SUMMARY
The use of blood-contaminated drug preparation equipment is believed to be associated with the transmission of hepatitis C virus (HCV) among injection drug users (IDUs), but the extent of HCV infection risk is unclear. The objective of this review was to appraise the evidence regarding HCV incidence associated with the use of drug preparation equipment such as drug mixing containers, filters and water. In June 2007, cohort and case–control studies examining the association of HCV incidence with the sharing of drug preparation equipment were identified by searching electronic reference databases as well as the reference lists of published papers. Ten studies (seven cohort and three nested case–control) met the inclusion criteria for the review. The relative risk of HCV infection associated with drug preparation equipment were mainly between 2.0 and 5.9; however, the precision of the estimates from individual studies were marked by wide confidence intervals. Few studies exist to allow an adequate assessment of the individual contributions of containers, filters and water to HCV incidence. The major methodological limitations of reviewed studies were short follow-up times, inadequate control of confounders and lack of exclusion of periods when IDUs were not at risk for HCV infection through drug injection. Current evidence implicating the association of drug preparation equipment with HCV incidence is limited by several methodological concerns.
Keywords: hepatitis C, HIV, injecting paraphernalia, injection drug use, syringes
INTRODUCTION
Worldwide, between 123 and 170 million people are chronically infected with the hepatitis C virus (HCV) [1]. Injection drug users (IDUs) constitute the largest group of persons infected with HCV and those most afflicted by new infections in developed countries [2,3]. Prevalence and incidence rates for HCV infection in general IDU populations (different rates in younger or older IDUs) are as high as 91% and 45.8 per 100 person-years, respectively [4,5]. There is evidence that many IDUs become infected with HCV early in their injecting career [6–8] and that the highest rates of HCV infection are observed among those who report a history of sharing drug preparation or injection equipment [3,8].
While the sharing of contaminated syringes is regarded as a major contributor to HCV infection, syringes may be only one source of injection-related infection. Drug mixing containers, cotton filters and rinse water, have garnered support in recent years as additional sources of bloodborne infection [9,10]. Opportunities for viral transmission through drug preparation equipment may exist at various stages of the injection process. For example, water used to prepare a drug solution can become contaminated with HCV if the solution is mixed with a syringe previously used for injection. Clean syringes can become contaminated when a drug solution is drawn from a container or through a filter previously used by an HCV-infected injector. Cleaning a syringe, container or filter with contaminated rinse water may also lead to viral cross-contamination of drug preparation and injection equipment.
There remains continuing debate about the relative contribution of syringes compared with drug preparation equipment in relation to HCV transmission [11,12]. Biologically, needles and syringes have the greatest potential for carrying HCV because of their direct contact with blood during venipuncture. However, the persistence of the hepatitis C virus in used drug preparation equipment is supported by laboratory evidence which shows that between 25% and 40% of filters, spoons and rinse water samples may harbour HCV RNA [13]. While the epidemiologic evidence for the association between HCV infection and drug preparation equipment sharing is not well established, support for ancillary injection materials is mounting as additional studies are carried out. A recent simulation study found that the probability of HCV infection was higher for syringes compared with other equipment during the first 5 years of injecting but was similar after 25 years [14]. Consequently, the authors of the study suggested that there may be more rapid spread of HCV through syringes during the initial years of injecting after which continued exposure to contaminated drug preparation equipment can lead to an elevated risk of HCV seroconversion.
To our knowledge, a review of the risk of HCV infection associated with drug preparation equipment has not yet been published in the medical literature. Therefore, the objective of this review was to critically appraise the evidence regarding HCV transmission risk from shared drug preparation equipment and to comment on whether the risk of HCV infection differs according to the type of drug preparation equipment shared.
METHODS
On June 20, 2007 a search of MEDLINE (1990-present), EMBASE (1990-present), BIOSIS (1969-present), Google Scholar and Thomson Scientific Web of Science databases was performed to identify cohort and case–control studies on the link between HCV seroconversion and the use of drug preparation equipment. The keyword search strategies used were (‘hepatitis C’or ‘HCV’) and (‘injection drug use’ or ‘intravenous drug use’ or ‘injection drug user’ or ‘intravenous drug user’) and (‘paraphernalia’ or ‘injection equipment’ or ‘drug equipment). Additional relevant papers were found through reference lists of identified articles.
Inclusion and exclusion criteria
We selected all peer-reviewed publications in English or French language published since 1990 (when antibody testing for hepatitis C became available). Abstracts of articles were used for the initial assessment of appropriateness for inclusion. Studies were included if they examined HCV incidence and its association to drug preparation equipment. For multiple publications using the same data, only the most recent article was selected for review. Reports on IDUs from developing countries were excluded because of the greater frequency of medically transmitted HCV [15]. In addition, reports on IDUs in prisons were not selected for the review because incarcerated persons may experience elevated risk for HCV infection from several noninjection routes and as a result of different injecting dynamics compared with street-recruited IDUs [16].
Definitions
For the purpose of this review, drug preparation equipment referred to drug mixing containers (i.e. cookers), drug filters (e.g. cotton, Kleenex and cigarette filter) and water or other liquid for rinsing drug equipment. Whenever possible, the method of sharing – borrowing and/or lending – is specified according to the definition provided by the source article.
Study assessment
Studies were assessed by three reviewers (PD, ER and JFB), with disagreement resolved by discussion until consensus was reached. The following information was sought for each article: author identification, year of publication, geographical location of study, study design, HCV prevalence in the source population and incidence in the sample, study sample size and demographic characteristics, definition of an injection drug user, method of HCV assessment, frequency of assessment of injection risk behaviours, methods to account for losses to follow-up and methods for statistical analysis. The quality of the studies was based on whether the study provided: (i) a clear definition of HCV-positive status, including support from laboratory test results, (ii) a description of the sampling method, with appropriate discussion of selection bias, (iii) the number of subjects enrolled into the study, (iv) the demographic and HCV serostatus profile of participants at baseline, (v) a clearly defined and appropriate comparison group, (vi) adequate follow-up relative to incident HCV infection and (vii) a description of and statistical adjustment for potential confounders.
A meta-analysis of the data was not performed because of the heterogeneity of study populations and methodologies. The results are reported in the form of a narrative systematic review, which is a method deemed to be appropriate in reporting results of observational studies [17].
RESULTS
Search results
The initial search identified 21 longitudinal studies which examined HCV incidence in the context of drug preparation equipment use. Of these, 10 studies (seven cohort and three nested case–control) met the inclusion criteria for review. Of the excluded studies, most (n = 6) were descriptive reports of equipment sharing or did not use multivariate methods for comparing HCV-seroconverters with subjects who remained HCV-negative. Another two studies did not adequately describe the materials examined (i.e. whether syringes or drug preparation equipment only). Finally, three studies were excluded because they were secondary reports of previously published data.
Study characteristics
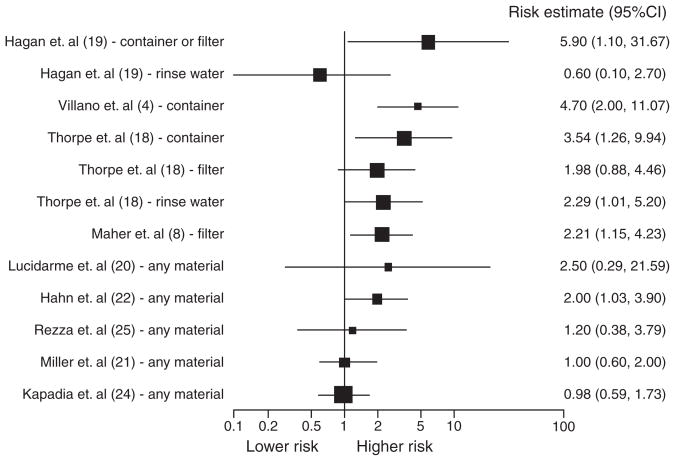
Studies were primarily reported for North American IDU populations and ranged in size from 106 to 543 subjects. Subjects were predominantly under 30 years of age and community-based samples. Reported HCV prevalence was up to 91.1% and HCV incidence was up to 37.3 per 100 person-years. Summaries of the reviewed studies are presented in Table 1 and the distribution of risk estimates are shown in Fig. 1. As shown in the figure, the relative risk estimates tended towards higher risk, with most of the larger studies showing risk estimates between 2.0 and 5.9. However, the large uncertainty in risk was evidenced by the wide confidence intervals of the risk estimates.
Table 1.
Published studies investigating the association between HCV seroconversion and sharing of drug preparation equipment
| Reference | Setting | Year subjects recruited |
Design, recruitment setting, sample size, testing, total follow-up time | Sample HCV prevalence (%) | Sample HCV incidence (per 100 person-years) | Multivariate results |
Definition of IDU |
Adjustment for confounders |
Study objectives and review comments | |
|---|---|---|---|---|---|---|---|---|---|---|
| Syringes | Nonsyringe equipment |
|||||||||
| Hagan et al. [19] | Seattle, USA | 1994–97 | Prospective cohort n = 317 (of 507 eligible) Drug treatment and street-recruited Age ≥ 14 years Serologic testing Seroconversion: 53 subjects Follow-up: 12 months |
– | 16.7 | – | RR: cooker or cotton (among syringe sharers) (past year): (not sig.; results not shown) Cooker or cotton (among syringe nonsharers) (past year): 5.9 (1.1–31.7)** Rinse water (among syringe nonsharers) (past year): 0.6 (0.1–2.7) |
Injected in past year | Stratified by syringe sharing status Frequency of injecting at baseline |
Objective: to assess drug equipment sharing as risk for HCV Follow-up at 1 year (questionnaire + blood at baseline and 12 months) 53 seroconversions observed, with 11 among syringe sharers Behaviours prior to cohort entry (including sharing behaviours) were not included in analysis as potential confounders Large number of eligible subjects not accounted for Information bias (errors in questionnaire data) is well discussed (esp. concerning rinse water and backloading) Discussion of impact of losses to follow-up not well supported Sharing prevalence (lifetime): Syringes: 77% Cooker or cotton: 17% (when no syringes shared) |
| Hahn et al. [22] | San Francisco, USA | 2000–01 | Prospective cohort n = 195 (of 291 eligible and 776 in cohort) Age < 30 years (median = 22) Recruitment by outreach and word of mouth seronegative at Baseline Serologic testing seroconversion: 48 subjects Follow-up: at least one extra visit after baseline |
37 (of entire cohort) | 25.1 | HR: syringe (borrowed past 3 months from nonsex partner): 2.6 (1.2–5.6)** Syringe (borrowed past 3 months from HCV+ sex partner): 2.2 (0.8–5.7) Syringe (borrowed past 3 months from HCV− or unknown status sex partner): 0.7 (0.3–1.5) |
HR: Cooker, cotton and water: 2.0 (1.0–3.8)** | Injected in past month | #Persons with whom pooled money to buy drugs (past 3 months) #Persons exchanged sex for money (past 3 months) Other variables in model |
Objective: to assess risk factors for HCV seronversion from equipment sharing, backloading, injecting others and being injected and other partner characteristics Follow-up every 3 months (questionnaire + blood) Sample of young IDUs only, whose sharing behaviour may differ from older IDUs High proportion of equipment sharing drug preparation equipment defined as cooker, cotton, rinse water, injecting residue from cooker or cotton Excluded periods of no injecting risk The result for borrowing needle from HCV-positive sex partner (past 3 months) became 3.3 (1.4–7.9) when sharing nonsterile drug prep equipment was excluded from model Sharing prevalence: Syringe: 67% Cooker, cotton, water: 85% |
| Kapadia et al. [24] | Baltimore, Chicago, Los Angeles, New Orleans, NY, USA | 1997–99 | Matched case–control (5:1 matching) n = 468 (78 cases, 390 matched controls) Age = 18–30 years Community-based Serologic testing Follow-up: 12 months |
36 (of entire cohort of 2198) | – | OR: Syringe (past 6 months): 1.49 (0.87–2.57) | OR: Drug prep materials (lifetime): 0.98 (0.59–1.73) Drug prep materials (past 4 months): 0.91 (0.59–1.42) |
Injected in past 6 months | Stratified by syringe disinfection Others unspecified |
Objective: to assess effect of bleach disinfection of syringes on risk of HCV seroconversion associated with nonsyringe materials Follow-up every 6 months (questionnaire + blood at baseline and every 6 months) With syringe disinfection, HCV risk from nonsyringe materials was significantly reduced, suggesting a strong risk of HCV with syringes Multivariate analysis of syringe sharing with adjustment or exclusion of drug prep materials was not assessed Estimate varies according to various models presented by the authors, but all consistent with 0.42 Sharing prevalence: Syringes: 27% Cooker: 41% Cotton: 33% Rinse water: 30% |
| Lucidarme et al. [20] | North/East, France | 1999–2000 | Prospective cohort n = 165 Mean age = 26.5 years Recruited from drug treatment Saliva and serologic testing Seroconversion: 16 subjects Follow-up: 12 months |
– | 9.0 | RR: Syringe (past 3 months): 6.82 (1.25–37.26) | RR: Any syringe and drug prep equipment (container, filter and water) (past 3 months): 2.50 (0.29–21.62) | Injected once in lifetime | Gender Geographical regions Substitutive treatment Use of condoms Daily cocaine injection Duration of injecting Other variables in model |
Objective: to assess incidence and risk factors for HCV from drug equipment sharing among persons who injected at least once in their lifetime Follow-up every 3 months (questionnaire + saliva) Large confidence intervals for all risk estimates leads to uncertainty of risk measure |
| Maher et al. [8] | New South Wales, Australia | 1999–2002 | Prospective cohort n = 368 Age ≥15 years (80% under 30 years) Street and treatment recruited PCR testing Follow-up: 3–6 months |
17–36 | 30.8 | HR: Syringe (past 6 months): 0.95 (0.49–1.85) | HR: Shared filter (past 6 months): 2.21 (1.15–4.23) | Injected in past 6 months | Age Gender Duration Injecting Drugs injected Recruitment location Ethnic minority status |
Objective: to report on incidence of and identify risk factors for HCV seroconversion Follow-up every 3 to 6 months (questionnaire + blood at baseline and every 3–6 months) Relatively minimal losses to follow-up Equal number of subjects reporting syringe, container, filter, water sharing No multivariate results provided for container, filter, water |
| Miller et al. [21] | Vancouver, Canada | 1996–2001 | Prospective cohort n = 232 Age = 13–24 years (median = 21) Street-recruited Serologic testing Seroconversion: 37 subjects Follow-up: unspecified |
46 | 37.3 | RR: Syringe (past 6 months): 1.13 (0.47–2.73) |
Baseline unadjusted OR: cooker, cotton and/or rinse water (past 6 months): 1.0 (0.6–2.0) |
Injected in past month | unspecified | Objective: to assess prevalence, incidence and risk factors for HCV among young IDUs Follow-up every 6 months (questionnaire + blood) Sample of young IDUs only, whose sharing behaviour differ from older IDUs |
| Rezza et al. [25] | Naples, Italy | 1991–93 | Nested case-control n = 106 (21 cases, 85 controls) Age = unspecified in-treatment Serologic testing Follow-up: 0.69 year (mean) |
63 (of entire cohort of 713) | 28.6 | OR: Syringe (past 6 months): 3.7 (0.1–129.1) |
OR: Nonsyringe materials (past 6 months): 1.2 (0.4–4.0) |
Heroin user (not further specified) | Unspecified | Objective: to assess prevalence, incidence and risk factors for HCV and HIV seroconversion among IDUs in drug treatment Follow-up every 6 months (questionnaire + blood at baseline and one more time) Statistical power limited by small number of seroconversions and sample size Small proportion of needle sharers but not other drug paraphernalia Several of the eligible subjects were not followed-up Adjustment variables for logistic model are not provided Behaviours prior to cohort entry (including sharing behaviours) were not included in analysis as potential confounders Information bias (errors in ques tionnaire data) is well discussed |
| Roy et al. [23] | Quebec province and city of Ottawa, Canada | 1997–2003 | Retrospective cohort n = 543 Mean age = 31.8 years Street-recruited Saliva testing Follow-up: ≥6 months |
60.4 | 27.1 | HR: Syringe borrowed (past 6 months): 1.82 (1.19–2.78) |
HR: Not significant (results not shown) |
Injected in past 6 months | Age injecting experience Borrowed used syringe Drug injected Prostitution District of recruitment |
Objective: to report on prevalence and incidence of HCV Follow-up every 6–12 months (questionnaire + saliva) Intervals for re-assessment may be too long as cases could be re-tested at up to 12 months Mostly cocaine injecting IDUs No details about other injecting equipment considered |
| Thorpe et al. [18] | Chicago, USA | 1997–99 | Prospective cohort n = 353 (of 510 eligible in 702 in cohort) Age = 18–30 years Street-recruited Serologic testing Seroconversion: 48 subjects Follow-up: 12 months |
27 (of entire cohort) | 10.0 | HR: Syringe (borrowed past 6 months): 2.10 (0.90–4.90) |
HR: Cooker: 4.07 (1.41–11.78)** Cotton: 2.38 (1.14–4.98)** Rinse water: 2.68 (0.86–8.35) HR (adjusted for syringe sharing): Cooker: 3.54 (1.26–9.94)** Cotton: 1.98 (0.88–4.46) Rinse water: 2.29 (1.01–5.20)** |
Injected in past 6 months | Education Homelessness Place of residence Frequency of injecting Cocaine injecting Syringe use Drug prep equipment use |
Objective: to assess risk factors for HCV seroconversion from equipment sharing Follow-up every 6 months (questionnaire + blood) Sharing defined as injecting with previously equipment when injecting at same time as another IDU Sample of young IDUs only, whose sharing behaviour may differ from older IDUs Adjusting for syringe sharing shows effects of each of other equipment, not done in most other studies Follow-up time inconsistently reported Adjusted Sharing prevalence: Syringe: 50% Cooker: 62% Cotton: 45% Rinse Water: 54% |
| Villano et al. [4] | Baltimore, USA | 1988–96 | Matched case–control (4:1 matching) n = 142 (43 cases, 99 controls) Age ≥ 18 years Street-recruited Serologic testing Follow-up: 6.5 years median (range 2.4–7.8 years) |
91.1 (of entire cohort of 1593) | 6.4 | OR: Syringe (past 6 months, compared with no drug use): 5.7 (2.2–14.4)** Syringe (past 6 months, no sharing of syringes: 3.0 (1.2–7.1) Similar results when adjusted for age |
OR: Cookers (past 6 months compared with no drug use): 4.7 (2.0–11.1)** No sharing of cookers (past 6 months compared with no drug use): 2.9 (1.1–7.5)** |
Injected in past 10 years | Age at enrolment | Objective: to assess incidence and risk factors for HCV among persons with a history of injection drug use in past 10 years Follow-up every 6 months (questionnaire + blood) Multivariate analysis only adjusted for age at enrolment and matched by time of visit, with no positive finding for syringe or container sharing Positive trend in risk estimate for sharing any equipment Crude statistical analysis (no multivariable adjustments other than for age and the matching variables) Potential confounding due to behaviours other than those occurring during the previous 6 months (including sharing behaviours) not taken into account |
OR, odds ratio; HR, hazard ratio; RR, relative risk.
P < 0.05.
Fig. 1.
Forest plot of risk estimates from multivariate analyses of drug preparation equipment sharing and its association to HCV seroconversion.
Incident HCV infection through drug preparation equipment in cohort studies
Among the studies reviewed, four of seven cohort studies found a positive association with the sharing of at least one type of drug preparation equipment. A prospective cohort of IDUs in New South Wales, Australia, showed a positive association between HCV seroconversion and filter sharing. In this analysis, which adjusted for syringe sharing in the 6 months prior to HCV testing, drug mixing containers, water and tourniquets were not found to be statistically significant predictors of seroconversion [8]. Although the study found positive associations of HCV infection with containers, water and tourniquet in univariate analyses, there was no discussion about the magnitude of these associations or reasons for excluding these materials from the final multivariate model of HCV risk.
In a prospective cohort study in Chicago, USA, HCV seroconversion was strongly associated with sharing a container or cotton filter during 6 months of follow-up [18]. In adjusted analyses, IDUs who shared containers were nearly four times more likely to become HCV-infected and filter sharers were twice as likely to seroconvert. On the other hand, sharing rinse water showed no significant risk for HCV infection. After adjusting for syringe sharing in the past 6 months, HCV incidence remained strongly associated with sharing containers but no longer associated with filters. Furthermore, the positive association with sharing rinse water showed a marginal statistical association with HCV incidence.
Another prospective cohort study in Seattle, USA, similarly showed a higher incidence of HCV among those who shared containers or cotton filters (but no concomitant syringe sharing) during a one-year follow-up compared with those who did not share these materials [19]. The study also found a lack of association for rinse water sharing among those who did not report sharing syringes.
In a study of IDUs in France, the sharing of any drug preparation equipment in the 3 months prior to HCV testing was a strong predictor of incident infection among those who were seronegative at baseline [20]. Follow-up occurred every 2 months and was accompanied by saliva testing for HCV antibodies.
Of the studies which found no or limited association with drug preparation equipment, a study of young IDUs (<30 years of age) in Vancouver, Canada, found no statistically significant link between the sharing of containers, filters or rinse water and HCV seroconversion [21]. Similarly, a study of young IDUs in San Francisco, USA, showed that borrowing previously used drug preparation equipment (container, filter and rinse water in the past 3 months before HCV screening) from a person who was not a sexual partner was associated with an elevated, but statistically nonsignificant, risk for HCV seroconversion [22]. Finally, the SurvUDI surveillance network which monitors a cohort of IDUs in the province of Quebec and city of Ottawa, Canada, found no association of drug preparation equipment with HCV seroconversion [23]. The study subjects were assessed every 6 months or more and were assessed for HCV infection using saliva testing.
Incident HCV infection through drug preparation equipment in case–control studies
The evidence for the link between drug preparation equipment sharing and HCV incidence is less convincing in case–control studies. Only one of three case–control studies found a positive association between HCV incidence and the sharing of any drug preparation equipment.
A case–control analysis, nested within a prospective cohort of injectors in Baltimore, USA, examined container sharing in the 6 months prior to HCV testing [4]. Although the study found a positive association, it compared equipment sharers with IDUs who did not use drugs in the past 6 months, rather than with a group who did not share injecting equipment. The authors also found a positive association between not sharing containers and no drug use, which suggests that containers alone may not account for HCV incidence.
The second case–control study consisted of a multi-site analysis of young IDUs and new injectors in the US. This study showed that sharing containers, cotton filters and rinse water were each associated with nearly a twofold greater risk of HCV infection in unadjusted analyses compared with the absence of sharing of these materials in the past 6 months prior to HCV testing [24]. However, when adjusted for the concomitant use of bleached syringes, the sharing of any drug preparation equipment was no longer statistically significant in relation to HCV infection, regardless of the period of risk considered (lifetime or past 4 months).
Finally, a study of an in-treatment sample of IDUs in Italy also showed a lack of association with HCV infection. The study found that participants who were HCV negative were no more likely than HCV positive IDUs in the study to share drug preparation equipment in the past 6 months before study enrolment [25].
DISCUSSION
Summary of findings
The paucity of longitudinal studies on the relation between HCV incidence and drug preparation equipment sharing has rendered it difficult to assess the relative contribution of these materials to the HCV epidemic among IDUs. Overall, the risk estimates from the reviewed studies suggest a positive association between HCV seroconversion and drug equipment sharing, but precision of the estimates appeared to be a concern. The second purpose of this review was to highlight some of the methodological concerns with existing studies. There appears to be a trend in the epidemiological literature in recent years towards more rigorous study designs and analyses, but as the following discussion highlights, there remains several common limitations across the reviewed studies.
Methodological issues
Study design
First, the small sample size and differential losses to follow-up brings into question the statistical power and validity of some studies. The retention of subjects in cohort studies was problematic as some studies lost up to half of eligible subjects during follow-up [19,21,25].
Several studies were also limited by a small number of seroconversions, which questions the adequacy of follow-up. Short follow-up times of less than 2 years in several of the reviewed cohort studies may not have allowed for sufficient time for observing HCV seroconversion in relation to risk behaviours [21,22,25]. In fact, limited power because of few seroconversions was a limitation commonly acknowledged by authors for the inability to detect small differences in HCV risk in relation to equipment sharing [18,22,24]. A lack of precision in risk estimates is shown by large confidence intervals for the association between HCV incidence and drug equipment sharing. The description of lost subjects in the study from Australia provides evidence that participants who continue in the study may be different with regard to their injection risk practices, which can influence the direction of the observed association with HCV seroconversion [8]. In addition to describing the subjects who were lost to follow-up, the study demonstrated the importance of specifying and controlling for known and potential confounders, providing a thorough definition of seroconversion cases, using short intervals of 3–6 months between subject assessments to reduce recall bias and using a complementary ethnographic approach to examine the syringe-mediated injection practices of participants. The study had a relatively large pool of HCV-uninfected individuals at baseline and used PCR testing for HCV RNA to identify recent infection compared with testing for HCV antibodies.
Confounding by injecting equipment and other covariates
In most of the studies, confounders were not explicitly stated or were inadequately controlled for. This may play an important role in the observed outcomes because the reviewed studies showed large heterogeneity with respect to the definition of measures, prevalence of risk behaviours, demographic characteristics of subjects and location of subject recruitment.
The sharing of drug preparation equipment is typically more widespread in IDU populations than of syringes alone and the sharing of different types of equipment is often not mutually exclusive [18]. Although some of the reviewed studies adjusted for syringe sharing as a confounder, others did not provide any description of syringe sharing to allow assessment of concomitant risk from syringes.
Other potential confounders such as age and location of recruitment were rarely adjusted for. For example, some studies suggest that younger injectors may be more likely to share used injecting equipment than older IDUs and, as a result, are at higher risk of bloodborne infections [26,27]. On the other hand, studies also show that the relatively short injecting career of younger IDUs may not correlate well with HCV infection in comparison with older IDUs whose more prolonged exposure to used equipment is likely to contribute to a greater cumulative risk of infection [26]. Second, subjects recruited from drug treatment and out-of-treatment settings may differ in their frequency of injection and extent of injection risk behaviours [28]. There was no evidence of adjustment for location of recruitment as a potential confounder in studies in which subjects originated from multiple sources.
The composition of drug injecting networks is an additional consideration that could impact the probability of infection, because the number of equipment sharing partners and the infection status of partners can be related to the risk of HCV infection [29].
Definition of drug injector
The definition of an injection drug user seemed to differ substantially across studies, particularly with regard to the duration of injecting and current status as an active IDU. Comparisons across studies were problematic because injecting careers encompassed timeframes that ranged from ‘ever injecting’, ‘injecting in the past year’ to ‘injecting in the past month’. For example, in the Baltimore case–control study, any injection in the past 10 years was a contentious issue because it is conceivable that injecting may have ceased or risk behaviours may have changed during the assessed period of risk [4]. Similarly, the study from Italy did not provide a clear definition of an IDU [25]. Participants were simply called heroin users without further specification about the history of injecting or frequency of current injecting [25].
The direction (borrowing vs lending) and frequency of equipment sharing was also rarely stated in several studies even though this information may have proven helpful in assessing the extent and frequency of exposure of study subjects to injection-related risk activities. Finally, information of the type of drug used may have better defined the relative risk of exposure to risk behaviours. For example, some studies have suggested that cocaine injection is associated with higher risk of HCV and HIV because of the profile and drug-related risk behaviours of users [30,31].
Prevalence of HCV infection
Appropriate comparisons across studies were hampered by varying HCV prevalence among IDU populations in the various study samples. For example, in high HCV prevalence settings, low rates of equipment sharing may be associated with high rates of HCV transmission because fewer sharing episodes are needed for transmission of infection [12].
Serologic testing was the most common method for identifying HCV-positive individuals across most studies. The use of saliva testing, which has lower sensitivity of 83% [32], for identifying HCV cases or selecting subjects for subsequent serologic testing may have underestimated the total number of incident infections in two studies [20,23]. It is unclear, how this underestimation may have attenuated the association with the sharing of drug preparation equipment.
Period of risk
Our review found that the period of risk assessment was not always relevant to HCV incidence. For example, longer periods of risk behaviour assessment can lead to a greater likelihood of misclassification of subjects with regard to their risk behaviours and, consequently, reduce the association between equipment sharing and HCV infection. In the Chicago [18] and Seattle [19] studies, risk behaviours for the prior 12 months were examined in relation to HCV seroconversion. However, historical risk behaviours might not be representative of more recent HCV-related risk behaviours because of possible changes in sharing habits or intensity of drug use.
In contrast, risk behaviour information which is restricted to a short period may preclude high-risk sharing episodes that occur prior to the period of interest. In other cases, periods of injecting may be intermittent and, thus, overestimate HCV infection risk during periods of noninjecting. Except for one study [22], there was little evidence for periods of noninjecting to be accounted for. The inclusion of lower-risk periods can lead to misclassification of equipment sharing and underestimation of the association between sharing and HCV infection.
Implications for future research and prevention
Researchers should strive to improve on several methodological aspects in order to better address the limitations identified in this review. First, additional prospective studies are needed to distinguish the relative contribution of the various types of drug preparation materials to HCV infection. This would allow for more specific identification of materials which carry the highest risk of HCV infection. Second, future research should aim to increase comparability of results across studies by a more justified choice of risk periods and consistency in the definitions of injection drug use and equipment sharing. Finally, water for drug preparation, unlike water for equipment rinsing, was seldom considered as a potential risk factor for HCV seroconversion. Other materials such as swabs and tourniquets were also rarely examined. Nevertheless, our review of the literature reinforces the need to recommend the use of sterile injecting equipment for all episodes of drug injecting.
CONCLUSION
The current evidence regarding drug preparation equipment sharing and HCV incidence is not overwhelming. To further differentiate the impact of various injecting equipment on HCV risk, additional longitudinal studies are needed that have longer and more rigorous follow-up, more appropriately control for potential and known confounders, adequately adjust for periods of noninjecting and use consistent definitions of risk behaviour.
Abbreviations
- HCV
hepatitis C virus
- IDUs
injection drug users
References
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Heintges T, Wands JR. Hepatitis C virus: epidemiology and transmission. Hepatology. 1997;26:521–526. doi: 10.1002/hep.510260338. [DOI] [PubMed] [Google Scholar]
- 3.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 4.Villano SA, Vlahov D, Nelson KE, et al. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol. 1997;35:3274–3277. doi: 10.1128/jcm.35.12.3274-3277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maher L, Li J, Jalaludin B, et al. High hepatitis C incidence in new injecting drug users: a policy failure? Aust N Z J Public Health. 2007;31:30–35. doi: 10.1111/j.1753-6405.2007.00007.x. [DOI] [PubMed] [Google Scholar]
- 6.Garfein RS, Vlahov D, Galai N, et al. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86:655–661. doi: 10.2105/ajph.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garten RJ, Lai S, Zhang J, et al. Rapid transmission of hepatitis C virus among young injecting heroin drug users in Southern China. Int J Epidemiol. 2004;33:182–188. doi: 10.1093/ije/dyh019. [DOI] [PubMed] [Google Scholar]
- 8.Maher L, Jalaludin B, Chant KG, et al. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction. 2006;101:1499–1508. doi: 10.1111/j.1360-0443.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 9.Koester S, Booth RE, Zhang Y. The prevalence of additional injection-related HIV risk behaviors among injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:202–207. doi: 10.1097/00042560-199606010-00015. [DOI] [PubMed] [Google Scholar]
- 10.Needle RH, Coyle S, Cesari H, et al. HIV risk behaviors associated with the injection process: multiperson use of drug injection equipment and paraphernalia in injection drug user networks. Subst Use Misuse. 1998;33:2403–2423. doi: 10.3109/10826089809059332. [DOI] [PubMed] [Google Scholar]
- 11.Koester S, Heimer R, Baron AE. Risk of hepatitis C among young adult injection drug users who share injection equipment. Am J Epidemiol. 2003;157:376. doi: 10.1093/aje/kwf197. [DOI] [PubMed] [Google Scholar]
- 12.Crofts N, Aitken CK, Kaldor JM. The force of numbers: why hepatitis C is spreading among Australian injecting drug users while HIV is not. Med J Aust. 1999;170:220–221. [PubMed] [Google Scholar]
- 13.Crofts N, Caruana S, Bowden S, et al. Minimising harm from hepatitis C virus needs better strategies. BMJ. 2000;321:899. [PMC free article] [PubMed] [Google Scholar]
- 14.Mathei C, Shkedy Z, Denis B, et al. Evidence for a substantial role of sharing of injecting paraphernalia other than syringes/needles to the spread of hepatitis C among injecting drug users. J Viral Hepat. 2006;13:560–570. doi: 10.1111/j.1365-2893.2006.00725.x. [DOI] [PubMed] [Google Scholar]
- 15.Prati D. Transmission of hepatitis C virus by blood transfusions and other medical procedures: a global review. J Hepatol. 2006;45:607–616. doi: 10.1016/j.jhep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Holsen DS, Harthug S, Myrmel H. Prevalence of antibodies to hepatitis C virus and association with intravenous drug abuse and tattooing in a national prison in Norway. Eur J Clin Microbiol Infect Dis. 1993;12:673–676. doi: 10.1007/BF02009378. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Altman D. Systematic Reviews in Health Care: Meta Analysis in Context. 2. London: BMJ Publishing Group; 2001. [Google Scholar]
- 18.Thorpe LE, Ouellet LJ. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol. 2002;155:645–653. doi: 10.1093/aje/155.7.645. [DOI] [PubMed] [Google Scholar]
- 19.Hagan H, Thiede H, Weiss NS, et al. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health. 2001;91:42–46. doi: 10.2105/ajph.91.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucidarme D, Bruandet A, Ilef D, et al. Incidence and risk factors of HCV and HIV infections in a cohort of intravenous drug users in the North and East of France. Epidemiol Infect. 2004;132:699–708. doi: 10.1017/s095026880400247x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller CL, Johnston C, Spittal PM, et al. Opportunities for prevention: hepatitis C prevalence and incidence in a cohort of young injection drug users. Hepatology. 2002;36:737–742. doi: 10.1053/jhep.2002.35065. [DOI] [PubMed] [Google Scholar]
- 22.Hahn JA, Page-Shafer K, Lum PJ, et al. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. J Infect Dis. 2002;186:1558–1564. doi: 10.1086/345554. [DOI] [PubMed] [Google Scholar]
- 23.Roy E, Alary M, Morissette C, et al. High hepatitis C virus prevalence and incidence among Canadian intravenous drug users. Int J STD AIDS. 2007;18:23–27. doi: 10.1258/095646207779949880. [DOI] [PubMed] [Google Scholar]
- 24.Kapadia F, Vlahov D, Des Jarlais DC, et al. Does bleach disinfection of syringes protect against hepatitis C infection among young adult injection drug users? Epidemiology. 2002;13:738–741. doi: 10.1097/00001648-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Rezza G, Sagliocca L, Zaccarelli M, et al. Incidence rate and risk factors for HCV seroconversion among injecting drug users in an area with low HIV seroprevalence. Scand J Infect Dis. 1996;28:27–29. doi: 10.3109/00365549609027145. [DOI] [PubMed] [Google Scholar]
- 26.Becker Buxton M, Vlahov D, Strathdee SA, et al. Association between injection practices and duration of injection among recently initiated injection drug users. Drug Alcohol Depend. 2004;75:177–183. doi: 10.1016/j.drugalcdep.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Fennema JSA, van Ameijden EJC, Van den Hoek A, et al. Young and recent-onset injecting drug users are at higher risk for HIV. Addiction. 1997;92:1457–1465. [PubMed] [Google Scholar]
- 28.Baker A, Kochan N, Dixon J, et al. HIV risk-taking behaviour among injecting drug users currently, previously and never enrolled in methadone treatment. Addiction. 1995;90:545–554. doi: 10.1046/j.1360-0443.1995.9045458.x. [DOI] [PubMed] [Google Scholar]
- 29.Latkin C, Mandell W, Vlahov D, et al. People and places: behavioral settings and personal network characteristics as correlates of needle sharing. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:273–280. doi: 10.1097/00042560-199611010-00010. [DOI] [PubMed] [Google Scholar]
- 30.Anthony JC, Vlahov D, Nelson KE, et al. New evidence on intravenous cocaine use and the risk of infection with human immunodeficiency virus type 1. Am J Epidemiol. 1991;134:1175–1189. doi: 10.1093/oxfordjournals.aje.a116021. [DOI] [PubMed] [Google Scholar]
- 31.Chaisson R, Bacchetti P, Osmond D, et al. Cocaine use and HIV infection in intravenous drug users in San Francisco. JAMA. 1989;261:561–565. [PubMed] [Google Scholar]
- 32.Cameron SO, Wilson KS, Good T, et al. Detection of antibodies against hepatitis C virus in saliva: a marker of viral replication. J Viral Hepat. 1999;6:141–144. doi: 10.1046/j.1365-2893.1999.00142.x. [DOI] [PubMed] [Google Scholar]