90007-3. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Science of the United States of America. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem F, Rougier A, Claverie B. Short- and long-delay intracranial ERP repetition effects dissociate memory systems in the human brain. Journal of Cognitive Neuroscience. 1999;11(4):437–458. doi: 10.1162/089892999563526. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations for decreased medial temporal activity. Journal of Cognitive Neuroscience. 2005;17(1):84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Habib R, Nyberg L, Tulving E. Hemispheric asymmetries of memory: the HERA model revisited. Trends Cogn Sci. 2003;7(6):241–245. doi: 10.1016/s1364-6613(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Clarke M. Spatio-temporal stages in face and word processing. Depth recorded potentials in the human occipital, temporal and parietal lobes. Journal of Physiology. 1994;88(1):1–50. doi: 10.1016/0928-4257(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: An event-related functional magnetic resonance imaging study. Journal of Neuroscience. 1999;19(10):3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: Application to words versus non-words and initial versus repeated face presentations. Neuroimage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Moritz CH, Meyerand ME, Rowley HA, Alexander AL, Hansen KW, Gleason CE, Carlsson CM, Ries ML, Asthana S, Chen K, Reiman EM, Alexander GE. Activation of brain regions vulnerable to Alzheimer’s disease: The effect of mild cognitive impairment. Neurobiology of Aging. 2006;27(11):1604–1612. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AD, Cho RY, Nystrom LE, Cohen JD, Braver TS. A computational model of anterior cingulate function in speeded response tasks: effects of frequency, sequence, and conflict. Cognitive, Affective, and Behavioral Neuroscience. 2002;2(4):300–317. doi: 10.3758/cabn.2.4.300. [DOI] [PubMed] [Google Scholar]
- Hansen KW, Gleason CE, Carlsson CM, Ries ML, Asthana S, Chen K, Reiman EM, Alexander GE. Activation of brain regions vulnerable to Alzheimer’s disease: The effect of mild cognitive impairment. Neurobiology of Aging. 2006;27(11):1604–1612. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Pascual-Leone A, Theoret H, Fregni F, Clark D, Wagner AD. Transient disruption of ventrolateral prefrontal cortex during verbal encoding affects subsequent memory performance. Journal of Neurophysiology. 2005;94(1):688–698. doi: 10.1152/jn.01335.2004. [DOI] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Jones C, Brown GM, Houle S, Tulving E. Functional role of the prefrontal cortex in retrieval of memories: a PET study. Neuroreport. 1995;6(14):1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. Journal of Neuroscience. 2000;20(16):6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Buckner RL. Preserved neural correlates of priming in old age and dementia. Neuron. 2004;42(5):865–875. doi: 10.1016/j.neuron.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O’Brien PC, Petersen RC, Boeve BF, Knopman D, Tang-Wai DF, Ivnik RJ, Smith GE, Tangalos EG, Jack CR., Jr. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61(4):500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Brouwers P, Cox C, Fedio P. On the nature of the verbal memory deficit in Alzheimer’s disease. Brain and Language. 1985;25(2):323–341. doi: 10.1016/0093-934x(85)90088-4. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Nobre AC, Bentin S, Spencer DD. Language-related field potentials in the anterior-medial temporal lobe: I. Intracranial distribution and neural generators. Journal of Neuroscience. 1995;15(2):1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Van Petten C, Paller K, Salmon DP, Iragui VJ, Kutas M. Word repetition in amnesia: Electrophysiological measures of impaired and spared memory. Brain. 2000;123(Pt 9):1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Morris SK, Ochoa C, Salmon DP, Thal LJ, Kutas M, Iragui VJ. Abnormal verbal event-related potentials in mild cognitive impairment and incipient AD. Journal Neurology, Neurosurgery, and Psychiatry. 2002;73:377–384. doi: 10.1136/jnnp.73.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Iragui VJ, Salmon DP, Riggins BR, Morris SK, Kutas M. Absent event-related potential (ERP) word repetition effects in mild Alzheimer’s disease. Clinical Neurophysiology. 2006;117(6):1319–1330. doi: 10.1016/j.clinph.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Gatherwright J, Taylor JR, Salmon DP, Kutas M, Iragui VJ. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology. 2008;70(19 Pt 2):1763–70. doi: 10.1212/01.wnl.0000281689.28759.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Taylor JR, Hillert DG, Chan S, Salmon DP, Gatherwright J, Iragui VJ, Kutas M. fMRI congruous word repetition effects reflect memory variability in normal elderly. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2008.10.010. (in-press) Epub ahead of print, doi:10.1016/j.neurobiolaging.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariente J, Cole S, Henson R, Clare L, Kennedy A, Rossor M, Cipoloti L, Puel M, Demonet JF, Chollet F, Frackowiak RSJ. Alzheimer’s patients engage an alternative network during a memory task. Annals of Neurology. 2005;58(6):870–879. doi: 10.1002/ana.20653. [DOI] [PubMed] [Google Scholar]
- Remy F, Mirrashed F, Campbell B, Richter W. Verbal episodic memory impairment in Alzheimer’s disease: a combined structural and functional MRI study. Neuroimage. 2005;25(1):253–266. doi: 10.1016/j.neuroimage.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Ries ML, Schmitz TW, Kawahara TN, Torgerson BM, Trivedi MA, Johnson SC. Task-dependent posterior cingulate activation in mild cognitive impairment. Neuroimage. 2006;29(2):485–492. doi: 10.1016/j.neuroimage.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Goekoop R, Stam CJ, Barkhof F, Scheltens P. Delayed rather than decreased BOLD response as a marker for early Alzheimer’s disease. Neuroimage. 2005a;26(4):1078–1085. doi: 10.1016/j.neuroimage.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: An fMRI study. Human Brain Mapping. 2005b;26(4):231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging Working Group Consensus Report of the Working Group on: “Molecular and Biochemical Markers of Alzheimer’s Disease”. Neurobiology of Aging. 1998;19:109–116. [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: Successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20(2):1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. Journal of Comparative Neurology. 1994;350(4):497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Taylor JR, Olichney JM. From amnesia to dementia: ERP studies of memory and language. Clin EEG Neurosci. 2007;38(1):8–17. doi: 10.1177/155005940703800106. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proceedings of the National Academy of Sciences of the United States of America. 1994a;91(6):2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Markowitsch HJ, Craik FI, Habib R, Houle S. Neuroanatomical correlates of retrieval in episodic memory: Auditory sentence recognition. Proceedings of the National Academy of Sciences of the United States America. 1994b;91(6):2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, et al. Dynamics of gray matter loss in Alzheimer’s disease. Journal of Neuroscience. 2003;23(3):994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of Neurophysiology. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: The anterior executive and posterior evaluative regions. Cerebral Cortex. 1992;2(6):435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998a;281(5380):1188–91. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Desmond JE, Glover GH, Gabrieli JD. Prefrontal cortex and recognition memory. Functional-MRI evidence for context-dependent retrieval processes. Brain. 1998b;121(Pt 10):1985–2002. doi: 10.1093/brain/121.10.1985. [DOI] [PubMed] [Google Scholar]
- Wagner AD. Working memory contributions to human learning and remembering. Neuron. 1999;22(1):19–22. doi: 10.1016/s0896-6273(00)80674-1. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Koutstaal W, Maril A, Schacter DL, Buckner RL. Task-specific repetition priming in left inferior prefrontal cortex. Cerebral Cortex. 2000;10(12):1176–1184. doi: 10.1093/cercor/10.12.1176. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng F-H, Bayne W, Mori S, Schad L, Mueller S, Du A-T, Kramer JH, Yaffe K, Chui H, Jagust WJ, Miller BL, Weiner MW. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68(1):13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental E-Figure 1. Timing of sample single trial (Event-related fMRI data acquisition). Each trial beings with the presentation of a fixation crosshair (“+”) and an auditory category statement (total duration of audio file = 3 s, including brief inter-stimulus interval), then a visual target word is presented for 500 ms, followed by a blank screen for a variable inter-trial interval of 2.5, 5, 7.5 or 10 s to produce trials of variable duration (7.5, 10, 12.5 or 15 s in total). Since the target word is presented 3 seconds following trial onset, the 3- and 4-TR latencies capture the rising/peak (~4.5 s) and falling (~7 s) phases, respectively, of the expected BOLD response to the target word (based on Cohen’s gamma-function (Cohen, 1997)).