Abstract
Objective
Increased pro-inflammatory cytokines and reactive oxygen and nitrogen species (RONS) occur in osteoarthritis (OA). Oxygen tension can alter the levels of RONS induced by interleukin-1 (IL1). RONS such as nitric oxide (NO) can alter energy metabolism. The aim of this study was to determine if oxygen tension alters energy metabolism, in articular cartilage, in response to IL1 or NO and to determine if cell death occurred.
Design
Porcine articular chondrocytes were incubated with IL1 or the NO donor NOC18 for 48 hrs in either 1, 5 or 20% O2. ATP levels were measured and immunoblots for AMPK were done. Protein translation was measured by S6 activation. Senescence and autophagy were determined by increased caveolin or conversion of LC3-I to LC3-II respectively.
Results
1% O2 significantly reduced ATP levels compared with 20% O2. 5% O2 significantly increased ATP levels compared with 20% O2. 1% O2 significantly increased pAMPK protein expresion compared with 5 or 20% O2. Oxygen tension had no effectcs on pS6, caveolin or LC3-II levels. IL-1 induced NO production was significantly reduced with decreased oxygen tension, and significantly reduced ATP levels at all oxygen tensions, but pAMPK was only significantly increased at 5% O2. IL1 significantly reduced pS6 at all oxygen tensions. IL-1 had no effects on caveolin and significantly increased LC3-II at 20% O2 only. NOC18 significantly reduced ATP levels at all oxygen tensions, and significantly increased pAMPK at 5% O2 only, and significantly decreased pAMPK at 1% O2. NOC18 significantly reduced pS6 at 1% O2 and significantly increased caveolin at 5% O2, and LC3-II at 1% O2.
Conclusion
Our data suggest 5% O2 is optimal for energy metabolism and protective to some effects of IL1 and NO. NO has the greatest effects on ATP levels and the induction of autophagy at 1% O2.
Introduction
Reactive oxygen and nitrogen species (RONS) are increased in osteoarthritis (OA) 1,2. RONS can be produced in response to inflammation and mechanical stress 3-5, both of which are risk factors for OA 6. RONS can have multiple effects on chondrocytes, but are associated with oxidative damage to DNA, proteins and lipids, resulting in a net loss of the extracellular matrix, with little cell death 7-11. One of the most direct effects of nitric oxide (NO) is inhibition of ATP production, by competing with oxygen to bind to cytochrome oxidase on the mitochondria, so inhibiting the electron transport chain and the generation of ATP 12.
The interaction of NO with oxygen is of particular interest to articular cartilage since articular cartilage is an avascular tissue. Therefore oxygen and other nutrients must diffuse into the tissue from the synovial fluid surrounding the joint, resulting in an oxygen gradient, ranging from 5% O2 on the surface to 1% O2 in the deep zone 13,14. It is unknown how NO can alter energy metabolism in articular chondrocytes at the physiological oxygen tensions that exist in articular cartilage.
5% O2 appears optimal for articular cartilage metabolism 15. The culture of articular cartilage explants at 5% O2 maintains the material properties of cartilage close to those observed in vivo, whereas culturing cartilage explants at 1 or 20% O2 reduces the material properties compared with those of articular cartilage in vivo 15. Similarly, 5% O2 is also associated with a significant increase in the rates of proteoglycan, collagen and hyaluronan synthesis compared with 20 or 1% O215,16. Oxygen tension can alter many aspects of chondrocyte metabolism 17,18. Although articular chondrocytes are highly glycolytic, they also show mitochondrial respiration in vitro 12,19-21. It is possible 5% O2 may be optimal for energy metabolism in articular cartilage, by providing an environment in which both glycolysis and oxidative phosphorylation can function. Inadequate levels of ATP are sensed by adenosine monophosphate – activated protein kinase (AMPK), which acts as a cellular energy sensor. Under normal physiological conditions, when ATP levels fall, AMPK becomes phosphorylated 22, and more ATP is generated.
Decreased ATP is often associated with cell death. However little cell death occurs in OA articular chondrocytes 23, or in response to RONS 10,11. OA articular chondrocytes show signs of stress induced senescence as indicated by reduced replicative capacity, telomere shortening, increased expression of the senescence regulator caveolin-124 and senescence-associated β-galactosidase expression, compared with non-OA chondrocytes 25-28. RONS can also induce senescence in chondrocytes in vitro at 20% O2 25,26. Alternatively, decreased ATP availability can initiate the conservation of ATP by the inhibition of energy demanding processes such as protein translation 29. When there is a severe lack of ATP availability, survival mechanisms such as induction of autophagy or stress induced cell senescence can occur. Autophagy can generate more energy substrates by ingestion of intracellular organelles and the recycling of amino acids. Autophagosomes are observed in OA chondrocytes 30.
In this study we determine how oxygen tension alters ATP production in articular chondrocytes in the presence of IL1, or an NO donor. We then investigated whether decreased availability of ATP is associated with initiation of energy conservation mechanisms and the induction of cell senescence or autophagy.
Materials and Methods
Antibodies
Rabbit monoclonal anti-phospho-AMPKα (Thr172), rabbit polyclonal anti-AMPKα, rabbit monoclonal anti-phospho-S6 ribosomal protein (Ser235/236), rabbit monoclonal anti-S6 ribosomal protein, and rabbit polyclonal anti-LC3 were obtained from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal anti-caveolin-1 was obtained from Novus Biologicals (Littleton, CO). Under normal conditions phosphorylation of AMPK occurs when ATP levels decrease. Phosphorylation of S6 (pS6) ribosomal protein correlates with an increase in translation of mRNA transcripts that contain an oligopyrimidine tract in their 5’ untranslated regions 31. These particular mRNA transcripts (5’TOP) encode proteins involved in cell cycle progression as well as ribosomal proteins and elongation proteins necessary for translation 31,32. Caveolin-1 is an integral membrane protein that serves as a scaffold and can regulate cell-signaling pathways involved in senescence 24. Autophagy or Type II cell death can be a cell survival response to nutrient deprivation. Autophagy is a catabolic process involving the regulated digestion of cellular macromolecules and even whole organelles. During autophagy a portion of the cytoplasm is sequestered in double-membrane vesicles called autophagosomes, which then fuse with vacuoles or lysosomes where hydrolysis of the contents occurs. Increased formation of LC3-II compared with LC3-I is an indication of autophagy. The LC3-1 form migrates as a 16 kDa band but is lipidated in autophagy to produce a 14 kDa LC3-II form and incorporated into growing autophagosomes 33.
Explant culture
Full thickness explants of articular cartilage were harvested from the femoral condyles of 2-year old female pigs within 4 hours of death. Cartilage explants were cultured in low glucose Dulbecco's Modified Eagle Medium (LG-DMEM) (Gibco, Gaithersburg, MD) with 10% heat inactivated FBS (Sigma-Aldrich, St. Louis, MO), 0.1 mM non-essential amino acids (Gibco), 37.5 μg/ml ascorbate-2-phosphate (Sigma-Aldrich), 10 mM HEPES (Gibco), 100 U/ml penicillin and streptomycin (Gibco). After 72 hrs in culture, the media were removed and replaced with media containing 0 - 10 ng/ml recombinant porcine IL1α (R & D Systems, Minneapolis) or 0 - 2 mM NOC18 (DETA-NONOate, A.G. Scientific Inc., San Diego, CA) and the explants incubated at 20%, 5% or 1% O2, with the residual gas composed of 5% CO2 and nitrogen, for a further 48 hours. NOC18 is a stable NO-amine complex, with a long half-life that spontaneously releases NO, without cofactors, under physiological conditions. Due to the limitation of the number of explants that could be obtained from one pig joint, experiments were only carried out at two oxygen tensions for each pig and repeated on three pigs. Statistical analysis was only carried out on data from the same pig.
NO Assay
NO production was assessed by determining the levels of total nitrate and nitrite in the media. Nitrate was first converted to nitrite using nitrate reductase and the total nitrite was measured using the Griess reagent 3.
ATP extraction from cartilage explants
The explants were dried and weighed under the same oxygen tension as the experimental condition and rapidly placed in 300 μl of 5% HClO3 and 2 mM EDTA. The explants were freeze thawed 3 times and neutralized with 65 μl of 40% KOH (pH 13) to pH 7.6-8.0, which is optimal for luciferase function. After centrifugation at 500g for 5 minutes, the supernatant was diluted 1:50 and 1:250 with PBS and the ATP levels measured, using the ATP Bioluminescence Assay Kit HS II (Roche Applied Bioscience). ATP standards were prepared ranging from 0 – 10000 ng/ml in PBS. 20 μl of luciferase was added to 180 μl of sample or standard and bioluminescence measured after a 1 second delay and integrated over 1-10 seconds, using a Veritas microplate luminometer (Turner Biosystems, Sunnyvale, CA). Samples were measured at 2 dilutions to ensure that no inactivation of the luciferase by the acid had occurred.
Live-Dead Assay
In the recovery experiments, chondrocyte viability was determined using the Live/Dead assay (Molecular Probes, Carlsbad, CA) after treatment.
Chondrocyte Cell Culture in Alginate Beads
Articular chondrocytes were enzymatically isolated from full thickness slices of articular cartilage from the femoral condyles of skeletally mature 2-3 year old pigs, using pronase for 1 hour and collagenase type II for 2 hours. The chondrocytes were resuspended in 1.2% alginate at a concentration of 4 × 106 cells/ml 34. Alginate was used to maintain the chondrocytes in a rounded shape and ensure collagen II expression associated with chondrocytes. If chondrocytes are grown as a monolayer, dedifferentiation to fibrochondrocytes occurs. The alginate beads were cultured in low glucose DMEM (Gibco, Gaithersburg, MD), containing the same additives as the explants were grown in, at 37° C, 5% CO2, 95% air for 72 hours prior to treatment. The media were then removed and the chondrocytes encapsulated in alginate beads and incubated with either control media or media containing 1 ng/ml recombinant porcine IL1α or 0.5 mM NOC18 for a further 48 hrs, at either 20, 5 or 1% O2, with the residual gas made up of 5% CO2 and N2. Sufficient chondrocytes could be obtained from pig joint that all treatments could be carried out at the three oxygen tensions for one joint and immunoblots performed. The experiments were then repeated three times.
Immunoblots
Chondrocytes were released from the alginate beads with calcium chelation in 55 mM sodium citrate containing protease and phosphotase inhibitors. Chondrocytes were resuspended in RIPA buffer containing protease and phosphotase inhibitors and protein extracted. 20 μg of protein was loaded on a 4-20% Tris-HCl SDS gel and separated by electrophoresis and transferred to PVDF membrane. The membrane was stained with amido black to confirm transfer of the protein samples and even loading of the lanes. For the detection of phosphorylated proteins Protein Free Block (Pierce Biotechnology Inc., Rockford, IL) was used as the blocking and incubation buffer for the primary antibody, and 5% non-fat milk in TBST was used for the secondary antibody. For total proteins 5% non-fat milk in TBST was used in the blocking and incubation buffers for primary and secondary antibodies. The PVDF membranes were blocked for 2 hrs at room temperature and incubated overnight at 4°C with primary antibody (1:5000 dilution), washed and incubated with secondary antibody at a 1:5000 dilution for 2 hrs with shaking at room temperature. Blots were washed and developed using Western Lightning Plus ECL (Perkin Elmer, Waltham, MA).
Statistical analysis
Statistical analysis was performed using ANOVA with Duncan's post-hoc comparison, with significance reported at the 95% confidence level.
Results
Effects of oxygen tension
Oxygen tension had no significant effects on NO levels (Table 1), but altered ATP levels in articular cartilage explants (Table 2). Incubation of articular cartilage explants at 1% O2, caused a significant (30-50%) reduction in ATP levels compared with 20% O2 (Tables 2a, b). Incubation of explants at 5% O2 caused a significant (10-15%) increase in ATP levels compared with incubation at 20% O2 (Tables 3a, b).
Table 1.
Articular cartilage explants were incubated with 0.1 ng/ml IL1, for 72 hrs at 20, 5 or 1% O2. Nitrite + nitrate were measured in the media. Data shown are means with 95% confidence intervals (lower limit, upper limit), N=9.
| IL-1 (ng/ml) | 20% O2 NOx (μM/mg) | 5% O2 NOx (μM/mg) | 1% O2 NOx (μM/mg) | p |
|---|---|---|---|---|
| 0 | 191 (144, 239) | 158 (66, 250) | 113 (85, 140) | 20 v 5 = 0.91, 20 v 1 = 0.77, 5 v 1 = 0.85 |
| 0.1 | 3163 (2663, 3664) | 2192 (1694, 2690) | 1254 (1033, 1475) | 20 v 5 = 0.001 20 v 1 = <0.0001 5 v 1 = 0.003 |
| p | < 0.0001 | <0.0001 | 0.0003 |
Table 2.
Articular cartilage explants were cultured with either IL1 (a) or NOC18 (b) for 48 hrs in either 20 or 1% O2. ATP was extracted from the explants and measured. Data shown are means with 95% confidence intervals (lower limit, upper limit), N=18.
| (a) | |||
|---|---|---|---|
| IL1 (ng/ml) | 20% O2 ATP (ng/mg) | 1% O2 ATP (ng/mg) | P |
| 0 | 77 (63, 92) | 59 (51, 67) | 0.005 |
| 0.1 | 49 (39, 59) | 46 (39, 53) | 0.70 |
| 1 | 36 (28, 45) | 35 (26, 44) | 0.97 |
| 10 | 41 (33, 50) | 37 (26, 47) | 0.33 |
| 0 v 0.1, 1,10 | <0.0001, <0.0001, <0.0001 | 0.05, 0.0004, 0.0005 | |
| 0.1 v 1, 10 | 0.04, 0.26 | 0.09, 0.09 | |
| 1 v 10 | 0.31 | 0.90 | |
| (b) | |||
|---|---|---|---|
| NOC18 (mM) | 20% O2 ATP (ng/mg) | 1% O2 ATP (ng/mg) | P |
| 0 | 78 (58, 99) | 40 (26, 53) | <0.0001 |
| 0.5 | 45 (32, 57) | 24 (6, 42) | 0.045 |
| 1 | 50 (33, 68) | 18 (12, 25) | 0.007 |
| 2 | 42 (31, 54) | 19 (15, 24) | 0.97 |
| 0 v 0.5, 1, 2 | 0.0004, 0.0004, <0.0001 | 0.11, 0.03, 0.03 | |
| 0.5 v. 1, 2 | 0.89, 0.68 | 0.49, 0.48 | |
| 1 v 2 | 0.61 | 0.97 | |
Table 3.
Articular cartilage explants were cultured with IL1 (a) or NOC18 (b) for 48 hrs in 5% O2. ATP was extracted from the explants and measured. Data shown are means with 95% confidence intervals (lower limit, upper limit), N=15.
| (a) | |
|---|---|
| IL1 (ng/ml) | ATP (ng/ml) |
| 0* | 68 (54, 82) |
| 0 | 82 (66, 98) |
| 0.1 | 72 (62, 82) |
| 1 | 50 (43, 57) |
| 10 | 40 (36, 45) |
| 0 v 0.1, 1, 10 | 0.21, 0.0004, <0.0001 |
| 0.1 v 1, 10 | 0.01, 0.0004 |
| 1 v 10 | 0.23 |
| (b) | |
|---|---|
| NOC18 (mM) | ATP (ng/ml) |
| 0* | 54 (45, 63) |
| 0 | 72 (58, 86) |
| 0.5 | 69 (58, 80) |
| 1 | 50 (42, 58) |
| 2 | 55 (45, 65) |
| 0 v 0.5, 1, 2 | 0.70, 0.01, 0.04 |
| 0.5 v 1, 2 | 0.02, 0.07 |
| 1 v 2 | 0.56 |
represents articular cartilage explants obtained from the same pigs cultured at 20% O2. p value for 20% v 5% O2 without IL1 = 0.11. p value for 20% v 5% O2 without NOC18 = 0.04.
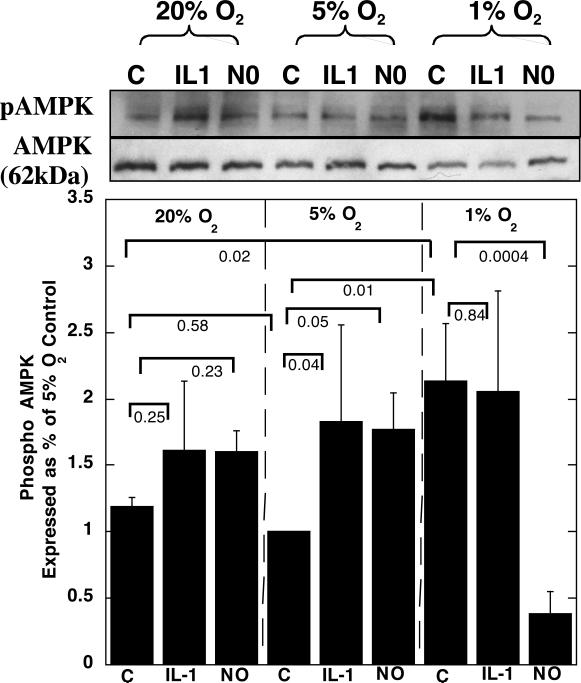
Incubation of articular chondrocytes in alginate beads at 1% O2 caused a significant increase in phospho-AMPK (pAMPK) protein expression compared with 5% or 20% O2 (Fig. 1). There was no difference in pAMPK protein levels between 20 or 5% O2 conditions.
Figure 1.
Articular chondrocytes were grown in alginate and cultured with either 0.1 ng/ml IL1 or 0.5 mM NOC18 for 48 hrs, at 20, 5 or 1% O2. The chondrocytes were released from the alginate and the protein extracted. Immunoblots were carried out for pAMPK and AMPK. Representative blots for pAMPK and AMPK are shown. Data shown are means with 95% confidence intervals, N=3.
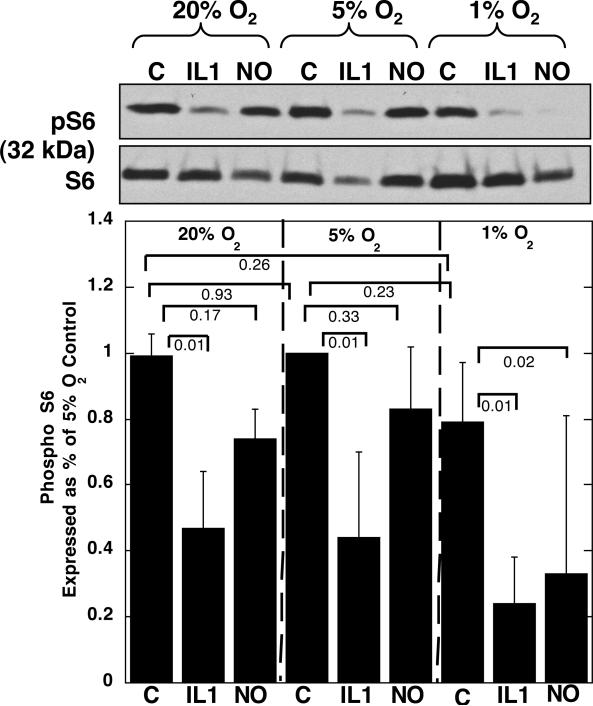
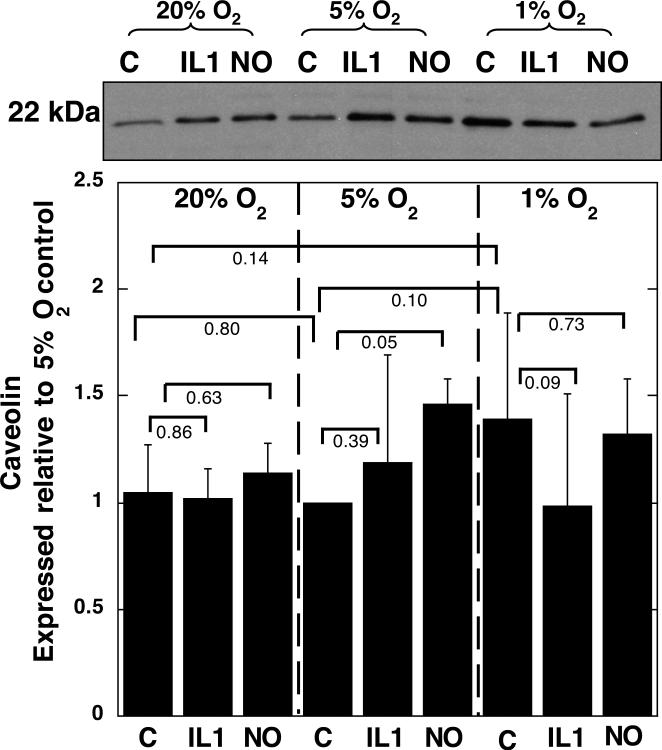
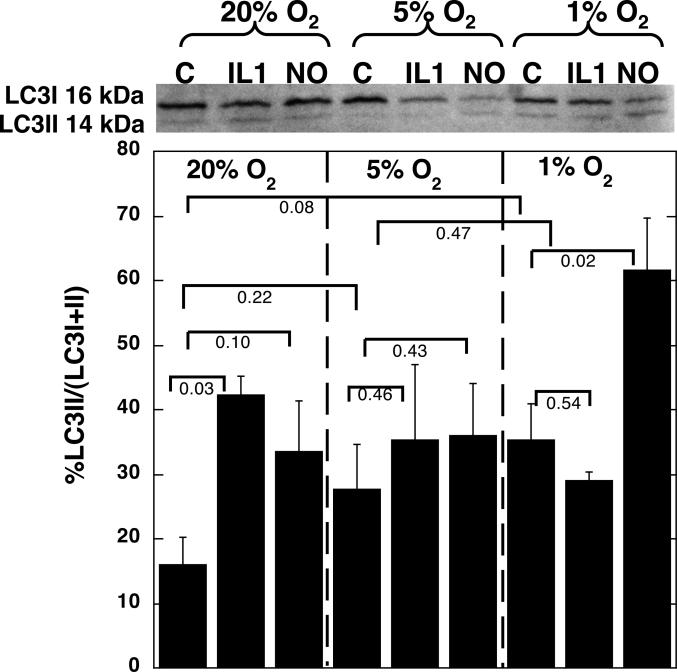
No effects of oxygen tension ocurred on pS6 (Fig. 2), or caveolin protein expression, (Fig. 3), although there was a trend towards increased caveolin expression at 1% O2 compared with 20 or 5% O2. A decrease in oxygen tension was associated with some conversion of LC3-I to LC3-II, as indicated by an increase in the ratio of LC3-II to the total LC3 (LC3-I + II) (Fig. 4).
Figure 2.
Articular chondrocytes were grown in alginate and cultured with either 0.1 ng/ml IL1 or 0.5 mM NOC18 for 48 hrs, at 20, 5 or 1% O2. The chondrocytes were released from the alginate and the protein extracted. Immunoblots were carried out for pS6 and S6. A representative blot is shown. Data shown are means with 95% confidence intervals, N=3.
Figure 3.
Articular chondrocytes were grown in alginate and cultured with either 0.1 ng/ml IL1 or 0.5 mM NOC18 for 48 hrs, in either 20, 5 or 1% O2. The chondrocytes were released from the alginate and the protein extracted. Immunoblots were carried out for caveolin. A representative blot is shown. Data shown are means with 95% confidence intervals, N=3.
Figure 4.
Articular chondrocytes were grown in alginate and cultured with either 0.1 ng/ml IL1 or 0.5 mM NOC18 for 48 hrs, in either 20, 5 or 1% O2. The chondrocytes were released from the alginate and the protein extracted. Immunoblots were carried out for LC3. A representative blot is shown. Data shown are means with 95% confidence intervals, N=3.
Effects of oxygen tension on the response to IL-1
0.1 ng/ml IL1 caused a significant increase in NO production at all oxygen tensions tested (Table 1). IL1 induced NO production was significantly less at 5 or 1% O2 than at 20% O2, and the IL1-induced NO levels were significantly less at 1 than at 5% O2.
Incubation with IL1 reduced ATP levels at all oxygen tensions tested (Tables 2 and 3). A higher concentration of IL1 (1 ng/ml) was necessary to cause a significant reduction in ATP at 5% O2 than at 20 or 1% O2 (0.1 ng/ml). 0.1 ng/ml IL1 caused approximately a 30% decrease in ATP levels when investigated at 20% O2, whereas 0.1 ng/ml IL1 only caused approximately a 15% decrease in ATP levels at when investigated at 1% O2. No changes in ATP were observed when the explants were cultured in DMEM high glucose media, under the same experimental conditions (data not shown).
IL1 caused a significant increase in pAMPK, compared with controls at the same oxygen tensions, when chondrocytes were incubated in 5% O2, but not at 1 or 20% O2 (Fig. 1). IL1 caused a significant decrease in pS6 ribosomal protein expression at all three oxygen tensions tested, compared with untreated chondrocytes incubated at the same oxygen tensions (Fig. 2). IL1 had no effect on caveolin expression compared with untreated chondrocytes incubated at the same oxygen tension (Fig. 3). Treatment of chondrocytes with IL1 at 20% O2 caused a significant increase in LC3-II to LC3-I+II, but IL1 had no significant effects when the experiments were carried out at 5 or 1% O2 (Fig. 4).
Effects of oxygen tension on the response to NOC18
The addition of the NO donor NOC18 reduced ATP levels at all oxygen tensions tested (Tables 2b, 3b). A greater concentration of NOC18 (1 mM) was needed to cause a significant reduction in ATP levels when articular cartilage explants were cultured at 5 or 1% O2, whereas explants cultured at 20% O2 required only 0.5 mM NOC18. No changes in ATP were observed when the explants were cultured in DMEM high glucose media, under the same experimental conditions (data not shown).
At 20% O2, 0.5 mM NOC18 showed some increase on pAMPK protein expression compared to controls incubated at 20% O2 (Fig. 1). At 5% O2 NOC18 caused a significant increase in pAMPK protein expression compared to controls incubated at 5% O2. Incubation at 1% O2 in the presence of NOC18 significantly decreased pAMPK protein expression compared with all other groups.
NOC18 reduced pS6 ribosomal protein expression at all three oxygen tensions tested, compared with untreated chondrocytes incubated at the same oxygen tension. (Fig. 2), which was significant when carried out at 1% O2. The levels of pS6 protein expression were significantly lower when NOC18 was added at 1% O2 than when NOC18 was added to the chondrocytes incubated at 20 or 5% O2.
NOC18 caused a significant increase in caveolin expression at 5% O2, but had no effect at 20 or 1% O2 compared with the controls incubated at the same oxygen tensions (Fig. 3). However, at 1% O2 the caveolin expression was already increased compared with 20% and 5% O2 controls (Fig. 3). The effects of NOC18 on caveolin expression were increased at 5 and 1% O2 compared with NOC18 added to chondrocytes in 20% O2. Oxygen tension did not have significantly different effects on the levels of NOC-18 induced caveolin protein expression.
Treatment of chondrocytes with NOC18 caused some increase in LC3II to LC3I+II at all oxygen tensions (Fig. 4). NOC18 caused a significant increase in the ratio of LC3II to LC3I+II at 1% O2, compared with all other groups.
Effects of oxygen tension, NO and IL1 on cell viability
No cell death was observed at the end of the treatments carried out at all three oxygen tensions, using the live-dead assay (data not shown), for both explants or chondrocytes grown in alginate.
Discussion
Oxygen tension had significant effects on the metabolism of articular cartilage in vitro. 5% O2 appeared optimal for ATP synthesis in articular cartilage in vitro, compared with the other oxygen tensions tested. The optimal environment at 5% O2 was suggested by the highest ATP availability, and lowest levels of pAMPK. At 5% O2 the AMPK energy sensor is not detecting the need to generate more ATP, and S6 activation is highest, suggesting high levels of protein translation, compared with the other oxygen tensions studied. These findings correlate with findings that 5% O2 was optimal for maintaining the material properties of articular cartilage in vitro closest to those found in vivo 15. Others showed proteoglycan synthesis in chondrocytes is closely related to cellular ATP levels 35. 20% O2 is representative of a hyper-physiological oxygen tension for articular cartilage in vivo, and appeared sub-optimal for ATP generation, compared with 5% O2. However, the levels of pAMPK and pS6 were similar for both 20 and 5% O2. Although cell culture is frequently carried out at 20% O2, these hyper-physiological levels of oxygen can induce oxidative stress in many tissues 36, causing alterations to DNA, proteins and lipids leading to their impaired functions. Potentially longer exposure to 20% O2 may result in greater oxidative stress than the 48 hour exposure used in this study. Increased oxidative DNA damage occurred in chondrocytes passaged as a monolayer when they were cultured at 20% O2 compared with 5% O2 37. At 1% O2, the least amount of ATP was detected, which was associated the greatest activation of AMPK and the lowest levels of protein translation, as indicated by decreased pS6 protein levels. These data suggest the high AMPK activation remains insufficient to maintain protein translation and further energy conservation measures were needed to avoid cell death, as indicated by increased markers of senescence. Decreased ATP levels at 1% O2 were also observed in other cell types 29,38.
Inflammation is a risk factor for OA and is associated with increased production of RONS in articular cartilage. We therefore wanted to determine if oxygen tension could alter RONS production and alter the metabolism of chondrocytes in response to pro-inflammatory cytokines. Previously we showed that hyper-physiological levels of IL1 (10 ng/ml) caused less NO production at 1% than at 20% O24,39. Our data confirmed that lower oxygen tensions reduce the amount of NO induced by physiological levels of IL1 (0.1 ng/ml). Therefore the levels of IL1 induced pro-inflammatory mediators in vivo are likely to be less in the deep zone of cartilage than the superficial zone, due to the reduced oxygen tension. Others show a lower concentration of IL1 is necessary to inhibit proteoglycan synthesis on superficial zone chondrocytes compared with deep zone chondrocytes 40.
The effects of IL1 were more severe at 20% O2 than 5 or 1% O2, as shown by the combination of the lack of activation of AMPK and increased LC3-II at 20% O2, suggesting that the ATP levels were so low that there was insufficient energy to activate AMPK. Further measures were needed to conserve energy that led to autophagy. At 5 or 1% O2, the levels of ATP were still sufficient to activate AMPK and hence there was no need for further energy conservation measures by induction of autophagy. The difference in response to IL1 with oxygen tension may be because IL1 induces more NO at 20% than at 5 or 1% O2. In contrast, IL1 caused down-regulation of S6 at all oxygen tensions, suggesting either activation of S6 is very sensitive to IL1 or that other signaling effects associated with IL1 alter S6 activation. Therefore, we used an NO donor to better understand the effects of NO on energy metabolism in articular chondrocytes. The NO donor chosen was NOC18, which is a stable NO-amine complex that spontaneously releases NO, without cofactors, under physiological conditions.
When ATP levels are optimal, at 5% O2, some resistance to the catabolic effects of pro-inflammatory cytokines and mediators on energy metabolism was observed. The more protective environment of 5% O2 is indicated by the greater concentration of IL1 or NO that was needed to cause significant inhibition of ATP levels at 5% O2, than at 20% or 1% O2. The addition of IL1 or NOC18 at 5% O2 showed ATP depletion and AMPK activation occurred as well as increased caveolin expression, the marker of cell senescence, but no indications of autophagy. These findings agree with others that activation of AMPK can activate conservation measures and lead to senescence 41. In chondrocytes grown in monolayer at 20% O2, increased expression of caveolin was seen in response to 10 ng/ml IL1β 25 and markers of cell senescence were induced by ROS 26. Our data showed S6 could be activated at 5% O2 in the presence of NOC18, suggesting energy levels are still able to sustain protein translation. Activation of S6 correlates with senescent cells still being metabolically and biosynthetically active cells that can secrete inflammatory mediators 42. Therefore senescent chondrocytes can signal inappropriately to the neighboring cells, leading to altered synthesis of collagen, matrix metalloproteinases and tissue inhibitors of metalloproteinases, which also occurs in OA chondrocytes 28. The presence of senescent cells in vivo may influence tissue behavior through paracrine signaling and contribute to progressive deterioration, and promote age-related diseases such as osteoarthritis.
The addition of NOC18 at 1% O2 had profound effects on articular chondrocytes. The lowest levels of ATP were observed, but there was no activation of AMPK or S6. The lack of increase in these proteins suggested that the levels of ATP were too low to carry out the energy demanding process of AMPK and S6 protein activation. The low levels of ATP (18 ng/mg) observed in chondrocytes in the presence of NO at low oxygen tensions may initiate energy conservation measures for survival beyond the inhibition of protein translation, as shown by the induction of LC3-II, a marker of autophagy. The process of autophagy would generate more ATP to avoid cell death, by ingestion of intracellular organelles and the recycling of amino acids to provide building blocks for macromolecular synthesis that may support cellular metabolism for further protein synthesis and secretion. LC3-II increased with decreased oxygen tensions, and was increased further in the presence of NOC18. Again, the effects of IL1 at 1% O2 are not as great as those of NOC18 at 1% O2, which maybe due to low oxygen tension reducing levels of IL1 induced NO at 1% O2.
Evidence of autophagy in articular cartilage has been observed by others both in vitro 43 and in vivo 30,44,45. Some studies suggest autophagy is a protective mechanism in non-OA articular cartilage and this ability is lost with the progression of OA in spontaneous aging-related and surgically induced OA models44, while others suggest autophagy is increased in the superficial and mid zones with OA progression, when OA is induced by partial menisectomy followed by high impact load45. Our in vitro studies suggest the greatest autophagy in response to NO in non-OA cartilage is seen at the lowest oxygen tension, likely found in the deep zone of cartilage, and some protective effects at 5% O2 compared to 1% O2. There are many differences between in vitro studies in non-OA cartilage to in vivo studies on OA cartilage, including species differences. The mechanical environment, oxygen tension and the zone may influence autophagy.
Autophagy can be regulated by the transcription factor hypoxia inducible factor (HIF), both HIF-1 and HIF-2 are found in articular cartilage and are important in maintaining cartilage homeostasis 46. HIF-1 activates autophagy and HIF-2 is increased in OA and can inhibit autophagy43. Understanding the role of HIF-1 is complex since HIF activation occurs in response to decreased oxygen tension, and also by IL-1 or NO 47.
In conclusion, our data indicate that the oxygen tension of the environment of an articular chondrocyte is important in determining its ability to cope with exposure to the RONS that are associated with the pathogenesis of OA.
Acknowledgements
Mr. Adrian Paz, Mr. Robert Nielsen and Mr. Stephen Johnson for excellent technical assistance. The project described was supported by Grant Numbers AR49790 and AR50245 from NIAMS, and a NSF Graduate Fellowship.
Contract grant sponsor: NIH; Contract grant numbers AR49790, AR50245; NSF Graduate Fellowship
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests None of the authors have conflicts of interest.
REFERENCES
- 1.Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747–55. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg J, Fermor B, Guilak F. Nitric oxide synthase and cyclooxygenase interactions in cartilage and meniscus: relationships to joint physiology, arthritis, and tissue repair. In: Harris R, editor. Inflammation in the Pathogenesis of Chronic Diseases: The COX-2 Controversy - Subcellular Biochemistry. Vol. 42. Springer; New York: 2007. pp. 31–62. [DOI] [PubMed] [Google Scholar]
- 3.Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Banes AB, Guilak F. The effects of static and intermittent compression on nitric oxide production in articular cartilage explants. J Orthop Res. 2001;19:72–80. doi: 10.1016/S0736-0266(00)00049-8. [DOI] [PubMed] [Google Scholar]
- 4.Cernanec J, Guilak F, Weinberg JB, Pisetsky DS, Fermor B. Influence of hypoxia and reoxygenation on cytokine-induced production of proinflammatory mediators in articular cartilage. Arthritis Rheum. 2002;46:968–75. doi: 10.1002/art.10213. [DOI] [PubMed] [Google Scholar]
- 5.Smith R, Carter D, Schurman D. Pressure and shear differentially alter human articular chondrocyte metabolism: a review. Clin Orthop Rel Res. 2004;427(Suppl):S89–95. [PubMed] [Google Scholar]
- 6.Abramson S, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11:227–35. doi: 10.1186/ar2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lotz M, Hashimoto S, Kuhn K. Mechanisms of chondrocyte apoptosis. Osteoarthritis Cartilage. 1999;7:389–91. doi: 10.1053/joca.1998.0220. [DOI] [PubMed] [Google Scholar]
- 8.Pelletier JP, Jovanovic D, Fernandes JC, Manning P, Connor JR, Currie MG, et al. Reduction in the structural changes of experimental osteoarthritis by a nitric oxide inhibitor. Osteoarthritis Cartilage. 1999;7:416–8. doi: 10.1053/joca.1998.0229. [DOI] [PubMed] [Google Scholar]
- 9.Studer RK, Levicoff E, Georgescu H, Miller L, Jaffurs D, Evans C. Nitric oxide inhibits chondrocyte response to IGF1: inhibition of IGF-1Rbeta tyrosine phosphorylation. Am J Physiol. 2000;279:C961–9. doi: 10.1152/ajpcell.2000.279.4.C961. [DOI] [PubMed] [Google Scholar]
- 10.Davies CM, Guilak F, Weinberg JB, Fermor B. Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthritis Cartilage. 2008;16:624–30. doi: 10.1016/j.joca.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen AF, Davies CM, De Lin M, Fermor B. Oxidative DNA damage in osteoarthritic porcine articular cartilage. J Cell Physiol. 2008;217:828–33. doi: 10.1002/jcp.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefanovic-Racic M, Stadler J, Georgescu HI, Evans CH. Nitric oxide and energy production in articular chondrocytes. J Cell Physiol. 1994;159:274–80. doi: 10.1002/jcp.1041590211. [DOI] [PubMed] [Google Scholar]
- 13.Silver IA. Measurement of pH and ionic composition of pericellular sites. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 1975;271:261–72. doi: 10.1098/rstb.1975.0050. [DOI] [PubMed] [Google Scholar]
- 14.Zhou S, Cui Z, Urban JP. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: a modeling study. Arthritis Rheum. 2004;50:3915–24. doi: 10.1002/art.20675. [DOI] [PubMed] [Google Scholar]
- 15.Fermor B, Christensen SE, Youn I, Cernanec JM, Davies CM, Weinberg JB. Oxygen, nitric oxide and articular cartilage. Eur Cell Mater. 2007;13:56–65. doi: 10.22203/ecm.v013a06. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto K, Fukuda K, Yamazaki K, Yamamoto N, Matsushita T, Hayakawa S, et al. Hypoxia-induced hyaluronan synthesis by articular chondrocytes: the role of nitric oxide. Inflamm Res. 2006;55:72–7. doi: 10.1007/s00011-005-0012-6. [DOI] [PubMed] [Google Scholar]
- 17.Milner P, Fairfax T, Browning JA, Wilkins R, Gibson J. The effect of oxygen tension on pH homeostasis in equine carticular chondrocytes. Arthritis Rheum. 2006;54:3523–32. doi: 10.1002/art.22209. [DOI] [PubMed] [Google Scholar]
- 18.Peansukmanee S, Vaughan-Thomas A, Carter S, Clegg P, Taylor S, Redmond C, et al. Effects of hypoxia on glucose transport in primary equine chondrocytes in vitro and evidence of reduced GLUT1 gene expression in pathologic cartilage in vivo. J Orthop Res. 2009;27:529–35. doi: 10.1002/jor.20772. [DOI] [PubMed] [Google Scholar]
- 19.Bywaters EGL. The metabolism of joint tissues. J Pathol Bact. 1937;44:247–68. [Google Scholar]
- 20.Tushan FS, Rodnan GP, Altman M, Robin ED. Anaerobic glycolysis and lactate dehydrogenase (LDH) isoenzymes in articular cartilage. J Lab Clin Med. 1969;73:649–50. [PubMed] [Google Scholar]
- 21.Johnson K, Jung A, Murphy A, Andreyev A, Dykens J, Terkeltaub R. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheum. 2000;43:1560–70. doi: 10.1002/1529-0131(200007)43:7<1560::AID-ANR21>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 22.Taylor CT. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem J. 2008;409:19–26. doi: 10.1042/BJ20071249. [DOI] [PubMed] [Google Scholar]
- 23.Aigner T, Hemmel M, Neureiter D, Gebhard PM, Zeiler G, Kirchner T, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44:1304–12. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 24.Muller M. Cellular senescence: molecular mechanisms, in vivo significance, and redox considerations. Antioxid Redox Signal. 2009;11:59–98. doi: 10.1089/ars.2008.2104. [DOI] [PubMed] [Google Scholar]
- 25.Dai SM, Shan ZZ, Nakamura H, Masuko-Hongo K, Kato T, Nishioka K, et al. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 2006;54:818–31. doi: 10.1002/art.21639. [DOI] [PubMed] [Google Scholar]
- 26.Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):106–10. doi: 10.2106/00004623-200300002-00014. [DOI] [PubMed] [Google Scholar]
- 27.Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1:57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 28.Yudoh K, Nguyen T, Nakamura H, Hongo-Masuko K, Kato T, Nishioka K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther. 2005;7:R380–91. doi: 10.1186/ar1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Molecular Cell. 2006;21:521–31. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roach H, Aigner T, Kouri JB. Chondroptosis: A variant of apoptotic cell death in chondrocytes? Apoptosis. 2004;9:265–77. doi: 10.1023/b:appt.0000025803.17498.26. [DOI] [PubMed] [Google Scholar]
- 31.Peterson RT, Schreiber SL. Translation control: connecting mitogens and the ribosome. Curr Biol. 1998;8:R248–50. doi: 10.1016/s0960-9822(98)70152-6. [DOI] [PubMed] [Google Scholar]
- 32.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5'TOP mRNA translation through inhibition of p70S6K. Embo J. 1997;16:3693–704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Carlo M, Jr., Loeser RF. Nitric oxide-mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum. 2002;46:394–403. doi: 10.1002/art.10056. [DOI] [PubMed] [Google Scholar]
- 35.Baker MS, Feigan J, Lowther DA. The mechanism of chondrocyte hydrogen peroxide damage. Depletion of intracellular ATP due to suppression of glycolysis caused by oxidation of glyceraldehyde-3-phosphate dehydrogenase. J Rheumatol. 1989;16:7–14. [PubMed] [Google Scholar]
- 36.Davies KJ. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50:279–89. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- 37.Martin JA, Klingelhutz AJ, Moussavi-Harami F, Buckwalter JA. Effects of oxidative damage and telomerase activity on human articular cartilage chondrocyte senescence. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2004;59:324–37. doi: 10.1093/gerona/59.4.b324. [DOI] [PubMed] [Google Scholar]
- 38.Shohet RV, Garcia JA. Keeping the engine primed: HIF factors as key regulators of cardiac metabolism and angiogenesis during ischemia. J Mol Med. 2007;85:1309–15. doi: 10.1007/s00109-007-0279-x. [DOI] [PubMed] [Google Scholar]
- 39.Cernanec JM, Weinberg JB, Batinic-Haberle I, Guilak F, Fermor B. Influence of oxygen tension on interleukin 1-induced peroxynitrite formation and matrix turnover in articular cartilage. J Rheumatol. 2007;34:401–7. [PubMed] [Google Scholar]
- 40.Fukuda K, Kumano F, Takayama M, Saito M, Otani K, Tanaka S. Zonal differences in nitric oxide synthesis by bovine chondrocytes exposed to interleukin-1. Inflamm Res. 1995;44:434–7. doi: 10.1007/BF01757700. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, Yang X, Lopez de Silanes I, Carling D, Gorospe M. Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J Biol Chem. 2003;278:27016–23. doi: 10.1074/jbc.M300318200. [DOI] [PubMed] [Google Scholar]
- 42.White E, Lowe S. Eating to exit:autophagy-enabled senescence revealed. Genes Dev. 2009;23:784–7. doi: 10.1101/gad.1795309. [DOI] [PubMed] [Google Scholar]
- 43.Bohensky J, Terkhorn SP, Freeman TA, Adams CS, Garcia JA, Shapiro IM, et al. Regulation of autophagy in human and murine cartilage: hypoxia-inducible factor 2 suppresses chondrocyte autophagy. Arthritis Rheum. 2009;60:1406–15. doi: 10.1002/art.24444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carames B, Taniguchi N, Otsuki S, Blanco F, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis & Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almonte-Becerril M, Navarro-Garcia F, Gonzalez-Robles A, Vega-Lopez MA, Lavalle C, Kouri JB. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of Osteoarthritis within an experimental model. Apoptosis. 2010 doi: 10.1007/s10495-010-0458-z. [DOI] [PubMed] [Google Scholar]
- 46.Pfander D, Swoboda B, Cramer T. The role of HIF-1alpha in maintaining cartilage homeostasis and during the pathogenesis of osteoarthritis. Arthritis Res Ther. 2006;8:104. doi: 10.1186/ar1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yudoh K, Nakamura H, Masuko-Hongo K, Kato T, Nishioka K. Catabolic stress induces expression of hypoxia-inducible factor (HIF)-1alpha in articular chondrocytes; involvement of HIF-1alpha in the pathogenesis of osteoarthritis. Arthritis Res Ther. 2005;7:R904–R14. doi: 10.1186/ar1765. [DOI] [PMC free article] [PubMed] [Google Scholar]