Abstract
Background
The current therapies for alcohol abuse disorders are not effective in all patients and continued development of pharmacotherapies is needed. One approach that has generated recent interest is the antagonism of ghrelin receptors. Ghrelin is a gut-derived peptide important in energy homeostasis and regulation of hunger. Recent studies have implicated ghrelin in alcoholism, showing altered plasma ghrelin levels in alcoholic patients as well as reduced intakes of alcohol in ghrelin receptor knock-out mice and in mice treated with ghrelin receptor antagonists. The aim of this study was to determine the neuroanatomical locus/loci of the effect of ghrelin receptor antagonism on alcohol consumption using the ghrelin receptor antagonist, D-Lys3-GHRP-6.
Methods
In Experiment 1, male C57BL/6J mice were injected with saline and allowed access to 15 % (v/v) ethanol or water for 2 h, three hours into their dark cycle, in a 2-bottle choice experiment. On test day, the mice were injected with either saline or 400 nmol of the ghrelin receptor antagonist, D-Lys3-GHRP-6, and allowed to drink 15 % ethanol or water for 4 h. The preference for alcohol and alcohol intake were determined. In Experiment 2, the same procedure was followed as in Experiment 1 but mice were only allowed access to a single bottle of 20 % ethanol (v/v) and alcohol intake was determined. Blood ethanol levels were analyzed and immunohistochemistry for c-Fos was carried out to investigate changes in neural activity. To further elucidate the mechanism by which D-Lys3-GHRP-6 affects alcohol intake, in Experiment 3, the effect of D-Lys3-GHRP-6 on the neural activation induced by intraperitoneal ethanol was investigated. For the c-Fos studies, brain regions containing ghrelin receptors were analyzed, i.e. the perioculomotor urocortin population of neurons (pIIIu), the ventral tegmental area (VTA) and the arcuate nucleus (Arc). In Experiment 4, to test if blood ethanol concentrations were affected by D-Lys3-GHRP-6, blood samples were taken at two time-points after D-Lys3-GHRP-6 pre-treatment and systemic ethanol administration.
Results
In Experiment 1, D-Lys3-GHRP-6 reduced preference to alcohol and in a follow-up experiment (Experiment 2), also dramatically reduced alcohol intake when compared to saline-treated mice. The resulting blood ethanol concentrations were lower in mice treated with the ghrelin receptor antagonist. Immunohistochemistry for c-Fos showed fewer immunopositive cells in the pIIIu of the antagonist-treated mice but no difference was seen in the VTA or Arc. In Experiment 3, D-Lys3-GHRP-6 reduced the induction of c-Fos by intraperitoneal ethanol in the pIIIu but had no effect in the VTA. In the Arc, there was a significant increase in the number of c-Fos immunopositive cells after D-Lys3-GHRP-6 administration but the antagonist had no effect on ethanol-induced expression of c-Fos. D-Lys3-GHRP-6-pretreatment also did not affect the blood ethanol concentrations observed after a systemic injection of ethanol when compared to saline-pretreated mice (Experiment 4).
Conclusions
These findings indicate that the action of ghrelin on the regulation of alcohol consumption may occur via the pIIIu.
Keywords: Drinking in the dark, Edinger-Westphal nucleus, subgriseal, growth hormone secretagogue receptor
Recent reports showing an overlap in the neural pathways responsible for obesity and those involved in excessive alcohol use (for review see Thiele et al., 2003) suggest that orexigenic peptides are promising targets for pharmacotherapy of alcoholism.
The orexigenic peptide ghrelin, produced in the gastric fundic mucosa (Korbonits et al., 2004; Nakazato et al., 2001), causes the secretion of growth hormone, and acts to stimulate appetite and food intake (Locke et al., 1995; Wren et al., 2000). Ghrelin is also expressed in the hypothalamus, namely in the lateral hypothalamus, the arcuate nucleus (Arc), the ventromedial nucleus, dorsomedial nucleus and the paraventricular nucleus (Cowley et al., 2003; Hou et al., 2006; Kageyama et al., 2008; Kojima et al., 1999).
The receptor at which ghrelin acts to stimulate the release of growth hormone is the growth hormone secretagogue receptor 1a (GHSR 1a) (Kojima et al., 1999; van der Lely et al., 2004). Zigman et al. (2006) showed that in the mouse, high levels of GHSR 1a mRNA occur in the perioculomotor urocortin-containing neurons (pIIIu, formerly known as the Edinger-Westphal nucleus; Horn et al., 2008; May et al., 2008). GHSR 1a mRNA is also abundant in the Arc (Tannenbaum et al., 1998; Willesen et al., 1999; Zigman et al. 2006) where the main targets of ghrelin are the neuropeptide Y/Agouti-related protein neurons and the leptin-responsive neurons (Traebert et al., 2002). Other regions of the brain where GHS 1a receptor mRNA is expressed, albeit in substantially lesser amounts than the Arc and pIIIu, include the ventral tegmental area (VTA), the laterodorsal tegmental area and the hippocampus (Guan et al., 1997; Zigman et al., 2006).
Ghrelin has been studied extensively with regards to food intake and obesity (for review see Neary et al., 2003). Ghrelin is secreted in anticipation of a meal, and ghrelin levels decrease after food has been consumed (Cummings et al., 2001; Drazen et al., 2006). Central or systemic ghrelin increases food intake, and the effects of ghrelin on feeding and weight gain involve the Arc (Nakazato et al., 2001; Ruter et al., 2003; Wren et al., 2000). The peripheral administration of a GHSR antagonist, D-Lys3-GHRP-6, in contrast, reduced food intake as well as weight gain (Asakawa et al., 2003).
Recent studies have also started to implicate ghrelin in alcohol drinking. Increased plasma levels of ghrelin have been reported in active drinkers, alcohol-dependent female patients, in alcohol withdrawal groups and in alcohol abstainers (Kim et al., 2005; Kraus et al., 2005; Wurst et al., 2007). A single nucleotide polymorphism in the GHSR 1a gene was found to be associated with heavy alcohol drinking in a Spanish population (Landgren et al., 2008). In mice, centrally administered ghrelin increased alcohol drinking while the GHSR 1a antagonists, BIM28163 and JMV2959, reduced alcohol consumption. The increased alcohol intake in response to centrally administered ghrelin was also found to be absent in GHSR 1a knock-out mice (Jerlhag et al., 2009b), showing that ghrelin acts at the GHSR 1a to affect alcohol-seeking behavior.
However, conflicting findings showing reduced plasma and stomach ghrelin levels in alcohol-dependent patients have also been reported (Badaoui et al., 2008). In normal volunteers, plasma ghrelin levels were reduced after acute alcohol (Calissendorff et al., 2005; Zimmermann et al., 2007). Addolorato et al. (2006) showed decreased ghrelin levels in alcohol-dependent patients compared to healthy subjects, but the authors also found a positive correlation between ghrelin levels and craving for alcohol. Lyons et al. (2008) reported that systemic ghrelin does not affect alcohol consumption in a “drinking in the dark” (DID) procedure in food-deprived C57BL/6J mice. Thus, the relationship between ghrelin and alcohol consumption and alcoholism still needs to be clarified.
It may be important to understand the neural substrates of ghrelin to clarify this relationship. Jerlhag et al. (2009b) showed that administering ghrelin, either intracerebroventricularly or into the VTA increased alcohol intake in mice whereas administering GHSR 1a antagonists, centrally or systemically, reduced alcohol intake. Thus, the authors concluded that ghrelin acting in the tegmental regions was responsible for the rewarding effects of alcohol. However, the VTA is located physically close to the pIIIu, and as observed in the Allen Brain Atlas (http://www.brain-map.org) and reported by Zigman et al. (2006), it contains substantially lower levels of GHSR1a mRNA than pIIIu. Several studies, using c-Fos expression mapping, electrolytic lesion of the pIIIu and rodents selectively bred for differences in alcohol intakes, have shown that the pIIIu is involved in the regulation of alcohol-related phenotypes, including high alcohol intake (Bachtell et al., 1999; Bachtell et al., 2002a; Bachtell et al., 2002b; Bachtell et al., 2003; Bachtell et al., 2004; Fonareva et al., 2009; Ryabinin et al., 2001; Ryabinin et al., 2003; Sharpe et al., 2005; Topple et al., 1998; Turek et al., 2005; Weitemier et al., 2001; Weitemier and Ryabinin, 2005). The Arc contains GHSR 1a receptors at levels comparable to the pIIIu (Zigman et al., 2006). Lesion and microinjection studies have implicated the Arc in ethanol-induced locomotor activation (Pastor and Aragon, 2008; Sanchis-Segura et al., 2000). It is, therefore, important to understand whether ghrelin acts via the VTA, pIIIu or Arc to regulate alcohol-related behaviors.
In the current study, we investigated the neuroanatomical locus/loci through which ghrelin acts to regulate alcohol consumption. At first we confirmed that the ghrelin receptor antagonist that we used, D-Lys3-GHRP-6 acts to decrease alcohol preference in C57BL/6J mice in a similar fashion to the GHSR antagonists used by Jerlhag and colleagues (2009b). Following this confirmation, we investigated the effects of D-Lys3-GHRP-6 on c-Fos immunoreactivity induced in pIIIu, VTA and Arc by alcohol intake in the drinking in the dark procedure and by systemic administration of ethanol. We also determined if D-Lys3-GHRP-6 affects blood ethanol concentrations after systemic ethanol administration.
METHODS
Animals
Male C57BL/6J mice purchased from Jackson Laboratories (Bar Habor, ME) or bred in our laboratory, weighing 21–29 g, were used in all the studies detailed here. For the DID experiments (Experiments 1 and 2), the mice were housed 5 per cage for one week after arrival and then housed individually and acclimated to a shifted light/dark cycle (lights on at 9 pm, lights off at 9 am) before the experiment was begun. The DID procedure, leading to blood ethanol concentrations (BECs) greater than 100 mg% and behavioral signs of intoxication, was developed based on substantially higher alcohol intake at peak times of activity during the dark phase of the circadian cycle in mice (Rhodes et al., 2005; Ryabinin et al., 2003; Sharpe et al., 2005). Specifically, in Experiment 1 we used a two-bottle choice DID procedure, where the mice were given access to 15% ethanol and water, and in Experiment 2 we used a single-bottle (of 20% ethanol) no-choice procedure standardized as a model of excessive alcohol consumption by Rhodes et al. (2005; 2007). In a standard single-bottle DID procedure, 20% ethanol is the concentration of choice as it elicits high alcohol intakes being the only fluid available during the access period but in the modified two-bottle choice DID procedure assessing ethanol preference, a slightly lesser ethanol concentration, 15%, is used as mice in this procedure (Blednov and Harris, 2008; Dhaher et al., 2009) have a choice between water and ethanol and the 20% ethanol solution is not preferred. In both DID procedures, the mice were given access to ethanol for 2 h for days 4 to 6 and then on day 7 (test day), access was increased to 4 h. As has been reported by Rhodes et al. (2005; 2007) and Sparta et al. (2008), using this ‘escalation’ procedure allows for excessive alcohol intakes on day 7 which may then be more responsive to pharmacological manipulations. In Experiment 3 (the injection experiment), mice were housed 5 to a cage and each cage was treated as a separate group. In Experiment 4, the effect, if any, of D-Lys3-GHRP-6 on blood ethanol concentrations was assessed after systemic ethanol administration. All mice had ad libitum access to rodent chow during the experiment and water was available unless otherwise noted. All experimental procedures were approved by the OHSU Animal Care and Use Committee (IACUC) and complied with NIH ethical guidelines for the treatment of laboratory animals.
D-Lys-3-GHRP-6
The GHSR 1a antagonist that we used, D-Lys-3-GHRP-6 [His-D-Trp-D-Lys-Trp-D-Phe-Lys-NH2], has been used primarily to investigate the role of ghrelin in food intake studies. D-Lys3-GHRP-6 is a selective and potent inhibitor of GHSR 1a (Pinilla et al., 2003; Sethumadhavan et al., 1991) with an IC50 of 0.9 µM (Traebert et al., 2002; Tocris Bioscience literature).
At doses similar to the dose that we used, Asakawa et al. (2003) and Beck et al. (2004) reported decreased food intake in rodents after systemic treatment for up to 24 h post-administration. Asakawa et al. (2003) also observed that D-Lys3-GHRP-6 had similar effects on food intake when administered systemically and intracerebroventricularly, which shows that the drug crosses the blood brain barrier.
Experiment 1: The Effect of D-Lys3-GHRP-6 on preference to alcohol in a two-bottle choice experiment
All mice underwent habituation to injections, i.e. during the first three days, the mice were injected with 0.3 ml saline (Hospira, Inc., Lake Forest, IL) and water bottles were replaced 30 min later. On days 4 to 6, the mice were injected with saline and 30 min later, they were allowed access to water and 15 % ethanol (v/v, made in tap water). To control for preference to the location of the fluids, in half of the animals, the ethanol tube was placed on the right and in the other half, on the left. The volumes of the fluids were recorded after 2 h and the ethanol tube was then replaced with a water tube. On day 7, animals were divided into groups matched for alcohol intakes and injected with either 0.3 ml saline or 400 nmol of D-Lys3-GHRP-6 (vehicle: saline; Tocris Bioscience, Ellisville, MO) and 30 min later were allowed access to water and 15 % ethanol for 4 h. Volumes of the fluids consumed were recorded and the mice euthanized by an overdose of CO2. All injections were intraperitoneal and administered 2 h and 30 min into the dark cycle and drinking tubes replaced 30 minutes later, at 12 pm.
Experiment 2: The Effect of D-Lys3-GHRP-6 on alcohol intake
On days 1 to 3, as in Experiment 1, the mice were habituated to saline (0.3 ml) injections as in Experiment 1. On days 4 to 6, mice received saline injections and 30 min later were given access to 20 % ethanol (v/v, made in tap water) for 2 h. On day 7, the mice were divided into groups matched for alcohol intakes and either injected with 0.3 ml saline or 400 nmol D-Lys3-GHRP-6. Thirty minutes later, they were allowed access to 20 % ethanol for 4 h. The volumes consumed were recorded at the end of the access periods and on day 7, at the conclusion of the 4 h, the mice were euthanized as above and brains and blood samples were collected. As before, all injections were intraperitoneal and carried out 2 h and 30 min into the dark cycle and drinking tubes replaced 30 minutes later, at 12 pm.
Experiment 3: The Effect of D-Lys3-GHRP-6 on c-Fos induction by systemic ethanol
Mice were habituated to saline injections for 4 days. On day 5, either 0.3 ml saline or 400 nmol D-Lys3-GHRP-6 was injected intraperitoneally. Thirty minutes later, either saline or 2.5 g/kg ethanol was injected, also intraperitoneally, and after 90 min the mice were euthanized as above and brains were collected for immunohistochemical processing.
Experiment 4: Blood ethanol concentrations after D-Lys3-GHRP-6 pre-treatment and systemic ethanol
Mice were injected with either 0.3ml saline or 400 nmol D-Lys3-GHRP-6 which was followed 30 min later by 2.5 g/kg ethanol i.p. The mice were then euthanized at two time-points: 1 h or 2 h after the second injection. Blood samples were collected and blood ethanol concentrations (BECs) were determined.
Blood ethanol concentration (BEC) measurements
Samples from both Experiments 2 and 4 were treated similarly, i.e. blood samples were centrifuged and plasma removed and frozen at −20°C until analyzed. Blood ethanol concentrations (BECs in mg/dl) were obtained using the GL5 Analyser (Analox Instruments USA, Lunenburg, MA).
Immunochemistry (IHC) for c-Fos
Brains were stored in 2 % paraformaldehyde in 10 mM phosphate-buffered saline (PBS) overnight and then cryoprotected in a 30 % sucrose/10 mM PBS solution until sectioned. The brains were sliced on a cryostat and floating sections, 30 microns thick, were collected and processed for c-Fos immunohistochemistry using standard protocols (Bachtell et al., 1999; Ryabinin et al., 2001). Briefly, the sections underwent a 0.3% hydrogen peroxide incubation to quench endogenous peroxidase activity and then blocking was carried out using goat serum (Vector Laboratories, Burlingame, CA). The c-Fos antibody used was obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and used at a 1:2000 dilution. A standard ABC kit (Vector Laboratories, Burlingame, CA) was used for the immunoreaction and DAB (Thermo Scientific, Rockford, IL) was used for visualization. Cells immunopositive for c-Fos were counted manually at 20× objective magnification by an experimenter blind to the group of animals, in the perioculomotor urocortin-containing neuronal population (pIIIu), the arcuate nucleus (Arc) and the ventral tegmental area (VTA). The same sections contained the VTA and the pIIIu and encompassed bregma levels −2.80 to −3.88. According to the Allen Brain Atlas (www.brain-map.org), mRNA for GHSR 1a is present across the entire rostrocaudal extent of the pIIIu and therefore all sections containing the pIIIu were included in the analysis. Sections containing the Arc were taken from bregma levels −1.46 to −2.54. Since the pIIIu is a midline brain region, the presented cell counts are for the entire nucleus, whereas for the VTA and the Arc, the counts are for one hemisphere. At least two sections per region per animal were analyzed, and the counts were averaged resulting in a single data point for each animal in statistical analysis.
Data analysis
Data are presented as means ± S.E.M. Preference and alcohol intake for the first three experimental days were compared using ANOVA with repeated measures. Comparisons between drug and saline treatments were carried out using analysis of variance (ANOVA) with drug treatment as the variable. The c-Fos data in Experiment 2 were analyzed using ANOVAs comparing the different treatment groups. For Experiment 3, a multi-factor ANOVA was carried out, with the first injection and the second injection as separate variables. When the interaction between the two injections was significant, only then was there considered to be an effect of the drug on ethanol-induced c-Fos. When there was no significant interaction between the two injections, data from the saline pre-treated mice or the D-Lys3-GHRP-6 pre-treated mice was pooled and compared using a Fisher’s PLSD post-hoc test. An effect was considered to be statistically significant when p < 0.05.
RESULTS
Experiment 1: The Effect of D-Lys3-GHRP-6 on preference to alcohol in a two-bottle choice experiment
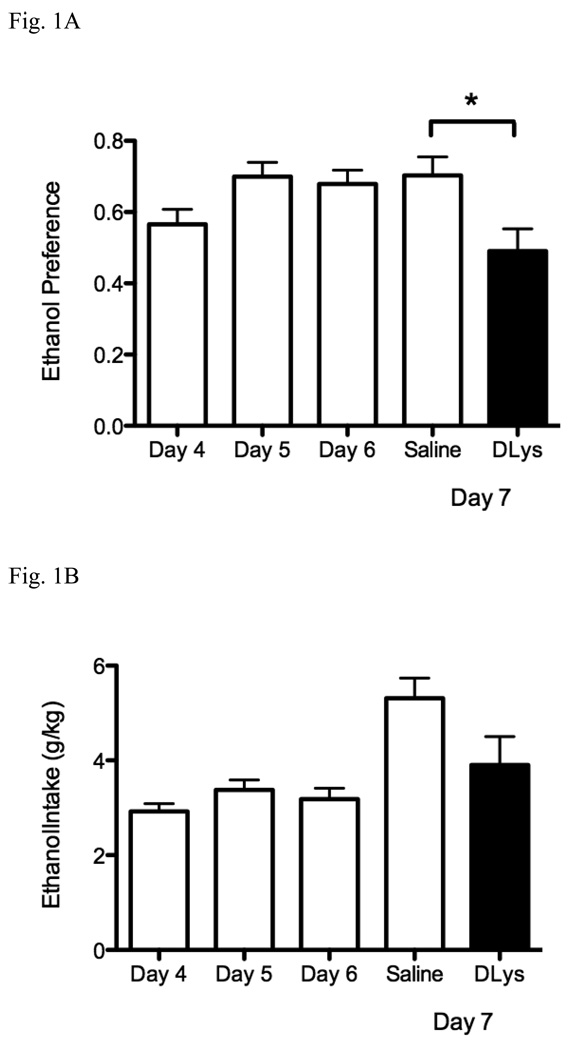
Previous studies have used the ghrelin receptor antagonists, BIM28163 and JMV2959, to demonstrate the effects of ghrelin antagonism on alcohol intake (Jerlhag et al., 2009b). Our first experiment tested whether the ghrelin receptor antagonist D-Lys3-GHRP-6 has an effect on alcohol preference. Mice were given access to water or 15 % ethanol for 2 h on days 4 to 6, 30 minutes after a saline injection. On day 7, the animals were administered either saline or the antagonist and allowed to drink either water or 15 % ethanol for 4 h, 30 minutes after the injection. Preference to ethanol is shown in Fig. 1A. Mice showed no difference in preference for ethanol over the first three experimental days. Ethanol was clearly preferred: preference on day 4 was 0.57 ± 0.04, on day 5 was 0.70 ± 0.04 and day 6 was 0.68 ± 0.04. On day 4, injection of D-Lys3-GHRP-6 blocked preference to ethanol (0.49 ± 0.06) [F(1,10) = 6.79, p = 0.026). Ethanol intakes also did not change over the first three experimental days. Intake on day 4 was 2.92 ± 0.17 g/kg, on day 5, 3.38 ± 0.21 g/kg and on day 6 was 3.18 ± 0.23 g/kg. D-Lys3-GHRP-6 showed a trend to decrease ethanol intake but this did not reach significance (p = 0.085) (Fig. 1B). Total volume of fluid consumed on day 4 was 1.08 ± 0.06 ml, on day 5, 1.01 ± 0.08 ml and on day 6, 0.97 ± 0.07 ml (no significant difference over the three days). There was no change in the total volume consumed between the group that was administered D-Lys3-GHRP-6 and the saline-treated group (Fig. 1C).
Fig. 1.
Effects of D-Lys3-GHRP-6 on 15 % ethanol preference in a drinking in the dark paradigm (A) Preference for ethanol (access to 15 % ethanol or water) (B) ethanol intake (g/kg) (C) total volume consumed (ml) for 2 h after saline injections (i.p) on days 4 to 6 and after saline or 400 nmol D-Lys3-GHRP-6 (i.p) on day 7 for 4 h. All values are means ± SEM. * p < 0.05.
Overall these data demonstrated that D-Lys3-GHRP-6 had similar effects to BIM28163 and JMV2959 in decreasing alcohol preference without affecting total fluid intake.
Experiment 2: The Effect of D-Lys3-GHRP-6 on alcohol intake
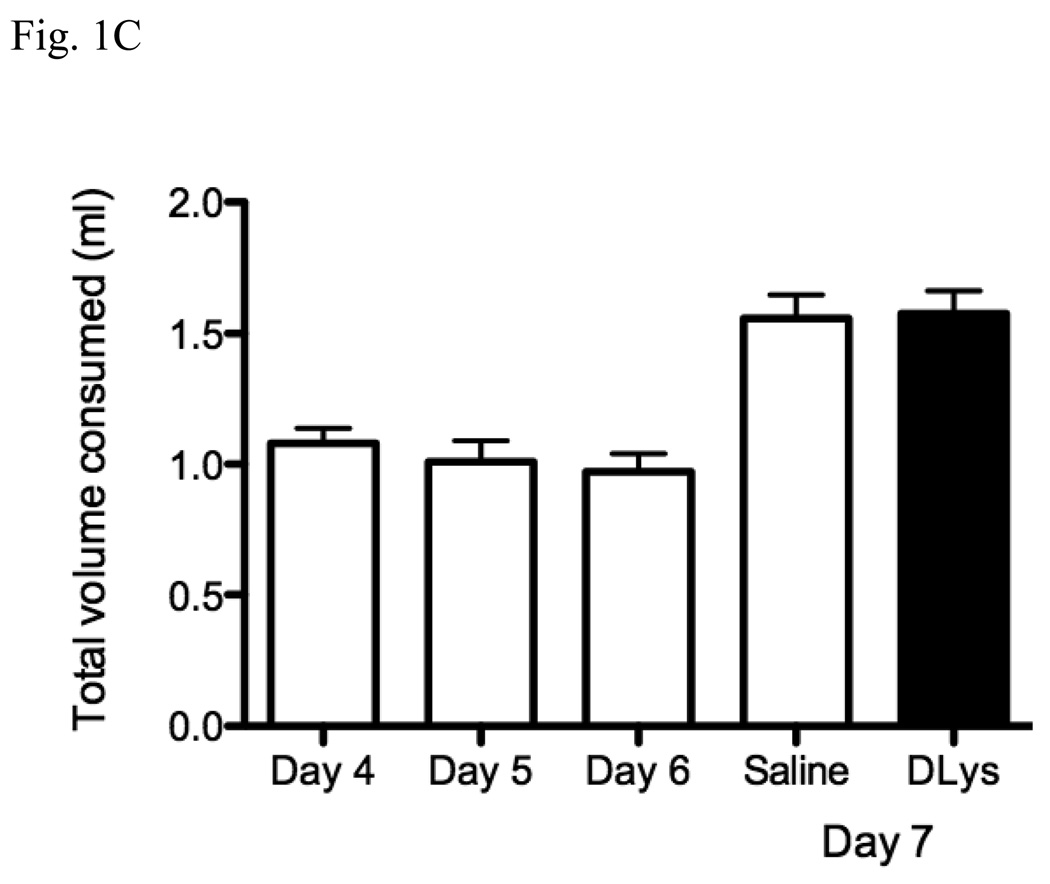
As the mice treated with D-Lys3-GHRP-6 clearly showed a reduced preference for alcohol when compared to the saline-treated animals, we next tested the effect of this drug in a single-bottle DID procedure known to produce substantial blood ethanol concentrations and ethanol-induced c-Fos expression. Mice were injected with saline for three days as before but allowed to drink only 20 % ethanol for 2 h (having access to water throughout the rest of the day) and then on day 7, either saline or D-Lys3-GHRP-6 was injected and the mice allowed to drink 20 % ethanol for 4 h. As shown in Fig. 2A, ethanol intakes were high during the 2 h access period: on day 4, the mice ingested 2.81 ± 0.20 g/kg, on day 5, 2.45 ± 0.21 g/kg and on day 6, 2.63 ± 0.16 g/kg. Mice injected with D-Lys3-GHRP-6 showed a significantly reduced intake of alcohol (2.49 ± 0.32 g/kg) when compared to the saline-injected mice (4.99 ± 0.38) [F(1,27) = 24.87, p < 0.0001] during the 4 h access period. The resulting blood ethanol concentrations after D-Lys3-GHRP-6 (63.64 ± 11.67 mg%) were also significantly lower than after saline injections (114.82 ± 21.28 mg%) [F(1,27) = 4.27, p = 0.0484] (Fig. 2B).
Fig. 2.
Effects of D-Lys3-GHRP-6 on intake of 20 % ethanol in a drinking in the dark paradigm (g/kg) (A) after saline injections (i.p) on days 4 to 6 and after saline or 400 nmol D-Lys3-GHRP-6 (i.p) on day 7 for 4 h (B) Blood ethanol concentrations (BECs) at the end of the 4 h access on day 7 (n = 14–15/group). All values are means ± SEM. * p < 0.001.
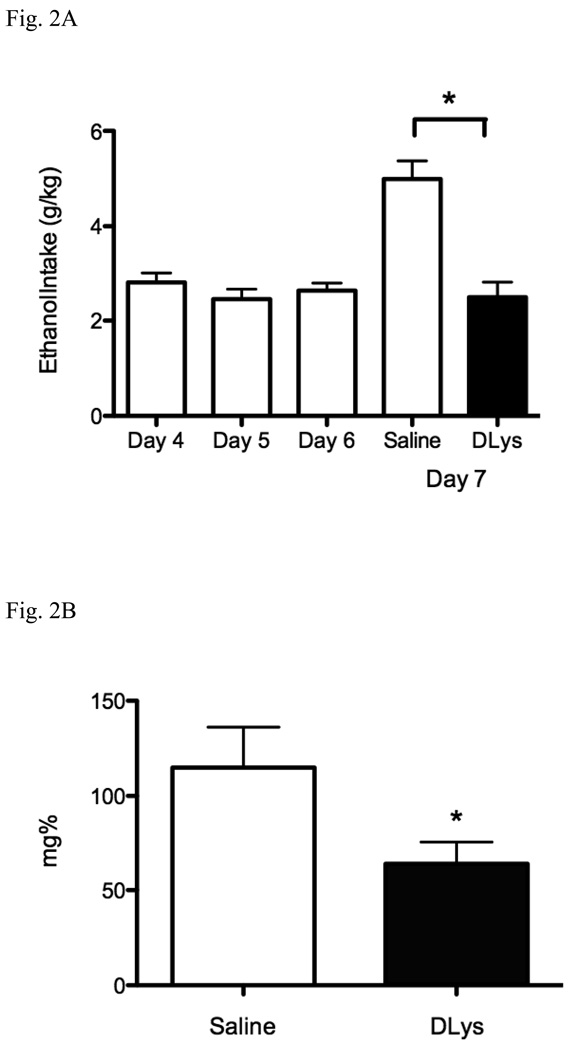
Immunohistochemistry for c-Fos showed significantly fewer c-Fos immunopositive cells in the pIIIu in the mice administered D-Lys3-GHRP-6 (3.90 ± 0.50) when compared to saline-treated mice (22.23 ± 2.52) [F(1,25) = 41.15, p < 0.0001] but no difference was seen in the VTA or the Arc (Fig. 3).
Fig. 3.
Effects of D-Lys3-GHRP-6 on c-Fos immunoreactivity following ethanol intake in a drinking in the dark paradigm (Experiment 2) in the pIIIu, Arc and VTA. Top row shows representative photomicrographs after saline pretreatment. Second row shows representative photomicrographs after pretreatment with 400 nmol of D-Lys3-GHRP-6. Scale bar represents 100 µm. The corresponding graphs, in the bottom row, show the number of c-Fos immunopositive cells in the pIIIu, Arc and VTA following ethanol intake in the drinking in the dark paradigm after saline treatment or 400 nmol D-Lys3-GHRP-6. All values are means ± SEM. * p < 0.0001 (n=12–15/group).
Experiment 3: The Effect of D-Lys3-GHRP-6 on c-Fos induction by systemic ethanol
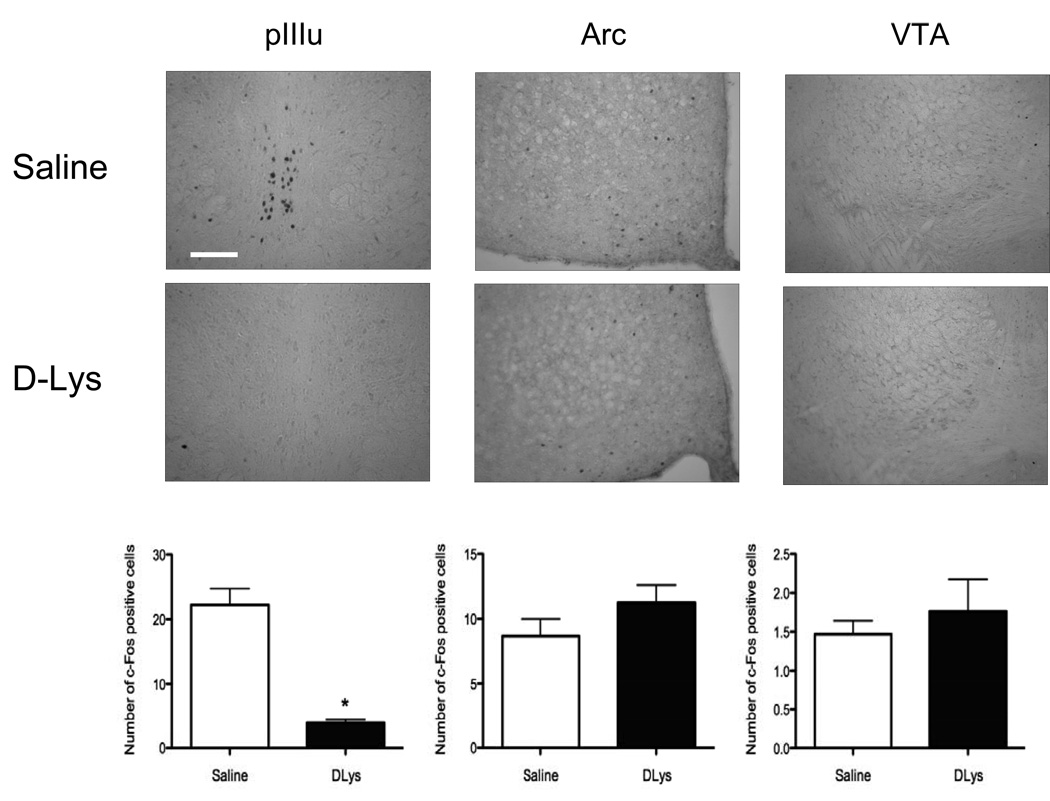
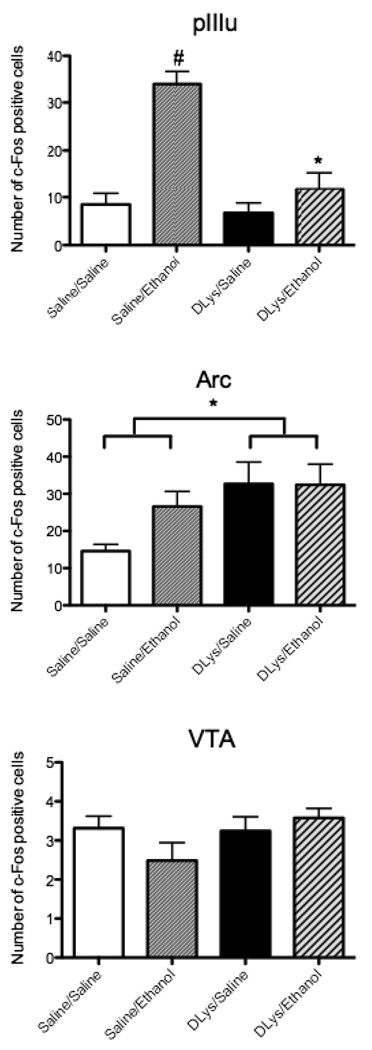
The selective reduction of c-Fos–immunoreactivity after D-Lys3-GHRP-6 observed in Experiment 2 could be due to direct actions of this drug on GHSR 1a in the pIIIu, or may be due to actions of this drug elsewhere leading to a reduction of ethanol intake and the resulting BECs, which in turn would result in lower activation in the pIIIu. To distinguish between these two possibilities, we tested whether D-Lys3-GHRP-6 could suppress ethanol injection-induced c-Fos in pIIIu. Saline or D-Lys3-GHRP-6 were injected followed 30 min later by either 2.5 g/kg ethanol or saline in male C57BL/6J mice. As published previously (Bachtell et al., 2002a; Hill et al., 2007; Spangler et al., 2009; Turek and Ryabinin, 2005), i.p. ethanol dramatically induced c-Fos in the pIIIu [F(3,15) = 18.86, p < 0.0001, ANOVA]. The number of c-Fos immunopositive cells seen after systemic ethanol in the pIIIu was dramatically reduced by D-Lys3-GHRP-6 as demonstrated by significant interaction between the effects of two injections [F(1,15) = 13.59, p = 0.002; Fisher’s PLSD post-hoc p < 0.0001 for the saline pre-treated ethanol-injected animals versus all other groups] (Fig. 4). This effect was specific for the pIIIu. For instance, D-Lys3-GHRP-6 did not have any effect on the expression of c-Fos seen after ethanol in the VTA (Fig. 4). In the Arc, there was no significant interaction between the two injections administered but there was a significant increase in the number of c-Fos cells in D-Lys3-GHRP-6-injected animals compared to saline as demonstrated by a significant effect of first injection [F(1,10) = 6.21, p = 0.032, ANOVA; Fisher’s PLSD post-hoc p =0.045 for all D-Lys3-GHRP-6-injected animals versus all saline-injected animals] (Fig. 4).
Fig. 4.
Number of c-Fos immunopositive cells in the pIIIu, Arc and VTA after saline or 400 nmol D-Lys3-GHRP-6 followed by either saline or 2.5 g/kg ethanol (i.p) in Experiment 3. Mice were euthanized 90 min after the second injection. All values are means ± SEM. # p < 0.001 for saline-pretreated ethanol-injected mice versus all other groups (in pIIIu), * p < 0.01 (interaction between injections in pIIIu) and * p < 0.05 (effect of the first injection only, in Arc; n = 3–5/group).
Experiment 4: Blood ethanol concentrations after D-Lys3-GHRP-6 pre-treatment and systemic ethanol
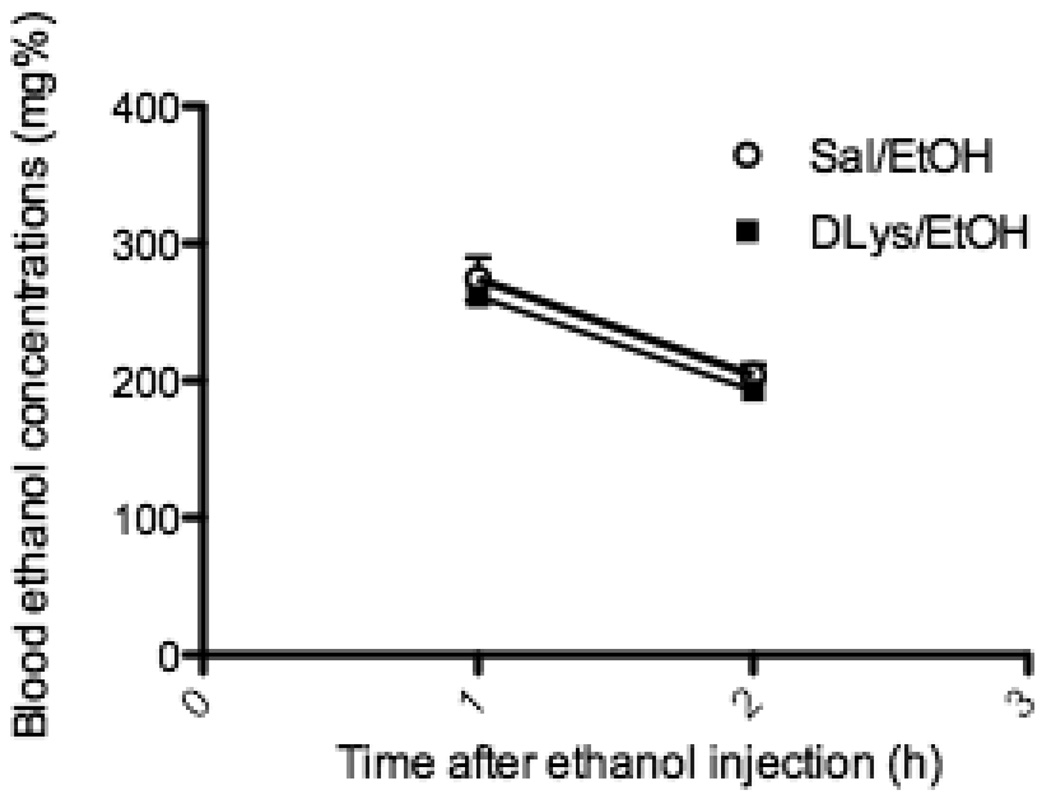
The decrease in number of c-Fos immunopositive cells in the pIIIu after ethanol drinking or injection could also theoretically have been due to changes in BECs caused by D-Lys3-GHRP-6. To address this possibility, we determined BECs at two time-points, 1 h and 2 h, after a systemic injection of 2.5 g/kg ethanol in mice that were pre-treated with either 400 nmol D-Lys3-GHRP-6 or with saline. As can be seen in Fig.5, BECs after D-Lys3-GHRP-6 (274.0 ± 15.71 at 1 h and 203.94 ± 7.85 at 2 h) were not different when compared to saline (261.54 ± 6.38 at 1h and 192.77 ± 6.52 at 2 h) at either time-point.
Fig. 5.
Blood ethanol concentrations at two time-points in mice pre-treated with saline or 400 nmol D-Lys3-GHRP-6 followed by 2.5 g/kg ethanol (i.p). Mice were euthanized 1 h or 2 h after the ethanol injection. All values are means ± SEM. (n = 14–15/group).
DISCUSSION
The results of this study support the involvement of endogenous ghrelin in regulation of alcohol consumption and shed light on the neuroanatomical substrate of this effect. First, we confirmed previous findings showing that ghrelin receptor antagonists reduce alcohol consumption. D-Lys3-GHRP-6, the GHSR 1a antagonist that we used, acted similarly to the antagonists used by Jerlhag et al. (2009b) and reduced preference to alcohol and alcohol intake in C57BL/6J mice in two DID paradigms. Second, immunohistochemical studies for c-Fos showed that D-Lys3-GHRP-6 decreased the neuronal stimulation induced by ethanol in the pIIIu only, while having no similar effects in the VTA or the Arc. D-Lys3-GHRP-6 did not affect blood ethanol concentrations after a systemic ethanol injection, thereby eliminating effects on metabolism as a possible explanation for our c-Fos data. These results indicate that endogenous ghrelin may act via the pIIIu to regulate alcohol consumption.
As D-Lys3-GHRP-6 decreased alcohol consumption in our studies, the first concern that must be considered is whether the drug and/or the dose tested have any non-specific effects on the activity of the animals. In preliminary experiments, we tested a lower dose, 200 nmol, of D-Lys3-GHRP-6 and saw no effect on the number of ethanol-induced c-Fos cells in the pIIIu (unpublished observations) and therefore, used a higher dose in the studies detailed here. In our preference experiment (Experiment 1), we showed no difference in the total volume consumed by the saline-treated mice or the mice treated with 400 nmol of D-Lys3-GHRP-6. We can, therefore, conclude that as the animals drank similar amounts of fluid, they were equally able to access the drinking tubes. The mice treated with the antagonist consumed more water whereas the saline-treated group consumed more ethanol.
The second consideration should be whether the ghrelin receptor antagonist shows selective effects on consumption of other rewarding substances. D-Lys3-GHRP-6 has been investigated in food intake studies and at doses similar to the dose we tested, decreased food intake in food-deprived rodents (Asakawa et al., 2003; Beck et al., 2004). In our preference studies, mice treated with D-Lys3-GHRP-6 did not show a decrease in the amount of water consumed suggesting that this drug shows at least some selectivity to ethanol consumption. However, Jerlhag and colleagues (2009a) showed that a GHSR 1a antagonist did decrease saccharin (a non-caloric reward) consumption. Of the two GHSR 1a antagonists tested by Jerlhag et al. (2009b), JMV2959 decreased food intake while BIM28163 increased it, indicating that the effect of ghrelin receptor antagonists on alcohol and saccharin are independent of their actions on food intake.
Moreover, while several reports suggested that alcohol in mice in the 24 hour access procedure is in part consumed (like food) for its caloric value (Dole and Gentry, 1985; McMillen and Williams, 1998), this does not appear to be the motivation for alcohol consumption in the DID paradigm. Data collected by Lyons et al. (2008) showing that systemic ghrelin administration, known to promote food consumption, does not increase alcohol intake in the DID procedure in food-deprived mice. An earlier study carried out by Sparrow et al. (2007), where mice given access to food during ethanol consumption drank significantly more than mice that did not have food available during access to ethanol, also disputes the hypothesis that the excessive alcohol intakes in DID procedures are due to caloric need. Thus, while a detailed understanding of the specificity of the effect of ghrelin receptor antagonist on alcohol intake would benefit from further studies, the data accumulated so far point to independence of the effects of ghrelin receptor antagonists on alcohol intake from effects of these drugs on food consumption.
The ability of ghrelin receptor antagonists demonstrated by Jerlhag et al. (2009b) and by us to decrease ethanol intake, together with decreased ethanol preference in GHSR 1a knockout mice (Jerlhag et al., 2009b), indicate that endogenous ghrelin promotes alcohol intake. In this sense, our results also concur with studies showing increased plasma ghrelin levels in alcoholic patients at various stages of alcoholism, i.e. active drinking, abstainers and in withdrawal (Kim et al., 2005; Kraus et al., 2005) and with results by Addolorato et al. (2006) showing that ghrelin was linked to craving for alcohol. On the other hand, the fact that peripherally-injected ghrelin did not promote alcohol intake in food-deprived mice (Lyons et al., 2008), while intracerebroventricular ghrelin did (Jerlhag et al., 2009b) suggest the intriguing possibility that the source of the endogenous ghrelin regulating alcohol intake is central and not peripheral. This possibility will be addressed in future studies.
In Experiment 2, we observed that the c-Fos immunoreactivity in the pIIIu of mice consuming alcohol was dramatically reduced following administration of systemic D-Lys3-GHRP-6, and that there was no effect in the VTA or Arc. This result suggested a specific effect of this antagonist in the pIIIu, which is in agreement with the ability of this nucleus to regulate alcohol intake (reviewed in Ryabinin and Weitemier, 2006) and the high levels of GHSR 1a receptor mRNA in this brain area. However, since activation of c-Fos in pIIIu is dose-dependently sensitive to the amount of administered or self-administered ethanol (Bachtell et al., 2002a; Sharpe et al., 2005), we needed to eliminate the potential confound that lower numbers of c-Fos positive cells after D-Lys3-GHRP-6 could be secondary to lower intakes of alcohol. Theoretically, the antagonist could have acted elsewhere in the brain leading lower intakes of alcohol, and hence less c-Fos immunoreactivity in pIIIu. To eliminate this possibility, we investigated the effects of D-Lys3-GHRP-6 on c-Fos immunoreactivity induced following systemic injections of 2.5 g/kg ethanol in Experiment 3. Systemic administration results in delivery of identical amounts of ethanol. This experiment showed that D-Lys3-GHRP-6 significantly reduced ethanol-induced c-Fos immunoreactivity in pIIIu despite the delivery of the same amounts of alcohol in saline and D-Lys3-GHRP-6-preinjected animals. The dose of ethanol, 2.5 g/kg, that we administered does not induce locomotor activity and thus, the increase in the number of c-Fos cells after ethanol administration and the decrease produced by D-Lys3-GHRP-6 in ethanol-induced c-Fos was also not secondary to any effects on locomotor activity. Also, previous genetic and lesion studies studies in mice indicate that the pIIIu is involved in regulation of alcohol consumption, but not locomotor activity in mice (Bachtell et al., 2002b; Bachtell et al., 2003; Bachtell et al., 2004; Weitemier and Ryabinin, 2005). Therefore, the result of this experiment indicates that the reduced activity of pIIIu is not secondary to decreased levels of ethanol or any effects on locomotor activity, and is a consequence of the antagonism of ghrelin receptors in the pIIIu.
The inducible transcription factor c-Fos, has been shown to selectively and consistently be induced in the pIIIu by various routes of administration of ethanol including systemic ethanol, inhalation of ethanol vapors or voluntary drinking of sweetened or unsweetened ethanol in both mice and rats (Bachtell et al., 1999; Bachtell et al., 2002a; Ryabinin et al., 2001; Ryabinin et al., 2003; Sharpe et al., 2005; Spangler et al., 2009; Topple et al., 1998; Weitemier et al., 2001) Furthermore, lesions of this nucleus decreased the robust alcohol consumption seen in C57BL/6J mice (Bachtell et al., 2004; Weitemier and Ryabinin, 2005). As further confirmation of the role of this nucleus in alcohol consumption, rodents selectively bred to prefer alcohol showed higher levels of urocortin immunoreactivity in the pIIIu when compared to control lines (Bachtell et al., 2002b; Bachtell et al., 2003; Fonareva et al., 2009; Turek et al., 2005). And as noted previously, high levels of the ghrelin receptor, GHSR 1a, mRNA are expressed in the pIIIu (Allen Brain Atlas, www.brain-map.org; Zigman et al., 2006), making this nucleus an important player in the regulation of alcohol consumption.
Jerlhag et al. (2009b) postulated that the sites of action through which ghrelin acts on alcohol consumption were the VTA and the laterodorsal tegmental area. However, we did not observe any changes in c-Fos immunoreactivity in the VTA after D-Lys3-GHRP-6 either in the DID or systemic studies. Jerlhag et al. (2009b) saw reduced alcohol drinking after injecting their antagonist, BIM28163 intracerebroventricularly. This route of administration does not allow for an exact locus of action, and thus it cannot be conclusively determined that the antagonist was acting in the tegmental areas to affect alcohol consumption. On the other hand, they observed that administering ghrelin, either intracerebroventricularly or into the VTA increased alcohol intake. Since the pIIIu and VTA are located in close proximity, the pIIIu has substantially higher levels of GHSR 1a mRNA expression than VTA, and they injected ghrelin in a relatively large volume (1 µl) it is possible that the injected ghrelin diffused from VTA and affected alcohol intake via the pIIIu.
GHSR 1a receptors are also highly expressed in the Arc (Zigman et al., 2006). However, D-Lys3-GHRP-6 did not affect c-Fos immunoreactivity following alcohol drinking in this nucleus arguing against actions of ghrelin on alcohol drinking though this nucleus. An unexpected finding in this study was the induction of c-Fos after D-Lys3-GHRP-6 in the Arc in Experiment 3. The direction of the effect on c-Fos levels is surprising because this drug is an antagonist of the receptors. Since, this increase in c-Fos positive cells was not observed in the DID study (Experiment 2), it is important to note that one of the differences of Experiment 3 versus Experiment 2, is that in Experiment 3 the mice underwent two systemic injections. Although mice were subjected to similar procedures prior to the experiment, it is presumed that it is more difficult to habituate to two injections versus one, and systemic injections are also accompanied by the stress of unexpected alcohol intoxication, which could in itself induce c-Fos in many areas. To extrapolate, it would appear that endogenous ghrelin may have anxiolytic effects which can be blocked by the antagonist resulting in higher c-Fos levels in the Arc. Lutter et al. (2008) has recently reported that this may be the case after injecting ghrelin and showing less anxiety in the elevated plus maze in mice. While further experimentation is needed to fully explain this phenomenon, this increase in c-Fos immunoreactivity following D-Lys3-GHRP-6 was not specific to the actions of alcohol as it was observed in both ethanol and saline-injected mice. Therefore, it cannot explain the effects of the antagonist on alcohol intake. No potential anxiety effects were observed in pIIIu, as there was a clear interaction between effects of D-Lys3-GHRP-6 and the effects of ethanol on c-Fos induction. This is in agreement with our previous studies showing that c-Fos expression in the pIIIu of C57BL/6J mice is not sensitive to stress or anxiety (Turek and Ryabinin, 2005; Spangler et al., 2009). We conclude therefore, that only the pIIIu, but not the VTA or Arc demonstrated a pattern of c-Fos activation consistent with the regulation of alcohol consumption.
In conclusion, our results indicate that ghrelin, acting through the pIIIu, plays an important role in the regulation of alcohol consumption. GHSR 1a antagonists may, therefore, have the potential to be used as novel pharmacotherapies for alcoholism.
ACKNOWLEDGEMENTS
This study is supported by NIH grant AA013738 and INIA West Consortium grant, AA013484. We thank Zuzzie Kapasova, Eleonora Juarez, Dawn Cote, Allison Anacker and William Giardino for their technical assistance.
REFERENCES
- Addolorato G, Capristo E, Leggio L, Ferrulli A, Abenavoli L, Malandrino N, Farnetti S, Domenicali M, D'Angelo C, Vonghia L, Mirijello A, Cardone S, Gasbarrini G. Relationship between ghrelin levels, alcohol craving, and nutritional status in current alcoholic patients. Alcohol Clin Exp Res. 2006;30:1933–1937. doi: 10.1111/j.1530-0277.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- Allen Brain Atlas. http://www.brain-map.org.
- Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, Kasuga M. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52:947–952. doi: 10.1136/gut.52.7.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 1999;847:157–165. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther. 2002a;302:516–524. doi: 10.1124/jpet.102.036046. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Strain differences in urocortin expression in the Edinger-Westphal nucleus and its relation to alcohol-induced hypothermia. Neuroscience. 2002b;113:421–434. doi: 10.1016/s0306-4522(02)00174-4. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Galvan-Rosas A, Tsivkovskaia NO, Risinger FO, Phillips TJ, Grahame NJ, Ryabinin AE. The Edinger-Westphal-lateral septum urocortin pathway and its relationship to alcohol consumption. J Neurosci. 2003;23:2477–2487. doi: 10.1523/JNEUROSCI.23-06-02477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Ryabinin AE. Lesions of the Edinger-Westphal nucleus in C57BL/6J mice disrupt ethanol-induced hypothermia and ethanol consumption. Eur J Neurosci. 2004;20:1613–1623. doi: 10.1111/j.1460-9568.2004.03594.x. [DOI] [PubMed] [Google Scholar]
- Badaoui A, De Saeger C, Duchemin J, Gihousse D, de Timary P, Starkel P. Alcohol dependence is associated with reduced plasma and fundic ghrelin levels. Eur J Clin Invest. 2008;38:397–403. doi: 10.1111/j.1365-2362.2008.01947.x. [DOI] [PubMed] [Google Scholar]
- Beck B, Richy S, Stricker-Krongrad A. Feeding response to ghrelin agonist and antagonist in lean and obese Zucker rats. Life Sci. 2004;76:473–478. doi: 10.1016/j.lfs.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Harris RA. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol. 2008;11:775–793. doi: 10.1017/S1461145708008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calissendorff J, Danielsson O, Brismar K, Rojdmark S. Inhibitory effect of alcohol on ghrelin secretion in normal man. Eur J Endocrinol. 2005;152:743–747. doi: 10.1530/eje.1.01905. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Finn DA, Oberbeck DL, Yoneyama N, Snelling CC, Wu W, Hitzemann RJ. Electrolytic lesions of the medial nucleus accumbens shell selectively decrease ethanol consumption without altering preference in a limited access procedure in C57BL/6J mice. Pharmacol Biochem Behav. 2009;92:335–342. doi: 10.1016/j.pbb.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Dole VP, Ho A, Gentry RT. Toward an analogue of alcoholism in mice: criteria for recognition of pharmacologically motivated drinking. Proc Natl Acad Sci U S A. 1985;82:3469–3471. doi: 10.1073/pnas.82.10.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazen DL, Vahl TP, D'Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147:23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- Fonareva I, Spangler E, Cannella N, Sabino V, Cottone P, Ciccocioppo R, Zorrilla EP, Ryabinin AE. Increased perioculomotor urocortin 1 immunoreactivity in genetically selected alcohol preferring rats. Alcohol Clin Exp Res. 2009;33:1956–1965. doi: 10.1111/j.1530-0277.2009.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Hill KG, Ryabinin AE, Cunningham CL. FOS expression induced by an ethanol-paired conditioned stimulus. Pharmacol Biochem Behav. 2007;87:208–221. doi: 10.1016/j.pbb.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn AK, Eberhorn A, Hartig W, Ardeleanu P, Messoudi A, Buttner-Ennever JA. Perioculomotor cell groups in monkey and man defined by their histochemical and functional properties: reappraisal of the Edinger-Westphal nucleus. J Comp Neurol. 2008;507:1317–1335. doi: 10.1002/cne.21598. [DOI] [PubMed] [Google Scholar]
- Hou Z, Miao Y, Gao L, Pan H, Zhu S. Ghrelin-containing neuron in cerebral cortex and hypothalamus linked with the DVC of brainstem in rat. Regul Pept. 2006;134:126–131. doi: 10.1016/j.regpep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Jerlhag E. Requirement of central ghrelin signaling for alcohol reward. Scand Neuropsychopharm. 2009a;2:21. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A. 2009b;106:11318–11323. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama H, Kitamura Y, Hosono T, Kintaka Y, Seki M, Takenoya F, Hori Y, Nonaka N, Arata S, Shioda S. Visualization of ghrelin-producing neurons in the hypothalamic arcuate nucleus using ghrelin-EGFP transgenic mice. Regul Pept. 2008;145:116–121. doi: 10.1016/j.regpep.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Yoon SJ, Choi B, Kim TS, Woo YS, Kim W, Myrick H, Peterson BS, Choi YB, Kim YK, Jeong J. Increased fasting plasma ghrelin levels during alcohol abstinence. Alcohol Alcohol. 2005;40:76–79. doi: 10.1093/alcalc/agh108. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin-a hormone with multiple functions. Front Neuroendocrinol. 2004;25:27–68. doi: 10.1016/j.yfrne.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Kraus T, Schanze A, Groschl M, Bayerlein K, Hillemacher T, Reulbach U, Kornhuber J, Bleich S. Ghrelin levels are increased in alcoholism. Alcohol Clin Exp Res. 2005;29:2154–2157. doi: 10.1097/01.alc.0000191753.82554.7e. [DOI] [PubMed] [Google Scholar]
- Landgren S, Jerlhag E, Zetterberg H, Gonzalez-Quintela A, Campos J, Olofsson U, Nilsson S, Blennow K, Engel JA. Association of pro-ghrelin and GHS-R1A gene polymorphisms and haplotypes with heavy alcohol use and body mass. Alcohol Clin Exp Res. 2008;32:2054–2061. doi: 10.1111/j.1530-0277.2008.00793.x. [DOI] [PubMed] [Google Scholar]
- Locke W, Kirgis HD, Bowers CY, Abdoh AA. Intracerebroventricular growth-hormone-releasing peptide-6 stimulates eating without affecting plasma growth hormone responses in rats. Life Sci. 1995;56:1347–1352. doi: 10.1016/0024-3205(95)00087-9. [DOI] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons AM, Lowery EG, Sparta DR, Thiele TE. Effects of food availability and administration of orexigenic and anorectic agents on elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:1962–1968. doi: 10.1111/j.1530-0277.2008.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen BA, Williams HL. Role of taste and calories in the selection of ethanol by C57BL/6NHsd and Hsd:ICR mice. Alcohol. 1998;15:193–198. doi: 10.1016/s0741-8329(97)00111-0. [DOI] [PubMed] [Google Scholar]
- May PJ, Reiner AJ, Ryabinin AE. Comparison of the distributions of urocortin-containing and cholinergic neurons in the perioculomotor midbrain of the cat and macaque. J Comp Neurol. 2008;507:1300–1316. doi: 10.1002/cne.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Neary NM, Small CJ, Bloom SR. Gut and mind. Gut. 2003;52:918–921. doi: 10.1136/gut.52.7.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor R, Aragon CM. Ethanol injected into the hypothalamic arcuate nucleus induces behavioral stimulation in rats: an effect prevented by catalase inhibition and naltrexone. Behav Pharmacol. 2008;19:698–705. doi: 10.1097/FBP.0b013e328315ecd7. [DOI] [PubMed] [Google Scholar]
- Pinilla L, Barreiro ML, Tena-Sempere M, Aguillar E. Role of ghrelin in the control of growth hormone secretion on pre-pubertal rats: Interactions with excitatory amino acids. Neuroendocrinology. 2003;77:83–90. doi: 10.1159/000068652. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Ruter J, Kobelt P, Tebbe JJ, Avsar Y, Veh R, Wang L, Klapp BF, Wiedenmann B, Tache Y, Monnikes H. Intraperitoneal injection of ghrelin induces Fos expression in the paraventricular nucleus of the hypothalamus in rats. Brain Res. 2003;991:26–33. doi: 10.1016/j.brainres.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Bachtell RK, Freeman P, Risinger FO. ITF expression in mouse brain during acquisition of alcohol self-administration. Brain Res. 2001;890:192–195. doi: 10.1016/s0006-8993(00)03251-0. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Galvan-Rosas A, Bachtell RK, Risinger FO. High alcohol/sucrose consumption during dark circadian phase in C57BL/6J mice: involvement of hippocampus, lateral septum and urocortin-positive cells of the Edinger-Westphal nucleus. Psychopharmacology (Berl) 2003;165:296–305. doi: 10.1007/s00213-002-1284-y. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Weitemier AZ. The urocortin 1 neurocircuit: ethanol-sensitivity and potential involvement in alcohol consumption. Brain Res Rev. 2006;52:368–380. doi: 10.1016/j.brainresrev.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Correa M, Aragon CM. Lesion on the hypothalamic arcuate nucleus by estradiol valerate results in a blockade of ethanol-induced locomotion. Behav Brain Res. 2000;114:57–63. doi: 10.1016/s0166-4328(00)00183-2. [DOI] [PubMed] [Google Scholar]
- Sethumadhavan K, Veeraragavan K, Bowers CY. Demonstration and characterization of the specific binding of growth hormone-releasing peptide to rat anterior pituitary and hypothalamic membranes. Biochem Biophys Res Comm. 1991;178:31–37. doi: 10.1016/0006-291x(91)91775-8. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Tsivkovskaia NO, Ryabinin AE. Ataxia and c-Fos expression in mice drinking ethanol in a limited access session. Alcohol Clin Exp Res. 2005;29:1419–1426. doi: 10.1097/01.alc.0000174746.64499.83. [DOI] [PubMed] [Google Scholar]
- Spangler E, Cote DM, Anacker AM, Mark GP, Ryabinin AE. Differential sensitivity of the perioculomotor urocortin-containing neurons to ethanol, psychostimulants and stress in mice and rats. Neuroscience. 2009;160:115–125. doi: 10.1016/j.neuroscience.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow AM, Lowery AG, Sparta DR, Thiele TE. Assessment of food availability on ethanol consumption with drinking in the dark (DID) procedures. Alcohol Clin Exp Res. 2007;31:211A. [Google Scholar]
- Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE . Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:259–265. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum GS, Lapointe M, Beaudet A, Howard AD. Expression of growth hormone secretagogue-receptors by growth hormone-releasing hormone neurons in the mediobasal hypothalamus. Endocrinology. 1998;139:4420–4423. doi: 10.1210/endo.139.10.6330. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Navarro M, Sparta DR, Fee JR, Knapp DJ, Cubero I. Alcoholism and obesity: overlapping neuropeptide pathways? Neuropeptides. 2003;37:321–337. doi: 10.1016/j.npep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Topple AN, Hunt GE, McGregor IS. Possible neural substrates of beer-craving in rats. Neurosci Lett. 1998;252:99–102. doi: 10.1016/s0304-3940(98)00574-6. [DOI] [PubMed] [Google Scholar]
- Traebert M, Riediger T, Whitebread S, Scharrer E, Schmid HA. Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. J Neuroendocrinol. 2002;14:580–586. doi: 10.1046/j.1365-2826.2002.00810.x. [DOI] [PubMed] [Google Scholar]
- Turek VF, Ryabinin AE. Expression of c-Fos in the mouse Edinger-Westphal nucleus following ethanol administration is not secondary to hypothermia or stress. Brain Res. 2005;1063:132–139. doi: 10.1016/j.brainres.2005.09.056. [DOI] [PubMed] [Google Scholar]
- Turek VF, Tsivkovskaia NO, Hyytia P, Harding S, Le AD, Ryabinin AE. Urocortin 1 expression in five pairs of rat lines selectively bred for differences in alcohol drinking. Psychopharmacology (Berl) 2005;181:511–517. doi: 10.1007/s00213-005-0011-x. [DOI] [PubMed] [Google Scholar]
- van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Woerner A, Bäckström P, Hyytiä P, Ryabinin AE. Expression of c-Fos in Alko alcohol rats responding for ethanol in an operant paradigm. Alcohol Clin Exp Res. 2001;25:704–710. [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Lesions of the Edinger-Westphal nucleus alter food and water consumption. Behav Neurosci. 2005;119:1235–1243. doi: 10.1037/0735-7044.119.5.1235. [DOI] [PubMed] [Google Scholar]
- Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- Wurst FM, Graf I, Ehrenthal HD, Klein S, Backhaus J, Blank S, Graf M, Pridzun L, Wiesbeck GA, Junghanns K. Gender differences for ghrelin levels in alcohol-dependent patients and differences between alcoholics and healthy controls. Alcohol Clin Exp Res. 2007;31:2006–2020. doi: 10.1111/j.1530-0277.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Buchmann A, Steffin B, Dieterle C, Uhr M. Alcohol administration acutely inhibits ghrelin secretion in an experiment involving psychosocial stress. Addict Biol. 2007;12:17–21. doi: 10.1111/j.1369-1600.2006.00026.x. [DOI] [PubMed] [Google Scholar]