Summary
Objective
The signaling protein p38 mitogen-activated protein kinase is required for inflammatory signaling in chondrocytes that regulates MMP production. We sought to determine the role of specific p38 isoforms in chondrocyte catabolic signaling in response to IL-1β and fibronectin fragments.
Methods
Human articular chondrocytes isolated from normal ankle cartilage from tissue donors or from osteoarthritic knee cartilage obtained during knee replacement were stimulated with IL-1β or fibronectin fragment (Fn-f), with or without pretreatment with p38 inhibitors (SB203580 or BIRB796) or growth factors (IGF-1 and OP-1). p38 isoform phosphorylation was measured by antibody array and immunoblotting. MMP-13 expression was measured by real-time PCR, ELISA, and immunoblotting. Chondrocytes were transfected with plasmids expressing constitutively active (CA) p38γ or with adenovirus expressing dominant negative (DN) p38γ.
Results
Stimulation of chondrocytes with either IL-1β or Fn-f led to enhanced phosphorylation of p38α and p38γ, with little phosphorylation of p38β or p38δ isoforms. p38α localized to the nucleus and p38γ to the cytosol. Inhibition of both p38α and p38γ with BIRB796 resulted in less inhibition of MMP-13 production in response to IL-1β or FN-f than did inhibition of only p38α with SB203580. Transfection with CAp38γ resulted in decreased MMP-13 production while transduction with DNp38γ resulted in increased MMP-13 production. IGF-1 and OP-1 pretreatment inhibited p38α phosphorylation but not p38γ phosphorylation.
Conclusions
p38γ is activated by catabolic stimulation of human articular chondrocytes, but interestingly suppresses MMP-13 production. Treatments that increase p38γ activation may be of therapeutic benefit in reducing chondrocyte production of MMP-13.
Keywords: chondrocyte, articular cartilage, integrins, cytokines, cell signaling, matrix metalloproteinase
Introduction
The p38 mitogen-activated protein kinases (MAPK) are serine/threonine kinases that, when activated, transmit extracellular signals to the cell nucleus. The p38 MAPK family is an important mediator of inflammatory processes including regulation of the signaling pathway activated by IL-1β1. There are four known p38 isoforms (p38α, p38β, p38γ, and p38δ). The first isoform to be described was p38α, which was shown to be involved in the production of the inflammatory cytokines IL-1β and TNF-α in response to LPS stimulation of human monocytes2. Subsequent reports identified p38β3, which shares 75% identity with p38α and the less similar p38γ4 and p38δ5, which both share ~60% identity with p38α. All isoforms are activated by dual phosphorylation at Thr180 and Tyr182 residues, but they differ in regards to the type of extracellular stimulus that results in activation, in tissue distribution, and in cellular function.
IL-1β stimulates chondrocytes to secrete a number of proteases that degrade articular cartilage matrix proteins. This matrix degradation leads to the production of matrix fragments that include fibronectin fragments (Fn-f) (reviewed in)6. These fragments can in turn stimulate chondrocytes via the α5β1 integrin to produce degradative enzymes, amplifying the already destructive stimulation received from IL-1β. One of the most important proteases involved in matrix degradation induced by IL-1β and Fn-f is MMP-13 (collagenase-3). This enzyme is responsible for degradation of type II collagen and is highly expressed in the pathological contexts of osteoarthritis7 and rheumatoid arthritis8. Stimulation of MMP-13 production by either IL-1β9 or Fn-f10–12 has been shown to require p38 activity. These earlier studies used chemical inhibitors and expression of dominant negative p38 constructs to inhibit p38 activity but did not specifically address which p38 isoforms were required.
A recent report has indicated the presence of all four p38 isoforms within inflamed synovial tissue but p38α and p38γ were the predominantly expressed isoforms and were the most highly phosphorylated suggesting they were more active13. To our knowledge, it has not been determined which particular p38 isoforms are activated in chondrocytes in response to inflammatory stimuli. One report has demonstrated the possible involvement of p38β in the synthesis of mPGES-1, an enzyme involved in the production of prostaglandin E2 in chondrocytes14. Another recent report has shown upregulation of p38δ gene expression in response to type II collagen fragment stimulation of chondrocytes15. But apart from these studies, we could not find evidence for p38 isoform specific activation in chondrocytes or information on the role of these isoforms in mediating downstream signaling that regulates MMP expression. Therefore, the initial aim of the present study was to determine which p38 isoforms are activated by inflammatory stimulation of articular chondrocytes. We found that p38α and p38γ were the predominant isoforms phosphorylated in response to IL-1β and FN-f. Since p38γ function had not been studied previously in chondrocytes, we focused further experiments on its involvement in MMP-13 production.
Methods
MATERIALS
Phospho-p38 (pan), p38α, phospho-MK2, phospho-HSP27 and HA antibodies were from Cell Signaling (Beverly, MA). p38γ antibody, recombinant IL-1β, pro-MMP-13 ELISA ( detects MMP-13 that has not been processed and activated), phospho-p38γ ELISA, and the Human phospho-MAPK Array kit were from R&D Systems (Minneapolis, MN). MMP-13 antibody was from Anaspec (San Jose, CA). Recombinant IGF-1 was from Austral Biologicals (San Ramon, CA) and recombinant OP-1 was a gift from Dr. Susan Chubinskaya at Rush University Medical School (Chicago, IL). Nuclear and cytoplasmic extracts were made using the NE-PER Nuclear and Cytoplasmic Extraction Reagents from Pierce Biotechnology (Rockford, IL). p38 inhibitor SB203580 was purchased from EMD Biosciences (San Diego, CA). p38 inhibitor BIRB79616 was purchased from the Medical Research Council Protein Phosphorylation Unit at the University of Dundee (Dundee, Scotland, UK). Real-time PCR primers for GAPDH, MMP-13, p38 isoforms, and SybrGreen PCR Mastermix were from SuperArray Biosciences (Frederick, MD). Recombinant fibronectin fragment (FN7-10) which contains the RGD α5β1 integrin binding site17 was provided by Dr. Harold Erickson of Duke University (Durham, NC).
TISSUE ACQUISITION AND CHONDROCYTE CELL CULTURE
Human ankle cartilage was obtained from tissue donors within 48 hours of death through the Gift of Hope Organ and Tissue Donor Network (Elmhurst, IL) or from the National Disease Research Interchange (Philadelphia, PA) in accordance with institutional protocol. Each donor specimen was graded for degenerative changes based on the 5-point Collins scale, as modified by Muehleman et al18. The OA cartilage was discarded tissue obtained after knee replacement surgery. Cartilage was dissected from the joints and digested in a sequential manner with Pronase and then overnight with collagenase, as previously described10. Viability of isolated cells was determined using trypan blue, and cells were counted using a hemocytometer. Monolayer cultures were established by plating cells in 6-well plates at 2 × 106 cells/ml in Dulbecco's modified Eagle's medium (DMEM)/Ham's F-12 medium supplemented with 10% fetal bovine serum. Plates were maintained for ~5–7 days, with feedings every 2 days until they reached 100% confluence prior to experimental use.
IMMUNOBLOTTING
Confluent cells in monolayer were switched to serum-free media. The following day cells were pretreated for 30 minutes with or without inhibitors before stimulation with either 10ng/mL IL-1β or 500nM Fn-f for 30 minutes. SB203580 was tested at concentrations of 1, 5, and 10µM and BIRB796 was used at concentrations of 0.1, 0.25, and 0.5µM. Cells were washed with PBS and treated with lysis buffer that contained 20mM Tris (pH 7.5), 150mM NaCl, 1mM EDTA, 1mM EGTA, 1% Triton X-100, 2.5mM tetrapyrophosphate, 1mM glycerol phosphate, 1mM Na3VO4, 1µl/ml leupeptin, and 1mM phenylmethylsulfonyl fluoride. Lysates were centrifuged to remove insoluble material, and the soluble protein concentration was determined with BCA reagent (Pierce). Samples containing equal amounts of total protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and probed with appropriate antibodies.
An antibody array that detects phosphorylated MAPK family members was also performed on chondrocyte lysates after stimulation with 10ng/mL IL-1β or 500nM Fn-f. Because the phosphorylation site is similar in the different p38 isoforms, this array uses isoform specific antibodies that are not phosphorylation-specific to capture the various p38 isoforms followed by a pan anti-phospho-p38 antibody to detect the phosphorylated protein.
For MMP-13 immunoblotting, cells were pretreated with inhibitors 30 minutes prior to stimulation with either IL-1β or Fn-f overnight. Media was then collected from cells and run on SDS-PAGE as above.
CYTOSOL AND NUCLEAR PREPARATIONS
Cells in monolayer were switched to serum-free media. The following day, cells were stimulated with IL-1β for 5 and 30 minutes. Cells were then removed from monolayer by scraping in ice cold PBS then cytosol and nuclear preparations were made using the NE-PER kit (Pierce). Protease and phosphatase inhibitors were included in lysis buffers when making fractions.
REAL TIME PCR ANALYSIS
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA). Two micrograms of RNA were reverse transcribed using an AMV reverse transcriptase and oligo dT primer at 42° C for 1 hour. 1µL of RT reaction was then combined in a reaction mixture with 1 µL of MMP-13 specific primer pair, 12.5µL 2X SybrGreen PCR Mastermix, and water to a final reaction volume of 25µL. Reactions were then run in duplicate with 40 cycles of amplification on an ABI Prism 7000 real-time PCR machine (Applied Biosystems). The amount of MMP-13 mRNA was normalized against levels of GAPDH mRNA using data from parallel reactions run with GAPDH primers. All data was analyzed using the Comparative CT Method.
PLASMIDS AND CELL TRANSFECTION
Plasmid expressing a constitutively active form of p38γ that is rendered active by mutation (p38γD179A) as described previously19 was kindly provided by Dr. David Engelberg (Hebrew University of Jerusalem). Plasmids were transfected into chondrocytes by nucleofection using the Amaxa system as described previously20. Cells were allowed to recover for 24 hours in media supplemented with 20% serum before being switched to media with 10% serum for subsequent experimentation.
ADENOVIRAL TRANDSDUCTION
Adenovirus expressing dominant negative p38γ was obtained from a commercial source (Cell Biolabs, San Diego, CA). Primary human chondrocytes were plated at a density of 1 × 106 cells per well in 12 well plates. The cells were then infected with adenovirus encoding p38γ dominant negative construct using calcium chloride to enhance transduction efficiency. Null control adenovirus was used as a negative control. Two hours after the transduction, the cells were fed with complete media and incubated for 48 hours at 37°C in a humidified environment containing 5% CO2. The cells were then changed to serum-free conditions and incubated overnight prior to stimulation.
STATISTICS
Student’s T-test (paired, two-tailed) was used for statistical analysis.
Results
PHOSPHORYLATION OF p38 ISOFORMS IN CHONDROCYTES IN RESPONSE TO IL-1β AND FIBRONECTIN FRAGMENT STIMULATION
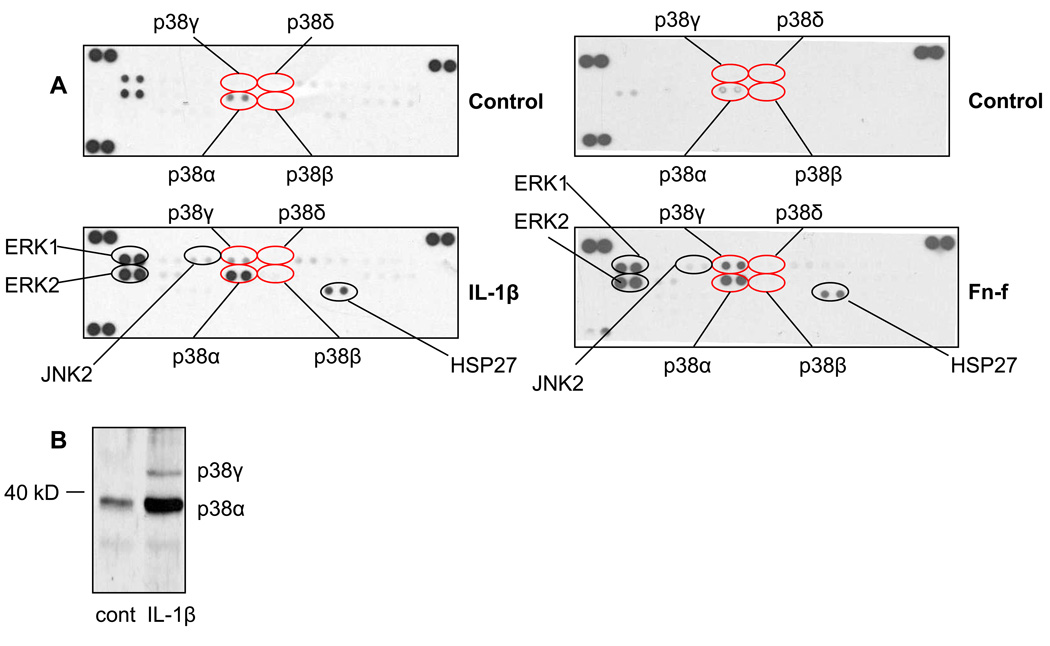
In initial experiments, we screened for the activation of multiple MAPKs, including the four isoforms of p38, by using an antibody array that detects phosphorylated forms of 27 different proteins in the MAPK pathway. Phosphorylation of both p38α and p38γ increased in chondrocytes following stimulation with either IL-1β or Fn-f (Fig. 1A). Conversely, we could not detect increased phosphorylation of either p38δ or p38β. ERK1, ERK2, JNK2, and HSP27 phosphorylation were also increased after stimulation. We used immunoblotting after separation of samples by SDS-PAGE as a second method for analyzing p38 isoform phosphorylation. p38γ runs at a slightly higher molecular weight (~ 45kD) than p38α (~ 38kD)16, 21. Using an antibody that recognizes the phosphorylated form of all four p38 isoforms (pan phospho-p38), we detected phosphorylation of two bands after IL-1β stimulation, consistent with the molecular weights of p38α and p38γ (Fig. 1B).
Fig. 1. p38α and p38γ are phosphorylated in chondrocytes following catabolic stimulation.
(A) Chondrocytes were stimulated for 30 minutes with either 10ng/mL IL-1β or 500nM Fn-f or with control media. Lysates were analyzed on a MAPK antibody array. Results are representative of three independent experiments. (B) Chondrocytes were stimulated with 10ng/mL IL-1β. Lysates were immunoblotted with pan phosphospecific p38 antibody that recognizes all isoforms. Anticipated locations of p38γ (above 40kD) and p38α (below 40kD) are marked. Results are representative of three independent experiments.
DIFFERENTIAL SUBCELLULAR LOCATION OF p38α AND p38γ AFTER STIMULATION WITH IL-1β AND FIBRONECTIN FRAGMENT
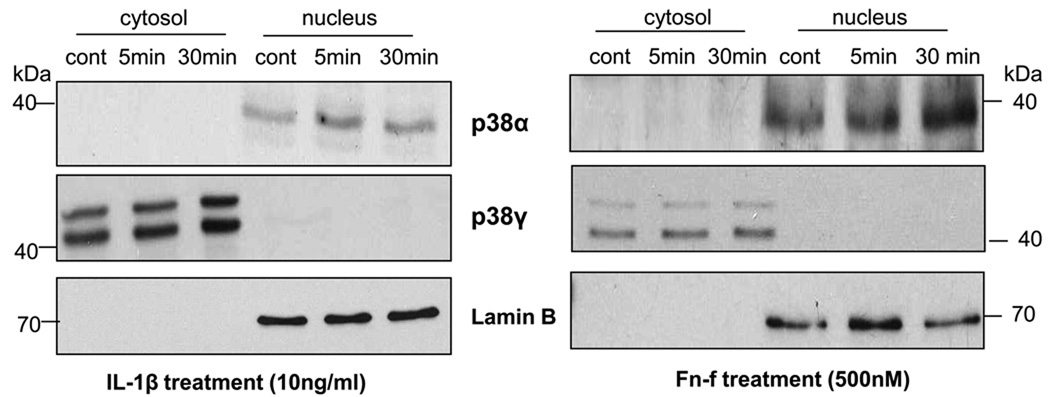
A previous study in cardiac myocytes demonstrated that p38α and p38γ may display different subcellular localizations, with p38α being both cytosolic and nuclear and p38γ found primarily in the cytosol22. This differential localization suggests that the two isoforms may have different cellular functions. We performed cytosol/nuclear protein fractionation on chondrocytes after stimulation with IL-1β or FN-f, followed by immunoblotting with isoform specific antibodies for p38α and p38γ that recognize both the phosphorylated and non-phosphorylated forms of the proteins. p38α was found within the nucleus of chondrocytes and the total level of p38α was increased slightly between 0–30 minutes of stimulation, while p38γ was confined to the cytosol and also showed a minimal increase in total protein after stimulation (Fig. 2). When using the isoform-specific antibody to total p38γ, two bands were consistently noted just below the 50kD marker (Fig. 2) and above the 40kD marker (Fig.1).
Fig. 2. p38α is localized to the nucleus and p38γ to the cytosol.
Chondrocytes were stimulated with IL-1β or Fibronectin fragment (FN-f) for 5 and 30 minutes. Cytosolic and nuclear fractions were immunoblotted with antibodies specific for total p38α or p38γ isoforms. Blots were stripped and immunoblotted with Lamin B as a nuclear marker protein to confirm separation of nuclear from cytosolic proteins. Results are representative of three independent experiments.
p38 INHIBITORS SB203580 AND BIRB796 DIFFERENTIALLY INHIBIT p38γ AND MMP-13 PRODUCTION IN CHONDROCYTES
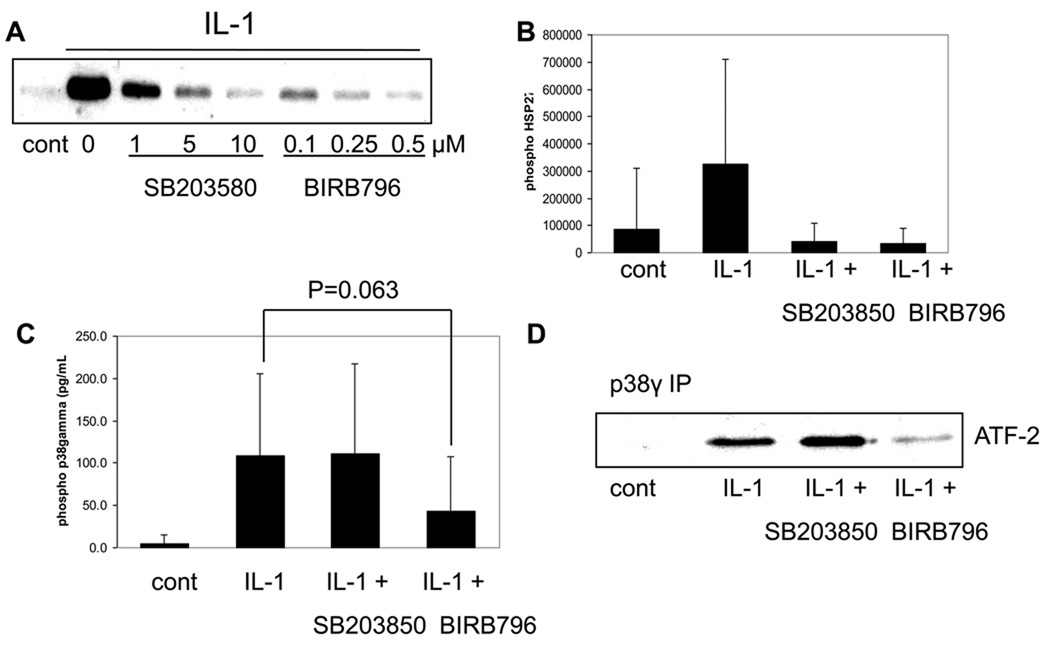
The p38 inhibitor SB203580 is a well characterized p38 inhibitor that inhibits p38α/β isoform activity without affecting the activity of p38δ/γ isoforms24. This inhibitor has been used to demonstrate that p38 is involved in the production of MMP-13 induced both by IL-19 and Fn-f10,12. There are, however, no commercially available chemical inhibitors that target p38γ specifically. A recent report has indicated that the p38 inhibitor BIRB796 can inhibit all four p38 isoforms and, when compared to SB203580, can be used to elucidate cellular functions of p38γ16. In order to determine functions of p38γ in chondrocytes using these inhibitors, we first sought to characterize their activities in our system. In initial experiments, we conducted a dose response curve for each inhibitor with a 30 minute inhibitor pretreatment prior to 30 minute IL-1β stimulation. To determine the activity of the p38α isoforms, we analyzed phosphorylation of HSP27 by immunoblotting. HSP27 is a substrate of MK2, which is downstream of p38α and not p38γ25 and is a marker of p38α activity. Increasing amounts of each inhibitor led to a decreased phosphorylation of HSP27 (Fig. 3A). Densitometric analysis showed that at a dose of 10µM SB203580 and 0.5µM BIRB796 phosphorylation of HSP27 was inhibited to the same degree (Fig.3B), confirming equal inhibition of p38α activity by each inhibitor at these doses. The selected doses were then used to test inhibitor effect on p38γ activity. We first checked phosphorylation of p38γ using an ELISA specific for phosphorylated p38γ. IL-1β stimulated phosphorylation of p38γ and this stimulation was not inhibited by SB203580 pretreatment, but was reduced by about 50% with BIRB796 pretreatment (Fig. 3C). We next performed a kinase assay with immunoprecipitated p38γ using recombinant ATF-2 as a substrate to determine inhibitor effect on p38γ activity. IL-1β stimulated p38γ kinase activity and this activity was not inhibited by SB203580 pretreatment, but was inhibited by BIRB796 pretreatment (Fig. 3D). We also checked phosphorylation of JNK2 to rule out the possibility of off target inhibitor effects. At the dose of BIRB796 used in the present studies (0.5µM), no effect on IL-1 stimulated JNK2 phosphorylation was noted (data not shown). Collectively, these experiments confirm that the inhibitors are performing as expected, with SB203580 inhibiting the activity of p38α but not p38γ and BIRB796 inhibiting the phosphorylation and activity of both p38α and p38γ.
Fig. 3. Effects of the p38 inhibitors SB203580 and BIRB796 on p38α and p38γ activity.
(A) Chondrocytes were pretreated with the indicated doses of SB203580 or BIRB796 prior to stimulation with IL-1β. Lysates were then immunoblotted with phosphospecific HSP27 antibody. (B) Immunoblots from two independent experiments were scanned and densitometric analysis was performed to determine intensity of the phosphorylated HSP27 band. Results are means and 95% confidence intervals. (C) Chondrocytes were pretreated with 10µM SB203580 or 0.5µM BIRB796 prior to IL-1β stimulation. Lysates were measured with an ELISA for phosphorylated p38γ. Results are mean and 95% confidence intervals of three independent experiments. (D) Chondrocytes were pretreated with 10µM SB203580 or 0.5µM BIRB796 prior to IL-1β stimulation. Lysates were immunopreciptated with p38γ specific antibody. Immunoprecipitates were then used in an in vitro kinase assay using recombinant ATF-2 as a substrate.
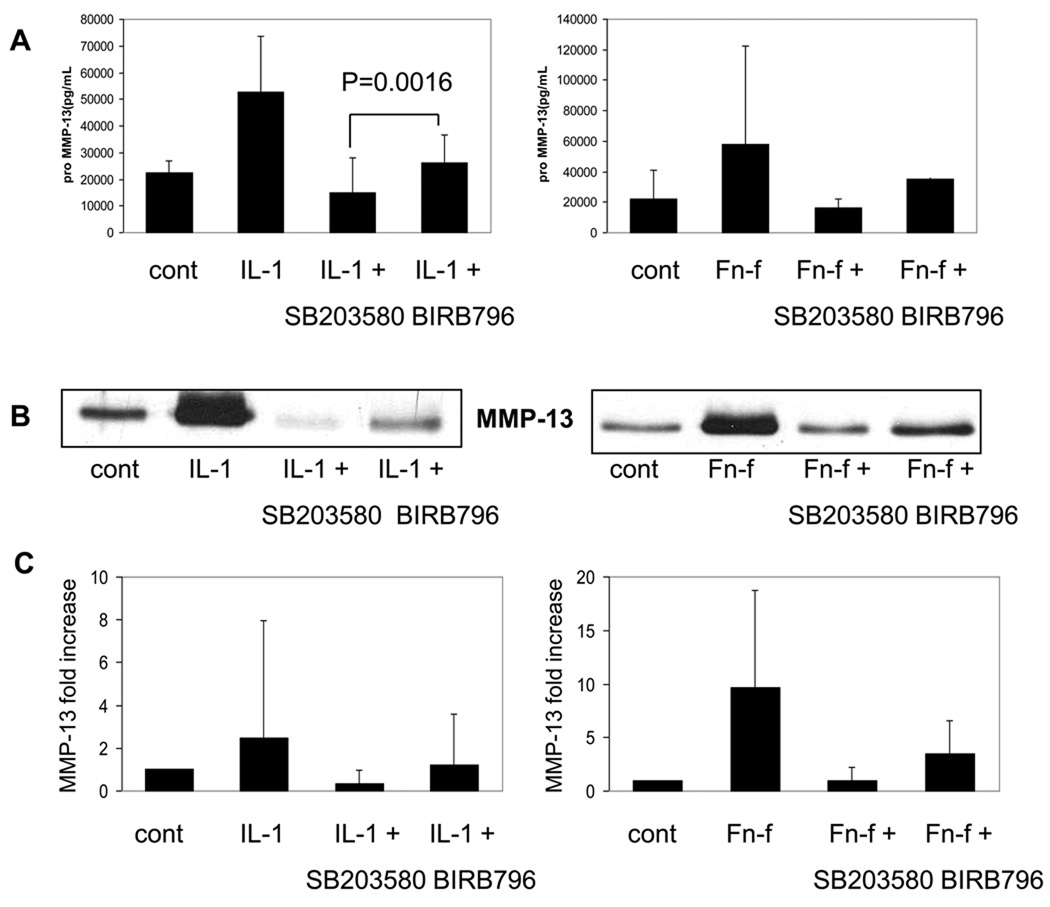
Chondrocytes were next pretreated with the inhibitors for 30 minutes prior to overnight stimulation with either IL-1β or Fn-f in order to determine the ability of the inhibitors to block MMP-13 expression and production. RNA was extracted for real-time PCR and the media supernatant was collected for ELISA and immunoblotting. p38α inhibition with SB203580 dramatically inhibited MMP-13 induced by either IL-1β or Fn-f at both the protein (Fig. 4A,B) and RNA levels (Fig. 4C). The combined inhibition of p38α and p38γ with BIRB796 also reduced MMP-13 expression and production but interestingly was less effective than inhibiting only p38α with SB203580. Inhibition of p38α without inhibition of p38γ might be more effective at inhibiting MMP-13 expression than inhibiting both isoforms if p38γ was serving as a negative regulator of MMP-13 expression rather than a positive regulator. This possibility was tested next.
Fig. 4. Effects of the p38 inhibitors SB203580 and BIRB796 on IL-1 and FN-f stimulation of MMP-13.
Chondrocytes were pretreated with either 10µM SB203580 or 500nM BIRB796 for 30 minutes prior to overnight stimulation with either 10ng/mL IL-1β or 500nM Fibronectin fragment. (A) Media was collected and an ELISA was run for pro-MMP-13 (n=5, mean±95% confidence intervals) or media was immunblotted with MMP-13 antibody (B). (C) RNA was harvested in parallel to media collection and real time PCR was run with MMP-13 primers in duplicate samples. Results were normalized to GAPDH as a housekeeping gene (n=2, mean±95% confidence intervals).
TRANSFECTION OF OSTEOARTHRITIC CHONDROCYTES WITH CONSTITUTIVELY ACTIVE p38γ REDUCES MMP-13 PRODUCTION
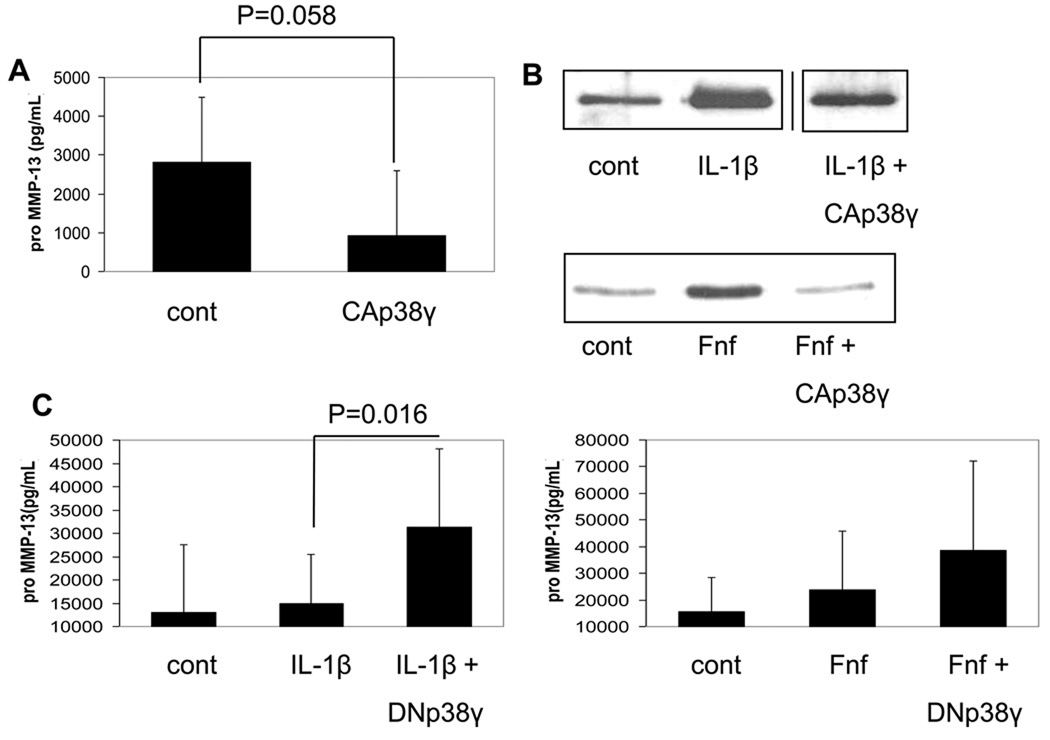
Since osteoarthritic chondrocytes produce high amounts of endogenous MMP-1326, we decided to use these cells to determine if expression of constitutively active p38γ19 could downregulate MMP-13 expression. Transfection of an HA-tagged construct was confirmed by immunoblotting transfected cells with HA antibody and phosphospecific p38 antibody (data not shown). Transfection with the active p38γ construct decreased MMP-13 production by OA chondrocytes (Fig. 5A). Since we showed that p38γ transfection was able to downregulate endogenous MMP-13 production in OA cells, we next wanted to see if p38γ transfection could inhibit the production of MMP-13 induced by either IL-1β or Fn-f in normal cells. Active p38γ transfection was able to downregulate MMP-13 production induced by either stimulus (Fig. 5B). We next obtained an adenovirus that expresses a dominant negative form of p38γ. We infected chondrocytes with this virus and 48 hours later stimulated cells with either fibronectin fragment or IL-1. Stimulated chondrocytes expressing dominant negative p38γ had increased MMP-13 production when compared to an adenovirus control (Fig. 5C) consistent with the hypothesis that p38γ serves as a negative regulator of MMP-13 expression.
Fig. 5. Effects on chondrocyte MMP-13 production after overexpression of constitutively active and dominant negative p38γ.
(A) Osteoarthritic chondrocytes were transfected with 3µg constitutively active (CA) HA-tagged p38γ plasmid or empty plasmid vector as a control. 48 hours after transfection, media was collected and pro-MMP-13 was measured by an ELISA. Results are the mean±95% confidence intervals of 3 independent experiments. (B) Normal human chondrocytes were transfected with 3µg constitutively active (CA) p38γ plasmid or empty plasmid vector as a control. 48 hours after transfection cells were stimulated with either 10ng/mL IL-1β or 500nM fibronectin fragment overnight. Media was collected and MMP-13 was measured by immunoblotting. Bands from the IL-1β experiment were from the same gel run at the same time and were spliced together (indicated by a line) due to being in noncontiguous lanes. (C) Chondrocytes were infected with adenovirus expressing dominant negative p38γ or non-expressing adenovirus as a control. 48 hours later cells were stimulated with either 10ng/mL IL-1β or 500nM Fibronectin fragment overnight. Media was collected and MMP-13 was measured by ELISA. Results are the mean and 95% confidence intervals of 3 independent experiments for IL-1β and 2 experiments for Fn-f.
THE GROWTH FACTORS IGF-1 AND OP-1 INHIBIT p38α BUT NOT p38γ PHOSPHORYLATION INDUCED BY IL-1β OR FIBRONECTIN FRAGMENT
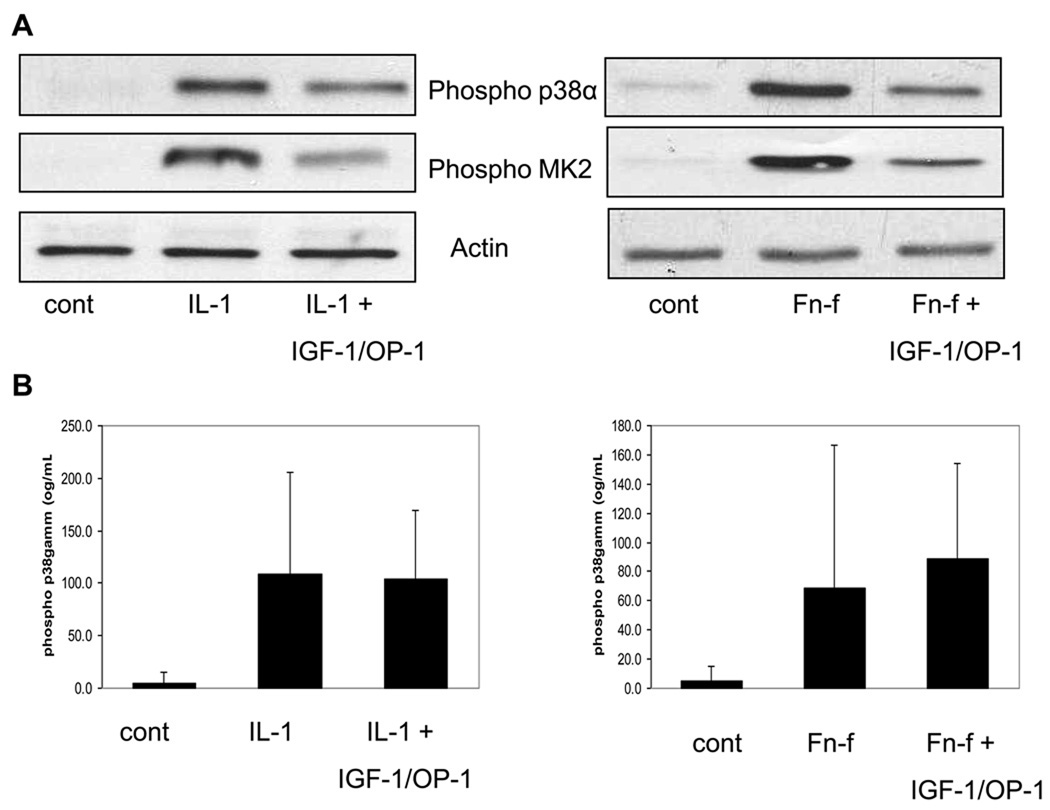
In a previous report27, we demonstrated that the growth factor combination of IGF-1 and OP-1 was able to significantly block chondrocyte MMP-13 production induced by IL-1β or Fn-f stimulation. We decided to determine if this growth factor combination might affect p38α and p38γ differentially. In the present study, pretreatment with the same growth factor combination decreased p38α phosphorylation induced by either IL-1β or Fn-f and reduced phosphorylation of MK2 which is downstream of p38α (Fig. 6A). Because of the lower amounts of phospho-p38γ in chondrocytes, we were not able to consistently measure effects of the growth factors on p38γ phosphorylation by immunoblotting and so used the more sensitive ELISA method. Unlike p38α, p38γ phosphorylation was not significantly different in cells pre-treated with the growth factors followed by either IL-1β or FN-f stimulation consistent with differential inhibition of p38α and p38γ by IGF-I plus OP-1(Fig. 6B).
Fig. 6. Effects of IGF-1 plus OP-1 on IL-1 and FN-f induced p38 and MK2 phosphorylation.
Chondrocytes were pretreated with 100ng/mL IGF-1 plus 100ng/mL OP-1 prior to 30 minute stimulation with either 10ng/mL IL-1β or 500nM Fibronectin fragment. (A) Samples were immunoblotted with pan phosphospecific p38 antibody and phosphospecific MK2 antibody. Blots were stripped and immunoblotted with actin antibody as a loading control. (B) Lysates were analyzed with an ELISA that measures phosphorylated p38γ. Results are the mean± 95% confidence intervals of 3 independent experiments.
Discussion
We show that both p38α and p38γ isoforms, but not p38β or p38δ isoforms, are phosphorylated in response to catabolic stimulation of human articular chondrocytes with either IL-1β or recombinant fibronectin fragment. Both IL-1 and FN-f have been previously shown to activate signaling pathways that require p38 activation for the increased production of MMPs9–12. In the present study, we found that the p38γ isoform may act as a negative feedback regulator of chondrocyte MMP-13 production in response to catabolic stimulation.
p38α is the predominant isoform expressed in many tissues whereas p38γ expression is more limited. A previous study comparing expression in heart, brain, placenta, lung, liver, skeletal muscle, kidney, and pancreas noted p38α in all the tissues studied but p38γ only in skeletal muscle4. Our immunoblotting results, using antibodies that recognize p38γ, with chondrocyte lysates demonstrated that chondrocytes do contain easily detectable amounts of the p38γ in addition to p38α. By real-time PCR we have detected expression of all four p38 isoforms with p38γ > p38α ≥ p38β > p38δ (unpublished observations).
The response of cells to a particular stimulus most often depends on a balance of activity in multiple signaling pathways. By immunoblotting, the relative amount of phosphorylated p38α in IL-1β and FN-f stimulated human articular chondrocytes was much greater than the amount of phosphorylated p38γ suggesting that these catabolic stimuli alter the balance in favor of p38α and this results in increased MMP-13 expression. p38α has been shown previously to be a positive regulator of MMP-13 production in carcinoma cells28 and the previous studies of IL-1 and FN-f stimulation in chondrocytes showed that inhibition of p38α with SB203580 or dominant negative constructs inhibited MMP-13 expression9–12. Of interest, when chondrocytes were pre-treated with IGF-I and OP-1, which inhibit IL-1 and FN-f induced MMP-1327, the phosphorylation of p38α but not p38γ was inhibited which would alter the balance towards p38γ which may at least partially contribute to inhibition of MMP-13 expression. Further studies would be needed to determine if IGF-I and OP-1 are affecting other pathways as well.
We were not able to determine the mechanism for the negative MMP-13 regulation by p38γ. Since p38γ appeared to be located in the cytosol and not the nucleus it is unlikely to be a direct effect on MMP-13 transcription. The balance of the phosphorylation of the two p38 isoforms could control MMP-13 expression through regulation of AP-1 activity. MMP-13 transcription induced by both IL-1β9 and Fn-f11 is dependent on AP-1 activation. Activation of p38γ has been found to decrease the activation of AP-1 in other cell types29, 30. Also consistent with a role for AP-1 modulation is the finding that the combination of IGF-1 plus OP-1 inhibited p38α but not p38γ phosphorylation. We had previously shown this growth factor combination inhibited AP-1 activation and subsequent MMP-13 expression27.
In previous reports, it has been demonstrated that p38α and p38γ phosphorylate different downstream targets. An early report indicated that MK2 is a downstream target of p38α, but not p38γ25. We found that IL-1β or FN-f stimulated MK2 phosphorylation and this was inhibited by blocking p38α with SB203580. Interestingly, several reports have implicated MK2 in the arthritis disease process. One report indicated MK2 deficient mice showed a resistance to collagen-induced arthritis31. Another report showed that suppression of MK2 expression in chondrocytes led to decreased production of MMP-13 and decreased prostaglandin E2 release32. If p38α and p38γ differentially phosphorylate and activate MK2 in chondrocytes, this may at least partially explain their different effects on MMP-13 production.
There are multiple kinases that can activate p38α33 while less is known about specific p38γ activation. However, selective activation of p38 isoforms has been reported and has been shown to be mediated by the complexes formed between the kinases that activate p38 (MAPK kinases or MKKs) and the specific p38 isoforms34. Although MKK6 can activate either p38α or p38γ, preferential activation of p38α has been noted such that lower levels of MMK6 activity result in activation of p38α and much higher levels are needed before p38γ is activated35.
In summary, our data show that activation of p38γ may decrease production of MMP-13 in human chondrocytes. Further studies are indicated to determine the mechanism for this effect which differs significantly from p38α. To date, the studies that have focused on p38 inhibition as a potential therapy for arthritis have not considered p38 isoform specificity. A recent study demonstrated more severe, rather than less severe, OA-like changes in mice expressing a dominant negative p38 construct under control of the type II collagen promoter36. The dominant negative construct used in that study had mutations in the p38 phosphorylation sites (Thr180 and Tyr182) that are required to activate p38. It was not determined if this construct specifically inhibited p38α activation or if p38γ, which shares the activating phosphorylation sites, may also have been inhibited. Based on our findings, a therapeutic approach to inhibit cartilage degradation would be to alter the balance in p38 activity to favor p38γ over p38α.
Acknowledgments
We are grateful to the National Disease Research Interchange (Philadelphia, PA) and the Gift of Hope Organ and Tissue Donor Network (Elmhurst, IL) and Drs. Arkady Margulis and Marcello DelCarlo for providing normal cartilage tissue. We would like to thank Dr. Lang and Dr. Martin from Wake Forest University Orthopedic Surgery Department for providing cartilage tissue from knee replacements. We also thank Dr. David Engelberg from Hebrew University of Jerusalem for providing p38 constitutively active plasmids, Dr. Harold Erickson at Duke University for recombinant fibronectin fragment, Dr. Susan Chubinskaya at Rush University for recombinant OP-1, and Rebecca Neiberg at Wake Forest University for help with statistical analysis.
Role of funding source
This work was supported by research grants from NIAMS (AR 49003) and the NIA (AG16697).The funding sources had no role in study design, collection of data, analysis of results or preparation of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
D. Long and R. Loeser both contributed to study design, analysis and interpretation of results, drafting of the article, and revisions. D. Long also contributed to data acquisition. Both authors have approved the final article.
Conflict of interest
The authors have no conflicts to disclose.
References
- 1.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 2.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, Chen C, Li Z, Guo W, Gegner JA, Lin S, et al. Characterization of the structure and function of a new mitogen-activated protein kinase (p38beta) J Biol Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Jiang Y, Ulevitch RJ, Han J. The primary structure of p38 gamma: a new member of p38 group of MAP kinases. Biochem Biophys Res Commun. 1996;228:334–340. doi: 10.1006/bbrc.1996.1662. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, et al. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J Biol Chem. 1997;272:30122–30128. doi: 10.1074/jbc.272.48.30122. [DOI] [PubMed] [Google Scholar]
- 6.Homandberg GA. Potential regulation of cartilage metabolism in osteoarthritis by fibronectin fragments. Front Biosci. 1999;4:D713–D730. doi: 10.2741/homandberg. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smeets TJ, Kraan MC, Galjaard S, Youssef PP, Smith MD, Tak PP. Analysis of the cell infiltrate and expression of matrix metalloproteinases and granzyme B in paired synovial biopsy specimens from the cartilage-pannus junction in patients with RA. Ann Rheum Dis. 2001;60:561–565. doi: 10.1136/ard.60.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002;46:2368–2376. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- 11.Loeser RF, Forsyth CB, Samarel AM, Im HJ. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J Biol Chem. 2003;278:24577–24585. doi: 10.1074/jbc.M304530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding L, Guo D, Homandberg GA. The cartilage chondrolytic mechanism of fibronectin fragments involves MAP kinases: comparison of three fragments and native fibronectin. Osteoarthritis Cartilage. 2008;16:1253–1262. doi: 10.1016/j.joca.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Korb A, Tohidast-Akrad M, Cetin E, Axmann R, Smolen J, Schett G. Differential tissue expression and activation of p38 MAPK alpha, beta, gamma, and delta isoforms in rheumatoid arthritis. Arthritis Rheum. 2006;54:2745–2756. doi: 10.1002/art.22080. [DOI] [PubMed] [Google Scholar]
- 14.Masuko-Hongo K, Berenbaum F, Humbert L, Salvat C, Goldring MB, Thirion S. Up-regulation of microsomal prostaglandin E synthase 1 in osteoarthritic human cartilage: critical roles of the ERK-1/2 and p38 signaling pathways. Arthritis Rheum. 2004;50:2829–2838. doi: 10.1002/art.20437. [DOI] [PubMed] [Google Scholar]
- 15.Ruettger A, Schueler S, Mollenhauer JA, Wiederanders B, Cathepsins BK. L are regulated by a defined collagen type II peptide via activation of classical protein kinase C and p38 MAP kinase in articular chondrocytes. J Biol Chem. 2008;283:1043–1051. doi: 10.1074/jbc.M704915200. [DOI] [PubMed] [Google Scholar]
- 16.Kuma Y, Sabio G, Bain J, Shpiro N, Marquez R, Cuenda A. BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J Biol Chem. 2005;280:19472–19479. doi: 10.1074/jbc.M414221200. [DOI] [PubMed] [Google Scholar]
- 17.Leahy DJ, Aukhil I, Erickson HP. 2.0 A crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84:155–164. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- 18.Muehleman C, Bareither D, Huch K, Cole AA, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5:23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- 19.Avitzour M, Diskin R, Raboy B, Askari N, Engelberg D, Livnah O. Intrinsically active variants of all human p38 isoforms. FEBS J. 2007;274:963–975. doi: 10.1111/j.1742-4658.2007.05644.x. [DOI] [PubMed] [Google Scholar]
- 20.Pulai JI, Chen H, Im HJ, Kumar S, Hanning C, Hegde PS, et al. NF-κB Mediates the Stimulation of Cytokine and Chemokine Expression by Human Articular Chondrocytes in Response to Fibronectin Fragments. J Immunol. 2005;174:5781–5788. doi: 10.4049/jimmunol.174.9.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratt PF, Bokemeyer D, Foschi M, Sorokin A, Dunn MJ. Alterations in subcellular localization of p38 MAPK potentiates endothelin-stimulated COX-2 expression in glomerular mesangial cells. J Biol Chem. 2003;278:51928–51936. doi: 10.1074/jbc.M309256200. [DOI] [PubMed] [Google Scholar]
- 22.Court NW, dos Remedios CG, Cordell J, Bogoyevitch MA. Cardiac expression and subcellular localization of the p38 mitogen-activated protein kinase member, stress-activated protein kinase-3 (SAPK3) J Mol Cell Cardiol. 2002;34:413–426. doi: 10.1006/jmcc.2001.1523. [DOI] [PubMed] [Google Scholar]
- 23.Qi X, Pohl NM, Loesch M, Hou S, Li R, Qin JZ, et al. p38alpha antagonizes p38gamma activity through c-Jun-dependent ubiquitin-proteasome pathways in regulating Ras transformation and stress response. J Biol Chem. 2007;282:31398–31408. doi: 10.1074/jbc.M703857200. [DOI] [PubMed] [Google Scholar]
- 24.Lee JC, Kassis S, Kumar S, Badger A, Adams JL. p38 mitogen-activated protein kinase inhibitors--mechanisms and therapeutic potentials. Pharmacol Ther. 1999;82:389–397. doi: 10.1016/s0163-7258(99)00008-x. [DOI] [PubMed] [Google Scholar]
- 25.Cuenda A, Cohen P, Buee-Scherrer V, Goedert M. Activation of stress-activated protein kinase-3 (SAPK3) by cytokines and cellular stresses is mediated via SAPKK3 (MKK6); comparison of the specificities of SAPK3 and SAPK2 (RK/p38) EMBO J. 1997;16:295–305. doi: 10.1093/emboj/16.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan Z, Bau B, Yang H, Soeder S, Aigner T. Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes, but less responsive to catabolic stimulation with interleukin-1beta. Arthritis Rheum. 2005;52:136–143. doi: 10.1002/art.20725. [DOI] [PubMed] [Google Scholar]
- 27.Im HJ, Pacione C, Chubinskaya S, Van Wijnen AJ, Sun Y, Loeser RF. Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1beta-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J Biol Chem. 2003;278:25386–25394. doi: 10.1074/jbc.M302048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Junttila MR, Ala-Aho R, Jokilehto T, Peltonen J, Kallajoki M, Grenman R, et al. p38alpha and p38delta mitogen-activated protein kinase isoforms regulate invasion and growth of head and neck squamous carcinoma cells. Oncogene. 2007;26:5267–5279. doi: 10.1038/sj.onc.1210332. [DOI] [PubMed] [Google Scholar]
- 29.Pramanik R, Qi X, Borowicz S, Choubey D, Schultz RM, Han J, et al. p38 isoforms have opposite effects on AP-1-dependent transcription through regulation of c-Jun. The determinant roles of the isoforms in the p38 MAPK signal specificity. J Biol Chem. 2003;278:4831–4839. doi: 10.1074/jbc.M207732200. [DOI] [PubMed] [Google Scholar]
- 30.Askari N, Diskin R, Avitzour M, Capone R, Livnah O, Engelberg D. Hyperactive variants of p38alpha induce, whereas hyperactive variants of p38gamma suppress, activating protein 1-mediated transcription. J Biol Chem. 2007;282:91–99. doi: 10.1074/jbc.M608012200. [DOI] [PubMed] [Google Scholar]
- 31.Hegen M, Gaestel M, Nickerson-Nutter CL, Lin LL, Telliez JB. MAPKAP kinase 2-deficient mice are resistant to collagen-induced arthritis. J Immunol. 2006;177:1913–1917. doi: 10.4049/jimmunol.177.3.1913. [DOI] [PubMed] [Google Scholar]
- 32.Jones SW, Brockbank SM, Clements KM, Le Good N, Campbell D, Read SJ, et al. Mitogen-activated protein kinase-activated protein kinase 2 (MK2) modulates key biological pathways associated with OA disease pathology. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Kang YJ, Seit-Nebi A, Davis RJ, Han J. Multiple activation mechanisms of p38alpha mitogen-activated protein kinase. J Biol Chem. 2006;281:26225–26234. doi: 10.1074/jbc.M606800200. [DOI] [PubMed] [Google Scholar]
- 34.Enslen H, Brancho DM, Davis RJ. Molecular determinants that mediate selective activation of p38 MAP kinase isoforms. EMBO J. 2000;19:1301–1311. doi: 10.1093/emboj/19.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alonso G, Ambrosino C, Jones M, Nebreda AR. Differential activation of p38 mitogen-activated protein kinase isoforms depending on signal strength. J Biol Chem. 2000;275:40641–40648. doi: 10.1074/jbc.M007835200. [DOI] [PubMed] [Google Scholar]
- 36.Namdari S, Wei L, Moore D, Chen Q. Reduced limb length and worsened osteoarthritis in adult mice after genetic inhibition of p38 MAP kinase activity in cartilage. Arthritis Rheum. 2008;58:3520–3529. doi: 10.1002/art.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]