Abstract
Acetaminophen (APAP) overdose can result in serious liver injury and potentially death. Toxicity is dependent on metabolism of APAP to a reactive metabolite initiating a cascade of intracellular events resulting in hepatocellular necrosis. This early injury triggers a sterile inflammatory response with formation of cytokines and innate immune cell infiltration in the liver. Recently, IL-1β signaling has been implicated in the potentiating of APAP-induced liver injury. To test if IL-1β formation through caspase-1 is critical for the pathophysiology, C57Bl/6 mice were treated with the pan-caspase inhibitor Z-VD-fmk to block the inflammasome-mediated maturation of IL-1β during APAP overdose (300 mg/kg APAP). This intervention did not affect IL-1β gene transcription but prevented the increase in IL-1β plasma levels. However, APAP-induced liver injury and neutrophil infiltration was not affected. Similarly, liver injury and the hepatic neutrophilic inflammation were not attenuated in IL-1-receptor-1 deficient mice compared to wild type animals. To evaluate the potential of IL-1β to increase injury, mice were given pharmacological doses of IL-1β after APAP overdose. Despite increased systemic activation of neutrophils and recruitment into the liver, there was no alteration in injury. We conclude that endogenous IL-1β formation after APAP overdose is insufficient to activate and recruit neutrophils into the liver or cause liver injury. Even high pharmacological doses of IL-1β, which induce hepatic neutrophil accumulation and activation, do not enhance APAP-induced liver injury. Thus, IL-1 signaling is irrelevant for APAP hepatotoxicity. The inflammatory cascade is a less important therapeutic target than intracellular signaling pathways to attenuate APAP-induced liver injury.
Keywords: Acetaminophen, Hepatotoxicity, Interleukin-1, Inflammasome, Neutrophils, Inflammation
INTRODUCTION
Acetaminophen (APAP) overdose is the most frequent cause of acute liver failure in the US and many European countries (Larson et al., 2005). Toxicity is dependent on the metabolic activation of APAP to the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) within hepatocytes (Nelson, 1990) and sinusoidal endothelial cells (Holt et al., 2010). NAPQI initiates a series of intracellular events critical for hepatotoxicity including glutathione depletion and covalent protein modifications leading to mitochondrial dysfunction with formation of reactive oxygen species and peroxynitrite (Nelson, 1990; Jaeschke and Bajt, 2006; Jaeschke et al., 2003). The oxidant stress is ultimately responsible for the opening of the mitochondrial membrane permeability transition (MPT) pore (Kon et al., 2004) and necrotic cell death (Gujral et al., 2002).
APAP-induced liver toxicity is accompanied by an inflammatory response involving activation of Kupffer cells and recruitment of neutrophils and mononuclear cells into the liver (Jaeschke, 2005; Liu and Kaplowitz, 2006; Laskin, 2009). The inflammatory response is initiated by release of cellular contents, some of which can function as damage associated molecular patterns (DAMPs) that can directly activate innate immune cells (Scaffidi et al., 2002; Martin-Murphy et al., 2010). During this sterile inflammation many cytokines and chemokines are up-regulated and innate immune cells begin to accumulate in the liver with neutrophils arriving early after the initial APAP-induced injury (Lawson et al., 2000). Among these pro-inflammatory cytokines, both interleukin-1α (IL-1α) (Blazka et al., 1995; Chen et al., 2007) and IL-1β (Imaeda et al., 2009), have been recently implicated as critical mediators of APAP hepatotoxicity.
Mature IL-1β is formed by post-translational modification caused by caspase-1 (Franchi et al., 2009). Caspase-1 (formerly known as interleukin-1 converting enzyme) is a pro-inflammatory caspase, which proteolytically cleaves pro-IL-β to generate the mature cytokine. The activity of this enzyme is controlled by the assembly of the Nalp3 inflammasome, which consists of the proteins Nalp3 (NACHT, LRR, and pyrin domain-containing protein 3), caspase-1, and ASC (apoptosis-associated speck-like protein containing a caspase recruiting domain [CARD]) (Franchi et al., 2009). It has been shown that DAMPs can signal via toll-like receptors (TLRs) and this can then activate the inflammasome (Park et al., 2004; Lamkanfi and Dixit, 2009). Upon TLR stimulation the Nalp3-inflammasome is assembled and the active caspase-1 is then capable of processing pro-IL-1β; only the processed form of IL-1β can be released from cells which is then capable of signaling through the IL-1 receptor-1 (IL-1R1) (Sims and Smith, 2010).
Previous studies implicating a central role of IL-1β (Imaeda et al., 2009) or IL-1α (Blazka et al., 1995; Chen et al., 2007) raise some concerns. The most important one is that IL-1 receptor signaling can not directly induce cell death due to the absence of a death domain (Sims and Smith, 2010). Thus, IL-1 acts mainly as a pro-inflammatory mediator activating and recruiting leukocytes, especially neutrophils, into the liver (Bajt et al., 2001). However, there is extensive evidence that neutrophils are not involved in the injury process after APAP overdose (Lawson et al., 2000; Cover et al., 2006; James et al., 2003; Williams et al., 2010). In addition, pancaspase inhibitors did not protect during the early injury phase (up to 6 h) of APAP hepatotoxicity (Lawson et al., 1999; Jaeschke et al., 2006). However, the purpose of these experiments was to assess the role of apoptosis not inflammation in this model (Lawson et al., 1999; Jaeschke et al., 2006). Thus, it remained unclear if these pancaspase inhibitors can actually affect IL-1β formation through modulation of caspase-1 activity and are able to reduce liver injury at later time points when neutrophils are more likely to be involved. Thus, the objective of this investigation was to evaluate the potential role of IL-1β in hepatic neutrophil recruitment and progression of liver injury after APAP overdose in a well-established murine model.
MATERIALS AND METHODS
Animals
Eight to twelve week old male C57BL/6J or IL-1 receptor 1 knock-out mice (B6.129S7-Il1r1tm1Imx/J), with an average weight of 18 to 22 g were purchased from Jackson Laboratory (Bar Harbor, Maine). All animals were housed in an environmentally controlled room with 12 h light/dark cycle and allowed free access to food (8604 Teklad Rodent, Harlan, Indianapolis, IN) and water. Experimental protocols were approved by the Institutional Animal Care and Use Committee of University of Kansas Medical Center and followed the criteria of the National Research Council for the care and use of laboratory animals in research. All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless stated otherwise.
Experimental design
Mice were intraperitoneally injected with 300 mg/kg APAP (dissolved in warm saline) after overnight fasting, 700 mg/kg D-galactosamine (Gal) and 100 μg/kg endotoxin (ET), 20 μg/kg recombinant mouse IL-1β containing <0.1 ng endotoxin/μg protein (GenScript, Piscataway, NJ), or equivalent volumes of saline. Additionally, some mice were injected (i.p.) with 10 mg/kg Z-VD-fmk (EP1013; a generous gift from Dr. S. X. Cai, Epicept Corp., San Diego, CA) (dissolved in Tris-buffered saline) two hours after APAP treatment or three hours after Gal/ET. The animals were sacrificed 6 or 24 h after APAP or 6 h after Gal/ET. Blood was drawn into heparinized syringes for measurement of alanine aminotransferase (ALT) activity (Kinetic Test Kit 68-B, Biotron Diagnostics, Inc., Hernet, CA) and cytokine concentrations. The liver was removed and was rinsed in ice-cold saline; liver sections were fixed in 10% phosphate buffered formalin for histological analyses. The remaining liver lobes were snap-frozen in liquid nitrogen and stored at −80 °C.
Histology
Formalin-fixed tissue samples were embedded in paraffin and 5 μm sections were cut. Sections were stained with hematoxylin and eosin (H&E) for blinded evaluation of the areas of necrosis by the pathologist. The percent of necrosis was estimated by evaluating the number of microscopic fields with necrosis compared to the entire cross section. Additional sections were stained for neutrophils using the anti-mouse neutrophil allotypic marker antibody (AbD Serotec, Raleigh, NC) as described in detail (Williams et al., 2010). Positively stained cells consistent with cellular morphology were quantified in 15 high power fields (HPF). Some sections were also stained for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) in situ cell death assay (Roche, Indianapolis, IN) as previously described (Gujral et al., 2002).
mRNA expression
Quantification of mRNA expression of several cytokines and chemokines was performed by real-time PCR (RT-PCR) analysis as described previously (Bajt et al., 2008). cDNA was generated by reverse transcription of total RNA by M-MLV reverse transcriptase in the presence of random primers (Invitrogen, Carlsbad, CA). Forward and reverse primers for the genes were designed using Primer Express software (Applied Biosystems, Foster City, CA). After normalization of cDNA concentration, SYBR green PCR Master Mix (Applied Biosystems) was used for real-time PCR analysis. The relative differences in expression between groups were expressed using cycle time (Ct) values generated by the ABI 7900 instrument (Applied Biosystems). All genes evaluated were first normalized to β-actin and then expressed as a fold increase relative to control which is arbitrarily set as 1.0. Calculations are made by assuming one cycle is equivalent to a two-fold difference in copy number which is the 2^(-ddCt) formula.
Plasma cytokine measurements
Plasma cytokine concentrations were quantified by the Bio-Plex bead-based multiplex assay following the kit instructions (Bio-Rad Laboratories, Hercules, CA) and analyzed on the Bio-Plex 200 instrument (Bio-Rad).
Tissue Caspase-3 Activity
Quantification of hepatic caspase-3 activity was performed as previously described in detail (Lawson et al., 1999; Jaeschke et al., 1998). Briefly, liver tissue was homogenized and protein concentration was normalized after BCA protein assay (Thermo Scientific, Rockford, IL). Homogenate was assayed with Acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (Ac-DEVD-AMC) (Enzo Life Sciences, Plymouth Meeting, PA) for change in fluorescence intensity of time using a Spectramax Gemini fluorescence plate reader (Molecular Devices, Sunnyvale, CA).
Flow Cytometric Analysis of CD11b Expression and Reactive Oxygen Formation: CD11b/Gr-1 staining
Neutrophil priming as measured by CD11b expression was performed as previously described in detail (Williams et al., 2010). Briefly, heparinized whole blood was stained with PE-Cy5-labeled-anti-Gr-1 (BioLegend, San Diego, CA) and PE-labeled-anti-CD11b (BioLegend). After red cell lysis samples were analyzed on the FACSCalibur (BD, Franklin Lakes, NJ).
Reactive Oxygen Species (ROS) Production (Smith and Weideman, 1993; Daley et al., 2008)
Neutrophil ROS priming was performed as previously described in detail (Williams et al., 2010). Briefly, whole blood was treated ex vivo for 10 minutes with phorbol 12-myristate 13-acetate (PMA) or saline followed by the addition of dihydrorhodamine-123 for 10 minutes at 37°C. Neutrophils were stained with PE-Cy5-labeled-anti-Gr-1 (BioLegend) then red blood cells were lysed. Samples were analyzed on the FACSCalibur and ROS production was evaluated in Gr-1bright neutrophils.
Statistics
All results were expressed as mean ± SE. Comparisons between multiple groups were performed with one-way ANOVA or, where appropriate, by two-way ANOVA, followed by a post hoc Bonferroni test. If the data were not normally distributed, we used the Kruskal-Wallis Test (nonparametric ANOVA) followed by Dunn’s Multiple Comparisons Test. P < 0.05 was considered significant.
RESULTS
Pharmacological inhibition of caspases in vivo during APAP toxicity
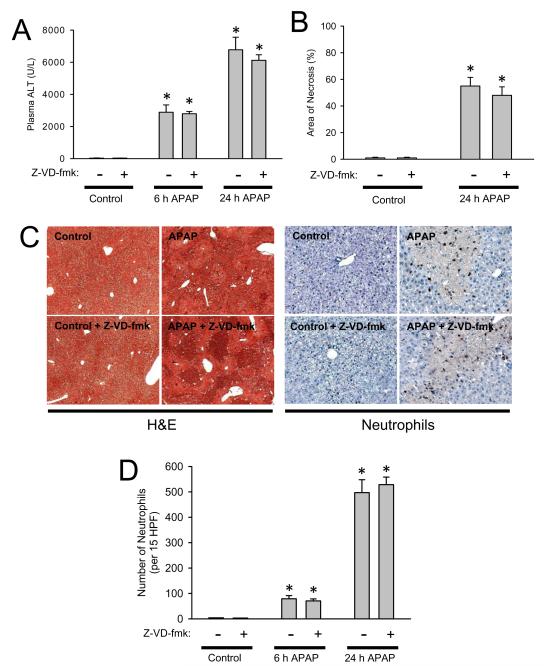
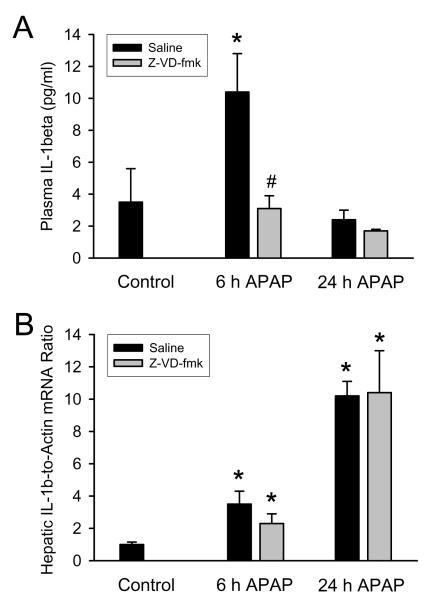
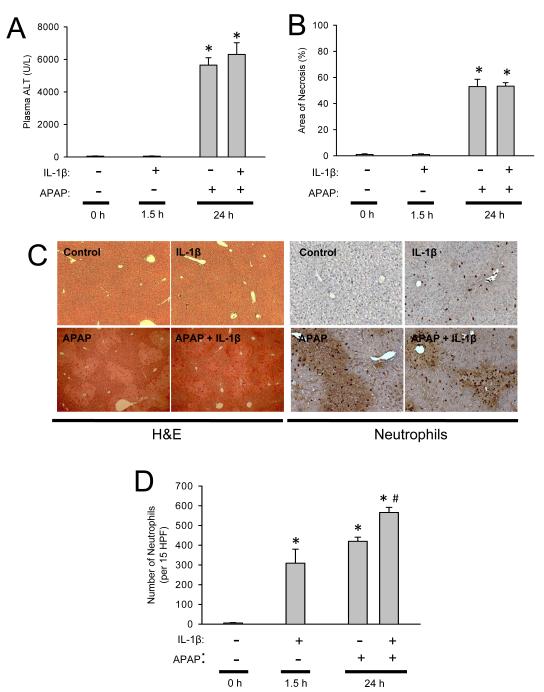
Treatment of fasted C57Bl/6 mice with 300 mg/kg APAP resulted in substantial liver injury as indicated by the increase in plasma ALT activities at 6 h (Figure 1A). During the following 18 h, the injury was further aggravated (Figure 1A). Liver injury was also confirmed by histology (Figure 1B,C), which showed extensive centrilobular necrosis (Figure 1C). Quantitatively, about 55% of hepatocytes were necrotic at 24 h (Figure 1B). APAP-induced liver injury caused a significant accumulation of neutrophils in the liver, which was only moderate at 6 h but dramatically increased by 24 h (Figure 1C,D). In order to inhibit IL-1β processing, animals were treated with the potent pan-caspase inhibitor Z-VD-fmk (Jaeschke et al., 2000) 2 h after APAP. Z-VD-fmk had no significant effect on plasma ALT activities or the area of necrosis (Figure 1 A-C). Z-VD-fmk had also no significant effect on hepatic neutrophil accumulation at 6 or 24 h (Figure 1C,D). Because previous studies ruled out relevant apoptotic cell death in this model (Gujral et al., 2002; Knight and Jaeschke, 2002; Lawson et al., 1999), no specific parameters of apoptosis were measured. To evaluate the efficacy of the pan-caspase inhibitor to modulate caspase-1 activity, plasma IL-1β protein levels were measured at 6 h and 24 h after APAP. Six hours after APAP the circulating levels of IL-1β were increased three-fold over baseline (Figure 2A). This increase in IL-1β protein levels was completely prevented with the pan-caspase inhibitor demonstrating the efficacy of Z-VD-fmk to block caspase-1 activity (Figure 2A). Consistent with the function of caspase-1 to process pro-IL-1β, the caspase inhibitor did not affect the increase in IL-1β mRNA (Figure 2B). Furthermore, the pan-caspase inhibitor did neither modulate the mRNA or plasma protein levels of several other cytokines and chemokines including TNF-α, IL-18, IL-10, IL-6, KC and MIP-2 (data not shown).
Figure 1.
Acetaminophen-induced liver injury in C57Bl/6 mice with or without caspase inhibitor. Animals were first treated with 300 mg/kg APAP and then two hours later with 10 mg/kg Z-VD-fmk or vehicle control. (A) Plasma ALT at 6 h and 24 h; (B) area of necrosis at 24 h. (C) Representative H&E-stained liver sections (x50 magnification) and immunohistochemistry for hepatic neutrophil accumulation (x200 magnification) are shown for controls and animals treated with APAP for 24 h. (D) Neutrophil numbers were quantified in 15 randomly selected high power fields (HPF; x400). Data represent means ± SE of n = 5 animals per group. *P<0.05 (compared to untreated controls).
Figure 2.
Interleukin-1β (IL-1β) protein levels in plasma (A) and hepatic IL-1β mRNA levels (B) were measured 6 and 24 h after administration of 300 mg/kg acetaminophen (APAP) in C57Bl/6 mice with or without the pan-caspase inhibitor (Z-VD-fmk). mRNA levels are expressed as IL-1β mRNA-to-β-actin mRNA ratio. The values of untreated controls were set as 1 and the fold change of the treated animals was calculated. Data represent means ± SE of n = 4-5 animals per group. *P<0.05 (compared to untreated control). #P<0.05 (compared to APAP-only equivalent time point)
Efficacy of Z-VD-fmk in the galactosamine/endotoxin liver injury model
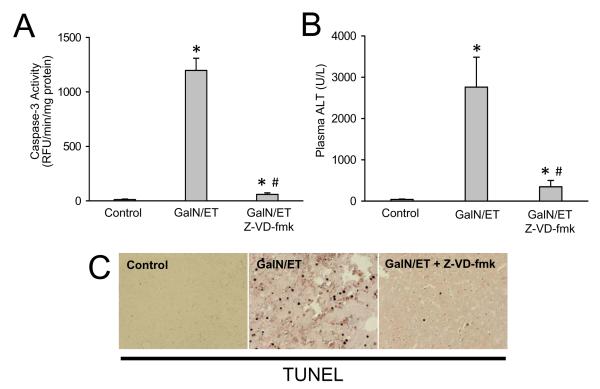
To further confirm the efficacy of the pan-caspase inhibitor, mice were treated with galactosamine/endotoxin (Gal/ET), which is a well established caspase-dependent model of hepatocellular apoptosis (Jaeschke et al., 1998). The 10 mg/kg dose and route of administration of Z-VD-fmk was effective in reducing 95% of the Gal/ET-induced increase in liver caspase-3 activity (Figure 3A). Blocking apoptosis also prevented secondary necrosis thereby reducing plasma ALT by nearly 90% (Figure 3B). To confirm the reduction in apoptotic cell death, liver sections were stained for DNA strand breaks with the TUNEL assay. Gal/ET-induced caspase activation causes massive DNA fragmentation as indicated by the extensive staining of TUNEL-positive hepatocytes (Figure 3C). Again, the caspase-inhibitor almost completely prevented any TUNEL staining in these livers (Figure 3C). Together, these data confirm the high efficacy of this pan-caspase inhibitor against TNF-mediated apoptosis in the Gal/ET model of liver injury.
Figure 3.
Effects of 10 mg/kg Z-VD-fmk on galactosamine/endotoxin (GalN/ET)-induced liver injury. Animals were treated with saline or 700 mg/kg GalN and 100 μg/kg ET and then three hours later with 10 mg/kg Z-VD-fmk or vehicle control. (A) Caspase-3 activity at 6 h; (B) Plasma ALT at 6 h. (C) Representative TUNEL staining of animals from each group. Data represent means ± SE of n = 3 animals per group. *P<0.05 (compared to saline-treated controls)
APAP toxicity in IL-1 receptor-1 knockout mice
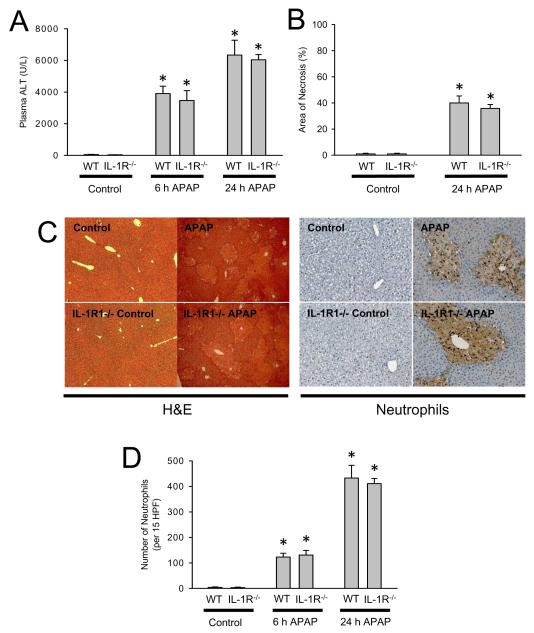
The caspase inhibitor data indicate that IL-1β maturation does not appear to be a critical factor in APAP-induced toxicity in contrast to previous reports. To confirm these findings and to exclude a potential role of IL-1α, which is formed independent of the inflammasome, IL-1R1-/- mice were treated with APAP. Similar to the caspase inhibitor data, there was no significant effect of APAP on plasma ALT activities, area of necrosis and hepatic neutrophil accumulation in IL-1R1-/- mice compared to wild type animals at 6 or 24 h after APAP (Figure 4 A-C). Despite similar neutrophil recruitment, both hepatic cytokine/chemokines mRNA production and plasma cytokine/chemokine levels were quantified to determine if the inflammatory response is altered or delayed in mice lacking IL-1 signaling. Transcription of inflammatory genes in the liver is unchanged between wild-type and knockout mice at 6 and 24 h (Table 1), however at the six hour time-point IL-6 and KC plasma protein levels are significantly reduced relative to the treated wild-type group (Table 1), however these differences are not seen at 24 h. In addition, protein levels of IL-1β (6 and 24 h) and IL-10 (6 h) did not increase to the same degree in IL-1R-/- mice as in wild type animals (Table 1). Despite this apparent delay in formation of some of the cytokine/chemokine, liver injury as well as the quantity and distribution of neutrophils are unchanged (Figure 4 C,D).
Figure 4.
Acetaminophen-induced liver injury in C57Bl/6 wild-type mice or IL-1R1-deficient mice. Animals were treated with 300 mg/kg APAP. (A) Plasma ALT at 6 h and 24 h; (B) area of necrosis at 24 h. (C) Representative H&E-stained liver sections (x50 magnification) and immunohistochemistry for hepatic neutrophil accumulation (x200 magnification) are shown for control and APAP-treated mice at 24 h; (D) Neutrophil numbers were quantified in 15 randomly selected high power fields (HPF; x400). Data represent means ± SE of n = 6 animals per group. *P<0.05 (compared to untreated controls).
Table 1.
Hepatic cytokine/chemokine mRNA induction and plasma protein concentrations after acetaminophen overdose in C57Bl/6 and IL-1R1-deficient mice.
| Liver mRNA expression (fold increase) |
Plasma Protein Concentration (pg/mL) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 6 h APAP |
24 h APAP |
Control |
6 h APAP |
24 h APAP |
|||||
| Cytokine Chemokine |
C57Bl/6 |
IL-1R1-/- |
C57Bl/6 |
IL-1R1-/- |
C57Bl/6 |
C57Bl/6 |
IL-1R1-/- |
C57Bl/6 |
IL-1R1-/- |
| IL-1β | 3.1 ± 0.6* | 2.4 ± 0.9* | 3.0 ± 0.9* | 2.8 ± 0.6* | 3.3 ± 1.0 | 12.5 ± 3.2* | 5.7 ± 0.7 | 2.4 ± 0.6 | 5.8 ± 1.4 |
| IL-10 | 65.7 ± 15.6* | 39.4 ± 10.8* | 10.0 ± 3.1* | 8.9 ± 3.5* | 11.1 ± 3.0 | 41.3 ± 6.8* | 26.0 ± 7.9 | 20.4 ± 3.7* | 6.8 ± 0.5 |
| IL-6 | 12.3 ± 6.1* | 5.6 ± 0.5* | 3.0 ± 0.6* | 3.5 ± 1.1* | 1.8 ± 0.7 | 52.8 ± 13.8* | 10.2 ± 2.7*# | 21.6 ± 5.6* | 29.8 ± 12.6* |
| KC | 6.1 ± 1.1* | 6.1 ± 2.6* | 10.8 ± 3.0* | 7.0 ± 1.7* | 4.9 ± 0.8 | 19.5 ± 3.4* | 4.2 ± 0.8# | 19.5 ± 2.5* | 19.3 ± 1.7* |
Hepatic mRNA levels and peripheral blood cytokine/chemokine concentrations were measured 6 h and 24 h after administration of 300 mg/kg APAP in C57Bl/6 wild type animals or IL-1R1-deficient mice. mRNA levels are calculated as the cytokine mRNA-to-β-actin mRNA ratio. For mRNA calculations the values of untreated controls were set as 1 and the fold change of the APAP-treated animals is shown. Data represent means ± SE of n = 5-6 animals per group.
P<0.05 (compared to control).
P<0.05 (compared to wild type equivalent time point)
Effects of supra-physiological levels of IL-1β during APAP toxicity
Although our experiments so far were unable to support a role of IL-1β in the pathophysiology of APAP hepatotoxicity, we can not rule out that the endogenous levels of IL-1β produced under our experimental conditions may have been too low to have a relevant impact on the injury mechanism. Therefore, we hypothesized that if IL-1β is critical for the injury via the recruitment of hepatic neutrophils or other leukocytes then an exacerbation of the injury should be observed when IL-1β is administered concurrently with APAP. Mice were injected i.p. with APAP and then given IL-1β or saline vehicle three hours later to mimic IL-1β, which would be produced during APAP-induced sterile inflammation. Administration of IL-1β alone caused recruitment of neutrophils into the liver within 90 minutes (Figure 5 D) without causing injury (Figure 5 A,B). When IL-1β was administered after APAP, the additional inflammatory mediators significantly enhanced APAP-induced hepatic neutrophil accumulation by 35% (Figure 5 D) but had no effect on plasma ALT values or the area of necrosis induced by APAP alone at 24 h (Figure 5 A-C). Because an impact of neutrophils on liver injury is generally observed between 6 h and 24 h after the insult (Ramaiah and Jaeschke, 2007) and the area of necrosis at 24 h represents the cumulative damage during the entire observation period, the lack of a difference in injury at 24 h makes it unlikely that there was a difference between the experimental groups at earlier time points. Thus, these data further support our findings that even excess IL-1β did not further aggravate APAP-induced liver injury.
Figure 5.
Acetaminophen-induced liver injury in C57Bl/6 mice with or without IL-1β treatment. Mice were treated with 300 mg/kg APAP and/or 20 μg/kg IL-1β or vehicle. (A) Plasma ALT levels at 24 h; (B) area of necrosis at 24 h; (C) Representative H&E-stained liver sections (x50 magnification) and immunohistochemistry for hepatic neutrophil accumulation (x200 magnification) are shown for control and treated mice. (D) Neutrophil numbers were quantified in 15 randomly selected high power fields (HPF; x400). Data represent means ± SE of n = 6 animals per group. *P<0.05 (compared to untreated controls). #P<0.05 (compared to APAP only).
Neutrophil activation during IL-1β treatment
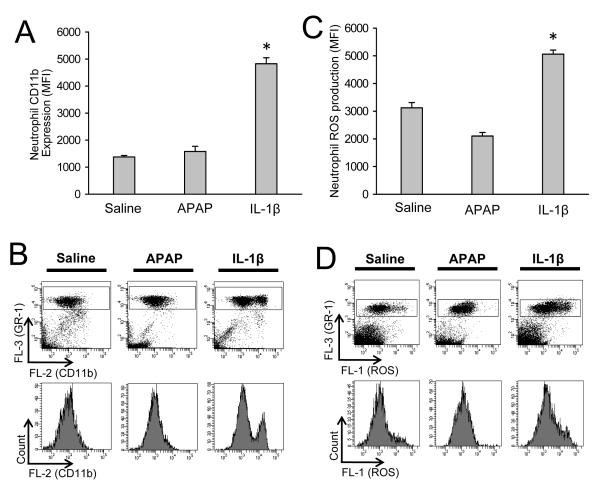
In order to verify that the dose of IL-1β was able to activate neutrophils, the enhanced expression of CD11b on neutrophils and priming for NADPH oxidase-derived reactive oxygen formation was evaluated on peripheral neutrophils. Our previous data showed a close correlation between the activation status of peripheral and hepatic neutrophils (Williams et al., 2010). C57Bl/6 mice were treated in vivo with saline (6 h), 300 mg/kg APAP (6 h), or 20 μg/kg IL-1β (1.5 h). Neutrophils were gated as Gr-1bright cells because previous literature demonstrated that Gr-1bright cells being the same population as Ly6G+ cells, which are neutrophils (Daley et al., 2008). APAP treatment caused no increase in CD11b expression versus control, and the very high dose of IL-1β caused only a partial activation of peripheral neutrophils resulting in two distinct cell populations expressing CD11b (Figure 6 A,B). Similarly, APAP did not cause increased ROS priming in peripheral neutrophils versus controls while supra-physiologic levels of IL-1β caused only a modest increase in ROS priming (Figure 6 C,D).
Figure 6.
Neutrophil priming in peripheral blood. C57Bl/6 mice (n=4 per group) were treated with 20 mL/kg saline (6 h), 300 mg/kg APAP (6 h), or 20 μg/kg IL-1β (1.5 h). Immediately after in vivo stimulation whole blood was stained for CD11b and Gr-1 surface expression and neutrophils (Gr-1bright cells) were analyzed by flow cytometry. (A) The mean CD11b fluorescent intensities of each treatment group are shown and (B) representative CD11b surface-expression dot plots and histograms for saline, APAP and IL-1β. To determine reactive oxygen species (ROS) priming after in vivo treatment, whole blood was stimulated ex vivo with PMA or saline. Upon PMA-induced ROS production DHR-123 is converted to R-123 and quantified in neutrophils by flow cytometry. (C) The mean ROS fluorescent intensities of each treatment group are shown and (D) representative ROS dot plots and histograms for saline, APAP and IL-1β. Data represent means ± SE of n = 4 animals per group. *P<0.05 (compared to saline-treated controls).
DISCUSSION
The primary objective of this study was to evaluate the formation and pathophysiological significance of IL-1β during APAP-induced hepatotoxicity. Although there were recent reports implicating the inflammasome (caspase-1) and IL-1β signaling in APAP overdose (Imaeda et al., 2009), there were some concerns whether a single pro-inflammatory mediator like IL-1β (Imaeda et al., 2009) or IL-1α (Blazka et al., 1995; Chen et al., 2007) could be such a dominant factor in the mechanism of injury as suggested by these authors.
Caspase-1 and IL-1β formation during APAP hepatotoxicity
The induction of IL-1α and IL-1β mRNA is well described after APAP overdose (Blazka et al., 1995; Gardner et al., 2003; Cover et al., 2006; Ishibe et al., 2009; Imaeda et al., 2009; Martin-Murphy et al., 2010). Our data are in agreement with the generally moderate transcriptional activation of IL-1β in response to APAP reported by these studies. In addition, we could demonstrate that there are significant but modest increases in mature IL-1β protein levels. Most importantly, this increase in IL-1β protein could be eliminated by inhibition of caspase-1 without affecting the mRNA levels. These data support the hypothesis that the increased formation of the mature IL-1β protein during APAP hepatotoxicity is dependent on caspases, most likely on caspase-1. Although previous efforts to directly measure changes in caspase-1 activity in the liver after APAP overdose were unsuccessful, this may have been due to the limited sensitivity of the assay and the selective activation of this enzyme in Kupffer cells (Lawson et al., 1999). Nevertheless, the observed effect of the pancaspase inhibitor on selective IL-1β processing is indirect evidence for the activation of caspase-1 and the inflammasome, which contributes to the formation of pro-inflammatory mediators after APAP. Previous studies indicated that the release of DAMPs from necrotic hepatocytes may contribute to the formation of cytokines by macrophages through activation of toll-like receptors (Imaeda et al., 2009; Martin-Murphy et al., 2010).
Effect of IL-1β on the inflammatory response during APAP hepatotoxicity
In contrast to TNF receptor 1 or the Fas receptor, the IL-1 receptor does not have a death domain and therefore can not directly trigger cell death. Thus, the only way IL-1β and the IL-1 receptor can be involved in cell injury is through the activation of cytotoxic leukocytes (Sims and Smith, 2010). During APAP-induced injury, the first leukocytes observed in the liver are neutrophils (Lawson et al., 2000), which have the capacity to severely aggravate liver injury (Jaeschke, 2006). IL-1α is a potent activator of neutrophils that can trigger substantial hepatic neutrophil recruitment (Bajt et al., 2001). Our data with IL-1β confirm these findings and additionally show that adding high doses of endogenous IL-1β during APAP toxicity has an additive effect on hepatic neutrophil accumulation. (Figure 5). Because both IL-1α and IL-1β at high doses can induce moderate TNF-α formation in vivo (Essani et al., 1995), this potent cytokine could also be involved in the observed effect of IL-1β injection on neutrophils. However, the peak levels of TNF after IL-1 are still less than 10% of peak levels after endotoxin (Essani et al., 1995). We have recently performed experiments where we injected endotoxin after APAP, which generated massive amounts of TNF and other cytokines (Williams et al., 2010). This activated circulating and hepatic neutrophils and increased the neutrophil numbers in the liver by 100% over APAP alone (Williams et al., 2010). Despite these effects of exogenous cytokines, eliminating the increase in endogenous IL-1β formation by a caspase inhibitor had no effect on the number of liver neutrophils. Thus, together these data suggest that the endogenous IL-1β formation during APAP hepatotoxicity is insufficient to have a relevant impact on hepatic neutrophil recruitment. Because IL-1R-deficient mice did not have less neutrophils in the liver compared to wildtype animals, it indicates that neither endogenous IL-1β nor IL-1α affect hepatic neutrophil accumulation. In addition, delayed treatment with high doses of IL-1β activated and primed circulating neutrophils, which closely reflect the activation status of liver neutrophils (Williams et al., 2010). APAP alone did not activate circulating or liver neutrophils as indicated by absence of enhanced CD11b expression and no priming for ROS formation (Figure 6 and Williams et al., 2010). This supports the conclusion that endogenously formed IL-1β is also insufficient to activate neutrophils. These findings, although opposite to what has been postulated (Imaeda et al., 2009), are not surprising given the substantial number of cytokines, chemokines and other mediators formed during the extensive cell damage caused by APAP (Lawson et al., 2000; Gardner et al., 2003; Cover et al., 2006; Ishibe et al., 2009; Imaeda et al., 2009; Martin-Murphy et al., 2010). In addition, the absolute cytokines levels observed after APAP overdose are not very high. Thus, the limited changes of individual mediators obviously have a limited impact on neutrophil activation and injury. We have previously shown that elimination of massively produced chemokines KC and MIP-2 during endotoxemia has no relevant impact on endotoxin-induced hepatic neutrophil infiltration or injury (Dorman et al., 2005). In contrast, elimination of the most upstream mediator of endotoxemia, TNF-α, completely eliminated hepatic neutrophil recruitment and injury (Schlayer et al., 1989; Essani et al., 1995). In this respect, elimination of a more upstream mediator of APAP-induced inflammation, high mobility group box protein-1, attenuated hepatic neutrophil accumulation but not the injury (Scaffidi et al., 2002). Thus, only changes in the most upstream mediators or initiators of the inflammatory cascade are likely to have a relevant impact on neutrophil recruitment into the liver.
Additional inflammatory cells activated during APAP hepatotoxicity include the resident macrophages of the liver (Kupffer cells) and newly recruited tissue macrophages derived from blood monocytes (Laskin, 2009). Emerging evidence indicates that Kupffer cells are mainly involved in the formation of pro- and anti-inflammatory cytokines but less in direct cytotoxicity (Ju et al., 2002; Martin-Murphy et al., 2010). The newly recruited tissue macrophages arrive mainly after the injury already peaked and appear to be involved in the resolution of inflammation and removal of necrotic cell debris (Dambach et al., 2002; Holt et al., 2008). Monocyte chemoattractant protein (MCP-1) rather than IL-1β is involved in the late monocyte recruitment (Dambach et al., 2002; Holt et al., 2008).
IL-1β and APAP-induced liver injury
The most critical question is whether IL-1β is involved in the injury process. In a previous paper it was reported that IL-1R-deficient mice are more than 90% protected against APAP hepatotoxicity based on plasma ALT activities (Chen et al., 2007). Using the same gene knockout mice, we did not find any evidence of protection at early or late time points after APAP overdose. When the authors used a combination of neutralizing antibodies against IL-1β, IL-1α, and IL-1R1, the protective effect was only 30% (Chen et al., 2007). Thus, even this study shows highly variable results on the role of IL-1. In addition, a second report claimed a critical role specifically for IL-1β due to the fact that gene knock-out mice of caspase-1 and different components of the inflammasome were partially protected (Imaeda et al., 2009). In this study, neutralizing IL-1β or deficiency of IL-18, Nalp3, caspase-1, and ASC showed reduced mortality and lower plasma ALT levels by 50-60% after APAP overdose (Imaeda et al., 2009). The authors concluded that the transcriptional activation of IL-1β and IL-18 together with the processing to the mature cytokine through caspase-1 is critical for APAP hepatotoxicity (Imaeda et al., 2009). Earlier studies also indicated that antibodies against IL-1α or TNFα protected against APAP toxicity in mice (Blazka et al., 1995,1996). In sharp contrast, our data are consistently showing no relevant effect of IL-1β (or IL-1α) in the pathophysiology. The amount of IL-1β generated was minor and insufficient to trigger activation and recruitment of neutrophils into the liver. In addition, we did not find significant upregulation of IL-18 mRNA after APAP treatment. A caspase inhibitor, which completely prevented the APAP-induced mature IL-1β formation, did neither affect hepatic neutrophil recruitment nor liver injury. Furthermore, the IL-1R1-deficient mouse was not protected or showed reduced inflammation. Finally, even treating the animals with extremely high doses of IL-1β, which by themselves can activate neutrophils and cause their accumulation in the liver, did only enhance the overall neutrophil count in the liver but had no effect on the injury. These findings are consistent with previous experiments where addition of endotoxin after APAP massively produced TNF-α, IL-1 and other cytokines and chemokines but did not enhance liver injury despite the doubling of hepatic neutrophil recruitment (Williams et al., 2010). It needs to be kept in mind that the objective of these experiments was to mimick the hypothesis that an inflammatory response triggered by the initial injury may impact the late phase of APAP-mediated liver injury. Therefore, a delayed treatment with IL-1β around the time of the initial injury is the most appropriate experimental design. However, the fact that we were not able to produce an aggravation of the APAP-mediated injury even with massive amounts of exogenous cytokines is consistent with a large number of different experiments that all demonstrated that the inflammatory response with hepatic neutrophil infiltration does not aggravate APAP-induced liver injury (Lawson et al., 2000; James et al., 2003; Cover et al., 2006; Williams et al., 2010). In these experiments, blocking or eliminating adhesion molecules which have been shown to be required for neutrophil cytotoxicity in the liver, i.e. CD18 and intercellular adhesion molecule-1 (ICAM-1) (Jaeschke and Smith, 1997), had no effect against APAP hepatotoxicity (Lawson et al., 2000; Williams et al., 2010). Furthermore, inhibiting or eliminating the capacity of neutrophils to generate reactive oxygen species, which is critical for neutrophil cytotoxicity in vivo (Jaeschke et al., 1999; Gujral et al., 2004; Hasegawa et al., 2005), had no effect on acetaminophen toxicity (James, et al., 2003; Cover et al., 2006). Most importantly, even when large amounts of pro-inflammatory mediators are either injected or generated through endotoxin during APAP-induced liver injury, the additional activated neutrophils do not affect the overall injury. Thus, even if sufficient IL-1 or IL-18 would have been generated during APAP overdose, there is no evidence that these phagocytes contribute to the injury.
Why is there such a difference in the results and conclusions between studies despite the use of the same mouse strain and similar doses of APAP? There is no easy answer. However, in the previous studies where extensive protection was shown in a large number of different gene knock-out mice, it was never tested if there are differences in metabolic activation or other changes in the intracellular signal transduction pathways (Chen et al., 2007; Imaeda et al., 2009). Furthermore, the cells that actually cause the toxicity were never identified. Thus, the mechanisms of APAP toxicity in these knock-out mice remained unclear and it was not excluded that off-target effects could have been responsible for the protection. In support of this hypothesis, it was recently shown that IL-1 receptor antagonist-deficient (IL-1ra-/-) mice showed reduced liver injury to APAP (Ishibe et al., 2009). Since IL-1ra blocks the signaling of IL-1α and IL-1β through IL-1R1 and prevents the intracellular recruitment of IL-1RAP (Sims and Smith, 2010), this finding is in direct contrast to the hypothesis that the IL-1 axis increases injury. Most importantly, this study demonstrated that IL-1ra-/- mice have decreased metabolic activity and therefore generate less reactive metabolite, i.e. the protection was caused by modulation of intracellular events rather than inflammation (Ishibe et al., 2009). However, consistent with our data, Imaeda et al. (2009) showed only a 6% increase of mature IL-1β in plasma after APAP. As we demonstrated, such a minute change in circulating IL-1β has no chance to cause neutrophil activation and recruitment into the liver and, therefore, it remained unclear how the activity of the inflammasome could have had any impact on APAP-induced liver injury.
Another set of earlier experiments demonstrated protective effects of antisera against IL-1α or TNF-α against APAP hepatotoxicity in mice (Blazka et al., 1995, 1996). However, there are also concerns with the interpretation of these data. First, the mechanism of injury involving both cytokines was not addressed. Second, the protective effect was observed as early as 4 h, i.e. at a time when little to no cytokine was produced. In addition, the hepatoprotection was no longer observed at 12 h (TNF) or 24 h (IL-1) (Blazka et al., 1995,1996). This is inconsistent with the sterile inflammation concept. Third, no inactive antiserum was used as control and potential endotoxin contamination of the reagents was not evaluated. Fourth, the effects were not reproducible in genetic models. Similar to the lack of protection in animals with IL-1R deficiency (Figure 4), there was no protection in TNF-α and lymphotoxin-α deficient mice against APAP hepatotoxicity (Boess et al., 1998). Fifth, the peak levels of IL-1α were <30 pg/ml (Blazka et al., 1995), i.e. in the same range as the IL-1β levels in our studies (Table 1). However, these cytokine levels are 3-4 orders of magnitude below the estimated levels after injection of 20 μg/kg of IL-1β, which resulted in only a partial activation of neutrophils and no effect on the APAP-induced liver injury (Figure 5). Taken together, similar to the recent papers (Chen et al., 2007; Imaeda et al., 2009), the earlier reports by Blazka et al. (1995, 1996) raise significant concerns whether IL-1α, IL-1β or both can have an impact on APAP-induced toxicity.
In summary, our comprehensive analysis of the formation and potential role of IL-1β in the pathophysiology of APAP hepatotoxicity revealed no support that this cytokine is generated in sufficient quantities to activate neutrophils and neither inhibition of caspases and the inflammasome nor deficiency of the IL-1R had any impact on APAP-induced inflammation and liver injury. Although these data are opposite to several papers (Blazka et al., 1995,1996; Chen et al., 2007; Imaeda et al., 2009), they are fully consistent with our previous findings that neutrophils do not aggravate liver injury after APAP overdose. Thus, the inflammatory cascade is a less relevant therapeutic target than the intracellular signaling pathways to attenuate APAP-induced liver injury.
ACKNOWLEDGMENT
This investigation was supported in part by National Institutes of Health Grants R01 DK070195 and R01 AA12916 to H.J., and by grants P20 RR016475 and P20 RR021940 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health. C.D. Williams was supported by the “Training Program in Environmental Toxicology” (T32 ES007079-26A2) from the National Institute of Environmental Health Sciences.
Footnotes
CONFLICT OF INTEREST STATEMENT The authors do not have any conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bajt ML, Farhood A, Jaeschke H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G1188–G1195. doi: 10.1152/ajpgi.2001.281.5.G1188. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Yan HM, Farhood A, Jaeschke H. Plasminogen activator inhibitor-1 limits liver injury and facilitates regeneration after acetaminophen overdose. Toxicol. Sci. 2008;104:419–427. doi: 10.1093/toxsci/kfn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazka ME, Elwell MR, Holladay SD, Wilson RE, Luster MI. Histopathology of acetaminophen-induced liver changes: role of interleukin 1 alpha and tumor necrosis factor alpha. Toxicol. Pathol. 1996;24:181–189. doi: 10.1177/019262339602400206. [DOI] [PubMed] [Google Scholar]
- Blazka ME, Wilmer JL, Holladay SD, Wilson RE, Luster MI. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 1995;133:43–52. doi: 10.1006/taap.1995.1125. [DOI] [PubMed] [Google Scholar]
- Boess F, Bopst M, Althaus R, Polsky S, Cohen SD, Eugster HP, Boelsterli UA. Acetaminophen hepatotoxicity in tumor necrosis factor/lymphotoxin-alpha gene knockout mice. Hepatology. 1998;27:1021–1029. doi: 10.1002/hep.510270418. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- Cover C, Liu J, Farhood A, Malle E, Waalkes MP, Bajt ML, Jaeschke H. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2006;216:98–107. doi: 10.1016/j.taap.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- Dorman RB, Gujral JS, Bajt ML, Farhood A, Jaeschke H. Generation and functional significance of CXC chemokines for neutrophil-induced liver injury during endotoxemia. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G880–G886. doi: 10.1152/ajpgi.00317.2004. [DOI] [PubMed] [Google Scholar]
- Essani NA, Fisher MA, Farhood A, Manning AM, Smith CW, Jaeschke H. Cytokine-induced upregulation of hepatic intercellular adhesion molecule-1 messenger RNA expression and its role in the pathophysiology of murine endotoxin shock and acute liver failure. Hepatology. 1995;21:1632–1639. [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner CR, Laskin JD, Dambach DM, Chiu H, Durham SK, Zhou P, Bruno M, Gerecke DR, Gordon MK, Laskin DL. Exaggerated hepatotoxicity of acetaminophen in mice lacking tumor necrosis factor receptor-1. Potential role of inflammatory mediators. Toxicol. Appl. Pharmacol. 2003;192:119–130. doi: 10.1016/s0041-008x(03)00273-4. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Hinson JA, Farhood A, Jaeschke H. NADPH oxidase-dervied oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G243–G252. doi: 10.1152/ajpgi.00287.2003. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol. Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G760–G767. doi: 10.1152/ajpgi.00141.2005. [DOI] [PubMed] [Google Scholar]
- Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J. Leukoc. Biol. 2008;84:1410–1421. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt MP, Yin H, Ju C. Exacerbation of acetaminophen-induced disturbances of liver sinusoidal endothelial cells in the absence of Kupffer cells in mice. Toxicol. Lett. 2010;194:34–41. doi: 10.1016/j.toxlet.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibe T, Kimura A, Ishida Y, Takayasu T, Hayashi T, Tsuneyama K, Matsushima K, Sakata I, Mukaida N, Kondo T. Reduced acetaminophen-induced liver injury in mice by genetic disruption of IL-1 receptor antagonist. Lab. Invest. 2009;89:68–79. doi: 10.1038/labinvest.2008.110. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Role of inflammation in the mechanism of acetaminophen-induced hepatotoxicity. Expert Opin. Drug. Metab. Toxicol. 2005;1:389–397. doi: 10.1517/17425255.1.3.389. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol. Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78:1670–1676. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Cai SX, Tseng BY, Bajt ML. Protection against TNF-induced liver parenchymal cell apoptosis during endotoxemia by a novel caspase inhibitor in mice. Toxicol. Appl. Pharmacol. 2000;169:77–83. doi: 10.1006/taap.2000.9035. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J. Immunol. 1998;160:3480–3486. [PubMed] [Google Scholar]
- Jaeschke H, Ho YS, Fisher MA, Lawson JA, Farhood A. Glutathione peroxidase-deficient mice are more susceptible to neutrophil-mediated hepatic parenchymal cell injury during endotoxemia: importance of an intracellular oxidant stress. Hepatology. 1999;29:443–450. doi: 10.1002/hep.510290222. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol. Lett. 2003;144:279–288. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J. Leukoc. Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- James LP, McCullough SS, Knight TR, Jaeschke H, Hinson JA. Acetaminophen toxicity in mice lacking NADPH oxidase activity: role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic. Res. 2003;37:1289–1297. doi: 10.1080/10715760310001617776. [DOI] [PubMed] [Google Scholar]
- Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem. Res. Toxicol. 2002;15:1504–1513. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- Knight TR, Jaeschke H. Acetaminophen-induced inhibition of Fas receptor-mediated liver cell apoptosis: mitochondrial dysfunction versus glutathione depletion. Toxicol. Appl. Pharmacol. 2002;181:133–141. doi: 10.1006/taap.2002.9407. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol. Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM. Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Laskin DL. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem. Res. Toxicol. 2009;22:1376–1385. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol. Sci. 2000;54:509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol. Appl. Pharmacol. 1999;156:179–186. doi: 10.1006/taap.1999.8635. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin. Drug. Metab. Toxicol. 2006;2:493–503. doi: 10.1517/17425255.2.4.493. [DOI] [PubMed] [Google Scholar]
- Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol. Lett. 2010;192:387–394. doi: 10.1016/j.toxlet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol. Pathol. 2007;35:757–766. doi: 10.1080/01926230701584163. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Schlayer HJ, Laaff H, Peters T, Woort-Menker M, Estler HC, Karck U, Schaefer HE, Decker K. Involvement of tumor necrosis factor in endotoxin-triggered neutrophil adherence to sinusoidal endothelial cells of mouse liver and its modulation in acute phase. J Hepatol. 1988;7:239–249. doi: 10.1016/s0168-8278(88)80488-4. [DOI] [PubMed] [Google Scholar]
- Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- Smith J, Weidemann M. Further characterization of the neutrophil oxidative burst by flow cytometry. J. Immunol. Methods. 1993;162:261–268. doi: 10.1016/0022-1759(93)90391-j. [DOI] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010 May 26; doi: 10.1111/j.1478-3231.2010.02284.x. in press. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]