Abstract
Mature CD4+Vβ5+ T cells that recognize a peripherally expressed endogenous superantigen are tolerized either by deletion or T cell receptor (TCR) revision. In Vβ5 transgenic mice, this latter tolerance pathway results in the appearance of CD4+Vβ5−TCRβ+ T cells, coinciding with Rag1, Rag2, and TdT expression and the accumulation of Vβ-DJβ recombination intermediates in peripheral CD4+ T cells. Because post-thymic RAG-dependent TCR rearrangement has remained controversial, we sought to definitively determine whether TCR revision is an extrathymic process that occurs in mature peripheral T cells. We now show that Rag deletion in post-positive selection T cells in Vβ5 transgenic mice blocks TCR revision in vivo, and that mature peripheral T cells sorted to remove cells bearing endogenous TCRβ chains can express newly generated TCRβ molecules in adoptive hosts. These findings unambiguously demonstrate post-thymic, RAG-dependent TCR rearrangement and define TCR revision as a tolerance pathway that targets mature peripheral CD4+ T cells.
Keywords: TCR revision, RAG1, RAG2, dLck-cre transgenic
Introduction
The precise control of RAG expression during T cell development in the thymus results in the ordered production of functional and self-MHC restricted T cell receptors for antigen (TCRs). During thymocyte development, RAG mediates TCRβ and then TCRα gene rearrangement (reviewed in Ref. 1). Pre-TCR and TCR signaling at the double-negative (DN) 3 and double-positive (DP) thymocyte stages, respectively, results in the rapid extinction of RAG expression, thereby ensuring allelic exclusion (reviewed in Ref. 2).
In Vβ5 transgenic (Tg) and non-transgenic (nonTg) C57BL/6 (B6) mice, chronic encounter of peripheral Vβ5+CD4+ T cells with an endogenous mammary tumor virus (Mtv)-encoded superantigen induces tolerance, either through deletion (3, 4), or TCR revision (5). Through TCR revision, CD4+Vβ5+ cells induce RAG expression and undergo TCRβ rearrangement, resulting in the generation of post-revision CD4+Vβ5−TCRβ+ T cells expressing a diverse repertoire of newly generated TCRs (5, 6). Peripheral CD4+ T cells from Vβ5 Tg mice express Rag1 and Rag2 and carry TCR Vβ-DJβ recombination intermediates (5, 7). Since the initial discovery of RAG+ peripheral T cells and TCR revision in Vβ5 Tg mice, several groups have demonstrated RAG expression and TCR gene recombination in peripheral T cells in both mouse and human (reviewed in Refs. 8, 9).
Despite extensive studies demonstrating TCR revision in several different experimental systems, the idea of post-thymic RAG-mediated TCR rearrangement in peripheral T cells remains controversial. Given the tight developmental control of RAG expression and the rigorous selection imposed on expressed TCRs, can RAG re-expression and TCR rearrangement be allowed outside the highly specialized thymic environment? To address this controversy, we devised a strategy for the conditional deletion of Rag2 in peripheral T cells, to clearly delineate the timeframe for RAG expression during TCR revision in Vβ5 Tg mice. Using enhanced yellow fluorescent protein (YFP) to report Cre-mediated recombination in mice transgenic for cre driven by the distal Lck promoter (dLck) that delete a floxed Rag2 allele after positive selection in the thymus, we show that excision of the Rag2 gene in adoptively-transferred YFP+ peripheral CD4+ T cells blocks TCR revision.
Materials and Methods
Mice
Vβ5 Tg and nonTg littermates on the B6 background were bred under specific-pathogen free conditions at the University of Washington. Rag2fl/fl mice (10) on the B6 background were a gift from K. Rajewsky (Harvard Medical School, Boston, MA). R26R-YFP reporter mice carrying a floxed transcriptional stop element that precedes the YFP reporter gene targeted to the endogenous ROSA26 locus (11) were provided by A. Rudensky (Sloan-Kettering Institute, New York, NY). Mice bearing the R26R-YFP reporter were hemizygous for this allele, except where indicated. Line 3779 dLck-icre Tg mice (12) were a gift from N. Killeen (University of California, San Francisco, CA). All mice were backcrossed to the B6 background >10 generations and intercrossed to generate Vβ5 Tg and nonTg B6 mice that were dLck-icreRag2fl/flR26R-YFP, hereafter referred to as dLck-creRag2fl/flYFP mice. Controls with the wild-type (WT) Rag2 gene are referred to as dLck-creRag2+/+YFP mice. B6 Ly5.2+ and B6.SJL (B6.SJL-PtprcaPepcb/BoyJ) Ly5.1+ mice and their F1 generation (Ly5.1+Ly5.2+) were purchased from The Jackson Laboratory or bred in house. Thymectomy was performed on 7-week-old mice as previously described (13). All experiments were conducted in accordance with the University of Washington Institutional Animal Care and Use Committee.
Cell preparation, enrichment, flow cytometry and sorting
Single-cell suspensions were prepared from thymus, spleen, and a pool of brachial, axillary, and inguinal lymph nodes. RBCs were removed from spleen and blood by water lysis. For flow cytometry, Fc receptors were blocked using anti-CD16/32 (2.4G2; BD Pharmingen). Cells were surface stained in HBSS containing 1% BSA with fluorochrome-conjugated or biotinylated antibodies (followed by fluorochrome-conjugated streptavidin), all purchased from BD Biosciences or eBioscience. Antibodies recognize mouse CD4 (RM4-5), CD8α (53-6.7), CD45R/B220 (RA3-6B2), CD45.1/Ly5.1 (A20), CD45.2/Ly5.2 (104), CD69 (H1.2F3), panTCRβ (H57-597.13), Vβ2 (B20.6), Vβ3 (KJ25), Vβ4 (KT4-10), Vβ5 (MR9-4), Vβ6 (RR4-7), or Vβ8.1/8.2 (KJ16). Flow cytometry data were collected using a FACSCanto (Becton Dickinson) and analyzed with FlowJo software (Treestar). “Untouched” T cell enrichments were performed using an EasySep Mouse T cell Enrichment Kit (Stem Cell Technologies). Cell sorting was done using a FACSAria (Becton Dickinson). For adoptive transfers, sorted cells were i.v. injected into the lateral tail vein in a 200 μl volume of HBSS. Recipient mice were prepared 17 days prior to adoptive transfer by injection of depleting anti-Thy-1 antibody (T24), as indicated.
Real-time PCR
Genomic DNA from sorted cell populations was RNase treated and processed using the PUREGENE DNA Purification Kit and protocol (Qiagen). DNA was used as a template for quantitative PCR using the Power SYBR Green PCR Master Mix (Applied Biosystems) and primers to amplify either the intact Rag2fl/fl product: (Forward) 5′-CAAACGTGCGAAGGGACTA-3′ and (Reverse) 5′-GCGCTCCTCCTGATACTCT-3′, or the Cd43 gene fragment: (Forward) 5′-CAAGCCTCAGGAAGAACTGG-3′ and (Reverse) 5′-CCTGGCCTTCATTCATTGTT-3′. PCR conditions were as follows: 10-min denaturation at 95°C, followed by 40 cycles of 15 s at 95°C, 30 s at 60°C, and 30 s at 72°C. All quantitative PCR was conducted using an ABI 7300 Real Time PCR System (Applied Biosystems). Reactions were run in triplicate and values for each sample averaged and normalized to the Cd43 control.
Results and Discussion
Conditional deletion of Rag2 in post-positive selection T cells
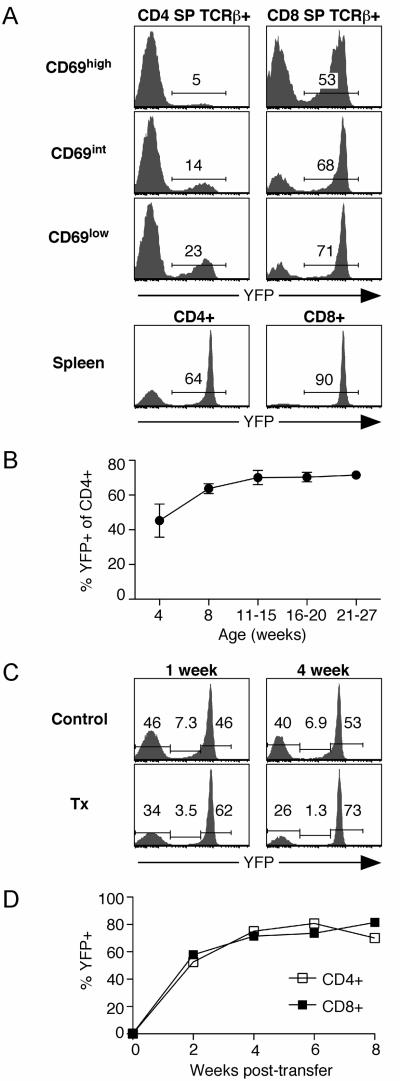
We devised an experimental system to conditionally delete floxed Rag2 alleles in peripheral T cells without interfering with RAG-mediated TCR gene rearrangement in the thymus. We made use of the previously described dLck-cre Tg line in which cre recombinase expression, regulated by the distal Lck promoter, is initiated following thymic positive selection (12). Cre activity was reported by removal of a floxed stop element to allow expression of a YFP reporter gene targeted to a ubiquitously expressed locus (11). As expected (12), YFP expression in dLck-creRag2fl/flYFP mice was undetectable in non-T cell populations in the bone marrow and spleen, and in DN and DP thymocytes (data not shown), and only appeared after CD4 and CD8 single-positive (SP) thymocytes had upregulated TCR and CD69 expression following positive selection (Fig. 1A). Maturation of post-selection TCRβ+ CD4 SP and CD8 SP thymocytes to the CD69low compartment was accompanied by an increase in the YFP+ fraction (Fig. 1A). Importantly, Vβ5 Tg and nonTg WT and dLck-creRag2fl/flYFP mice had equivalent percents and absolute cell numbers of DN, DP, CD4 SP, and CD8 SP thymocytes and splenic T cells, suggesting that neither Rag2 gene deletion nor expression of Cre or YFP resulted in cell toxicity or altered thymocyte development (data not shown).
Figure 1. Gradual upregulation of YFP expression as a reporter for Cre-mediated recombination.
A. YFP reporter expression by post-selection TCRβ+ CD4 SP and CD8 SP thymocytes of the indicated CD69 phenotype and CD4+ and CD8+ splenocytes from 12-week old dLck-creRag2fl/flYFP mice. Data are representative of 4 mice. B. YFP reporter expression by CD4+ PBL from dLck-creRag2fl/flYFP mice of the indicated ages. Data points represent the mean with error bars indicating SD for at least 4 mice for each indicated age group. C. YFP reporter expression by CD4+ PBL from Vβ5 Tg dLck-creRag2fl/flYFP euthymic controls and littermate mice thymectomized 1 or 4 weeks previously. Data are representative of 6 mice for each group. D. YFP expression by peripheral T cells from dLck-creRag2fl/flYFP mice sorted as YFP− to >99% purity and transferred into B6 Ly5.1+ recipients that had been pre-treated with a depleting anti-Thy-1 antibody. Donor CD4+ and CD8+ T cells were analyzed to identify the percent that had converted to YFP+ at 2, 4, 6 (from blood) and 8 (spleen) weeks after transfer. Data points are the average value from 2 recipient mice, and are representative of 5 independent experiments for a total of 8 mice.
Compared to the most mature CD69low SP thymocyte subset, splenic CD4+ and CD8+ T cells had a much higher percent of YFP+ cells, suggesting that some YFP− cells become YFP+ after exit from the thymus (Fig. 1A). This apparent conversion is most obvious in the CD4+ T cell compartment. In the lymphoid periphery, 11-15-week-old mice had a higher percent of YFP+ peripheral CD4+ T cells compared to 4-week-old mice (Fig. 1B), an age at which a majority of the peripheral T cell compartment is comprised of recent thymic emigrants (13).
To test whether CD4+YFP− cells in the periphery were enriched for recent thymic emigrants, we thymectomized mice and analyzed peripheral CD4+ T cells 1 and 4 weeks later. In thymectomized compared to euthymic control mice, there was a reduction in the percent of YFP− cells and a corresponding increase in the percent of YFPhigh cells. The proportion of CD4+ T cells expressing intermediate levels of YFP (and therefore had most recently initiated YFP reporter expression) was reduced approximately 5-fold within 4 weeks of thymectomy. These data indicate that a large proportion of the YFP− population becomes YFP+ in the first several weeks after exiting the thymus in dLck-creRag2fl/flYFP mice (Fig. 1C). However, not all peripheral YFP− cells become YFP+, even 12 months after thymectomy (data not shown). To directly test whether YFP− peripheral T cells convert to YFP+, sorted YFP− cells were transferred into adoptive hosts. Within 2 weeks, a majority of CD4+ and CD8+ cells had activated YFP expression (Fig. 1D). Combined, these data demonstrate that YFP-reported Cre activity within the CD4+ T cell lineage in dLck-cre Tg mice increases gradually over time, beginning in the thymus at the SP stage and progressing as the cells age in the lymphoid periphery.
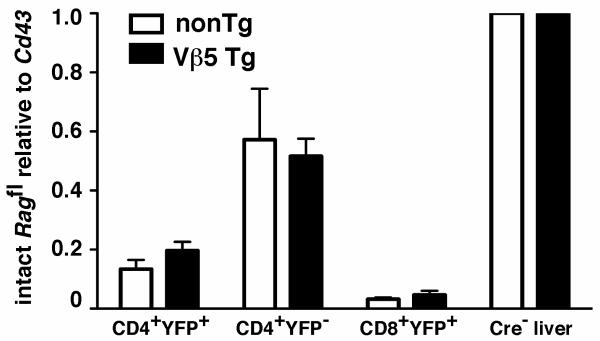
Given the dynamic nature of YFP reporter expression in dLck-creRag2fl/flYFP mice, we next tested whether YFP faithfully reports Cre-mediated activity in peripheral T cells. Using quantitative PCR on genomic DNA from sorted cells, we determined that most CD4+YFP+ and CD8+YFP+ cells had removed the floxed Rag2 target gene (Fig. 2). Surprisingly, nearly half of the floxed Rag2 alleles had also been excised in CD4+YFP− T cells. Similar patterns were observed for both Vβ5 Tg and TCR nonTg dLck-creRag2fl/flYFP mice (Fig. 2). Thus, the YFP signal does not identify all cells that have undergone Cre-mediated recombination. Such disparate recombination frequencies between target and reporter loci are a known complication for conditional gene removal systems (14). Importantly for our subsequent experiments, most YFP+ cells have deleted the floxed Rag2 target. These data, combined with the complicated dynamics of peripheral T cell YFP− to YFP+ conversion (Fig. 1) prevent equating YFP+ and YFP− T cells for comparison within the same mouse as Rag2-deficient and Rag2-sufficient counterparts, respectively. Instead, we used an adoptive transfer system to track sorted populations of YFP+ cells from Rag2fl/fl and Rag2+/+ mice.
Figure 2. The majority of YFP+ cells have excised the floxed Rag2 gene.
Genomic DNA was isolated from CD4+YFP+, CD4+YFP−, and CD8+YFP+ T cells sorted to >98% purity from 7-9 week old TCR nonTg and Vβ5 Tg dLck-creRag2fl/flYFP mice. Real-time PCR was used to quantify the abundance of the intact floxed Rag2 allele relative to Cd43. Shown are the mean values with error bars indicating the SD of data normalized to values obtained using liver DNA from Rag2fl/fl mice that were cre nonTg. (TCR nonTg N=4, Vβ5Tg N=5).
YFP and Cre fail to induce rejection of transferred cells
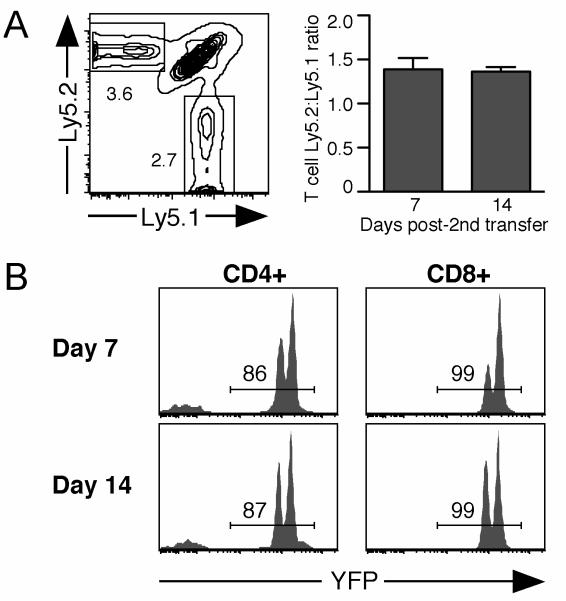
To establish adoptive transfer as a useful model for analyzing the impact of Rag2 deletion on TCR revision, we determined whether YFP and/or Cre serve as transplantation antigens. Splenocytes from dLck-creRag2+/+YFP mice hemizygous for the YFP reporter allele were injected into allelically marked B6 recipients to prime any potential response against YFP or Cre. Two weeks later, a second inoculum was administered, containing equal numbers of congenically marked WT and YFP homozygous dLck-creRag2+/+YFP splenocytes. One week after the second transfer, Ly5.2+ T cells of dLck-creRag2+/+YFP origin were recovered at higher frequencies compared to WT Ly5.1+ T cells (Fig. 3A). Analysis of both CD4+ and CD8+ T cells showed the absence of selection against the YFP+ cells at days 7 and 14 following the second injection (Fig. 3B). Two distinct YFP+ peaks are apparent, the dimmer signal representing YFP hemizygous cells from the initial injection, and the brighter signal from the second injection of YFP homozygous cells (Fig. 3B). The continued presence of cells from the initial injection can account for the higher Ly5.2:Ly5.1 ratio of donor T cells at days 7 and 14 (Fig. 3A). Thus, neither YFP nor Cre act as minor histocompatibility antigens to trigger rejection of transferred cells in B6 recipients.
Figure 3. YFP and Cre fail to trigger rejection of adoptively transferred cells.
Ly5.1+Ly5.2+ B6 recipients were injected with 15×106 splenocytes from YFP hemizygous Ly5.2+ dLck-creRag2+/+YFP mice. Two weeks later, recipients were given a second injection of 18×106 YFP homozygous Ly5.2+ dLck-creRag2fl/flYFP splenocytes mixed with an equal number of Ly5.1+ B6 splenocytes. Recipient splenocytes were analyzed 7 and 14 days later for the presence of Ly5.2+ dLck-creRag2+/+YFP and Ly5.1+ B6 cells. A. Representative Ly5.1/Ly5.2 plot gated on combined CD4+ and CD8+ T cells from spleen of recipients 7 days after the second injection. Chart indicates the Ly5.2:Ly5.1 ratio of donor T cells with error bars indicating SD. B. YFP expression by Ly5.2+ CD4+ and CD8+ donor cells from dLck-creRag2+/+YFP mice on days 7 and 14 after the second injection. Data are representative of 4 recipients.
RAG-dependent TCR revision in peripheral T cells
To determine whether the appearance of post-revision CD4+Vβ5−TCRβ+ T cells requires Rag expression in peripheral T cells, we sorted YFP+ and YFP− peripheral T cells from young Vβ5 Tg dLck-creRag2fl/flYFP (experimental) and Vβ5 Tg dLck-creRag2+/+YFP (control) mice, and transferred them into congenically-marked recipients. Rag2-sufficient CD4+Vβ5+YFP+ cells transferred from Rag2+/+ control donors gave rise to post-revision CD4+Vβ5−TCRβ+ T cells, while YFP+ Rag2-deficient cells from Rag2fl/fl donors remained Vβ5+ (Fig. 4A and 4B). Adoptively-transferred CD4+Vβ5+YFP− cells from both Rag2+/+ and Rag2fl/fl donors that remained YFP− after transfer gave rise to a similar fraction of post-revision Vβ5− cells. Of the CD4+Vβ5+YFP− donor cells that became YFP+ after adoptive transfer, only Rag2+/+ control cells generated post-revision CD4+Vβ5−TCRβ+ cells, while the Rag2fl/fl cells failed to undergo TCR revision (Fig. 4A and 4B). Therefore, Rag2 expression in peripheral T cells is required for the appearance of CD4+Vβ5−TCRβ+ T cells in Vβ5 Tg mice.
Figure 4. Rag2 deletion in peripheral T cells prevents TCR revision.
Splenic and lymph node T cells from 7-8-week old control Vβ5 Tg dLck-creRag2+/+YFP and experimental Vβ5 Tg dLck-creRag2fl/flYFP mice were sorted to obtain YFP+ and YFP− cells that were >99% Vβ5+. 3x106 CD4+YFP+ or CD4+YFP− cells were injected into separate Ly5.1+ recipients that had been pretreated with depleting anti-Thy-1 antibody. Seven weeks after adoptive transfer, donor Ly5.2+CD4+ cells were analyzed for the appearance of post-revision Vβ5−TCRβ+ cells. A. Analysis of YFP and Vβ5 expression by donor CD4+ T cells on the day of sort (left column), and 7 weeks after adoptive transfer (right column). B. Vβ5 and TCRβ expression by Ly5.2+CD4+YFP+ donor cells 7 weeks after adoptive transfer.
In these experiments, the YFP reporter for Cre activity was critical for identifying Rag2-sufficient and Rag2-deficient cells. It is surprising that such a complete block in TCR revision occurred among transferred CD4+YFP+ T cells from Rag2fl/fl donors (Fig. 4), given that 20-40% of CD4+YFP+ T cells from young mice carry at least one intact floxed Rag2 gene (Fig. 2). As post-revision T cells of Rag-sufficient origin first appeared more than 3 weeks after adoptive transfer (data not shown), it is likely that ongoing Cre-mediated excision further reduced the intact floxed Rag2 target in Ragfl/fl YFP+ cells post-adoptive transfer, before Rag expression was needed for TCR revision. Providing further support for this explanation, the amount of intact floxed Rag2 is much lower in CD4+YFP+ T cells from old compared to young mice (data not shown).
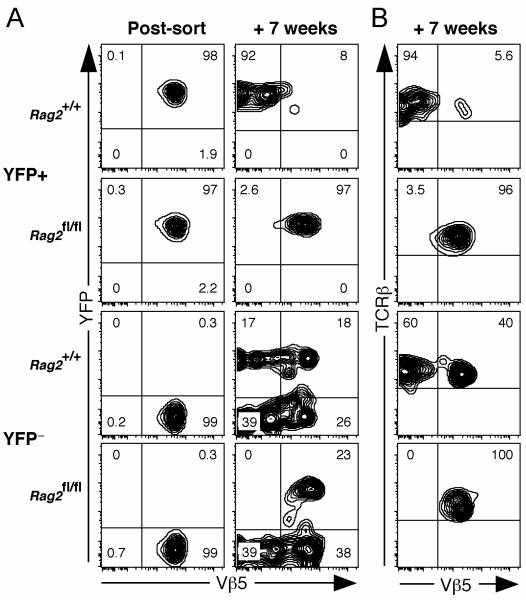
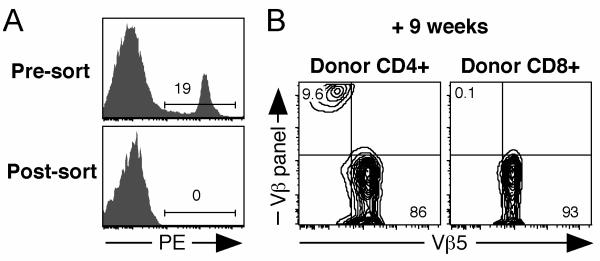
To provide further evidence for post-thymic, RAG-mediated TCR rearrangement in mature CD4+ T cells from Vβ5 Tg mice that do not carry such extensive genetic alterations, we stained peripheral T cells from young Vβ5 Tg B6 mice with a panel of Vβ-specific antibodies, followed by a “dump” sort to remove all T cells expressing any of these TCRβ chains (Fig. 5A). After transfer into congenic recipients, donor CD4+ T cells gave rise to a population of Vβ5− cells that expressed newly formed TCRβ chains recognized by the Vβ-specific panel of antibodies used for the original dump sort, while donor CD8+ T cells remained uniformly Vβ5+ and negative for expression of the Vβ panel (Fig. 5B). These data demonstrate de novo TCRβ expression by peripheral CD4+ T cells from Vβ5 Tg mice.
Figure 5. De novo generation of TCRβ chains by peripheral T cells.
Spleen and lymph node cells from 8-13 week old Ly5.2+ Vβ5 Tg mice were negatively selected using MHC class II and FcR antibody-coated beads. The remaining cells were stained with PE-conjugated antibodies specific for B220, CD11b, Ter119, NK1.1, Vβ2, Vβ3, Vβ4, Vβ6, Vβ8.1, and Vβ8.2, and sorted to obtain a PE− population enriched for T cells. CD4+ and CD8+ sorted cells were >99% Vβ5+ and were transferred into thymectomized Ly5.1+ recipients that had been pretreated with anti-Thy-1 antibody. Splenocytes from recipients were stained 9 weeks later and donor Ly5.2+ CD4+ and CD8+ T cells were analyzed for surface expression of Vβ5 and a panel of Vβs including Vβ2, Vβ3, Vβ4, Vβ6, and Vβ8. A. Pre- and post-sort analysis of cells showing the PE “dump” gate. B. Analysis of donor CD4+ and CD8+ cells for expression of Vβ5 and the Vβ panel (Vβ2, Vβ3, Vβ4, Vβ6, and Vβ8) 9 weeks after transfer.
Prior to this study, extensive data have suggested that RAG-dependent TCR revision is an extrathymic event in Vβ5 Tg mice (reviewed in Refs. 8, 9). To this collective body of evidence, we add new definitive data showing that peripheral CD4+ T cells from Vβ5 Tg mice that have deleted Rag2 after positive selection are unable to generate Vβ5−TCRβ+ CD4+ post-revision T cells. This study clearly demonstrates that TCR revision is a post-thymic process that requires RAG expression in peripheral T cells for the generation of novel TCRs. The germinal center localization of RAG+ revising T cells (15) likely provides the proper microenvironment for selecting an appropriate TCR repertoire outside the thymus.
Acknowledgements
We thank Drs. Nigel Killeen for dLck-icre Tg mice, Klaus Rajewsky for Rag2fl/fl mice, and Alexander Rudensky for R26R-YFP mice.
Grant support
This work was supported by the National Institutes of Health (grant RO1 AG 13078 to P.J.F), the Cancer Research Institute's Predoctoral Emphasis Pathway in Tumor Immunology Program (to J.S.H.), and the National Cancer Institute Basic and Cancer Immunology Grant (grant T32CA09537 to J.S.H). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the CRI, or the NCI.
Non-standard abbreviations
- PE
Phycoerythrin
- WT
wild-type
- Tg
transgenic
- DN
double-negative
- DP
double-positive
- SP
single-positive
- dLck
distal Lck promoter
- Mtv
mammary tumor virus
- Cre
Cre recombinase
- YFP
enhanced yellow fluorescent protein
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Schlissel MS. Regulating antigen-receptor gene assembly. Nat. Rev. Immunol. 2003;3:890–899. doi: 10.1038/nri1225. [DOI] [PubMed] [Google Scholar]
- 2.Malissen M, Trucy J, Jouvin Marche E, Cazenave PA, Scollay R, Malissen B. Regulation of TCR α and β gene allelic exclusion during T-cell development. Immunol. Today. 1992;13:315–322. doi: 10.1016/0167-5699(92)90044-8. [DOI] [PubMed] [Google Scholar]
- 3.Fink PJ, Swan K, Turk G, Moore MW, Carbone FR. Both intrathymic and peripheral selection modulate the differential expression of Vβ5 among CD4+ and CD8+ T cells. J. Exp. Med. 1992;176:1733–1738. doi: 10.1084/jem.176.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fink PJ, Fang CA, Turk GL. The induction of peripheral tolerance by the chronic activation and deletion of CD4+Vβ5+ cells. J. Immunol. 1994;152:4270–4281. [PubMed] [Google Scholar]
- 5.McMahan CJ, Fink PJ. RAG reexpression and DNA recombination at T cell receptor loci in peripheral CD4+ T cells. Immunity. 1998;9:637–647. doi: 10.1016/s1074-7613(00)80661-5. [DOI] [PubMed] [Google Scholar]
- 6.McMahan CJ, Fink PJ. Receptor revision in peripheral T cells creates a diverse Vβ repertoire. J. Immunol. 2000;165:6902–6907. doi: 10.4049/jimmunol.165.12.6902. [DOI] [PubMed] [Google Scholar]
- 7.Cooper CJ, Orr MT, McMahan CJ, Fink PJ. T cell receptor revision does not solely target recent thymic emigrants. J. Immunol. 2003;171:226–233. doi: 10.4049/jimmunol.171.1.226. [DOI] [PubMed] [Google Scholar]
- 8.Hale JS, Fink PJ. T-cell receptor revision: friend or foe? Immunology. 2010;129:467–473. doi: 10.1111/j.1365-2567.2010.03250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner DH., Jr. Re-shaping the T cell repertoire: TCR editing and TCR revision for good and for bad. Clin. Immunol. 2007;123:1–6. doi: 10.1016/j.clim.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Hao Z, Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J. Exp. Med. 2001;194:1151–1163. doi: 10.1084/jem.194.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srinivas S, Watanabe T, Lin CS, William CM, Tanage Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4–8. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang DJ, Wang Q, Wei J, Baimukanova G, Buchholz F, Stewart AF, Mao X, Killeen N. Selective expression of the Cre recombinase in late-stage thymocytes using the distal promoter of the Lck gene. J. Immunol. 2005;174:6725–6731. doi: 10.4049/jimmunol.174.11.6725. [DOI] [PubMed] [Google Scholar]
- 13.Boursalian TE, Golub J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat. Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat. Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- 15.Cooper CJ, Turk G, Sun M, Farr AG, Fink PJ. Cutting Edge: TCR revision occurs in germinal centers. J. Immunol. 2004;173:6532–6536. doi: 10.4049/jimmunol.173.11.6532. [DOI] [PubMed] [Google Scholar]