Abstract
Awareness of hepatitis C virus (HCV) infection status is expected to influence risk behaviors. In 2004–2005, injection drug users (IDUs) recruited from syringe exchange programs (SEPs) and methadone clinics in Montreal, Canada, were interviewed on drug use behaviors (past 6 months) and HCV testing. Subjects (n = 230) were classified as low/intermediate risk (20.4% borrowed drug preparation equipment only) and high risk (19.6% borrowed syringes), and 54.5% reported being HCV positive. Logistic regression modeling showed that compared to no risk (60% borrowed nothing), low/intermediate risk was associated with fewer noninjecting social network members, poor physical health, and problems obtaining sterile injecting equipment. High risk was associated with all of these factors except social networks. HCV status was not associated with any level of risk. Improved access to sterile injecting equipment may be more important than knowledge of HCV status in reducing injection risks among this IDU population. The study limitations are noted and recommendations discussed.
Keywords: Hepatitis C, paraphernalia sharing, screening, counseling, guidelines, receptive sharing, social network, risk hierarchy, syringe exchange program (SEP)
Introduction
Hepatitis C virus (HCV) infection rates continue to be high among injection drug users (IDUs). In Montreal, Canada, recent surveillance data estimate HCV prevalence and incidence rates to be 60.4% and 27.1 per 100 person-years, respectively (Roy et al., 2007). While syringe sharing is considered to carry the highest risk of infection, ancillary materials such as drug cookers, cotton, and rinse water which are used in the preparation and injection of drugs have also been implicated in HCV risk (Hagan et al., 2001; Hahn et al., 2002; Thorpe et al., 2002). Increasing awareness of HCV infection status through screening so that risk behaviors can be avoided is expected to be a useful public health intervention for reducing the overall population burden of HCV. However, in spite of the high prevalence of HCV infection in the drug-injecting population, many IDUs may be unaware of their HCV status (whether positive or negative) as a result of not being screened for HCV, not receiving their test result, or misunderstanding the results of a screening test (Best et al., 1999; Heimer et al., 2002; Stein, Maksad, and Clarke, 2001).
The borrowing of used injection equipment poses a health risk for both HCV positive and negative IDUs. More specifically, HCV positive individuals are at risk of reinfection with HCV or superinfection with different genotypes of HCV (Bowden, McCaw, White, Crofts, and Aitken, 2005; Herring, Page-Shafer, Tobler, and Delwart, 2004). HCV negative IDUs would be expected to avoid borrowing equipment to remain free of infection. Additionally, the borrowing of injecting equipment poses a risk of infection with other bloodborne pathogens such as human immunodeficiency virus (HIV) and hepatitis B virus among HCV positive as well as negative individuals. However, given that injection behaviors are related to perceptions of risk, the risk practices related to HCV may be different from those of HIV. The lower perceived risk related to HCV may lead to higher risk behaviors among HIV negative IDUs because their understanding of hepatitis C infection and associated risk behaviors may be very different within the context of HIV infection (Davis, Rhodes, and Martin, 2004; Rhodes, Davis, and Judd, 2004).
The identification of HCV-infected persons has the potential to limit HCV spread through counseling about risk factors for infection, reinfection, and transmission to others. Counseling of uninfected persons may succeed in changing high-risk behavior and lead to the initiation of treatment for substance use. The type, content, and approach to risk reduction counseling, in addition to the patient’s state of readiness and self-efficacy for undertaking behavioral changes, are other important determinants of ongoing risk practices (Joseph et al., 1984; Prochaska, DiClemente, and Norcross, 1992).
However, equivocal evidence on changes in morbidity, mortality, and posttest injection behaviors have cast doubts on whether HCV screening and posttest counseling achieves safer injecting among IDUs. Despite a scarcity of studies on this subject and a lack of available data to evaluate the efficacy of screening on long-term outcomes, the Canadian Consensus Conference on Viral Hepatitis Management, the US Centers for Disease Control and Prevention, and the US National Institutes of Health have recommended the serologic testing of people at increased risk of HCV infection, particularly those who currently inject or have a history of injecting illicit drugs (Centers for Disease Control and Prevention [CDC], 1998; National Institutes of Health [NIH] Expert Panel, 2002; Sherman et al., 2004). Whereas recommendations for routine, voluntary screening for HIV are made on the basis of clinical efficacy and cost-effectiveness, the same recommendations cannot be currently made for HCV screening. The inconsistency in positive behavioral outcomes among IDUs following an HCV screening test has prompted the US Preventive Services Task Force not to recommend for or against routine HCV screening in at-risk, asymptomatic adults (US Preventive Services Task Force, 2004).
The objective of the current study is to address the ongoing debate regarding the potential impact of knowing one’s HCV status on injection risk behaviors. In particular, we examine the relative contribution of HCV infection status and other factors associated with borrowing drug injection equipment in a sample of IDUs in Montreal.
Methods
Study Design and Population
The current analysis is part of a cross-sectional study designed to assess HCV-related knowledge, perceptions, and risk behaviors among IDUs in Montreal, Canada. Active IDUs (i.e., those who injected at least once during the past 6 months) were recruited from three major syringe exchange programs (SEPs) and two methadone maintenance treatment (MMT) clinics in Montreal between April 2004 and January 2005. SEPs are one of the primary sources of sterile syringes and drug preparation equipment (i.e., containers for drug mixing, filters, water for drug preparation, preinjection and postinjection swabs, and tourniquets) in Montreal for a target population of over 12,000 IDUs (Remis, Bruneau, and Hankins, 1998). Syringes were distributed based on client need without exchange limits and with a view toward encouraging the use of sterile materials for every injection (Direction de la santé publique de Montréal, 2006). The SEP recruitment sites were located in downtown areas close to locations of drug use and easily accessible by public transportation. Together, the SEPs operated 7 days a week during daytime and overnight hours. MMT clinic subjects were new clients to treatment (with a median time in treatment of 2 months), whose frequency of injecting risk behaviors did not differ significantly from SEP clients. Previous research suggests that while the majority of MMT patients eventually reduce drug use, some continue to inject during the early months of treatment and maintain pretreatment risk behaviors (Hartel et al., 1997).
The study sample was drawn from an IDU population that frequently participates in research studies in the Montreal region and is well represented by the SurvUDI surveillance study (Hankins et al., 2002; Roy et al., 2007). The population is described as a mainly marginalized, street-recruited group of injectors. Approximately two thirds are male, and cocaine is the drug most often injected. Access to screening for HIV and HCV infection is relatively high with almost three quarters of the SurvUDI study sample having had at least one test for HIV or HCV during the past 2 years. However, of HCV-infected IDUs, only 2% had ever received medical therapy for HCV infection compared to 10% of HCV-infected cases in Quebec (Leclerc and Morissette, 2006a; Leclerc, Morissette, Roy, Tremblay, and Alary, 2006b). The close and well-established collaboration between the Montreal Public Health Department in which the study was initiated and the recruitment sites allows for more effective exchange of research findings.
Inclusion and Exclusion Criteria
Four study interviewers, aged between 23–34 years, were recruited for their prior experience in working with marginalized groups such as IDUs. Prior to enrolment in the study, study participants were explained the purpose and contribution of the study to improving prevention activities for IDUs. Eligibility for participation was verified by the presence of injection marks or through the subject’s knowledge of typical injection procedures and about community services offered to IDUs. Participants were at least 18 years of age, provided informed consent, and were reimbursed C$20 for their time. In addition, all persons approached for participation were referred to community prevention services for IDUs such as SEPs, HCV screening, counseling, and education resources.
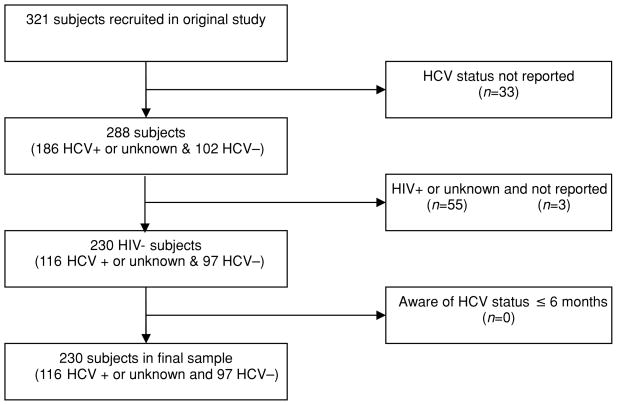
The analysis for this study was restricted to participants who self-reported their HCV infection as being positive, negative, or unknown. Participants who were unaware of their HCV status (n = 13) were grouped with HCV positive subjects based on their small number and research suggesting a high probability of HCV positive status for those who are unaware (Stein et al., 2001). The sample was also restricted to those who reported being HIV negative because their risk behaviors were assumed to be motivated by their perceived susceptibility to HCV infection only. Finally, participants who were aware of their HCV status for at least 6 months prior to the interview were chosen to ensure that the reporting of information on risk behaviors occurred after HCV screening. The selection of final study participants is documented in Figure 1. This subsample of 230 participants was similar to the original study sample with regard to demographics, drug use characteristics, and health status.
Figure 1.
Derivation of study sub sample from subjects recruited into the HCV-related Knowledge, Perceptions, and Risk Behaviors study in Montreal, Canada.
The study procedures and documents were approved by the McGill University Faculty of Medicine Institutional Review Board for Research on Human Subjects.
Data Collection
Participants underwent an anonymous, face-to-face interview that lasted on average 1.5 hours. Interviews were conducted in the English or the French language by study interviewers in a private room at the recruitment site or arranged in a private location at the Montreal Public Health Department, whenever appropriate. The interview was administered using a structured questionnaire which collected information on sociodemographics (age, gender, self-identified ethnicity, marital status, education, income, housing), health status (self-reported HCV testing and infection status, self-rated physical and mental health, access to and use of sterile injecting equipment, receipt of risk reduction information), drug use practices (years injecting, drugs injected, frequency and place of injecting), and social network characteristics (number of injecting partners, size of non-drug-injecting network, size of IDU network). Drug use and harm reduction practices pertained to the 6 months or the 1 month prior to the interview. The 1-month timeframe of risk behavior assessment was used for questions most likely to be affected by recall. Questions were developed using information from the recruitment sites and expert opinion and were also modeled on several sources such as previously validated questionnaires about injection drug use and determinants of HCV risk among IDUs (Cox et al., 2002; Koester, Booth, and Zhang, 1996; Needle et al., 1998). The initial questionnaire was pilot tested with 86 subjects in the target population prior to the start of the study and adjusted according to participant feedback.
We used self-reported HCV infection status rather than serologic testing because perceived infection status is likely sufficient for the decision to engage in risk behaviors (Best et al., 1999). While self-reported HCV status and injection practices may be susceptible to socially desirable response, some studies have shown that self-reported information about infection status (especially positive status) and risk behaviors are of acceptable validity and reliability (Darke, 1998; Dowling-Guyer et al., 1994; Stein et al., 2001). A recent study confirmed our assumption that the predictive value of self-reported HCV status among IDUs is adequately high among HCV positive (94%) and HCV negative (72%) IDUs (Hagan et al., 2006).
Definitions
For the purpose of this study, the most commonly injected drug was defined as the drug that was injected at least half the time in the past 6 months, typically cocaine and heroin in our sample. Public or semipublic places of injecting were defined as areas without privacy, such as the street, cars, parks, abandoned buildings, or public toilets. Private injection settings referred to one’s own home, the home of a friend or family member, or a hotel room. Housing in the past 6 months was classified as unstable if a subject resided in a single-room occupancy hotel, shelter, jail, or had no fixed address.
The social network of a subject was determined by asking each study participant to anonymously identify up to 10 individuals with whom there was regular contact during the month prior to the interview. Two types of networks were considered, namely, (i) noninjecting networks composed of sexual and/or social support individuals and (ii) drug-injecting networks composed of IDUs who were present during injecting or with whom drugs and/or injecting materials were shared.
Borrowing, also referred to in the literature as receptive sharing, comprised injections with any drug equipment previously used by another IDU during a common injecting episode or when the participant injected alone. In addition to syringes, we considered the borrowing of drug injection and preparation equipment such as drug-mixing containers (including syringes), filters (e.g., cotton), drug preparation water, swabs, and tourniquets.
Dependent Variable
The outcome of interest was a trichotomous variable of drug equipment borrowing, representing a risk hierarchy for HCV transmission and described as no risk, low intermediate-risk, and high risk borrowings. The hierarchy was based on substantive knowledge from the published literature about injection-related risk for HCV and typical patterns of drug equipment sharing among IDUs. However, unlike many epidemiologic studies which examine the practice of equipment sharing without differentiating borrowing from lending, we chose to examine only the borrowing of used syringes and drug preparation equipment. This approach presents a more specific understanding of the potential for HCV transmission and HCV acquisition by HCV positive and negative IDUs, respectively. In preliminary analyses, we found that borrowing and lending of injecting equipment occurred with equal frequency among study participants, thereby further motivating our decision to examine only one direction of sharing.
Syringes were assigned the highest risk of HCV infection due to their direct contact with intravenous blood. Drug preparation materials were considered to represent relatively lower risk, as they are less likely to have direct contact with blood by intravenous means. Thus, our risk hierarchy designated participants as having (i) no risk if they did not share any drug injection equipment, (ii) low/intermediate risk if they shared only drug preparation equipment, and (iii) high risk if they shared syringes (and possibly drug preparation equipment) in the past 6 months.
Statistical Analysis
Variables were examined with respect to HCV infection status in univariate analyses using Pearson’s chi-square or Fisher’s exact test as appropriate. Statistical significance was set at p < .05 (two-tailed).
Polytomous logistic regression analysis was used to assess the relationship of each risk category (i.e., no, low/intermediate, high) with HCV infection status and other independent variables of interest. Variables found to be statistically significant at p < .20 in the univariate analysis were considered in a multivariate model. The most parsimonious model was chosen by sequentially removing variables and examining the change in the likelihood ratio of the resulting model (Hosmer, Jr., and Lemeshow, 1989). The odds ratios (ORs) and 95% confidence intervals (CIs) are reported for each risk category in the final model and adjusted for potential confounders. Interactions of HCV status with age and gender were also examined.
Results
Among the 230 participants, 187 (81%) were recruited from SEPs and 43 (19%) from MMT programs (Table 1). The majority of subjects were male (69%), over the age of 30 years (55%), single (87%), and self-identified as Canadian (91%). With regard to testing, 79% of participants self-reported as being tested for HCV at least once in the past year (range = 1–3 tests). Approximately 55% of subjects reported they were HCV positive and had known their positive status for 6 years on average. HCV transmission risk during the past 6 months was categorized as no risk for 138 (60%) participants, low/intermediate for 47 (20%), and high for 45 (20%).
Table 1.
Univariate results of HCV risk factors by level of risk from drug equipment borrowing
| Total (n = 230) | No [risk] (n = 138) | Low/intermediate risk (n = 47) |
High [risk] (n = 45) |
|||
|---|---|---|---|---|---|---|
| n (%) | n (%) | p | n (%) | p | ||
| Recruitment site | .70 | .82 | ||||
| SEP | 187 (81.3) | 112 (81.2) | 40 (85.1) | 35 (77.8) | ||
| Methadone clinic | 43 (18.7) | 26 (18.8) | 7 (14.9) | 10 (22.2) | ||
| Sociodemographic | ||||||
| Age | .93 | .30 | ||||
| <30 years | 103 (44.8) | 65 (47.1) | 20 (42.6) | 18 (40.0) | ||
| ≥30 years | 127 (55.2) | 73 (52.9) | 27 (57.4) | 27 (60.0) | ||
| Gender | .95 | .75 | ||||
| Male | 158 (69.0) | 94 (68.6) | 32 (68.1) | 32 (71.1) | ||
| Female | 71 (31.0) | 43 (31.4) | 15 (31.9) | 13 (28.9) | ||
| Ethnic origin | .29 | .32 | ||||
| Canadian | 209 (90.9) | 125 (90.6) | 41 (87.2) | 43 (95.6) | ||
| First nations, Métis, Inuit, Cree | 7 (3.0) | 5 (3.6) | 2 (4.3) | 0 (0) | ||
| European | 8 (3.5) | 6 (4.3) | 1 (2.1) | 1 (2.2) | ||
| Haitian, Jamaican | 2 (0.9) | 1 (0.7) | 1 (2.1) | 0 (0) | ||
| Othera | 4 (1.7) | 1 (0.7) | 2 (4.3) | 1 (2.2) | ||
| Marital status | .50 | .54 | ||||
| Single | 201 (87.4) | 116 (84.1) | 44 (93.6) | 41 (91.1) | ||
| Married | 2 (0.9) | 1 (0.7) | 0 (0) | 1 (2.2) | ||
| Common law | 12 (5.2) | 9 (6.5) | 1 (2.1) | 2 (4.4) | ||
| Divorced, separated, widowed | 15 (6.4) | 12 (8.7) | 2 (4.3) | 1 (2.2) | ||
| Education | .83 | .32 | ||||
| ≤High school | 173 (75.5) | 102 (74.5) | 35 (74.5) | 36 (80.0) | ||
| > High school | 56 (24.5) | 35 (25.5) | 12 (25.5) | 9 (20.0) | ||
| Income (past year) | .89 | .21 | ||||
| < $20,000 | 169 (73.5) | 101 (73.2) | 34 (72.3) | 34 (75.6) | ||
| ≥$20,000 | 61 (26.5) | 37 (26.8) | 13 (27.7) | 11 (24.4) | ||
| Unstable housingb | .41 | .01 | ||||
| Unstable | 84 (36.7) | 45 (32.6) | 18 (39.1) | 21 (46.7) | ||
| Stable | 145 (63.3) | 93 (67.4) | 28 (60.9) | 24 (53.3) | ||
| Health status | ||||||
| Tested for HCV (past year) | 170 (79.1) | 104 (81.3) | 31 (68.9) | .22 | 35 (83.3) | .88 |
| Self-reported HCV status | .74 | .06 | ||||
| Positive | 116 (54.5) | 64 (50.8) | 22 (48.9) | 30 (71.4) | ||
| Negative | 97 (45.5) | 62 (49.2) | 23 (51.1) | 12 (28.6) | ||
| Self-rated physical health | .05 | <.001 | ||||
| Poor/average | 106 (46.3) | 49 (35.8) | 25 (53.2) | 32 (71.1) | ||
| Good/excellent | 123 (53.7) | 88 (64.2) | 22 (46.8) | 13 (28.9) | ||
| Self-rated mental health | .07 | .007 | ||||
| Poor/average | 109 (47.6) | 54 (39.4) | 24 (51.1) | 31 (68.9) | ||
| Good/excellent | 120 (52.4) | 83 (60.6) | 23 (48.9) | 14 (31.1) | ||
| Drug overdose (past year) | .79 | .10 | ||||
| Yes | 46 (20.0) | 13 (28.9) | 9 (19.1) | 24 (17.4) | ||
| No | 184 (80.0) | 114 (82.6) | 38 (80.9) | 32 (71.1) | ||
| Injecting practices | ||||||
| Years injecting | .22 | .10 | ||||
| ≤ 10years | 116 (50.4) | 71 (51.4) | 28 (59.6) | 17 (37.8) | ||
| > 10 years | 114 (49.6) | 67 (48.6) | 19 (40.4) | 28 (62.2) | ||
| Most common drug injectedb | .69 | .04 | ||||
| Cocaine | 154 (76.6) | 93 (74.4) | 30 (73.2) | 31 (88.6) | ||
| Heroin | 47 (23.4) | 32 (25.6) | 11 (26.8) | 4 (11.4) | ||
| Frequency of injectingc | .21 | .95 | ||||
| < Daily | 124 (61.4) | 66 (56.9) | 29 (69.0) | 29 (65.9) | ||
| Daily | 78 (38.6) | 50 (43.1) | 13 (31.0) | 15 (34.1) | ||
| Place of injectingc | .07 | .03 | ||||
| Public/semipublic | 70 (40.0) | 33 (32.0) | 19 (51.4) | 18 (51.4) | ||
| Private | 105 (60.0) | 70 (68.0) | 18 (48.6) | 17 (48.6) | ||
| Social and drug-using network | ||||||
| Injecting partners‡ | .001 | .001 | ||||
| None | 55 (27.2) | 47 (40.5) | 3 (7.1) | 5 (11.4) | ||
| ≥1 | 147 (72.8) | 69 (59.5) | 39 (92.9) | 39 (88.6) | ||
| Mean size (SD) of noninjecting social networkc | 1.50 (1.72) | 1.80 (1.86) | 0.85 (1.10) | .003 | 1.39 (1.60) | .06 |
| Mean size (SD) of IDU networkc | 1.28 (1.30) | 0.97 (1.15) | 1.70 (1.32) | <.001 | 1.80 (1.42) | <.001 |
| Harm reduction practices | ||||||
| Asked someone to obtain sterile injecting equipment from an SEPc | < .001 | <.001 | ||||
| No | 153 (66.5) | 108 (78.3) | 24 (51.1) | 21 (46.7) | ||
| Yes | 77 (33.5) | 30 (21.7) | 23 (48.9) | 24 (53.3) | ||
| Asked someone to obtain sterile drug preparation equipment from an SEPc | <.001 | .001 | ||||
| No | 160 (69.9) | 109 (79.6) | 26 (55.3) | 25 (55.6) | ||
| Yes | 69 (30.1) | 28 (20.4) | 21 (44.7) | 20 (44.4) | ||
| Problems obtaining sterile injecting equipment from an SEP (hours of operation, distance, transportation)c | <.001 | <.001 | ||||
| No | 197 (87.6) | 127 (94.8) | 36 (76.6) | 34 (77.3) | ||
| Yes | 28 (12.4) | 7 (5.2) | 11 (23.4) | 10 (22.7) | ||
| Ever received HCV disease and risk factor information | .09 | .51 | ||||
| No | 44 (19.1) | 28 (20.3) | 5 (10.6) | 11 (24.4) | ||
| Yes | 186 (80.9) | 110 (79.7) | 42 (89.4) | 34 (75.6) | ||
SD = standard deviation.
Note: Percentages are among the respondents. No risk refers to no material sharing when injecting in the past 6 months (referent group); low/intermediate risk refers to sharing of at most nonsyringe materials (container, filter, water, postinjection swab, tourniquet); high risk refers to sharing of at least syringes; p value compares risk category to referent group (i.e., no risk).
Major groups include Hispanic, Oriental, and Middle Eastern.
Refers to the past 6 months.
Refers to the past month.
Univariate results (Table 1) show that compared to no risk, other risk categories were associated with poor physical health, having one or more injecting partners, greater IDU network size, asking someone to obtain sterile equipment, and having problems obtaining clean injecting materials from an SEP. Low/intermediate risk was associated with having a smaller non-IDU network consisting primarily of sex and social support individuals. High-risk borrowing was also associated with unstable housing, poor mental health, injecting cocaine, and injecting in public places and was only marginally associated with positive HCV status.
Table 2 documents the results of multivariable polytomous regression analysis of HCV transmission risk in which all significant variables from the univariate model were considered. Compared to no risk, factors associated with low intermediate risk and high risk borrowing included poor physical health, asking someone to obtain clean syringes or drug preparation equipment from an SEP, and problems obtaining sterile injection equipment. The latter referred to SEP accessibility issues such as hours of operation of the SEP and issues related to transportation or distance from the SEP, as reported by open-ended follow-up questions. Additionally, low intermediate risk IDUs were more likely to have fewer noninjecting individuals in their social networks compared to no risk individuals. HCV infection appeared to be associated positively with high risk borrowing and negatively with low intermediate risk borrowing, but these findings were not statistically significant. There was no association between injection risk and years of injecting, place of injecting, housing stability, physical health, number of injecting partners, size of one’s IDU network, or with asking someone to obtain sterile drug preparation equipment from an SEP. Furthermore, no interactions were found between HCV status and age or gender.
Table 2.
Results of multivariate polytomous regression of HCV risk factors by level of risk from drug equipment borrowing (n = 176)
| No risk adjusted OR | Low/intermediate risk adjusted OR (95% CI) | High risk adjusted OR (95% CI) | |
|---|---|---|---|
| Age < 30 years (vs. ≥ 30 years) | 1.00 | 0.92 (0.36–2.36) | 0.43 (0.14–1.32) |
| Male (vs. female) | 1.00 | 1.13 (0.42–3.04) | 0.82 (0.28–2.39) |
| HCV positive (vs. HCV negative status) status | 1.00 | 0.84 (0.35–2.01) | 1.93 (0.69–5.38) |
| Asked someone to obtain sterile syringes from an SEPa | 1.00 | 2.50 (1.05–5.95) | 3.70 (1.42–9.71) |
| Problems obtaining sterile injecting equipment from an SEPa | 1.00 | 15.87 (3.80–66.67) | 21.74 (4.57–100.00) |
| Poor/average (vs. good/excellent) self-rated physical health | 1.00 | 2.39 (1.02–5.61) | 5.14 (1.87–14.13) |
| Cocaine (vs. heroin) injecting | 1.00 | 1.41 (0.49–4.08) | 1.76 (0.44–6.96) |
| Size of noninjecting social network (per member)a | 1.00 | 0.55 (0.38–0.79) | 0.84 (0.61–1.17) |
Note: Final model adjusted for age, gender, and the drug most commonly injected, as shown. Significant results shown in bold. No risk refers to no material sharing when injecting in the past 6 months (referent group); low/intermediate risk refers to sharing of at most nonsyringe materials (container, filter, water, postinjection swab, tourniquet); high risk refers to sharing of at least syringes.
Refers to the past month.
Discussion
In this study of active IDUs, over three quarters of participants reported receiving an HCV screening test in the year before study enrolment, indicating a relatively high level of screening coverage. There was no significant difference in testing frequency across risk categories. Our findings indicate that issues related to poor access to sterile injecting equipment, the composition of an IDU’s social network, and personal health status rather than HCV infection status, were associated with risky injecting behavior. Access to clean equipment and supportive social networks are likely to create an environment which encourages safer injection and deters equipment sharing (Hagan, Jarlais, Friedman, Purchase, and Alter, 1995; Latkin, Forman, Knowlton, and Sherman, 2003).
Despite the uncertain effectiveness of HCV screening on behavioral change, some researchers have argued in support of the CDC recommendations as a means of decreasing the population burden of HCV and facilitating entry of IDUs into treatment (Alter et al., 2004). Numerous factors may account for the lack of association between awareness of HCV infection status and risk reduction. For example, the decision to adopt safer injecting practices can be influenced by misperceptions about the health risks and severity of HCV infection, confusion with other common infectious diseases, as well as feelings of helplessness toward HCV infection (Best et al., 1999; Craine, Walker, Carnwath, and Klee, 2004; Davis et al., 2004; Rhodes et al., 2004).
Previous Research Findings on HCV Status and Risk Behaviors
Our findings are consistent with previous studies on the impact of HCV testing and infection status on injection risk behaviors. A recent study from five US cities showed that although drug user treatment1 and SEP use were associated with greater awareness of HCV status, knowledge of one’s positive HCV status was not associated with safer injection practices (Hagan et al., 2006). Similarly, a cohort study of young IDUs (aged 18–30 years) in Baltimore found negligible differences between HCV seropositive and seronegative subjects in their reduction of equipment sharing following HCV screening, despite both groups reporting some reduction in sharing (Ompad, Fuller, Vlahov, Thomas, and Strathdee, 2002). Similarly, a cross-sectional study of IDUs in drug user treatment in France showed that HCV positive subjects did not differ from negative individuals in their frequency of lending or borrowing used injecting equipment (Vidal-Trécan, Coste, Varescon-Pousson, Christoforov, and Boissonnas, 2000). Among street-recruited IDUs in Denver, Colorado, participants who were aware of their HCV positive status at study enrolment were less likely to share used injection equipment than those who were unaware of their status (Kwiatkowski, Fortuin Corsi, and Booth, 2002). More importantly, a subsequent positive test among HCV negative enrolees had no effect on existing injecting practices.
In an ethnographic investigation of IDUs in the United Kingdom, receiving an HCV negative test was associated with a reduction in injection risk behaviors (Craine et al., 2004). A positive test result, on the other hand, was associated with varying responses ranging from ambivalence regarding risk behaviors to concern about limiting disease transmission. The authors attributed the lack of consistent behavior modification to the variability in the quality of counseling and the poor disclosure of infection status among injecting partners. Nonetheless, subjects reported that an awareness of injecting partners’ HCV infection status was more important than knowing their own HCV status in determining their risk reduction behaviors. Consistent with the current findings, the study also suggested that contextual factors such as the availability of and access to clean equipment, rather than the awareness of HCV serostatus, was important for determining injecting risk.
HCV Status Awareness in the Context of HIV
The current study also supports the notion that personal infection status may be less important than other factors for risk reduction. This may be explained by the lower prominence of HCV relative to other bloodborne viruses. For example, the greater attention attributed to HIV compared to HCV may mediate the approach to both risk reduction among IDUs and clinical counseling by health care providers. Given the relatively recent recognition of HCV as a prevalent pathogen associated with injection drug use, HCV-related knowledge among IDUs is likely more limited and its importance trivialized compared to HIV (Best et al., 1999; Rhodes et al., 2004; Stein et al., 2001). In other cases, IDUs may perceive themselves to be at greater risk of HCV, since it is more widespread among injectors (US Preventive Services Task Force, 2004). Greater recognition and emphasis on HIV by health care providers might also attenuate the importance of HCV infection during clinical encounters with IDUs.
Counseling for HCV
In the absence of national guidelines on HCV screening and management, clinicians may rely on modified HIV counseling that incorporates HCV epidemiological information. Existing HCV management recommendations in Canada and the United States are not specific to IDUs and, as a result, do not address the complex array of barriers faced by IDUs for accessing HCV screening, treatment and care (CDC, 1998; Health Canada and The College of Family Physicians of Canada, 2002). IDUs must often manage unstable lifestyles and concurrent health problems such as psychiatric illness, substance abuse, and HIV infection. The inadequacy of existing approaches to HCV management for IDUs is further evidenced by the limited physician referrals to HCV specialists, poor knowledge of treatment options among many clinicians, and limited recommendations for behavior change provided to patients (Shehab, Sonnad, Jeffries, Gunaratnum, and Lok, 1999). Indeed, a national survey of Canadian family physicians revealed the majority (97%) to have easy access to the tests necessary to evaluate a newly diagnosed HCV-infected patient; however, many physicians (51%) believe they do not have the time to provide adequate counseling (Graves, Cox, Lambert-Lanning, Stephenson, and Steben, 2005).
In a review of the management of bloodborne viruses among IDUs, Loxley, Bolleter, and Carruthers (2001) emphasized the potential for disease screening to be a psychosocial as well as medical intervention. Since IDUs are frequently screened for the presence of bloodborne pathogens, these encounters represent excellent opportunities for brief, targeted interventions. The clinical encounter can be envisioned as going beyond a conventional intervention focused on providing information on transmission risk behaviors and the natural history of disease to a counseling strategy based on identifying the client’s stage of risk reduction and readiness for change (Zimmerman, Olsen, and Bosworth, 2000). Such stage specific interventions could be used to optimize the screening encounter. Other factors that may improve these encounters is respecting that the decision to be tested belongs to the patient, ensuring that testing-related “discussions” are personalized, and providing small amounts of prevention information appropriate for the person’s life circumstances. Also, while most IDUs believe test providers to be helpful in supporting risk reduction, counseling must continue to be provided in a nonjudgemental manner (Loxley et al., 2001)
Study’s Limitations
Since the current study did not evaluate the direct impact of counseling on risk reduction, future studies are warranted in order to investigate the role of counseling on changes in drug injection equipment borrowing and lending. The cross-sectional design of the study precludes any conclusion regarding the direction of the associations found. However, by restricting the analysis to injection practices that follow the participant’s awareness of his or her HCV status, we have some reassurance regarding the probable causal association of infection status and injecting practices. Our study may have also been limited by a primarily SEP-recruited sample of IDUs that may not be representative of IDUs who do not use such services. However, the inclusion of subjects both in and out of drug user treatment as well as recruitment across multiple sites is expected to improve the generalizability of the results.
Conclusions and Recommendations
Improving access to sterile injecting equipment as a means of decreasing high-risk borrowing is a public health strategy that can have important implications for HCV prevention until we can better exploit screening for the control of HCV among IDUs. However, better uptake of sterile injection equipment comes with its own challenges, especially with regard to nonsyringe materials (Morissette et al., 2007). Enhancing the relevance of knowing one’s infection status as a means of motivating risk reduction should, nevertheless, continue to be regarded as one of several important goals for reducing the burden of HCV in drug-injecting populations. To this end, current recommendations regarding HCV screening and counseling for IDUs should be revised, with a consideration of barriers (both endogenous and exogenous) to behavior change.
Acknowledgments
The authors thank the study participants, study personnel, and the collaborating recruitment sites (CACTUS-Montréal, Spectre de rue, Relais Méthadone, Dopamine, Herzl Family Practice). Financial support for the study was provided by the Montreal Public Health Department and the Health Canada/Canadian Institutes of Health Research (CIHR) Research Initiative on Hepatitis C.
Glossary
- HCV counseling
Among other goals, posttest HCV counseling is intended to discuss the meaning of the test result, reinforce personal risk reduction strategies, emphasize the need for repeat testing if necessary, refer to appropriate infection management care, and provide counseling or referral to counseling for coping with the emotional consequences of testing positive
- HCV screening
The purpose of screening for HCV infection is to identify infection in people not complaining of symptoms associated with HCV but who are suspected of having infection, so that they may be managed accordingly. Screening for HCV is currently undertaken in a range of groups and settings and supported by several consensus statements internationally
- Methadone maintenance treatment (MMT)
Methadone is a synthetic opioid, used medically as an analgesic and in the treatment of narcotic addiction, typically heroin. Methadone is considered to be generally effective in management of heroin addiction and reduction of HIV rates from needle sharing
- Syringe exchange program (SEP)
Syringe exchange programs are one of the ideology-based strategies of harm reduction for preventing infection with bloodborne viruses such as HIV or hepatitis B and C viruses among injecting drug users. The goal of SEPs is to reduce the transmission of bloodborne infections associated with drug injection by providing sterile syringes in exchange for used, potentially contaminated syringes. Some SEPs also supply clients with other materials used in drug injection and preparation as well as a room in which to inject. In addition to being a source of clean injection equipment, SEPs can offer other prevention services including risk reduction education and counseling and drug user treatment referrals
Biographies

Dr. Robert Allard, MD, MSc, is a community medicine physician mostly involved in the surveillance and control of communicable diseases and in epidemiologic methodology. He is currently working on the application of space–time clustering detection methods to health care utilization and reportable diseases. He is an adjunct professor at McGill University and at Université de Montréal.

Dr. Joseph Cox, MD, MSc, FRCPC, is a public health specialist with experience in applied clinical and public health research. Dr. Cox conducts epidemiological research on populations at risk for bloodborne and sexually transmitted infections and drug dependency. He is the principal investigator on several studies focused on the psychosocial and behavioral aspects of HIV and hepatitis C infection. He is interested in the development of infection control strategies that exploit the clinic–population health interface.

Prithwish De, MHSc, PhD is an epidemiologist with an interest in the study of social networks and their impact on injection risk behaviors among drug users. His work on the psychosocial factors and risk behaviors of IDUs is carried out as an integral part of the population health research agenda of the Montreal Public Health Department and involves both community and academic collaborators. His previous experiences have been in the field of HIV/AIDS, hepatitis C, and STD research in vulnerable populations.

Dr. Lisa Graves is an assistant professor in the Department of Family Medicine at McGill University. She is the director of the Herzl Family Practice Methadone Maintenance Program and the director of undergraduate education of the Department of Family Medicine. Her research interests include vulnerable populations, problematic substance use in pregnancy, and medical education related to training with vulnerable populations.

Carole Morissette, MD, FRCPC, is a public health specialist with experience in prevention program at community level, organization of services, and public health research. Dr. Morissette is involved in research on street youth, drug users, and ethnocultural communities. She is interested in advocacy and development of collaborative partnership to promote transfer of knowledge into best practices and improved preventive interventions for marginalized populations.

Élise Roy, MD, MSc, is a professor in the Drug Addiction Service, Faculty of Medicine and Health Sciences, University of Sherbrooke. Since 1991, Dr. Roy has undertaken many research projects related to the health of marginalized youth, particularly the health consequences of drug abuse and injection drug use. She is the principal investigator of several studies looking at the prevalence and incidence of HIV and hepatitis C among street youth, the psychosocial determinants of initiation into injection drug use, and the sociological aspects of drug use and its health consequences in this population.

Randolph Stephenson obtained his PhD in clinical and research psychology from the Université du Québec à Montréal (Québec, Canada) and his postgraduate specialized diploma in addiction from the Université de Sherbrooke (Québec, Canada) and was part of the first cohort of the Canadian Health Institutes Research Post-doctoral Strategic Training Fellow in Primary Health Care from the University of Western Ontario (Ontario, Canada). His research interests range from addiction to primary care topics. He works as a clinical psychologist in the Methadone Maintenance Program of the Herzl Family Practice Centre of the SMBD Jewish General Hospital located in Montreal.

Claude Tremblay, MSc, is a research profesionnal who has worked on several projects involving sexually transmitted infections related to injection drug use and men who like to have sex with men (MSM) populations. Her interests are in statistical analysis applied to public health.
Footnotes
Treatment can be briefly and usefully defined as a planned, goal-directed change process, which is bound (by culture, place, time, etc.) and can be categorized into professional-based, tradition-based, mutual-help-based (AA, NA, and the like), and self-help (“natural recovery”) models. There are no unique models or techniques used with substance users—of whatever types—which aren’t also used with nonsubstance users. In the West, with the relatively new ideology of “harm reduction” and the even more recent “quality of life (QOL)” model there are now new sets of goals in addition to those derived from/associated with the older tradition of abstinence-driven treatment models. Editor’s note.
References
- Alter MJ, Seeff LB, Bacon BR, Thomas DL, Rigsby MO, Di Bisceglie AM. Testing for hepatitis C virus infection should be routine for persons at increased risk for infection. Annals of Internal Medicine. 2004;141:715–717. doi: 10.7326/0003-4819-141-9-200411020-00013. [DOI] [PubMed] [Google Scholar]
- Best D, Noble A, Finch E, Gossop M, Sidwell C, Strang J. Accuracy of perceptions of hepatitis B and C status: cross sectional investigation of opiate addicts in treatment. British Medical Journal. 1999;319:290–291. doi: 10.1136/bmj.319.7205.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden S, McCaw R, White PA, Crofts N, Aitken CK. Detection of multiple hepatitis C virus genotypes in a cohort of injecting drug users. Journal of Viral Hepatitis. 2005;12:322–324. doi: 10.1111/j.1365-2893.2005.00592.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recommendations and Reports. 1998;47(RR-19):1–39. [PubMed] [Google Scholar]
- Cox J, Stephenson R, Morissette C, Roy E, Graves L, Allard R, Mereb C. Development of a questionnaire on hepatitis C–related knowledge, attitudes and behaviors of injection drug users. Journal of Urban Health. 2002;79(4):S99. [Google Scholar]
- Craine N, Walker M, Carnwath T, Klee H. Hepatitis C testing and injecting risk behaviour: the results of a UK based pilot study. International Journal of Drug Policy. 2004;15:115–122. [Google Scholar]
- Darke S. Self-report among injecting drug users: a review. Drug and Alcohol Dependence. 1998;51:253–263. doi: 10.1016/s0376-8716(98)00028-3. [DOI] [PubMed] [Google Scholar]
- Davis M, Rhodes T, Martin A. Preventing hepatitis C: “common sense”, “the bug” and other perspectives from the risk narratives of people who inject drugs. Social Science and Medicine. 2004;59:1807–1818. doi: 10.1016/j.socscimed.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Direction de santé publique de Montréal. VIH/Hépatite C—Information aux professionnels. Appel à la vigilance—Août 2006: Recrudescence d’infections par le virus de l’immunodéficience humaine (VIH) et le virus de l’hépatite C (VHC) chez les utilisateurs de drogues injectables (UDI) de Montréal. 2006. Retrieved January 10, 2008, from http://www.santepub-mtl.qc.ca/Mi/vigilance/31082006.html.
- Dowling-Guyer S, Johnson ME, Fisher DC, Needle R, Waltters J, Andersen M, Williams M, Kotranski L. Reliability of drug users’ self-reported HIV risk behaviors and validity of self-reported drug use. Assessment. 1994;1:383–392. [Google Scholar]
- Graves L, Cox J, Lambert-Lanning A, Stephenson R, Steben M. Knowledge, perceptions and attitudes of Canadian family physicians about Hepatitis C. Poster session presented at the North American Primary Care Research Group Annual Meeting; Quebec City, Canada. 2005. Oct, Retrieved January 10, 2007, from http://www.fmdrl.org/index.cfm?event=c.AccessResourceandrid=213. [Google Scholar]
- Hagan H, Campbell J, Thiede H, Strathdee S, Ouellet L, Kapadia F, Hudson S, Garfein RS. Self-reported hepatitis C virus antibody status and risk behavior in young injectors. Public Health Reports. 2006;121:710–719. doi: 10.1177/003335490612100611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan H, Jarlais DC, Friedman SR, Purchase D, Alter MJ. Reduced risk of hepatitis B and hepatitis C among injection drug users in the Tacoma syringe exchange program. American Journal of Public Health. 1995;85:1531–1537. doi: 10.2105/ajph.85.11.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan H, Thiede H, Weiss NS, Hopkins SG, Cuchin JS, Alexander ER. Sharing of drug preparation equipment as a risk factor for hepatitis C. American Journal of Public Health. 2001;91:42–46. doi: 10.2105/ajph.91.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JA, Page-Shafer K, Lum PJ, Bourgois P, Stein E, Evans JL, Busch MP, Tobler LH, Phelps B, Moss AR. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. Journal of Infectious Diseases. 2002;186:1558–1564. doi: 10.1086/345554. [DOI] [PubMed] [Google Scholar]
- Hankins C, Alary M, Parent R, Blanchette C, Claessens C the SurvUDI Working Group. Continuing HIV transmission among injection drug users in Eastern Central Canada: the SurvUDI Study, 1995 to 2000. Journal of Acquired Immune Deficiency Syndromes. 2002;30:514–521. doi: 10.1097/00126334-200208150-00007. [DOI] [PubMed] [Google Scholar]
- Hartel DM, Schoenbaum EE, Selwyn PA, Friedland GH, Klein RS, Drucker E. Patterns of heroin, cocaine and speedball injection among Bronx (USA) methadone maintenance patients: 1978–1988. Addiction Research. 1997;8:394–404. [Google Scholar]
- Health Canada and The College of Family Physicians of Canada. Physicians’ desk reference: primary care management of hepatitis C. 2002. Retrieved January 10, 2007, from http://www.cfpc.ca/English/cfpc/programs/patient%20care/hepatitis%20c/primary/default.asp?s=1.
- Heimer R, Clair S, Grau LE, Bluthenthal RN, Marshall PA, Singer M. Hepatitis-associated knowledge is low and risks are high among HIV-aware injection drug users in three US cities. Addiction. 2002;97:1277–1287. doi: 10.1046/j.1360-0443.2002.t01-1-00211.x. [DOI] [PubMed] [Google Scholar]
- Herring BL, Page-Shafer K, Tobler LH, Delwart EL. Frequent hepatitis C virus superinfection in injection drug users. Journal of Infectious Diseases. 2004;190:1396–1403. doi: 10.1086/424491. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Jr, Lemeshow S. Applied logistic regression. New York: John Wiley; 1989. [Google Scholar]
- Joseph JG, Emmons CA, Kessler RC, Wortman CB, O’Brien K, Hocker WT, Schaefer C. Coping with the threat of AIDS. An approach to psychosocial assessment. American Psychologist. 1984;39:1297–1302. [PubMed] [Google Scholar]
- Koester S, Booth RE, Zhang Y. The prevalence of additional injection-related HIV risk behaviors among injection drug users. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1996;12:202–207. doi: 10.1097/00042560-199606010-00015. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski CF, Fortuin Corsi K, Booth RE. The association between knowledge of hepatitis C virus status and risk behaviors in injection drug users. Addiction. 2002;97:1289–1294. doi: 10.1046/j.1360-0443.2002.00208.x. [DOI] [PubMed] [Google Scholar]
- Latkin CA, Forman V, Knowlton A, Sherman S. Norms, social networks, and HIV-related risk behaviors among urban disadvantaged drug users. Social Science and Medicine. 2003;56:465–476. doi: 10.1016/s0277-9536(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Leclerc P, Morissette C. Situation épidémiologique des infections transmissibles sexuellement et par le sang (ITSS) pour la région de Montréal. Pascale Leclerc, Carole Morissette. Direction de santé publique de Montréal. 2006a. Retrieved January 10, 2007, from http://www.santepubmtl.qc.ca/Publication/mts/itsssituation.html.
- Leclerc P, Morissette C, Roy É, Tremblay C, Alary M. Accès au matériel stérile d’injection et aux soins médicaux pour les utilisateurs de drogues par injection de Montréal. Poster session presented at the Les journées annuelles de santé publique; Montreal, Quebec, Canada. 2006b. Oct, [Google Scholar]
- Loxley W, Bolleter A, Carruthers S. Talking about testing: opportunities for prevention in blood borne virus testing and vaccination with injectors. Australia: National Drug Research Institute, Perth; 2001. [Google Scholar]
- Morissette C, Cox J, De P, Tremblay C, Roy E, Allard R, Stephenson R, Graves L. Minimal uptake of sterile drug preparation equipment in a predominantly cocaine injecting population: implications for HIV and hepatitis C prevention. International Journal of Drug Policy. 2007;18:204–212. doi: 10.1016/j.drugpo.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Needle RH, Coyle S, Cesari H, Trotter R, Clatts M, Koester S, Price L, McLellan E, Finlinson A, Bluthenthal RN, Pierce T, Johnson J, Jones TS, Williams M. HIV risk behaviors associated with the injection process: multiperson use of drug injection equipment and paraphernalia in injection drug user networks. Substance Use and Misuse. 1998;33:2403–2423. doi: 10.3109/10826089809059332. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Expert Panel. NIH consensus statement on management of hepatitis C: 2002. NIH Consensus State Science Statements. 2002;19:1–46. Retrieved August 20, 2006, from http://consensus.nih.gov/2002/2002HepatitisC2002116PDF.pdf. [PubMed] [Google Scholar]
- Ompad DC, Fuller CM, Vlahov D, Thomas D, Strathdee SA. Lack of behavior change after disclosure of hepatitis C virus infection among young injection drug users in Baltimore, Maryland. Clinical Infectious Diseases. 2002;35:783–788. doi: 10.1086/342063. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: applications to addictive behaviors. American Psychologist. 1992;47:1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- Remis RS, Bruneau J, Hankins CA. Enough sterile syringes to prevent HIV transmission among injection drug users in Montreal? Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1998;18(Suppl 1):S57–S59. doi: 10.1097/00042560-199802001-00011. [DOI] [PubMed] [Google Scholar]
- Rhodes T, Davis M, Judd A. Hepatitis C and its risk management among drug injectors in London: renewing harm reduction in the context of uncertainty. Addiction. 2004;99:621–633. doi: 10.1111/j.1360-0443.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- Roy É, Alary M, Morissette C, Leclerc P, Boudreau JF, Parent R, Rochefort J, Claessens C The SurvUDI Working Group. High hepatitis C virus prevalence and incidence among Canadian intravenous drug users. International Journal of STD and AIDS. 2007;18:23–27. doi: 10.1258/095646207779949880. [DOI] [PubMed] [Google Scholar]
- Shehab TM, Sonnad SS, Jeffries M, Gunaratnum N, Lok AS. Current practice patterns of primary care physicians in the management of patients with hepatitis C. Hepatology. 1999;30:794–800. doi: 10.1002/hep.510300328. [DOI] [PubMed] [Google Scholar]
- Sherman M, Bain V, Villeneuve JP, Myers RP, Cooper C, Martin S, Lowe C. The management of chronic viral hepatitis: A Canadian consensus conference 2004. Canadian Journal of Infectious Diseases & Medical Microbiology. 2004;15:313–326. doi: 10.1155/2004/326964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Maksad J, Clarke J. Hepatitis C disease among injection drug users: knowledge, perceived risk and willingness to receive treatment. Drug and Alcohol Dependence. 2001;61:211–215. doi: 10.1016/s0376-8716(00)00144-7. [DOI] [PubMed] [Google Scholar]
- Thorpe L, Ouellet LJ, Hershow R, Bailey SL, Williams IT, Williamson J, Monterroso ER, Garfein RS. Risk of hepatitis C virus injection among young adult injection drug users who share injection equipment. American Journal of Epidemiology. 2002;155:645–653. doi: 10.1093/aje/155.7.645. [DOI] [PubMed] [Google Scholar]
- US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: recommendation statement. Annals of Internal Medicine. 2004;140:462–464. doi: 10.7326/0003-4819-140-6-200403160-00013. [DOI] [PubMed] [Google Scholar]
- Vidal-Trécan G, Coste J, Varescon-Pousson I, Christoforov B, Boissonnas A. HCV status knowledge and risk behaviours amongst intravenous drug users. European Journal of Epidemiology. 2000;16:439–445. doi: 10.1023/a:1007622831518. [DOI] [PubMed] [Google Scholar]
- Zimmerman GL, Olsen CG, Bosworth MF. A “stages of change” approach to helping patients change behavior. American Family Physician. 2000;61:1409–1416. [PubMed] [Google Scholar]