Abstract
Background
Since moderate wine drinking is atheroprotective it is clinically relevant to elucidate its possible mechanism/s of action/s. Our objective is to demonstrate the potential benefits of the wine components, quercetin and ethanol, on the development of aortic plaques with parallel changes in antiatherogenic factors.
Methods and Results
The effects of quercetin and ethanol on the development of aortic atherosclerotic lesions, liver PON1 gene expression, and serum PON1 activity were measured in LDLR-/- mice on an atherogenic diet for 4-weeks and 8-weeks. Depending on the duration and dosage of these modulators, 12.5-25 mg/dl quercetin (12.5Q-25Q) and 18-25% ethanol, the magnitude of decreases in aortic lesions caused by moderate ethanol and quercetin ranged from 20-70% (p<0.05-p<0.001) based on ultrasound biomicroscopy (UBM) analyses, and from 18-61% (p<0.05-p<0.001) based on morphometric analyses. The composite plot of all the UBM and morphometric data showed significant correlation between these two methods (p = 0.0001, Pearson r = 0.79 for 4-weeks treatment; p = 0.000004, Pearson r = 0.84 for 8-weeks treatment). Concomitantly, 4-week treatments with 12.5Q and 18% ethanol up regulated liver PON1 mRNA by 41% (p<0.05) and 37% (p<0.05), respectively, accompanied by 92% (p<0.001) and 61% (p<0.001) increases in serum PON1 activity, respectively. The corresponding values after 8-weeks treatment with 12.5Q and 18% ethanol were 23% (p<0.05) and 40% (p<0.02) with respect to the up regulation of liver PON1 mRNA expression while the stimulations of serum PON1 activity were 75% (p<0.001) and 90% (p<0.001), respectively.
Conclusions
Based on these findings, we conclude that quercetin and moderate ethanol significantly inhibit the progression of atherosclerosis by up regulating the hepatic expression of the antiatherogenic gene, PON1, with concomitant increased serum PON1 activity.
Keywords: atherosclerosis, paraoxonase, LDLR-/- mouse, ethanol, quercetin
Introduction
While numerous reports support the concept that moderate wine consumption has atheroprotective effects (Kloner and Rezkalla, 2009), the potential benefits of the moderate ethanol as well as the polyphenol components of red wine per se on these parameters remain to be established. Quercetin is a major polyphenol of red wine. Wine consumption in several countries shows a remarkable inverse correlation to local rates of coronary heart disease mortality (St Leger et al., 1979). Paraoxonase (PON) is a multifunctional antioxidant enzyme tightly associated with high density lipoprotein (HDL) that was shown to not only (i) inhibit low density lipoprotein (LDL) oxidation and hydrolyze oxidized LDL (Navab et al., 1997; Aviram et al., 1998; Shih et al., 1998; Mackness et al., 2003) but also (ii) detoxify the homocysteine metabolite, homocysteine thiolactone (HTL). HTL can pathologically cause protein damage by homocysteinylation of the lysine residues, thereby leading to atherosclerosis (Aviram and Rosenblat, 2004; Jakubowski, 2000). Studies by us (Lakshman et al., 2006) and others (Jakubowski, 2007) in Type II diabetics showed a strong correlation between decreased homocysteine thiolactonase (HCTL) activity with the severity of coronary artery disease (CAD). There have been reports showing the preservation of serum paraoxonase (PON1) activity by wine flavonoids in mice (Fuhrman and Aviram, 2002; Aviram et al., 1999). A recent clinical trial demonstrated the beneficial effects of grape extract on the susceptibility of LDL oxidation in heavy smokers (Vigna et al., 2003).
Accumulation of oxidized LDL (OxLDL) in the intima of the arteries leads to the pathogenesis of CAD, whereas HDL protects against the incidence of CAD (Witztum and Steinberg, 1991; Navab et al., 2001). A negative correlation of serum PON1 activity with the development of aortic lesion scores has been previously demonstrated in a mouse model when fed an atherogenic diet (Shih et al., 1996). Thus, one of the major reasons for the antiatherogenic property of HDL is attributed to its protective capacity against LDL oxidation catalyzed by its component enzyme PON1 (Durington et al., 2001; Aviram et al., 2000). While a clinical trial showed the increase in serum PON1 activity in moderate drinkers (van der Gaag et al., 1999), we were the first to show that moderate alcohol up regulates PON1 gene and PON1 activity (Rao et al., 2003). Therefore, it is logical to systematically pursue the possible effects of wine components, quercetin, and ethanol in the regulation of PON1 expression and activity in LDL receptor gene-knockout (LDLR-/-) mice, an ideal animal model to study the progression of atherosclerosis. Furthermore, Ultrasound Biomicroscopy (UBM) with its high resolution scan heads has been used successfully in determining the various stages of atherosclerotic lesions (Daugherty, 2002; Fitzgerald et al., 2003; Lewis et al., 2002; Wiesmann et al., 2003; Gan et al., 2007). In the present study, we have utilized UBM as well as morphometric analyses in LDLR-/- mouse model to convincingly demonstrate not only the abilities of dietary quercetin and moderate alcohol in inhibiting the progression of atherosclerotic lesions in the aorta but also show parallel positive alterations in liver PON1 gene expression and serum PON1 activity.
Materials and Methods
Animals
Animal studies were performed on LDLR-/- mice (body wt ∼25 g of either sex, Jackson Laboratory, strain B6.129S7-Ldlrtm1Her/J, Bar Harbor, ME) and protocol was reviewed and approved by the institutional IACUC. The animals were housed in groups of 4 per cage in plastic cages (40 × 24 × 18 cm) in a temperature controlled room at 25°C at a relative humidity of 50–60% with 12 hours of light:dark cycle. All the animals were fed a pellet commercial diet (Purina Rodent Chow, #500, TMI Nutrition, St. Louis, MO) for 1 week after arrival. They were then switched to an atherogenic liquid diet (Dyets Inc., Control #710059, Ethanol #710290, Bethlehem, PA) and sorted randomly to the following six experimental groups (6/group) for duration of 4-weeks and 8-weeks: Atherogenic (ATH) Control; ATH plus 18% ethanol calories (18% ETOH); ATH plus 25% ethanol calories (25% ETOH); ATH plus 12.5 mg/dl quercetin (12.5Q); ATH plus 18.75 mg/dl quercetin (18.75Q); and ATH plus 25 mg/dl quercetin (25Q). Thus, in the indicated Ethanol Diets, ethanol calories replaced equivalent amount of dextrin-maltose calories present in the corresponding Control Diet. All the diets were prepared thrice a week by mechanical mixing and stored in a cold room (4°C) until used. Since the animals in the 25% ETOH group consumed 20 ml of their respective liquid diet per day, all the animals in the rest of the various groups were pair-fed the same 20 ml of their respective liquid diets per day.
Ultrasound Biomicroscopy
This was carried out essentially as previously described (Gan et al., 2007). At the indicated experimental time intervals, each animal was maintained lightly anaesthetized with an isoflurane dose of 1.5% with 100% oxygen mix, resulting in a heart rate of approximately 350 beats/min. The body temperature was maintained at 37°C using an isothermal pad. A UBM system (Vevo 600, Visualsonics, Toronto, Canada) equipped with a 40 MHz mechanical transducer was used for all the examinations. The maximum penetration depth of this transducer is 1.2 cm with a centre focus at 6 mm. The 40 MHz scan head has a theoretic resolution of 40 m and operates with a frame rate of 32 Hz. After removing the chest hair, ultrasound transmission gel was applied liberally on the anterior chest wall before scanning. A right parasternal long axis view was used to visualize the ascending aorta, aortic arch, and the neck vessels within one plane. By adjusting the distance between the transducer and the target vascular site of interest, the intima-media complex thickness (IMT) or atherosclerotic lesions were visualized. A two-dimensional 10s CINE loop set to record 100 frames was stored digitally for off-line analysis using Image-Pro Plus (Version 6.1, Media Cybernetics, Inc., Bethesda, MD) which is a commercially available software allowing manual or semi-automated image analysis routine. By inspecting the 10s long loop, the frames representing the largest and smallest cross-sectional vessel area were selected as the systolic respective diastolic images. Three images at the same vascular site were measured and the average was taken for the atherosclerotic lesion area (mm2) from each mouse during diastole. All the images were analyzed by an operator blinded to the identities of the animals.
Other Analyses on the Experimental Animals
At the end of the final experimental period, all the animals in each group were exsanguinated via abdominal aorta under isoflurane anesthesia, and the blood serum and the entire aortas were collected for further analyses as described below. The liver from each animal was quick frozen in liquid nitrogen and processed for RNA isolation and PON1 mRNA analyses by real-time RT-PCR as described below.
En Face Preparation and Quantification of Atherosclerotic Lesions
This procedure was according to Tangirala et al. (1995). Briefly, each experimental mouse was anesthetized and the heart and the aorta were dissected from the thoracic cavity, cleared-off from adjacent tissue and were fixed in 4% formaldehyde/PBS (pH 7.4) for ≥ 3 days. Each aorta was dissected out from the heart until 3-5 mm after the iliac bifurcation under the dissection microscope (LW Scientific, Inc., Atlanta, GA). The entire aorta was cut longitudinally from the aortic arch including the innominate artery, left common carotid artery and left subclavian artery to the end of the descending aorta where it divides into the common iliac arteries. The aorta was then pinned onto a standard black wax dissection pan using 0.15 mm black anodized pins. The fixed aorta was briefly rinsed in 80% ethanol, stained for 30 min with Sudan IV (0.5% Sudan IV/35% ethanol/50% acetone), and destained for 5 min in 80% ethanol. Images were captured using an Olympus DP12 digital camera, and the red-stained lesion area corresponding to the plaques was quantified with Image-Pro Plus image analysis software. Total atherogenic lesions were expressed as percentage of total aortic lesion area divided by the total area of the aorta.
Quantification of PON1 gene expression
RNA Isolation
Briefly, a sample of each liver was homogenized in 1 ml of Tri-Reagent. Samples were left for 5 min at room temperature followed by addition of 0.2 ml of chloroform, shaken vigorously for few sec and again left at room temperature for additional 15 min. After centrifugation (12,000 × g for 20 min) at 4°C, the upper aqueous phase was carefully pipetted out into a sterile tube. The RNA was precipitated by addition of 0.5 ml of isopropanol and incubated at room temperature for 5 to 10 min. RNA was pelleted by centrifuging again at 12,000 × g at 4°C for 15 min. The precipitated RNA was washed in 70% ethanol, briefly air-dried, and then solubilized in Formazol (MRC, Cincinnati, OH). Total RNA concentrations were measured by absorbance reading at 260 nm using SpectroMAX 190 (Molecular Devices Co., Sunnyvale, CA). The purity and concentration of total RNA samples were examined by determining the A260/A280 ratio. Isolated RNA was used immediately or stored at -80°C until use.
Real-Time RT-PCR
PON1 mRNA was measured by RT-PCR essentially as described by us previously (Gong et al., 2009). Briefly, cDNA templates for use in real time PCR were synthesized from 5 μg of total RNA by in vitro transcription in 20 μl reaction containing 0.5 μg Oligo (dT), 10 μM dNTPs and 1 μl of Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) at 42°C for 50 min. Typical real time PCR reaction mixture included the same amount of cDNA templates from RT, 10 pmol of each primers, 25 μl iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), and sterile water in a reaction volume of 50 μl. The PCR conditions were: 3 min at 95°C follow by 40 cycles at 95°C for 30 seconds, 55°C for 30 seconds and 68°C for 1 min. The primer pairs for mouse PON1 were:
Forward primer 5′- TTCCTTTGTACACAGCAGCG -3′ and
Reverse primer 5′- TGCTGGCTCACAGATTTC -3′.
This primer pair was first tested by regular PCR to be highly effective and specific for amplification. Housekeeping gene β-Actin (Ambion, Austin, TX) was used as the internal control. Relative PON1 gene expression levels were calculated by subtracting the threshold cycle number (Ct) of the β-Actin gene from the Ct of PON1 and raising 2 to the power of this difference. Ct values are defined as the number of PCR cycles at which the fluorescent signal during the PCR reaches a fixed threshold.
Measurement of PON1 activity
PON1 activity was measured essentially as described by us previously (Gong et al., 2009) using paraoxon (Sigma, St. Louis, MO) as initial rate of substrate hydrolyzes to p-nitro phenol. PON1 activity in each experimental group is expressed as percent of the activity in the control group.
Statistical Analysis
All data are expressed as Mean ± SD using Excel office software (Microsoft Inc., Redmond, WA) and the significance of variance (ANOVA) followed by Tukey test.
Results
UBM Images and En Face Morphometry of aortas
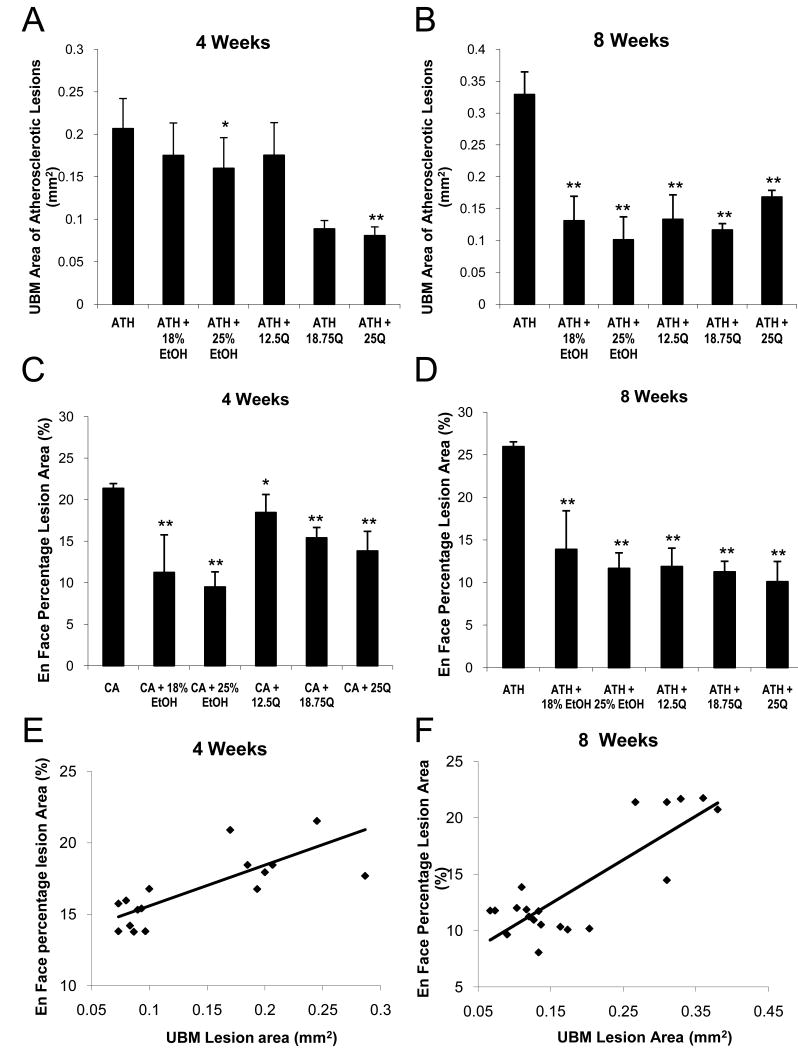
Representative UBM images and the corresponding images of en face quantification of the entire aortas of each experimental group are shown in Figure 1 (4-week and 8-week study). The initiation site of atherosclerotic lesions is known to be the region proximal to the minor side of the aortic arch (Gan et al., 2007). At this specific site, three images at the same vascular site were measured and the average was taken for the atherosclerotic lesion area (mm2) from each mouse during diastole.
Figure 1. Representative Morphometric and UBM images of aortas from the indicated groups.
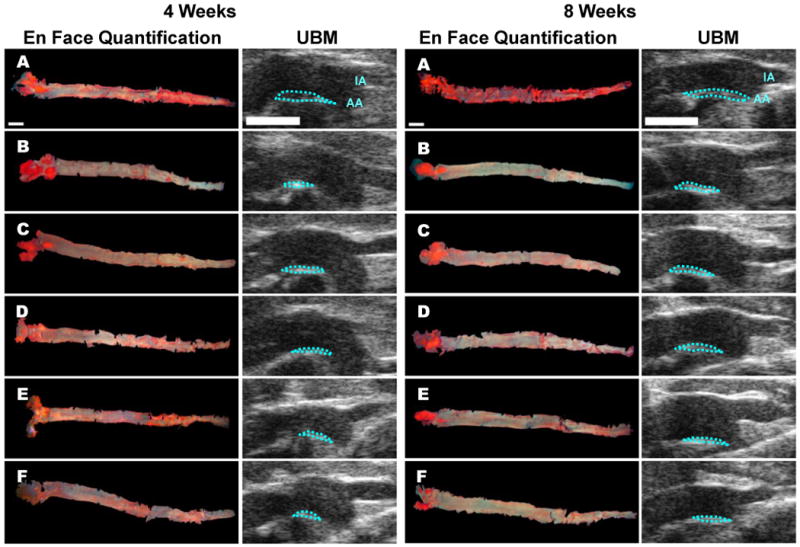
A series of comparative images displaying atherogenic lesions measurements in both En Face and UBM images from LDLR-/- mouse aorta following a 4-week and 8-week atherogenic diet protocol (A. ATH, B. ATH + 18% EtOH, C. ATH + 25% EtOH, D. ATH + 12.5Q, E. ATH + 18.75Q, and F. ATH + 25Q). UBM images show an overview of the ascending aorta including the innominate artery (IA) and aortic arch (AA). The blue dotted lines mark the borders of the atherosclerotic lesions. The white bar represents 2 mm.
Influence of Ethanol or Quercetin feeding to LDLR-/- mice on atherogenic diet on the Development of Atherosclerotic Lesions based upon UBM quantification
Figures 2A and 2B show that, compared to the untreated Control group, the amount of atherosclerotic plaques decreased by 20% (NS) and 60% (p<0.001) in 18% ETOH group while the plaques decreased by 24% (p<0.05) and 70% (p<0.001) in the 25% ETOH group after 4-weeks and 8-weeks of experimentation, respectively. Furthermore, the extent of decrease in aortic plaques decreased markedly by 58% (p<0.001) in the 12.5Q group compared to the Control group after 8-weeks although this decrease was marginal after 4-weeks. On the other hand, UBM area of atherosclerotic lesions decreased by 62% (p<0.001) and by 52%-65% (p<0.001) after 4-weeks and 8-weeks, respectively, in both 18.75Q and 25Q groups compared to the untreated Control group.
Figure 2. Morphometric and UBM analyses of the aorta for atherosclerotic lesions in various experimental groups.
LDLR-/- mice were maintained on the indicated atherogenic diet feeding regimen for 4-weeks and 8-weeks.
Panel 2A and 2B: The ascending aorta, aortic arch (AA), and the innominate artery (IA) from the UBM images data in Figure 1 were visualized within one plane by a right parasternal long-axis view using an Ultrasound Biomicroscopy system (Vevo 600, Visualsonics, Toronto, Canada). Three images at the same vascular site were measured and the average was taken for the atherosclerotic lesion area (mm2) from each mouse during diastole. The data are expressed as average UBM area of atherosclerotic lesion with error bars designating the SD.
Panel 2C and 2D: The aortas were analyzed for the presence of Sudan IV-stained lesions as described in the Methods. The data are expressed as average En Face percentage area of atherosclerotic lesions with error bars designating the SD.
Panel 2E and 2F: The plots of the correlation of the UBM (panel 2A and 2B) and the En Face (panel 2C and 2D) quantification of aortic lesion areas at 4- and 8-weeks of experimentation from the various experimental groups (p = 0.0001, Pearson r = 0.79 for 4 weeks treatment; p = 0.000004, Pearson r = 0.84 for 8 weeks treatment). *: p<0.05; **: p<0.001
Influence of Ethanol or Quercetin feeding to LDLR-/- mice on atherogenic diet on the Development of Atherosclerotic Lesions based upon Morphometry
Figures 2C and 2D clearly show that the extent of aortic lesions decreased with dietary moderate ethanol resulting eventually in 50% decrease (p<0.001) and 46% decrease (p<0.001) in aortic plaques after 4-weeks and 8-weeks, respectively, in the 18% ETOH group compared to the untreated Control group. Similarly, the extent of atherosclerotic lesions significantly decreased by 59% (p<0.001) and 53% (p<0.001) in the 25% ETOH group compared to the untreated Control group after 4-weeks and 8-weeks, respectively. Figure 2C also shows that after 4-weeks the extent of aortic lesions progressively decreased by 18% (p<0.05), 32% (p<0.001), and 35% (p<0.001) in 12.5Q, 18.75Q, and 25Q groups, respectively. The corresponding decreases in the aortic lesions for these three quercetin groups after 8-weeks (Figure 2D) were 52% (p<0.001), 57% (p<0.001), and 61% (p<0.001) respectively, compared to the untreated Control group. Thus, it is obvious that both quercetin and moderate ethanol do indeed cause significant decrease in the occurrence of aortic lesions in LDLR-/- mice when they are on an atherogenic diet.
Correlation between the UBM and Morphometric Analyses
As shown in Figures 2E and 2F, when all the experimental points in the ultrasound Biomicroscopy (UBM) lesion area measurements were correlated with all the morphometric measurements of En face percentage lesion area values, the composite plot showed a significant correlation (p = 0.0001, Pearson r = 0.79 for 4-weeks treatment; p = 0.000004, Pearson r = 0.84 for 8-weeks treatment). Thus, based on the composite plot, the mean decreases in aortic lesions, respectively after 4-weeks and 8-weeks of feeding an atherogenic diet in various experimental groups were as follows: 35% and 53% (p<0.001) in 18% ETOH group; 41% and 61% (p<0.001) in 25% ETOH group; 22% and 55% (p<0.001) in 12.5Q group; 47% and 62% (p<0.001) in 18.75Q group and finally 48% and 56% (p<0.001) in 25Q group.
Influence of dietary ethanol or quercetin on liver PON1 mRNA expression
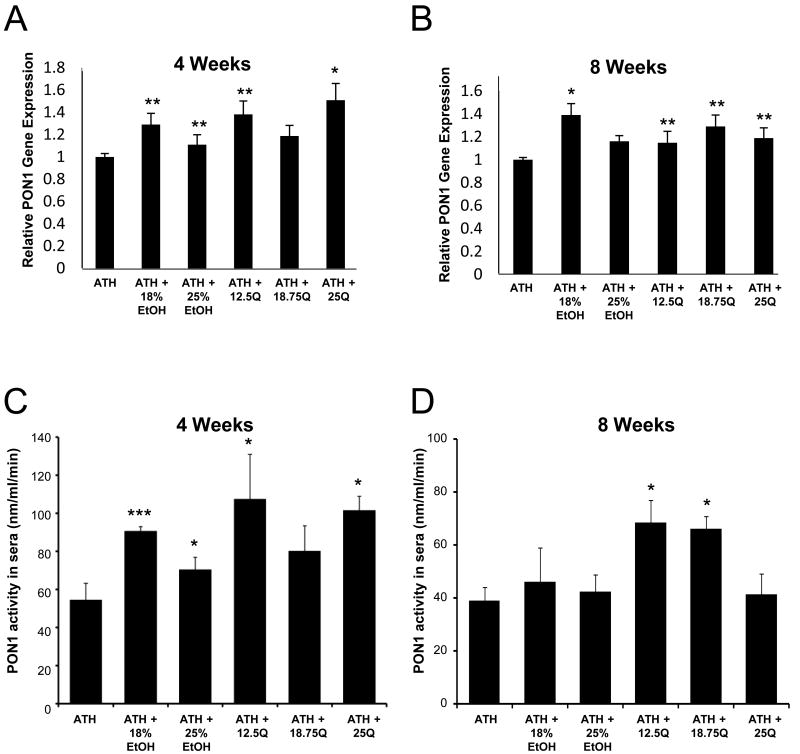
As shown in Figure 3A, after 4-weeks of the indicated treatments, 18% ETOH group had 31% (p<0.05) increase in liver PON1 mRNA expression while the 25% ETOH group showed a marginal 15% (p<0.05) increase. On the other hand the 12.5Q group showed a 39% (p<0.05) and a significant 51% (p<0.02) increase in liver PON1 mRNA expression in the 25Q group. Strikingly, after 8-weeks of the various treatments (Figure 3B), the same 18% ETOH group showed a substantial 40% (p<0.02) increase in PON1 mRNA expression while the increases in PON1 mRNA expressions ranged between 25% and 31% (p<0.05) in the various quercetin treatment groups.
Figure 3. Influence of ethanol and quercetin feeding on the expression of liver PON1 mRNA and serum PON1 activity in LDLR-/- mice from indicated groups.
(Panel 3A and 3B) Each liver sample from the respective groups were analyzed for PON1 mRNA and actin mRNA expression by Real-time RT-PCR and the relative abundance of PON1 mRNA was determined as described in the Materials and methods section. Each bar is the Mean ± SD. (Panel 3C and 3D) Serum PON1 activities were determined in the respective groups as described in the Materials and Methods section. Each value is the Mean ± SD. *: p<0.02; **: p<0.05; ***: p <0.001
Influence of dietary ethanol or quercetin on Serum PON activity
Serum PON1 activity increased by 64% (p<0.001) and 27% (p<0.02) in 18% ETOH and 25% ETOH groups, respectively in the 4-week experimental groups (Figure 3C) while these stimulatory effects of these ethanol dosages were less pronounced in the corresponding 8-week experimental groups (Figure 3D). Similarly, 12.5Q and 25Q 4-week groups showed 90% (p<0.02), and 82% (p<0.02), increases in serum PON1 activity, respectively (Figure 3C), whereas the corresponding increases in serum PON1 activity in these 8-week experimental Quercetin groups were 80% (p<0.02), and 70% (p<0.02), respectively (Figure 3D).
Discussion
LDLR-/- mouse is an excellent model to study atherosclerosis because it consistently develops atherosclerotic lesions in a time-dependent manner anywhere from 2 weeks to 12 weeks on a high cholesterol cholate-containing diet. This characteristic enables a systematic evaluation of the effects of wine components such as moderate ethanol or polyphenols like quercetin not only on the development of atherosclerotic lesions as a function of a reasonable time period of experimentation, but also on the PON status the antiatherogenic enzyme. Thus, this animal model offers an unique opportunity to evaluate and correlate with respect to the extent of atherosclerosis based on morphometric analysis of the autopsy samples of the entire aorta as well as on ultrasonic imaging of the aorta in live animals with the alterations in PON1 expression and PON1 activity. The present study provides strong evidences that both quercetin (up to 25 mg/dl of the diet) and moderate ethanol (up to 25% of dietary calories as ethanol) independently attenuate significantly the progression of atherosclerotic plaques in the LDLR-/- mouse model when these animals are maintained on an atherogenic diet based not only upon the ultrasound imaging (Figures 1, 2A, and 2B), but also upon the morphometric analyses (Figures 1, 2C, and 2D). More important, the coefficient of correlation of the results by these two independent methods was excellent (Figure 2E and 2F; 4-weeks r=0.79; 8-weeks r=0.84). What is remarkable is, we now show for the first time a direct correlation between these two methods indicating that Ultrasound Biomicroscopy is highly reliable in studying the in vivo temporal effects of any antiatherogenic modulator on the progression of atherosclerosis, unlike the morphometric analyses that can only be accomplished with the use of enormous number of animals for each time point after autopsy. Recent studies by Aviram's group (Rosenblat et al., 2008; Aviram et al., 2008) have reported the antiatherogenic capacities of green tea and pomegranate polyphenols in apolipoprotein E-deficient mice, another atherogenic mouse model. Interestingly, it has been elegantly shown (Kozarsky et al., 2000) that overexpression of the HDL class B scavenger receptor type I reduces atherosclerosis in cholesterol-fed LDLR-/- mice.
We have previously established in rats that increased serum PON1 activity mediated by quercetin (Gong et al., 2009) and moderate ethanol (Rao et al., 2003) is accompanied by increased capacity of the HDL to inhibit the oxidation of LDL to oxidized LDL, the atherogenic lipoprotein. Therefore, our present findings demonstrating the significant inhibitory actions of quercetin and moderate ethanol on the development of atherosclerotic lesions in LDLR-/- mice (Figures 1 and 2) are consistent with concomitant up regulation of liver PON1 mRNA and serum PON1 activity (Figure 3) by these two dietary modulators. The lesser magnitudes of up regulation of PON1 expression and its activity at 25% dietary ethanol compared to 18% ethanol (Figure 3) are consistent with our previous findings of the inhibitory effects of heavy ethanol (35% of the total dietary calories) on these parameters (Rao et al., 2003). At the same time, since PON1 expression is still significantly increased after 8 weeks (Figure 3B) it is possible that the apparent lack of significance in the stimulation of PON1 activity (Figure 3D) in the ethanol group may be because of wider variations in individual values in this group. A future study of longer term treatment should throw light on the beneficial effect of moderate ethanol.
It is significant to point out that 18% ethanol calories in the liquid diet amounts to ethanol consumption of 20 g/kg body wt/day (based upon an average consumption of 20 ml of the 2.5% (w/v) Ethanol liquid diet (equal to 18% of dietary calories) by a 25 g mouse per day. This equates to 1.5 drinks/day (1 drink is equal to ∼14 g ethanol), which is considered as moderate drinking in humans. Consequently, our findings on the beneficial effects of moderate ethanol in preventing the progression of atherosclerosis with concomitant up regulation of PON1 expression and activity in a well established atherogenic animal model such as the LDLR-/- mouse have clinical significance. Thus, our present findings strongly support the long accepted concept of “French Paradox” that moderate drinking is cardioprotective as demonstrated by innumerable human trials although this is the first time the direct beneficial effects of moderate ethanol as well as quercetin in inhibiting the progression of atherosclerotic lesions in the aorta by two independent sophisticated methods has been demonstrated. Similarly, the dietary quercetin concentrations of 12.5, 18.75, and 25 mg/dl of the respective groups amount to consumptions of 100, 150, and 200 mg of quercetin per kg body weight per day. A previous study (de Boer et al., 2005) in rats reported a plasma quercetin concentration of 24 μM after 11 weeks of feeding 800 mg quercetin per kg body weight per day. Since the quercetin dosage in the present study ranged from 100 to 200 mg/kg/day, it is reasonable that the plasma/tissue quercetin concentration in our study should range from 3-6 μM. Thus, it is remarkable that quercetin at such low concentrations, seems to exert its positive influence on PON1 status and protective effects on aortic plaque formation. Further detailed long-term duration and dosage studies are necessary to test the atheroprotective effects of both ethanol and quercetin.
Acknowledgments
This work was supported partially by a VA merit Review grant from the Department of Veterans Affairs (Raj Lakshman) and a R21 grant from NIH (Raj Lakshman).
References
- Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high- density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest. 1998;101:1581–1 590. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram M, Rosenblat M, Billeke S, La Du B. Human serum PON is inactivated by oxidized LDL and preserved by antioxidants. Free Rad Bio Med. 1999;26:892–904. doi: 10.1016/s0891-5849(98)00272-x. [DOI] [PubMed] [Google Scholar]
- Aviram M, Hardak E, Vaya J, Mahmood S, Milo S, Hoffman A, Billicke S, Draganov D, Rosenblat M. Human serum paraoxonases (PON1) Q and R selectively decrease lipid peroxides in human coronary and carotid atherosclerotic lesions: PON1 esterase and peroxide-like activities. Circulation. 2000;101:2510–2517. doi: 10.1161/01.cir.101.21.2510. [DOI] [PubMed] [Google Scholar]
- Aviram M, Rosenblat M. Paraoxonase 1, 2, 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med. 2004;37:1304–1316. doi: 10.1016/j.freeradbiomed.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Aviram M, Volkova N, Coleman R, Dreher M, Reddy MK, Ferreira D, Rosenblat M. Pomegranate phenolics from the peels, arils, and flowers are antiatherogenic: studies in vivo in atherosclerotic apolipoprotein e-deficient (E0) mice and in vitro in cultured macrophages and lipoproteins. J Agric Food Chem. 2008;56:1148–57. doi: 10.1021/jf071811q. [DOI] [PubMed] [Google Scholar]
- Daugherty A. Mouse models of atherosclerosis. Am J Med Sci. 2002;323(1):3–10. doi: 10.1097/00000441-200201000-00002. [DOI] [PubMed] [Google Scholar]
- de Boer VC, Dihal AA, van der Woude H, Arts IC, Wolffram S, Alink GM, Rietjens IM, Keijer J, Hollman PC. Tissue distribution of quercetin in rats and pigs. J Nutr. 2005;135(7):1718–25. doi: 10.1093/jn/135.7.1718. [DOI] [PubMed] [Google Scholar]
- Durington PN, Mackness B, Mackness PI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21(4):473–480. doi: 10.1161/01.atv.21.4.473. [DOI] [PubMed] [Google Scholar]
- Fitzgerald S, Gan L, Wickman-Tordby A, Bergström G. Cardiovascular and renal phenotyping of genetically modified mice—a challenge for traditional physiology. Clin Exp Pharmacol Physiol. 2003;30:207–216. doi: 10.1046/j.1440-1681.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- Fuhrman B, Aviram M. Preservation of Paraoxonase activity by wine flavonoids. Ann NY Acad Sci. 2002;957:321–324. doi: 10.1111/j.1749-6632.2002.tb02933.x. [DOI] [PubMed] [Google Scholar]
- Gan LM, Grönros J, Hägg U, Wikström J, Theodoropoulos C, Friberg P, Fritsche-Danielson R. Non-invasive real-time imaging of atherosclerosis in mice using ultrasound biomicroscopy. Atherosclerosis. 2007;190(2):313–20. doi: 10.1016/j.atherosclerosis.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Gong M, Garige M, Varatharajalu R, Marmillot P, Gottipati C, Leckey LC, Lakshman MR. Quercetin up regulates paraoxonase 1 gene expression with concomitant protection against LDL oxidation. Biochem Biophys Res Commun. 2009;379:1001–1004. doi: 10.1016/j.bbrc.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Jakubowski H. Calcium-dependent Human Serum Homocysteine Thiolactone Hydrolase. J Biol Chem. 2000;275:3957–3962. doi: 10.1074/jbc.275.6.3957. [DOI] [PubMed] [Google Scholar]
- Jakubowski H. The molecular basis of homocysteine thiolactone-mediated vascular disease. Clin Chem Lab Med. 2007;45:1704–1716. doi: 10.1515/CCLM.2007.338. [DOI] [PubMed] [Google Scholar]
- Kloner RA, Rezkalla SH. To drink or Not to drink? That is the question. Circulation. 2000;116:1306–1317. doi: 10.1161/CIRCULATIONAHA.106.678375. [DOI] [PubMed] [Google Scholar]
- Kozarsky KF, Donahee MH, Glick JM, Krieger M, Rader DJ. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler Thromb Vasc Biol. 2000;20(3):721–727. doi: 10.1161/01.atv.20.3.721. [DOI] [PubMed] [Google Scholar]
- Lakshman MR, Gottipati CS, Narasimhan SJ, Munoz J, Marmillot P, Nylon ES. Inverse correlation of serum paraoxonase and homocysteine thiolactonase activities and antioxidant capacity of high- density lipoprotein with the severity of cardiovascular disease in persons with type 2 diabetes mellitus. Metabolism. 2006;55:1201–1206. doi: 10.1016/j.metabol.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lewis JS, Achilefu S, Garbow JR, Laforest R, Welch MJ. Small animal imaging. Current technology and perspectives for oncological imaging. Eur J Cancer. 2002;38(16):2173–88. doi: 10.1016/s0959-8049(02)00394-5. [DOI] [PubMed] [Google Scholar]
- Mackness B, Durrington P, McElduff P, Yarnell J, Azam N, Watt M, Mackness M. Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation. 2003;107:2775–2779. doi: 10.1161/01.CIR.0000070954.00271.13. [DOI] [PubMed] [Google Scholar]
- Navab M, Hama-Levy S, Van Lenten BJ, Fonarow GC, Cardinez CJ, Castellani LW, Brennan ML, Lusis AJ, Fogelman AM, La Du BN. Mildly oxidized LDL induces an increased apolipoprotein J/paraoxonase ratio. J Clin Invest. 1997;99:2005–2019. doi: 10.1172/JCI119369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M, Berliner JA, Subbanagounder G, Hama S, Lusis AJ, Castellani LW, Reddy S, Shih D, Shi W, Watson AD, Van Lenten BJ, Vora D, Fogelman AM. HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2001;21:481–488. doi: 10.1161/01.atv.21.4.481. [DOI] [PubMed] [Google Scholar]
- Rao MN, Marmillot P, Palmer DA, Seeff LJ, Strader BD, Lakshman MR. Light, but not heavy alcohol drinking stimulates paraoxonase by up regulating liver PON mRNA in both rats and humans. Metabolism. 2003;52:1287–1294. doi: 10.1016/s0026-0495(03)00191-4. [DOI] [PubMed] [Google Scholar]
- Rosenblat M, Volkova N, Coleman R, Almagor Y, Aviram M. Antiatherogenicity of extra virgin olive oil and its enrichment with green tea polyphenols in the atherosclerotic apolipoprotein-E-deficient mice: enhanced macrophage cholesterol efflux. J Nutr Biochem. 2008;19:514–23. doi: 10.1016/j.jnutbio.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, Lusis AJ. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- Shih DM, Gu L, Hama S, Xia YR, Navab M, Fogelman AM, Luisis AJ. Genetic-Dietary regulation of serum paraoxonase expression and its role in atherogenic mouse model. J Clin Invest. 1996;97:1630–1639. doi: 10.1172/JCI118589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Leger AS, Cochrane AL, Moore F. Factors associated with cardiac mortality in developed countries with reference to the consumption of wine. Lancet. 1979;1(8124):1017–1020. doi: 10.1016/s0140-6736(79)92765-x. [DOI] [PubMed] [Google Scholar]
- Tangirala RK, Rubi EM, Palinski W. Quantitation of atherosclerosis in murine models: extent of lesions in LDL-/- and apolipoprotein E-deficient mice. J Lipid Res. 1995;36:2320–2328. [PubMed] [Google Scholar]
- van der Gaag MS, van Tol A, Scheek LM, James RW, Urgert R, Schaafsma G, Hendriks HF. Daily moderate alcohol consumption increases serum paraoxonase activity; a diet-controlled, randomized intervention study in middle-aged men. Atherosclerosis. 1999;147:405–410. doi: 10.1016/s0021-9150(99)00243-9. [DOI] [PubMed] [Google Scholar]
- Vigna GB, Costantini F, Aldini G, Carini M, Catapanod A, Schenaa, Tangerinia A, Bombardellie RE, Morazzonie P, Mezzettib A, Fellina R, Facinoc RM. Effect of Grape seed extract on low-density lipoprotein susceptibility to oxidation in heavy smokers. Metabolism. 2003;52:1250–1257. doi: 10.1016/s0026-0495(03)00192-6. [DOI] [PubMed] [Google Scholar]
- Wiesmann F, Szimtenings M, Frydrychowicz A, Illinger R, Hunecke A, Rommel E, Neubauer S, Haase A. High-resolution MRI with cardiac and respiratory gating allows for accurate in vivo atherosclerotic plaque visualization in the murine aortic arch. Magn Reson Med. 2003;50(1):69–74. doi: 10.1002/mrm.10500. [DOI] [PubMed] [Google Scholar]
- Witztum JL, Steinberg D. Role of oxidized low density lipoproteins in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]