Abstract
Lymphomas of the gall bladder and extrahepatic bile ducts are exceedingly rare. We present the clinicopathological features of 19 cases from our files; 14 patients had primary lymphoma (13 involving gall bladder and one involving common hepatic duct), while five had systemic lymphoma on further work-up. Most patients presented with symptoms mimicking cholecystitis. The most common primary lymphoma types were diffuse large B-cell lymphoma (DLBCL), extranodal marginal zone lymphoma (EMZL), B-lymphoblastic lymphoma (B-LBL) and follicular lymphoma (FL). Two cases had features of lymphomatous polyposis, one a case of FL and the second a case of mantle cell lymphoma (MCL), with disease limited to the mantle zones, so-called in situ MCL. Other rare lymphoma subtypes not previously described in this site included the extracavitary variant of primary effusion lymphoma (PEL) and plasmablastic lymphoma (PBL). Patients with DLBCL and EMZL were older (mean age 75.8 years) than those with other subtypes (mean age 47 years) and more likely to have gallstones (60% vs. 12.5%). A comprehensive literature review revealed 36 primary gall bladder and 16 primary extrahepatic bile duct lymphomas. When compared to primary gall bladder lymphomas, those involving the extrahepatic bile ducts present at a younger age (47 years vs. 63 years) usually with obstructive jaundice, and are less often associated with gallstones (17% vs. 50%) or regional lymph node involvement (6% vs. 31%). In conclusion, primary lymphomas of the gall bladder and extrahepatic bile ducts show a broad spectrum of disease types, but in many respects mirror the spectrum of primary lymphomas of the gastrointestinal tract.
Keywords: lymphoma, gallbladder, bile duct, hepatic duct, cystic duct, cholecystitis, cholelithiasis, extranodal, intestinal follicular lymphoma, in situ mantle cell lymphoma, HHV-8/KSHV, plasmablastic lymphoma, primary effusion lymphoma, lymphomatous polyposis
Introduction
Lymphoma of the gall bladder is exceedingly rare with only anecdotal cases reported in literature. We present the largest series of gall bladder lymphomas to date, based on a retrospective study of 19 cases in our files. Fourteen patients had only loco regional involvement (i.e. primary lymphoma), of which one had lymphoma involving the common hepatic duct without gall bladder involvement. Five patients who presented with gall bladder disease had systemic lymphoma on further work-up. In most instances the presenting symptoms mimicked cholecystitis. Histologically, there was a diverse spectrum, including many subtypes not previously documented in the gall bladder such as mantle cell lymphoma, plasmablastic lymphoma, primary effusion lymphoma, and post-transplantation lymphoproliferative disorder of T-cell type. In addition, we have also reviewed all the cases reported in literature and present the clinicopathological features of primary gall bladder and bile duct lymphomas.
Materials and methods
All cases with lymphomatous involvement of the gall bladder were retrieved from our consultation files. This study was approved by the Institutional Review Board of the National Cancer Institute (IRB protocol No. 10-C-N 074). Cases in which lymphoma was restricted to the gall bladder with or without regional spread were identified, based on available and follow-up information from referring pathologists and clinical charts. Additionally, we identified cases in which gall bladder involvement was the initial clinical manifestation of disease, even when subsequent evaluation revealed that the disease was not restricted to the gall bladder. However, cases with a known prior diagnosis of lymphoma were excluded from study.
Clinical details, gross descriptions, and histological slides were reviewed. All cases were classified according to the World Health Organization (WHO) classification (2008) for hematolymphoid neoplasms(61). Immunostains performed on all cases included CD20, CD3, Kappa and Lambda. Additional pertinent studies were performed in selected cases based on the histologic differential diagnosis and included immunohistochemistry (CD79a, PAX5, CD2, CD5, CD4, CD8, CD43, CYCLIN D1, CD10, BCL2, BCL6, Ki 67, IgD, IgG, IgA, IgM, CD30, CD15, MUM1, CD138, P53, TdT, CD99, HHV8), in situ hybridization for EBV (EBER), and molecular studies [IGH@ and TCR gamma gene rearrangements, fluorescence in situ hybridization (FISH) for t(14;18)(q32;q21), t(11;14)(q13;q32) and t(8;14)(q24;q32)). IGH@ and TCR gamma gene rearrangements were identified by PCR analysis of DNA extracted from formalin fixed paraffin embedded tissue. IGH@ PCR was performed using consensus primers to framework region (II and/or III) and the joining region of the immunoglobulin heavy chain gene(54), while TCR gamma gene PCR was performed using two separate primer sets with primers Vg101, Vg11 and Jg12 (set 1) and primers Vg 101, Vg11 and Jp12 (set 2)(42).
A literature search was performed in PubMed using search words including gall bladder, bile duct, cystic duct, and lymphoma, lymphoproliferative disorder or atypical lymphoid hyperplasia. Publications were retrieved and reviewed to identify cases of primary gall bladder/bile duct lymphoma. A few additional cases that had not been retrieved in the PubMed search were identified by cross-references within the retrieved publications. All available clinical and pathology data were tabulated.
Results
Clinical and pathological features – NIH cases
We identified 14 primary lymphomas, 13 involving the gall bladder and one involving the extrahepatic bile ducts (Table 1). Patients were 5 to 84 (mean 57) years in age and included 10 males and four females. Eleven of 12 cases (92%) with gall bladder lymphoma presented with symptoms of cholecystitis, cholelithiasis or jaundice, while one case was detected incidentally; no details were available in two cases. Three patients were immunocompromised (2 HIV positive; 1 post-transplant), while two additional patients had colon cancer. Three of the 14 patients (23%) had B-symptoms. Four of 11 cases (36%) had gallstones. Regional lymphadenopathy was intraoperatively identified in six cases. Histologically, regional lymph nodes were involved by lymphoma in three of these six (50%) cases, while local contiguous spread to surrounding soft tissue was present in two additional cases without nodal involvement. There was a single case (case # 4) with a common hepatic duct mass, without gall bladder involvement. This patient had undergone a gastric bypass surgery three years prior to presenting with obstructive jaundice; there were no gall stones or regional lymphadenopathy. None of the 14 cases had systemic disease.
Table 1.
Clinicopathologic Features of Primary Gall Bladder and Bile Duct Lymphomas.
| Case/Sex | Age | Clinical presentation |
Stones | Diagnosis | Regional node involvement |
Pertinent studies |
|---|---|---|---|---|---|---|
| 1/M | 5 | Cholecystitis | No | PTCL (PTLD) |
Yes | CD2+, CD4+,CD8−, BF1+, CD56−, EBER−, TRG monoclonal |
| 2/F | 37 | Cholecystitis | No | B-LBL | NA | CD79a+, PAX5+, CD10+, TdT+, CD20(focal) |
| 3/M | 37 | GB mass HIV+ |
No | PEL, Extracavitary variant |
No | PAX5+ (focal), MUM1+, EMA+, EBER+, HHV8+ |
| *4/F | 48 | Obstructive jaundice |
No | FL Grade 3B | No | CD20+, CD30+, CD43+ (focal), BCL2−, BCL6−, CD10− |
| 5/M | 51 | Cholecystitis and GB mass, HIV+ |
No | PBL | NA | CD79a+, CD138+, MUM1+, CD10+(focal), EBER+ |
| 6/M | 51 | NA | NA | FL Grade 1– 2 |
No | CD20+, CD10+, BCL2+, BCL6+ |
| 7/M | 51 | Cholelithiasis | Yes | FL Grade 1– 2 |
Yes | CD20+, CD10+, BCL2+ |
| 8/M | 65 | Incidental cholecystectomy; colon resection for adenocarcinoma |
No | MCL in situ | NA | CD20+, CD5−, CD43+, SOX11+, CYCLIN D1+, LAMBDA+ t(11;14)+ |
| 9/M | 72 | Cholecystitis | Yes | DLBCL | Yes | CD20+, CD10+, BCL2+, t(14;18)+ |
| 10/M | 74 | Cholelithiasis, h/o colon cancer |
Yes | EMZL | NA | CD20+(focal), CD79a+, KAPPA+, CD43−, IGH monoclonal |
| 11/F | 74 | Cholecystitis | No | DLBCL | NA | CD20+, CD10−, BCL6+, MUM1−, BCL2+ |
| 12/M | 74 | Cholecystitis | No | B-LBL | No | PAX5+, CD10+, CD99+, TdT+(focal), IGH monoclonal |
| 13/M | 75 | Cholecystitis | Yes | DLBCL | NA | CD20+, CD10−, BCL6−, BCL2+ |
| 14/F | 84 | Cholecystitis | No | EMZL | NA | CD20+, CD79a+, KAPPA+ (focal), IGH monoclonal |
case involving common hepatic duct, previously reported by Jho et al (32)
DLBCL – diffuse large B-cell lymphoma; EMZL – extranodal marginal zone lymphoma; FL – follicular lymphoma; GB – gall bladder; LBL – lymphoblastic lymphoma; MCL – mantle cell lymphoma; NA – not available; PBL – plasmablastic lymphoma; PEL – primary effusion lymphoma; PTCL – peripheral T-cell lymphoma; PTLD – post-transplantation lymphoproliferative disorder
Three cases of diffuse large B-cell lymphoma (DLBCL) were identified (3/14; 21.5%). Following the Hans immunohistochemical algorithm(26), two of three were of germinal center B-cell type, one of them with the t(14;18) involving the BCL2 and IGH@ genes. Two cases of follicular lymphoma (FL), Grade 1–2, were observed, one of which presented as a localized polypoid lesion of the mucosa (case #6). A third case of FL (case #4) presented in the common bile duct and was Grade 3B. Two cases (2/14; 14.3%) of extranodal marginal zone lymphoma (EMZL) were seen (Fig. 1), both of which showed extensive mucosal involvement. One case of mantle cell lymphoma (MCL) (case # 8) had features of lymphomatous polyposis (Fig. 2). It exhibited limited involvement of the mantle zones with neoplastic cells surrounding reactive germinal centers. It was CD5-negative but SOX11+, and was positive for the t(11;14) by FISH. A colonic resection performed at the same time for colonic adenocarcinoma showed no involvement by lymphoma.Two HIV-associated lymphomas were identified: a solid variant of primary effusion lymphoma (PEL) (case # 3) (Fig. 3) and plasmablastic lymphoma (PBL) (case # 5) (Fig. 4). Two patients presented with B-lymphoblastic lymphoma (B-LBL) as the sole manifestation of disease. The single patient with T-cell lymphoma was a 5-year-old boy (case #1) who was status post-cardiac transplant on tacrolimus and presented with cholecystitis. Histologically, there was transmural involvement by a clonal T-cell infiltrate, negative for EBV by EBER-ISH.
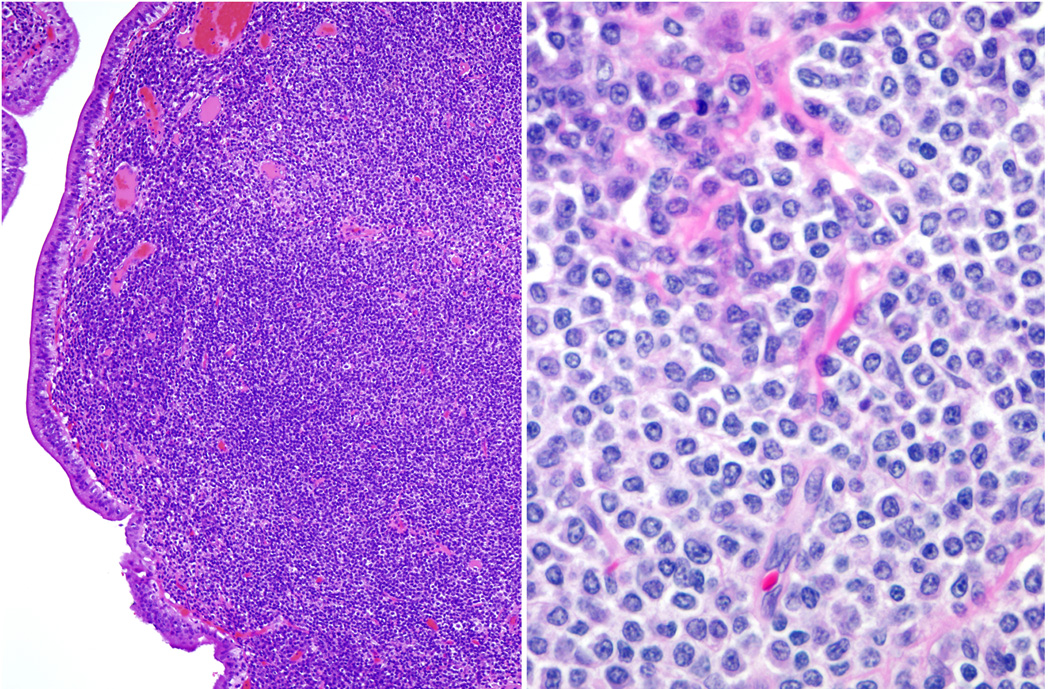
Fig. 1. Extranodal marginal zone lymphoma.
A. The gall bladder mucosa is involved by a dense lymphoid infiltrate. B. Many cells exhibit plasmacytoid differentiation whereas others have a rim of pale cytoplasm and a monocytoid appearance.
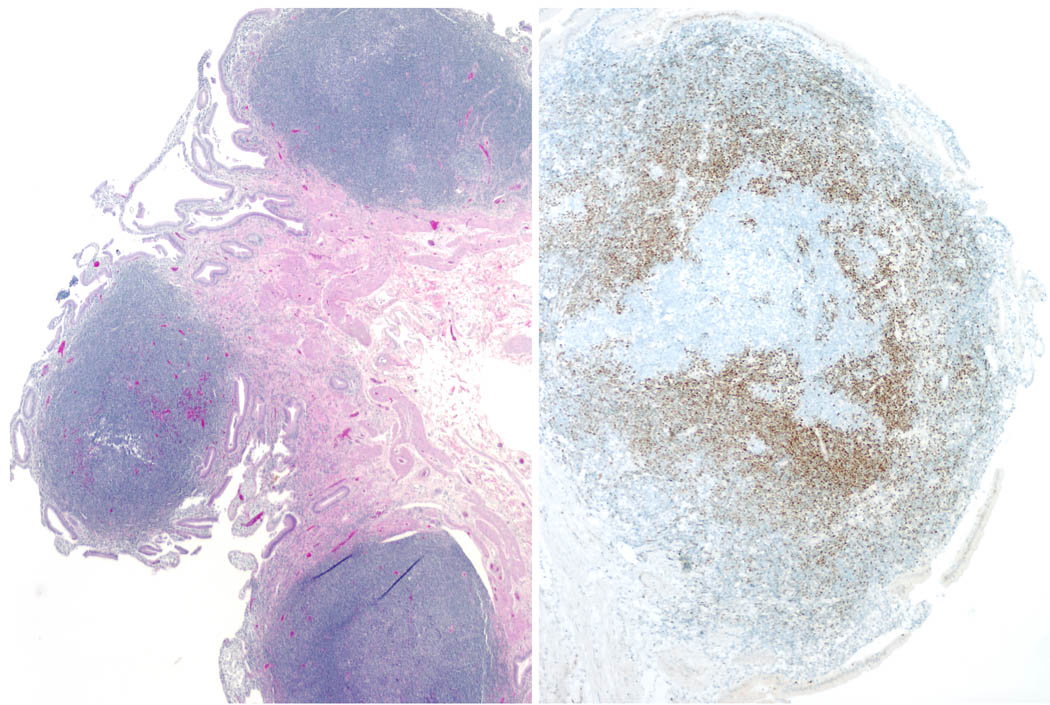
Fig. 2. Mantle cell lymphoma with features of lymphomatous polyposis.
A. A polypoid infiltrate is present within the gall bladder mucosa. B. Cyclin D1 immunostain highlights the neoplastic cells, which are largely confined to the mantle zones surrounding negative reactive germinal centers.
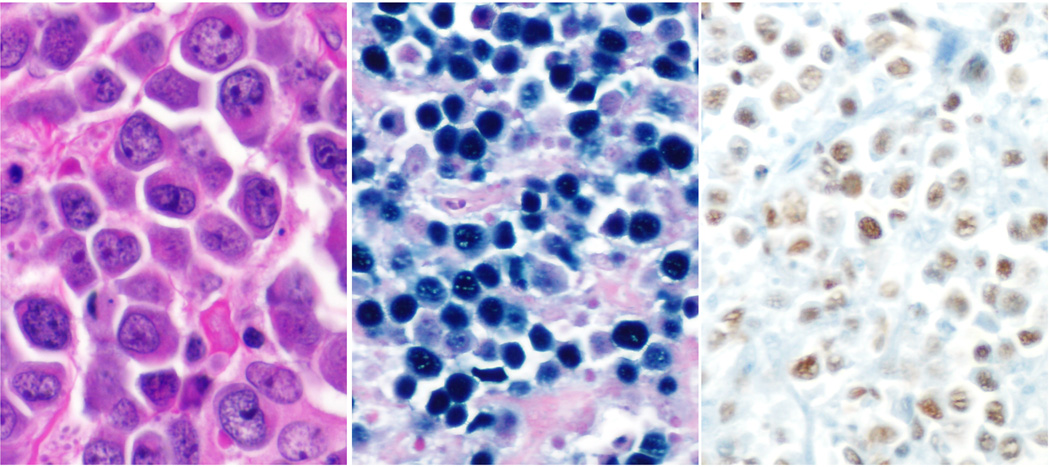
Fig. 3. Extracavitary variant of primary effusion lymphoma.
A. The cells are large and pleomorphic with ample cytoplasm and prominent central eosinophilic nucleoli. B. In-situ hybridization for EBV (EBER probe) and C. HHV-8 immunostain shows positivity in nuclei of neoplastic cells.
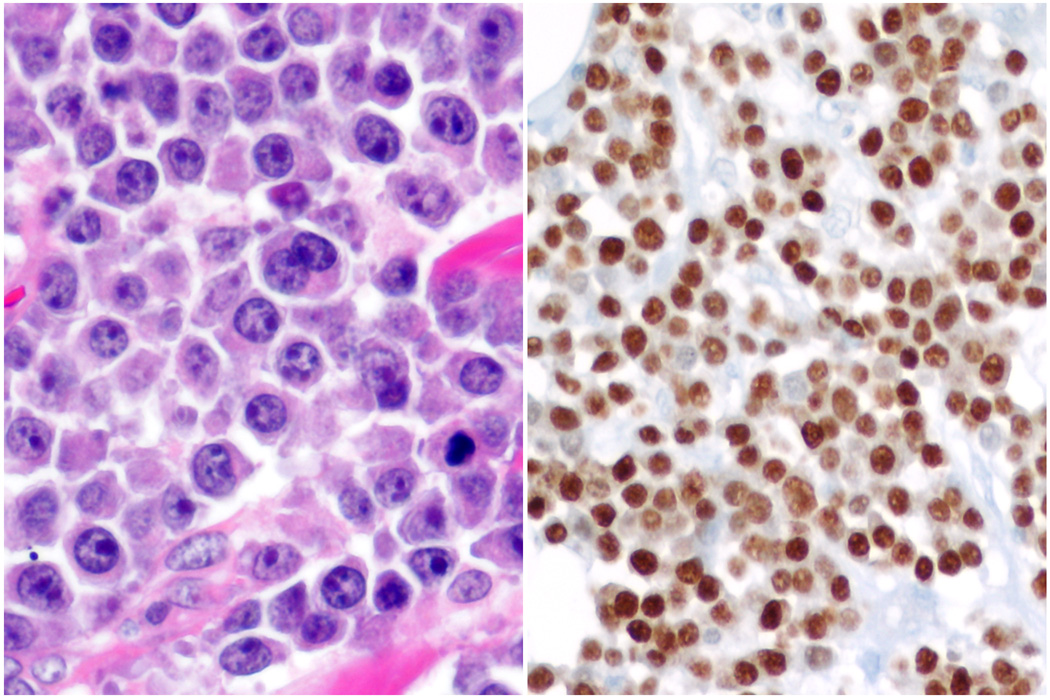
Fig. 4. Plasmablastic lymphoma.
A. There is a monomorphic infiltrate of cells with enlarged nuclei, prominent nucleoli and moderate amount of eosinophilic cytoplasm. B. Neoplastic cells are strongly and diffusely positive for MUM-1/IRF-4.
Histologically, there was transmural involvement by lymphoma in ten cases (71.4%), while four cases (28.6%) showed relative mucosal sparing with predominant smooth muscle and adventitial involvement. The four cases with relative mucosal sparing included two patients with DLBCL (case # 9 and 13) and one patient each with plasmablastic lymphoma (case # 5) and B-LBL (case # 12).
Patients with DLBCL (cases # 9, 11, 13) and EMZL (cases # 10, 14) were older (72 to 84 years, mean 75.8 years) than those with other subtypes (5 to 74 years, mean 46.6 years). Gallstones were present in two of three cases (66%) with DLBCL, one of two cases (50%) with EMZL, and only in one of eight (12.5%) cases with other lymphoma subtypes.
An additional five patients without a prior diagnosis of lymphoma presented with symptoms of gall bladder disease and were diagnosed with lymphoma following cholecystectomy (Table 2). Unlike the first group of patients wherein the disease was restricted to the gall bladder and regional lymph nodes, these five patients had widespread disease on further work-up. Thus, although they had systemic involvement by lymphoma, they had come to clinical attention due to gall bladder involvement. These five patients ranged in age from 38 to 85 years (mean 64.8 years) and included three females and two males. Four patients (80%) had B-symptoms. One patient had Sjogren syndrome; none were immunocompromised. Only one of four patients (25%) with available gross description had gall stones. Three patients (60%) had regional lymphadenopathy at surgery, while all had systemic involvement on follow-up. Histologically, one patient had classical Hodgkin lymphoma, while the remaining four had B-cell lymphomas. One case was a DLBCL with a double-hit translocation involving the BCL2 and MYC genes. All five cases (100%) showed transmural involvement of the gall bladder wall.
Table 2.
Systemic Lymphomas Presenting With Gall Bladder Disease
| Case/Sex | Age | Clinical presentation | Stones | Diagnosis | Pertinent studies |
|---|---|---|---|---|---|
| 1/M | 38 | Gallbladder mass | No | TCHRBCL | CD20+, CD30−, BCL6+, EBER− |
| 2/F | 59 | Abdominal lymphadenopathy |
Yes | DLBCL | CD20+, BCL2+, t(14;18)+, t(8;14)+ |
| 3/F | 60 | Lymphadenopathy | No | B-NHL | CD20+, BCL6+, EBER+, IGH monoclonal |
| 4/F | 82 | Incidental cholecystectomy |
NA | MCCHL | CD30+, CD15+ (weakly), EBER+ |
| 5/M | 85 | Cholecystitis | No | EBV+ DLBCL of the elderly |
CD20+(focal), CD30+(focal), EBER+ |
DLBCL – diffuse large B-cell lymphoma; MCCHL – mixed cellularity classical Hodgkin lymphoma; NA – not available; NHL – non-Hodgkin lymphoma; TCHRBCL – T-cell histiocyte rich large B-cell lymphoma
Clinical and pathological features – Literature cases
The review of literature identified 52 cases of primary biliary lymphoma including 36 involving the gallbladder and 16 involving only the extrahepatic bile ducts. Most patients had presented with features suggestive of acute cholecystitis or obstructive jaundice; five were detected incidentally on imaging or surgery for unrelated conditions. The 36 patients with primary lymphoma of the gallbladder (Table 3) included 23 women and 13 men, aged 4 to 91 (mean 63) years. The most common lymphoma type was EMZL (12 cases)(1, 6, 7, 14, 35, 43, 45, 49, 53, 62, 66, 67), with an additional case from older literature (29) reported as “pseudolymphoma”. Collectively, these 13 patients were predominantly women (11/13) with a mean age of 68 years (range 31–91 years). Of the cases with clinical information, gall stones were present in 54.5% (6/11) of cases and regional lymph nodes were involved in 20% (2/10) of cases. Of the eight cases with reported outcomes, six (75%) were alive at follow-up (from 6 months to 8 years), while two had died of unrelated diseases without recurrence of lymphoma. There were eight cases of DLBCL(13, 24, 28, 34, 51, 57, 70, 72), with an additional case of diffuse non-Hodgkin lymphoma (NHL), not further specified(67). These nine patients averaged 64 (range 40–78) years of age without gender predilection (4 women, 5men); 83.3% (5/6) cases had gallstones and 28.6% (2/7) cases had lymph node involvement. Only one of nine (11%) patients had no evidence of disease at 38 months(34), whereas others developed systemic disease, with three patients dying within three years of diagnosis. The four cases of follicular lymphoma (22, 31, 47, 69) were also predominantly women with a mean age of 71 (range 63–78) years; gallstones were present in two cases and one had regional lymph node involvement. All three patients with available follow up information (from 9 months to 3 years) were alive without evidence of disease. There were anecdotal reports of other lymphoma types including lymphoblastic lymphoma, angiotropic or intravascular lymphoma, low grade B-cell lymphoma, poorly differentiated lymphocytic lymphoma, classical Hodgkin lymphoma, and reports in older literature diagnosed as lymphosarcoma and reticulum cell sarcoma.
Table 3.
Primary Gall Bladder Lymphomas Reported in Literature
| Diagnosis | No. of cases |
Age range (mean) |
Gender | Gall stones | Regional adenopathy |
References |
|---|---|---|---|---|---|---|
| EMZL* | 13 | 31–91 (68) | 11F, 2M | 6/11, 2NA | 2/10, 3NA | (6, 35) (66) (53) (51) (62) (45) (43) (14) (7) (1) (49) (29) |
| DLBCL ** | 9 | 40–78 (63.7) | 4F, 5M | 5/6, 3 NA | 2/7, 1NA | (51, 57) (72) (70) (28, 34) (13) (24) (67) |
| LGBCL | 1 | 75 | F | 1/1 | NA | (18) |
| FL | 4 | 63–78 (71) | 3F, 1M | 2/4 | 1/4 | (47, 69) (22) (31) |
| B-LBL | 1 | 4 | M | 0/1 | 0/1 | (25) |
| T-LBL | 1 | 26 | F | 0/1 | 0/1 | (44) |
| PDLL | 1 | 54 | M | 0/1 | 1/1 | (9) |
| Angiotropic | 1 | 68 | F | 1/1 | NA | (38) |
| Intravascular | 1 | 79 | M | 0/1 | 0/1 | (37) |
| Lymphosarcoma | 2 | 44, 75 | 2F | 1/2 | 2/2 | (68) |
| Reticulum cell sarcoma |
1 | 29 | M | 0/1 | NA | (71) |
| MCCHL | 1 | 75 | M | 1/1 | 0/1 | (48) |
Includes a “pseudolymphoma” (29)
Includes a “diffuse mixed small and large cell NHL” (67)
DLBCL – diffuse large B-cell lymphoma; EMZL – extranodal marginal zone lymphoma; FL – follicular lymphoma; LBL – lymphoblastic lymphoma; LGBCL – low grade B-cell lymphoma; MCCHL – mixed cellularity classical Hodgkin lymphoma; NA – not available; NED – no evidence of disease; PDLL – poorly differentiated lymphocytic lymphoma
In 16 cases identified in the literature, the lymphoma involved only the extrahepatic bile ducts without gallbladder involvement (Table 4). These patients were 25 to 68 years old (mean 47 years) without gender predilection (9 women, 7 men) and had presented with obstructive jaundice (15/16 cases, 93.8%). Only one patient (1/15, 6.7%) had regional nodal involvement and gallstones (1/5, 20%). Of 12 patients with available follow-up information (from 6 months to 4 years), four died of progressive disease (4/12, 33%), while eight were alive and in remission. Preoperatively, all 16 patients were thought to have cholangiocarcinoma. Histologically, there were seven cases (43.8%) of high grade B-cell lymphomas (three with DLBCL, three with “diffuse small or medium cell lymphoma” and one with T-cell histiocyte rich large B-cell lymphoma (TCHRBCL). Four patients (25%) had follicular lymphoma. There was one case of EMZL, two EBV-PTLD in a post liver transplant setting, one T-cell NHL not further specified and one “NHL” not further specified. Analysis of our cases combined with those reported in literature identified certain clinicopathologic differences between lymphomas of the gall bladder and extrahepatic bile ducts. Patients with bile duct lymphoma presented at a younger age (mean 47 years) than those with gall bladder lymphoma (mean age 63 years). All patients with bile duct lymphoma were symptomatic, whereas gallbladder lymphoma was an incidental finding in a proportion of cases (8/47, 17%). There was no significant gender predilection, although women were slightly more commonly affected in both groups (59% of bile duct and 54% of gallbladder lymphomas). An association with gallstones was noted more often in gallbladder lymphomas (21/42, 50%) than in bile duct lymphoma (1/6, 17%). Regional lymph node involvement was also more frequent in gallbladder lymphomas (11/35, 31.4%, versus 1/16, 6.3%). Among histologic types, EMZL was distinctly uncommon in the bile ducts (1/17, 5.9% of cases) as compared to the gall bladder (15/50, 30% of cases).
Table 4.
Primary Extrahepatic Bile Duct Lymphomas Reported in Literature
| Diagnosis | No. of cases |
Age range (mean) |
Gender | Gall stones |
Regional adenopathy |
References |
|---|---|---|---|---|---|---|
| DLBCL and other aggressive B-NHLs* |
7 | 34 – 68 (49) |
4F, 3M | 1/4, 3NA |
1/6, 1NA | (33, 36) (41, 63) (41) (46) (21) (10) |
| FL | 4 | 33 – 53 (43) |
3F, 1M | 0/1, 3NA |
0/4 | (16, 60) (2) (40) |
| EBV-PTLD | 2 | 41, 59 | 1F, 1M | NA | 0/2 | (5) |
| EMZL | 1 | 59 | M | NA | 0/1 | (58) |
| T-NHL, NOS | 1 | 25 | F | NA | 0/1 | (15) |
| NHL, NOS | 1 | 48 | M | NA | 0/1 | (64) |
Includes one T-cell histiocyte rich large B-cell lymphoma (10)
DLBCL – diffuse large B-cell lymphoma; FL – follicular lymphoma; EBV-PTLD – EBV-related post-transplantation lymphoproliferative disorder; EMZL – extranodal marginal zone lymphoma; NA – not available; NHL – non-Hodgkin lymphoma; TCHRBCL –
Discussion
Lymphomas of the gallbladder and extrahepatic bile ducts are exceedingly rare. Of a total of 1452 gall bladder specimens examined over a five-year-period, Darmas et al. found only one case of lymphoma(18). Almost all documented cases of lymphomas at these sites have been case reports and the present study represents the largest series of gall bladder lymphomas to date. The majority of patients with gall bladder lymphoma present with symptoms of cholecystitis. In our series, patients with DLBCL and EMZL were older and were more likely to have gallstones than those with other lymphoma types. Even though the age distribution is likely to be skewed by the presence of unusual lymphoma variants occurring in immunocompromised hosts, all the patients with DLBCL and EMZL were older than 70 years of age.
It is notable that the spectrum of gall bladder lymphomas mirrors the spectrum of lymphomas observed in the gastrointestinal tract. In our study, the most common lymphoma types were DLBCL, EMZL, FL, B-LBL with additional instances of extracavitary PEL and PBL, all subtypes that may present with gastric or intestinal mucosal disease. When our cases are taken in combination with those reported in literature, the most common lymphoma types in the gall bladder/bile ducts are DLBCL and EMZL (16/66, 24% each) followed by FL (10/66, 15%) and LBL (4/66, 6%). Comparative clinical features for these subtypes are shown in Table 5. Primary lymphoma of the extrahepatic bile ducts is even more uncommon than gall bladder lymphoma. We encountered only one case of bile duct lymphoma, adding to the 16 previously reported cases. Bile duct lymphomas probably cause a clinically significant obstruction to bile flow, possibly resulting in patients presenting earlier and with less frequent nodal involvement.
Table 5.
Comparison of Clinicopathologic Features of Most Common Subtypes
| Type | No (%) | Mean Age |
M:F | Symptomatic | EHBD disease | Stones | Node + |
|---|---|---|---|---|---|---|---|
| EMZL | 16 (24%) | 68.8 | 4:12 | 14/15, 93% | 2/16#, 12.5% | 7/13, 54% | 2/11, 18% |
| DLBCL* | 16 (24%) | 60.4 | 8:8 | 12/15, 80% | 6/16, 37.5% | 8/12, 66% | 4/12, 33% |
| FL | 10 (15%) | 56.5 | 4:6 | 8/9, 89% | 4/10, 40% | 3/7, 43% | 2/10, 20% |
| LBL | 4 (6%) | 35.3 | 2:2 | 4/4, 100% | 0/4, 0% | 0/4, 0% | 0/3, 0% |
One case involved both gallbladder and common bile duct
Includes one case of T-cell histiocyte rich large B-cell lymphoma
DLBCL – diffuse large B-cell lymphoma; EHBD – extrahepatic bile duct; EMZL – extranodal marginal zone lymphoma; FL – follicular lymphoma; LBL – lymphoblastic lymphoma
Similar to EMZL of other anatomic sites, those in the gall bladder show a female preponderance. As in other sites, one can postulate that acquired mucosa associated lymphoid tissue (MALT) secondary to infection or chronic inflammation may produce the substrate for these lymphomas(30). In support of this hypothesis, Tomori et al have reported that although the gall bladder normally has sparse lymphoid tissue, MALT may develop in 11% of gallbladders in association with positive bile cultures and gallstones(65). On the other hand, Angelopoulou et al hypothesize that in EMZL, the malignant transformation of the original clone could have occurred outside the gall bladder with subsequent homing via an adhesion molecule mechanism(3). Their hypothesis is supported by a recent report of sequential development of a low grade MALT lymphoma affecting the stomach, the small intestine and the gallbladder eight and three years apart, respectively(59). Tumor cells from all three sites were shown to derive from a single clone by the demonstration of an identical immunoglobulin heavy chain gene rearrangement using the polymerase chain reaction technique. Genetic studies in one case showed a gall bladder EMZL to have the BIRC3/MALT1 translocation that is most often associated with gastric EMZL(7).
In general, DLBCL is the most common type of extranodal lymphoma. In the gallbladder/bile ducts, DLBCL occurs at a mean age of 60 years, without gender preference and is more likely than the other subtypes to be associated with gallstones and regional lymph node involvement. Gall bladder DLBCL has been postulated to evolve from follicular cholecystitis and low grade MALT lymphomas(43). Although this is a possible explanation of lymphomagenesis at this site, our cases with DLBCL were younger than those with EMZL, and had different clinicopathologic features suggesting that these DLBCLs were more likely to be de novo tumors rather than evolved from EMZL. In addition, we encountered a single case of FL, Grade 3B, a tumor that is felt to be biologically closely related to DLBCL, and typically lacks both BCL2 protein and the BCL2/IGH@ translocation(8).
Extranodal variants of DLBCL that we report for the first time in the gall bladder include PBL and PEL. Both PBL and PEL typically affect HIV-positive patients, as was true in our cases. The former most commonly involves the oral cavity(17), although involvement of other mucosal sites, bone and soft tissue is often seen(20). PEL usually presents with body cavity effusions without detectable mass lesions. However, the gastrointestinal tract is one of the more common sites of extracavitary PEL, and progression to positive effusions is not always seen(11, 12, 19). Our patient presented with an isolated gallbladder mass composed of large cells that expressed MUM1, EMA, HHV8 (LANA), EBER and CD3 (cytoplasmic). Aberrant expression of CD3 has been reported previously in instances of PEL, as well as other EBV-positive large B-cell lymphomas(27, 50). In addition to the cases of PEL and PBL, two cases of angiotropic lymphoma were reported in literature (37, 38) and occurred in older individuals.
Primary FL of the intestine differs clinically from primary nodal FL and was recognized in the 2008 WHO classification as a separate subtype(61). The duodenum is the most commonly affected site(52, 73). Similar to duodenal FL, we identified one case involving the gallbladder that presented as an isolated polypoid lesion with localized mucosal disease without evidence of systemic involvement. The single case of MCL also affected the gall bladder in a pattern similar to lymphomatous polyposis of the intestine and represents the first documentation of this lymphoma subtype at this site. Although the neoplastic cells expressed cyclin D1 and SOX11, the tumor cells were negative for CD5, an uncommon feature in mantle cell lymphoma, sometimes associated with indolent disease(23). Our case of MCL had disease limited to the mantle zones, surrounding residual reactive germinal centers, and conforms to what has been termed in situ MCL(4, 55).
There were two cases of B-LBL. B-LBL is more likely than T-LBL to present with disease outside of the hematolymphoid organs(39). In a large study of 25 cases of precursor B-LBL without leukemic involvement, Lin et al. identified bone, soft tissue, and skin as the most commonly involved sites, but two cases involved the gastrointestinal tract(39). In addition to our two cases, there has been one previous report each of B-LBL(25) and T-LBL(44) involving the gall bladder. None of the four cases had an associated gallstone or regional lymph node disease.
Secondary involvement of the gall bladder in a systemic lymphoma is also rare; in a review of 1269 cases, the gallbladder was secondarily invaded in only 30 (2.4%) cases(56). In the present study, five patients with systemic lymphoma initially presented with gallbladder disease. One patient had classical Hodgkin lymphoma, while others had various forms of B-cell lymphoma. Histologically, all five cases showed transmural involvement, similar to most of the cases with primary gallbladder lymphoma, suggesting that histological pattern of gallbladder involvement may not help differentiate primary and secondary lymphomas at this site.
In summary, we have detailed the clinicopathological spectrum of primary lymphomas of the gallbladder and extrahepatic bile ducts based on an analysis of 14 cases from our files and a review of 52 cases reported in literature. Gall bladder lymphomas present with non-specific features of cholecystitis, whereas obstructive jaundice is more likely with bile duct disease. In general, these lymphomas appear to recapitulate the spectrum of extranodal lymphomas affecting the gastrointestinal tract, with a predominance of DLBCL, EMZL, and FL, particularly of the primary intestinal or duodenal type. Although the inclusion of a number of rare entities in our series, including in-situ MCL, PBL and solid PEL, relates to the nature of our consultation practice, these subtypes also have been reported to occur in the intestine. The very broad spectrum of disease types encountered underscores the need for detailed immunophenotypic analysis of gall bladder lymphocytic infiltrates for accurate diagnosis and management.
Acknowledgement
This research was supported by the Intramural Research Program of the Center for Clinical Research, National Cancer Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abe Y, Takatsuki H, Okada Y, et al. Mucosa-associated lymphoid tissue type lymphoma of the gallbladder associated with acute myeloid leukemia. Intern Med. 1999;38:442–444. doi: 10.2169/internalmedicine.38.442. [DOI] [PubMed] [Google Scholar]
- 2.Andre SB, Farias AQ, Bittencourt PL, et al. Primary extranodal non-Hodgkin lymphoma of the extrahepatic bile duct mimmicking Klatskin tumor. Rev Hosp Clin Fac Med Sao Paulo. 1996;51:192–194. [PubMed] [Google Scholar]
- 3.Angelopoulou MK, Kontopidou FN, Pangalis GA. Adhesion molecules in B-chronic lymphoproliferative disorders. Semin Hematol. 1999;36:178–197. [PubMed] [Google Scholar]
- 4.Aqel N, Barker F, Patel K, et al. In-situ mantle cell lymphoma--a report of two cases. Histopathology. 2008;52:256–260. doi: 10.1111/j.1365-2559.2007.02906.x. [DOI] [PubMed] [Google Scholar]
- 5.Baron PW, Heneghan MA, Suhocki PV, et al. Biliary stricture secondary to donor B-cell lymphoma after orthotopic liver transplantation. Liver Transpl. 2001;7:62–67. doi: 10.1053/jlts.2001.20781. [DOI] [PubMed] [Google Scholar]
- 6.Bickel A, Eitan A, Tsilman B, et al. Low-grade B cell lymphoma of mucosa-associated lymphoid tissue (MALT) arising in the gallbladder. Hepatogastroenterology. 1999;46:1643–1646. [PubMed] [Google Scholar]
- 7.Bisig B, Copie-Bergman C, Baia M, et al. Primary mucosa-associated lymphoid tissue lymphoma of the gallbladder: report of a case harboring API2/MALT1 gene fusion. Hum Pathol. 2009;40:1504–1509. doi: 10.1016/j.humpath.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Bosga-Bouwer AG, van Imhoff GW, Boonstra R, et al. Follicular lymphoma grade 3B includes 3 cytogenetically defined subgroups with primary t(14;18), 3q27, or other translocations: t(14;18) and 3q27 are mutually exclusive. Blood. 2003;101:1149–1154. doi: 10.1182/blood.V101.3.1149. [DOI] [PubMed] [Google Scholar]
- 9.Botha JB, Kahn LB. Primary lymphoma of the gall bladder. Case report and review of the literature. S Afr Med J. 1974;48:1345–1348. [PubMed] [Google Scholar]
- 10.Brouland JP, Molimard J, Nemeth J, et al. Primary T-cell rich B-cell lymphoma of the common bile duct. Virchows Arch A Pathol Anat Histopathol. 1993;423:513–517. doi: 10.1007/BF01606544. [DOI] [PubMed] [Google Scholar]
- 11.Carbone A, Gloghini A, Vaccher E, et al. Kaposi's sarcoma-associated herpesvirus/human herpesvirus type 8-positive solid lymphomas: a tissue-based variant of primary effusion lymphoma. J Mol Diagn. 2005;7:17–27. doi: 10.1016/S1525-1578(10)60004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chadburn A, Hyjek E, Mathew S, et al. KSHV-positive solid lymphomas represent an extra-cavitary variant of primary effusion lymphoma. The American journal of surgical pathology. 2004;28:1401–1416. doi: 10.1097/01.pas.0000138177.10829.5c. [DOI] [PubMed] [Google Scholar]
- 13.Chatila R, Fiedler PN, Vender RJ. Primary lymphoma of the gallbladder; case report and review of the literature. Am J Gastroenterol. 1996;91:2242–2244. [PubMed] [Google Scholar]
- 14.Chim CS, Liang R, Loong F, et al. Primary mucosa-associated lymphoid tissue lymphoma of the gallbladder. Am J Med. 2002;112:505–507. doi: 10.1016/s0002-9343(01)01132-9. [DOI] [PubMed] [Google Scholar]
- 15.Chiu KW, Changchien CS, Chen L, et al. Primary malignant lymphoma of common bile duct presenting as acute obstructive jaundice: report of a case. J Clin Gastroenterol. 1995;20:259–261. doi: 10.1097/00004836-199504000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Christophides T, Samstein B, Emond J, et al. Primary follicular lymphoma of the extrahepatic bile duct mimicking a hilar cholangiocarcinoma: case report and review of the literature. Hum Pathol. 2009;40:1808–1812. doi: 10.1016/j.humpath.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Colomo L, Loong F, Rives S, et al. Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. The American journal of surgical pathology. 2004;28:736–747. doi: 10.1097/01.pas.0000126781.87158.e3. [DOI] [PubMed] [Google Scholar]
- 18.Darmas B, Mahmud S, Abbas A, et al. Is there any justification for the routine histological examination of straightforward cholecystectomy specimens? Ann R Coll Surg Engl. 2007;89:238–241. doi: 10.1308/003588407X168361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DePond W, Said JW, Tasaka T, et al. Kaposi's sarcoma-associated herpesvirus and human herpesvirus 8 (KSHV/HHV8)-associated lymphoma of the bowel. Report of two cases in HIV-positive men with secondary effusion lymphomas. The American journal of surgical pathology. 1997;21:719–724. doi: 10.1097/00000478-199706000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Dong HY, Scadden DT, de Leval L, et al. Plasmablastic lymphoma in HIV-positive patients: an aggressive Epstein-Barr virus-associated extramedullary plasmacytic neoplasm. The American journal of surgical pathology. 2005;29:1633–1641. doi: 10.1097/01.pas.0000173023.02724.1f. [DOI] [PubMed] [Google Scholar]
- 21.Eliason SC, Grosso LE. Primary biliary malignant lymphoma clinically mimicking cholangiocarcinoma: a case report and review of the literature. Ann Diagn Pathol. 2001;5:25–33. doi: 10.1053/adpa.2001.21483. [DOI] [PubMed] [Google Scholar]
- 22.Ferluga D, Luzar B, Gadzijev EM. Follicular lymphoma of the gallbladder and extrahepatic bile ducts. Virchows Arch. 2003;442:136–140. doi: 10.1007/s00428-002-0734-6. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez V, Salamero O, Espinet B, et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer research. 2010;70:1408–1418. doi: 10.1158/0008-5472.CAN-09-3419. [DOI] [PubMed] [Google Scholar]
- 24.Friedman EP, Lazda E, Grant D, et al. Primary lymphoma of the gallbladder. Postgrad Med J. 1993;69:585–587. doi: 10.1136/pgmj.69.813.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gravel J, Lallier M, Garel L, et al. Primary non-Hodgkin lymphoma of the extrahepatic biliary tract and gallbladder in a child. J Pediatr Gastroenterol Nutr. 2001;32:598–601. doi: 10.1097/00005176-200105000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 27.Hollingsworth HC, Stetler SM, Gagneten D, et al. Immunodeficiency-associated malignant lymphoma. Three cases showing genotypic evidence of both T- and B-cell lineages. The American journal of surgical pathology. 1994;18:1092–1101. [PubMed] [Google Scholar]
- 28.Huang WT, Chuang SS, Huang CC, et al. Primary diffuse large B-cell lymphoma of the gallbladder with cholelithiasis masquerading as acute cholecystitis: case report and literature review. N Z Med J. 2007;120:U2470. [PubMed] [Google Scholar]
- 29.Hussain SA, English WE, Lytle LH, et al. Pseudolymphoma of the gallbladder. Am J Gastroenterol. 1976;65:152–155. [PubMed] [Google Scholar]
- 30.Isaacson PG. Gastrointestinal lymphoma. Hum Pathol. 1994;25:1020–1029. doi: 10.1016/0046-8177(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 31.Jelic TM, Barreta TM, Yu M, et al. Primary, extranodal, follicular non-Hodgkin lymphoma of the gallbladder: case report and a review of the literature. Leuk Lymphoma. 2004;45:381–387. doi: 10.1080/10428190310001597919. [DOI] [PubMed] [Google Scholar]
- 32.Jho DH, Jho DJ, Chejfec G, et al. Primary biliary B-cell lymphoma of the cystic duct causing obstructive jaundice. Am Surg. 2007;73:508–510. [PubMed] [Google Scholar]
- 33.Kaplan LD, Kahn J, Jacobson M, et al. Primary bile duct lymphoma in the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1989;110:161–162. doi: 10.7326/0003-4819-110-2-161. [DOI] [PubMed] [Google Scholar]
- 34.Kato H, Naganuma T, Iizawa Y, et al. Primary non-Hodgkin's lymphoma of the gallbladder diagnosed by laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg. 2008;15:659–663. doi: 10.1007/s00534-007-1283-9. [DOI] [PubMed] [Google Scholar]
- 35.Koshy M, Zhao F, Garofalo MC. Primary MALT lymphoma of the gallbladder. Case report. J Gastrointestin Liver Dis. 2008;17:207–210. [PubMed] [Google Scholar]
- 36.Kosuge T, Makuuchi M, Ozaki H, et al. Primary lymphoma of the common bile duct. Hepatogastroenterology. 1991;38:235–238. [PubMed] [Google Scholar]
- 37.Kuroda N, Mizobuchi M, Shimamura Y, et al. An Asian variant of intravascular lymphoma: unique clinical and pathological manifestation in the gallbladder. Apmis. 2007;115:371–375. doi: 10.1111/j.1600-0463.2007.apm_578.x. [DOI] [PubMed] [Google Scholar]
- 38.Laurino L, Melato M. Malignant angioendotheliomatosis (Angiotropic lymphoma) of the gallbladder. Virchows Arch A Pathol Anat Histopathol. 1990;417:243–246. doi: 10.1007/BF01600140. [DOI] [PubMed] [Google Scholar]
- 39.Lin P, Jones D, Dorfman DM, et al. Precursor B-cell lymphoblastic lymphoma: a predominantly extranodal tumor with low propensity for leukemic involvement. The American journal of surgical pathology. 2000;24:1480–1490. doi: 10.1097/00000478-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Machado MC, Abdo EE, Penteado S, et al. Lymphoma of the biliary tract: report of 2 cases. Rev Hosp Clin Fac Med Sao Paulo. 1994;49:64–68. [PubMed] [Google Scholar]
- 41.Maymind M, Mergelas JE, Seibert DG, et al. Primary non-Hodgkin's lymphoma of the common bile duct. Am J Gastroenterol. 1997;92:1543–1546. [PubMed] [Google Scholar]
- 42.McCarthy KP, Sloane JP, Kabarowski JH, et al. A simplified method of detection of clonal rearrangements of the T-cell receptor-gamma chain gene. Diagn Mol Pathol. 1992;1:173–179. [PubMed] [Google Scholar]
- 43.McCluggage WG, Mackel E, McCusker G. Primary low grade malignant lymphoma of mucosa-associated lymphoid tissue of gallbladder. Histopathology. 1996;29:285–287. doi: 10.1111/j.1365-2559.1996.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 44.Mitropoulos FA, Angelopoulou MK, Siakantaris MP, et al. Primary non-Hodgkin's lymphoma of the gall bladder. Leuk Lymphoma. 2000;40:123–131. doi: 10.3109/10428190009054889. [DOI] [PubMed] [Google Scholar]
- 45.Mosnier JF, Brousse N, Sevestre C, et al. Primary low-grade B-cell lymphoma of the mucosa-associated lymphoid tissue arising in the gallbladder. Histopathology. 1992;20:273–275. doi: 10.1111/j.1365-2559.1992.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen GK. Primary extranodal non-Hodgkin's lymphoma of the extrahepatic bile ducts. Report of a case. Cancer. 1982;50:2218–2222. doi: 10.1002/1097-0142(19821115)50:10<2218::aid-cncr2820501041>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 47.Ono A, Tanoue S, Yamada Y, et al. Primary malignant lymphoma of the gallbladder: a case report and literature review. Br J Radiol. 2009;82:e15–e19. doi: 10.1259/bjr/30768802. [DOI] [PubMed] [Google Scholar]
- 48.Orton DF, Saigh JA. CT of Hodgkin's lymphoma limited to the gallbladder. Abdom Imaging. 1996;21:238–239. doi: 10.1007/s002619900054. [DOI] [PubMed] [Google Scholar]
- 49.Pelstring RJ, Essell JH, Kurtin PJ, et al. Diversity of organ site involvement among malignant lymphomas of mucosa-associated tissues. Am J Clin Pathol. 1991;96:738–745. doi: 10.1093/ajcp/96.6.738. [DOI] [PubMed] [Google Scholar]
- 50.Petitjean B, Jardin F, Joly B, et al. Pyothorax-associated lymphoma: a peculiar clinicopathologic entity derived from B cells at late stage of differentiation and with occasional aberrant dual B- and T-cell phenotype. The American journal of surgical pathology. 2002;26:724–732. doi: 10.1097/00000478-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Podbielski FJ, Pearsall GF, Jr, Nelson DG, et al. Lymphoma of the extrahepatic biliary ducts in acquired immunodeficiency syndrome. Am Surg. 1997;63:807–810. [PubMed] [Google Scholar]
- 52.Poggi MM, Cong PJ, Coleman CN, et al. Low-grade follicular lymphoma of the small intestine. J Clin Gastroenterol. 2002;34:155–159. doi: 10.1097/00004836-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 53.Rajesh LS, Nada R, Yadav TD, et al. Primary low-grade B-cell lymphoma of the mucosa-associated lymphoid tissue of the gallbladder. Histopathology. 2003;43:300–301. doi: 10.1046/j.1365-2559.2003.01655.x. [DOI] [PubMed] [Google Scholar]
- 54.Ramasamy I, Brisco M, Morley A. Improved PCR method for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell neoplasms. J Clin Pathol. 1992;45:770–775. doi: 10.1136/jcp.45.9.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richard P, Vassallo J, Valmary S, et al. "In situ-like" mantle cell lymphoma: a report of two cases. J Clin Pathol. 2006;59:995–996. doi: 10.1136/jcp.2005.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenberg SA, Diamond HD, Jaslowitz B, et al. Lymphosarcoma: a review of 1269 cases. Medicine (Baltimore) 1961;40:31–84. doi: 10.1097/00005792-196102000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Sato THG, Masuo K, Mochizuki T, Sanada Y. A Case Of Malignant Lymphoma Of The Gallbladder. Jpn J Gastroenterol Surg. 2001;34:1308–1311. [Google Scholar]
- 58.Shimura T, Kuwano H, Kashiwabara K, et al. Mucosa-associated lymphoid tissue lymphoma of the extrahepatic bile duct. Hepatogastroenterology. 2005;52:360–362. [PubMed] [Google Scholar]
- 59.Stephen MR, Farquharson MA, Sharp RA, et al. Sequential malt lymphomas of the stomach, small intestine, and gall bladder. J Clin Pathol. 1998;51:77–79. doi: 10.1136/jcp.51.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugawara G, Nagino M, Oda K, et al. Follicular lymphoma of the extrahepatic bile duct mimicking cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2008;15:196–199. doi: 10.1007/s00534-007-1248-z. [DOI] [PubMed] [Google Scholar]
- 61.Swerdlow SHCE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of tumors of haematopoietic and lymphoid tissues. Lyon: IARC; 2008
- 62.Takano Y, Katsurada J, Kawamura M, Sakamoto N, Watanabe Y, Kanno S. A Case Of Primary Low-Grade B-Cell Lymphoma Of Mucosa-Associated Lymphoid Tissue (MALT) Of Gallbladder. Yakuri To Rinsho. 2004;14:397. [Google Scholar]
- 63.Takehara TMH, Naitou M, et al. A case report of primary extranodal non-Hodgkin lymphoma of the extrahepatic bile duct. Acta Hepatol Jpn. 1989;88:247–252. [Google Scholar]
- 64.Tartar VM, Balfe DM. Lymphoma in the wall of the bile ducts: radiologic imaging. Gastrointest Radiol. 1990;15:53–57. doi: 10.1007/BF01888735. [DOI] [PubMed] [Google Scholar]
- 65.Tomori H, Nagahama M, Miyazato H, et al. Mucosa-associated lymphoid tissue (MALT) of the gallbladder: a clinicopathological correlation. Int Surg. 1999;84:144–150. [PubMed] [Google Scholar]
- 66.Tsuchiya T, Shimokawa I, Higami Y, et al. Primary low-grade MALT lymphoma of the gallbladder. Pathol Int. 2001;51:965–969. doi: 10.1046/j.1440-1827.2001.01299.x. [DOI] [PubMed] [Google Scholar]
- 67.Tzanakakis GN, Vezeridis MP, Jackson BT, et al. Primary extranodal non-Hodgkin's lymphoma of the extrahepatic biliary tract. R I Med J. 1990;73:483–486. [PubMed] [Google Scholar]
- 68.Vaittinen E. Sarcoma of the gall-bladder. Ann Chir Gynaecol Fenn. 1972;61:185–189. [PubMed] [Google Scholar]
- 69.Willingham DL, Menke DM, Satyanarayana R. Gallbladder lymphoma in primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2009;7:A26. doi: 10.1016/j.cgh.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto T, Kawanishi M, Yoshiba H, et al. Primary non-Hodgkin's lymphoma of the gallbladder. AJR Am J Roentgenol. 2005;184:S86–S87. doi: 10.2214/ajr.184.3_supplement.01840s86. [DOI] [PubMed] [Google Scholar]
- 71.Yasuma T, Yanaka M. Primary sarcoma of the gallbladder--report of three cases. Acta Pathol Jpn. 1971;21:285–304. doi: 10.1111/j.1440-1827.1971.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 72.Yokoe M, Mizuno Y, Hattori K, et al. A case of malignant lymphoma of the gallbladder. Nippon Shokakibyo Gakkai Zasshi. 2003;100:1219–1223. [PubMed] [Google Scholar]
- 73.Yoshino T, Miyake K, Ichimura K, et al. Increased incidence of follicular lymphoma in the duodenum. The American journal of surgical pathology. 2000;24:688–693. doi: 10.1097/00000478-200005000-00007. [DOI] [PubMed] [Google Scholar]