Abstract
There are considerable data addressing sex-related differences in cardiovascular system aging and disease risk/progression. Sex differences in cardiovascular aging are evident during resting conditions, exercise, and other acute physiological challenges (e.g., orthostasis). In conjunction with these sex-related differences—or perhaps even as an underlying cause—the impact of cardiorespiratory fitness and/or physical activity on the aging cardiovascular system also appears to be sex-specific. Potential mechanisms contributing to sex-related differences in cardiovascular aging and adaptability include changes in sex hormones with age as well as sex differences in baseline fitness and the dose of activity needed to elicit cardiovascular adaptations. The purpose of the present paper is thus to review the primary research regarding sex-specific plasticity of the cardiovascular system to fitness and physical activity in older adults. Specifically, the paper will (1) briefly review known sex differences in cardiovascular aging, (2) detail emerging evidence regarding observed cardiovascular outcomes in investigations of exercise and physical activity in older men versus women, (3) explore mechanisms underlying the differing adaptations to exercise and habitual activity in men versus women, and (4) discuss implications of these findings with respect to chronic disease risk and exercise prescription.
Keywords: Gender differences, Cardiorespiratory fitness, Exercise, Cardiovascular risk
Introduction
There is growing awareness on the part of both investigators and clinicians about sex-related differences in cardiovascular aging and disease risk/progression in humans, as demonstrated by a number of recent reviews on these topics (Baylis 2009; Fu et al. 2008; Meyer et al. 2008; Ostadal et al. 2009; Huxley 2007; McBride et al. 2005). Sex differences in cardiovascular system aging are evident at rest, during acute physiological challenges (exercise, orthostasis, environmental stress), and in response to pharmacological drug infusions. In addition, there appear to be differences between the sexes in the plasticity of various cardiovascular system components and risk factors in response to chronic physical activity and exercise with advancing age. This heterogeneity between aging women and men is evident in the aerobic exercise-induced adaptive responses of the heart (Spina et al. 1996), the arterial vasculature (Black et al. 2009; Martin et al. 1991; Parker et al. 2007, 2008), and autonomic control of the circulation (Fu et al. 2008; Ridout et al. 2010). Differences in the responsiveness of the cardiovascular system to physical activity and exercise in men versus women may in fact underlie some of the observed sex differences in cardiovascular aging. While the possible mechanisms contributing to such sex-related differences in cardiovascular adaptability and disease risk invariably include age-related alterations in sex hormones (particularly the loss of estrogen in women), the role of other factors such as baseline fitness and physical activity levels and differing dose–response relationships in women and men are also important to consider. The primary purpose of the present paper is to review findings from investigations of cardiovascular system aging and adaptation in older adults. Recognition of sex differences in cardiovascular aging, adaptability, and disease/dysfunction has important implications for understanding the biology of aging, and for the development of interventions (perhaps sex-specific) to ameliorate cardiovascular deconditioning and disease progression.
Observed age differences in the cardiovascular system between the sexes
Resting characteristics
Changes in certain cardiac and autonomic characteristics are sex-specific in older adults; specifically, older women appear to exhibit greater declines in these resting parameters that may augment their cardiovascular disease risk. For example, a recent study of 1,333 healthy individuals (ages 10–89) free of heart disease and hypertension revealed that peak early mitral annular velocity (E′) deteriorates more significantly with age in women than in men (Okura et al. 2009). A reduced E′ reflects reduced left ventricular relaxation (i.e., impaired diastolic filling) and is correlated with decreased exercise capacity in both healthy adults as well as patients with cardiovascular disease (Okura et al. 2000; Terzi et al. 2007). Authors of the study speculated that the greater age-related decline observed in older women could explain in part sex differences in cardiovascular disease risk and the greater number of cardiovascular deaths in U.S. women than men (Rosamond et al. 2008). Similarly, it is well-documented that muscle sympathetic nerve activity increases to a greater extent with age in women than men, and this finding has also been proposed as a factor contributing to the greater influence of advancing age on hypertension and cardiovascular disease progression in women (Matsukawa et al. 1998; Narkiewicz et al. 2005). Lipsitz et al. (2006; Sevre et al. 2001) also found evidence for increased sympathetic activation during an orthostatic challenge, as elderly hypertensive women demonstrate greater systemic vascular resistance and low-frequency systolic pressure variability (an indicator of sympathetic vascular control) during tilt. The relationship between impaired autonomic function and hypertension in older women has also been observed with studies of baroreflex responsiveness, as Sevre et al. (2001) observed that middle-aged hypertensive women—but not men—exhibited reduced cardiovagal baroreflex sensitivity, which was correlated to a higher systolic blood pressure. Recently, Fu et al. (2008; Lloyd-Jones et al. 2005) reviewed these three studies and proposed that these sex differences in autonomic function could be a potential mechanism underlying the higher prevalence of hypertension in older women. These collective resting data indicate that older women exhibit greater declines in indices of both cardiac and autonomic cardiovascular function, which may contribute to the greater cardiovascular morbidity and mortality observed in older postmenopausal women.
Exercising central hemodynamics
There are also sex differences in the effect of age on central (i.e., cardiac and pressor) responses to large muscle mass dynamic exercise. For example, Weiss et al. (2006) reported that maximal cardiac output decreases more steeply in the later decades (ages 60–90) in older men (≥age 60) than older women, as the slope of the decrease in cardiac output is over two-fold higher in older men versus women. A similar trend was observed with respect to maximal oxygen uptake in the same study. Similarly, Goldspink et al. (2009) reported that maximal cardiac power output and reserve decrease by 20–25% in men across the ages 20–75, correlating with a similar (~21%) decrease in left ventricular mass, whereas the same variables are preserved in aging women. In contrast, older women exhibit an exaggerated pressure response to exercise relative to their younger counterparts (Ogawa et al. 1992) that has also been observed during small muscle mass leg exercise (Martin et al. 1991; Parker et al. 2008). This exaggerated pressor response is not as pronounced in older men.
Exercising peripheral hemodynamics
Our laboratory has noted sex differences in peripheral hemodynamic responses to leg exercise. Although leg blood flow and vascular conductance responses during graded leg cycling exercise are reduced with age in both chronically endurance-trained (Proctor et al. 1998; Wahren et al. 1974) or very sedentary (Beere et al. 1999; Lawrenson et al. 2003) men, we have reported that normally active older men (i.e., men who are neither extremely sedentary nor highly trained, as defined by oxygen uptake values falling within the 20–80th percentile of ageand sex-specific norms) do not exhibit attenuated leg blood flow responses when compared with young men. However, a demographically similar group of normally active older women demonstrated blunted leg hyperemic and vascular conductance responses relative to their young counterparts during the same mode of submaximal exercise (Proctor et al. 2003). We confirmed evidence of an age–sex interaction in the leg hyperemic response to dynamic exercise in normally active adults utilizing an exercise model not thought to be limited by age-related reductions in the central pumping capacity of the heart. Specifically, during single leg knee extensor exercise, older women, but not men, exhibit blunted hyperemic and vasodilator responses to graded exercise relative to their young counterparts, a finding not explained solely by age-associated differences in quadriceps muscle mass or the mechanics of the knee extensor exercise model (Parker et al. 2007, 2008). Interestingly, Goldspink et al. (2009) recently reported the opposite trend with respect to forearm hyperemic responses, as authors reported a greater decrease in peak reactive hyperemic forearm blood flow in older men than women. Unfortunately, to date, there is substantial heterogeneity in the effect of human aging alone on peripheral hemodynamic responses to exercise. That is, leg blood flow during knee extensor exercise is reduced with age in completely sedentary men (Lawrenson et al. 2003) while forearm hemodynamic responses to handgrip exercise are well preserved with age in normally active men (Donato et al. 2006; Jasperse et al. 1994), both findings in contrast to the work presented above. This disparity in the literature on men limits our ability at present to discern whether there exist fundamental differences in the impact of biological aging on blood flow to exercising muscle between women and men, especially when the effect of training status and chronic activity are taken into account.
Balance between central and peripheral hemodynamics
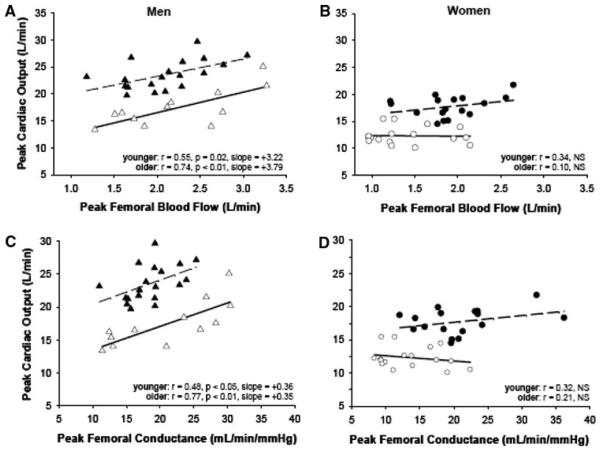
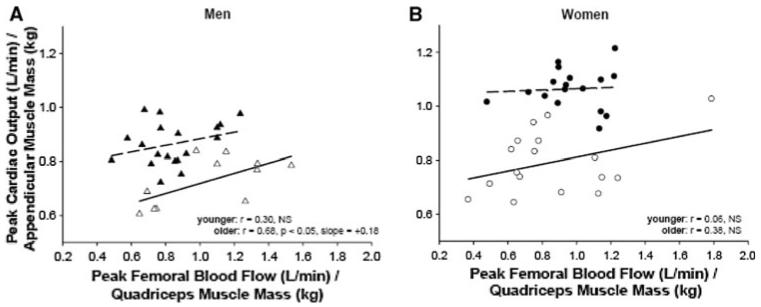
Recently, our laboratory also reported sex-specificity in the effect of age on the balance between maximal cardiac output and peripheral vascular reserve (Ridout et al. 2010). Young and older healthy men and women performed treadmill and knee extensor exercise to fatigue on separate days to assess maximal cardiac output and peak femoral blood flow, respectively. Interestingly, maximal cardiac output was positively correlated with peak femoral blood flow in young and older men but not in women (in either age group) (Fig. 1). Furthermore, normalization of outcomes to muscle mass (i.e., cardiac output to appendicular muscle mass and peak femoral blood flow to quadriceps mass) reduced the correlation between these variables in younger men but not older men, with no change in women (Fig. 2). We thus concluded that aging is associated with a more pronounced sex difference in the relationship between the pumping capacity of the heart and peripheral vascular reserve (i.e., a much stronger association between leg vasodilator reserve and systemic aerobic capacity in older men than women), a finding also in line with data investigating the relationship between maximal aerobic capacity and calf vasodilatory capacity (Martin et al. 1991).
Fig. 1.
Peak cardiac output during treadmill exercise versus peak quadriceps blood flow during knee extensor exercise; filled triangle younger men (n = 19), open triangle older men (n = 12; a); filled circle younger women (n = 17), open circle older women (n = 17; b). Peak cardiac output during treadmill exercise versus femoral artery conductance during peak knee extensor exercise; filled triangle younger men, open triangle older men (c); filled circle younger women, open circle older women (d). From Ridout et al. (2010). Reproduced with permission from the American Physiological Society
Fig. 2.
Peak cardiac output during treadmill exercise normalized to appendicular muscle mass versus femoral blood flow during peak knee extensor exercise normalized to quadriceps muscle mass; filled triangle younger men (n = 19), open triangle older men (n = 12; a); filled circle younger women (n = 17), open circle older women (n = 17; b). From Ridout et al. (2010). Reproduced with permission from the American Physiological Society
Vascular responses to non-exercise stimuli
Finally, while there are well-documented sex differences in young adults in responses to intra-arterial infusions of vasodilators and vasoconstrictors (Kneale et al. 1997, 2000), similar findings have only recently been established in older adults. Stauffer et al. measured forearm blood flow responses to infusions of the vasoconstrictor endothelin-1 as well as endothelin A and B (ETA and ETB, respectively) receptor antagonists in middle-aged and older men and women. Older men demonstrated significantly greater dilation to the ETA receptor antagonist, suggesting that men are under greater ETA-mediated vasoconstrictor tone than older women (Stauffer et al. 2010). This finding is particularly relevant given that ETA is the predominant endothelin receptor subtype located in the coronary vasculature, perhaps contributing to sex differences in coronary artery disease. It also suggests that age-related changes in vasoconstrictor tone and the regulation of peripheral vascular resistance are sex-specific in older adults, given the work presented in previous paragraphs regarding heightened sympathetic nervous system activation in older women in contrast to the augmented influence of ETA in older men.
Evidence for a sex-specific modulatory influence of fitness and physical activity on the cardiovascular system
Resting characteristics
Emerging cross-sectional and longitudinal evidence suggests that just as the effect of age on the cardiovascular system differs in men versus women, the influence of acute and chronic physical activity on modifying certain biomarkers of cardiovascular aging also demonstrates sexspecificity in older humans. With respect to resting cardiovascular outcomes, for example, Wilund et al. reported an inverse association between VO2 max and the number of coronary artery calcified lesions in older men but not older women, and in the same investigation, fetuin-A, a systemic inhibitor of coronary calcification, was positively correlated with VO2 max in older men but again not in older women. In contrast, Havlik et al. (2005) found that increased walking frequency measured over a 24-month period in the Activity Counseling Trial was predictive of reduced aortic pulse wave velocity, an index of arterial stiffness, in older women but not in men. The authors hypothesized that there might be a threshold effect of walking on pulse wave velocity such that the lower fitness of the older women relative to older men at the beginning of the study placed women in a specific range of central vascular adaptability. Collier (2008) also reported that 4 weeks of resistance training lowered resting diastolic blood pressure to a greater extent in middle-aged, hyper-tensive women than men. Interestingly, other age-related structural vascular characteristics, such as increased carotid artery compliance and femoral artery intima-medial thickness, appear beneficially influenced by chronic aerobic fitness in both men and women (Moreau et al. 2006a, b; Tanaka et al. 1998, 2000). However, it is notable that even in these latter studies that have shown favorable effects of chronic aerobic fitness on cardiovascular outcomes, the magnitude of the effect may be sex-dependent. For example, Moreau et al. (2006a, b) found that age-associated increase in IMT was 33% smaller in endurance-trained compared with sedentary men, whereas the magnitude of the effect of chronic physical activity was half as great in older women (i.e., IMT was 15% smaller in endurance-trained compared with sedentary postmenopausal women). In contrast, Tanaka and colleagues (Tanaka et al. 1998, 2000) observed that habitual physical activity abolished age-related differences in central arterial stiffness in older women but only attenuated the effect of age on carotid compliance in older men.
Exercising central hemodynamics
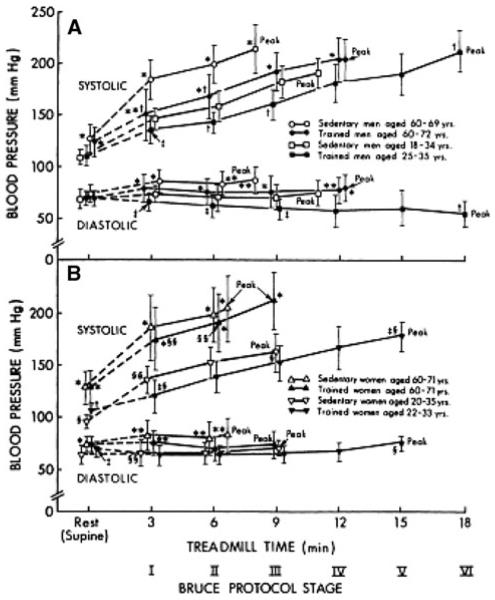
Certain central hemodynamic responses to exercise in older adults also have sex-specific relationships to physical activity and fitness, with several studies reporting a more beneficial effect of fitness and physical activity in older men than women. For example, chronic physical training is associated with a lower blood pressure response during submaximal treadmill exercise in young and older men and young women, but this is often less apparent in older women (Martin et al. 1991) (Fig. 3). Improvements in peak calf conductance in older men and women in the study of Martin et al. were also differentially mediated through weight loss (men) and adipose tissue loss (women). Spina (1999) provided the first evidence that left ventricular remodeling and changes in filling dynamics observed in older men following exercise training are absent in women. This sex difference in cardiac remodeling with exercise training has also been observed in middle-aged men and women with Type 2 diabetes (McGavock et al. 2004; Poirier et al. 2000) and in older adults with isolated diastolic dysfunction (Haykowsky et al. 2004). However, it should again be noted that several studies have shown either no sex difference in adaptations (Ishikawa et al. 1999; Kohrt et al. 1991; blood pressure and maximal oxygen uptake, respectively) to exercise training in older adults or, conversely, a beneficial effect of female sex on improvements associated with exercise training. For example, with respect to the latter, Soto et al. (2008) demonstrated that older women, but not older men, exhibited improved myocardial fatty acid metabolism in response to beta-adrenergic stimulation (dobutamine infusion) following 11 months of aerobic exercise training.
Fig. 3.
Plots of systolic and diastolic blood pressures at rest, during the final 30 s of each stage of the Bruce Protocol, and at peak exercise is sedentary and trained younger and older men and women. Data are mean ± SD. Peak, peak exercise. *p < 0.01, **p < 0.05 versus younger subjects of corresponding gender and training status at the same stage of exercise. From Martin et al. (1991). Reproduced with permission from the American Heart Association
Exercising peripheral hemodynamics
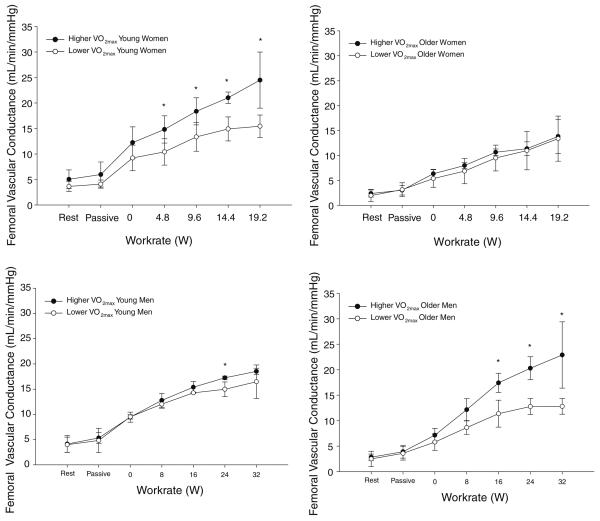
Sex-specificity of peripheral vasodilatory responsiveness with chronic fitness and/or acute exercise training is also evident. For example, Martin et al. (1990) found that aerobic training significantly increased peak calf blood flow and vascular conductance in older men while only marginally increasing these responses in older women, suggesting that the influence of fitness and/or physical activity on leg blood flow in older adults may be sex-specific. Similarly, in our cross-sectional investigation of blood flow and vascular conductance responses to increasing knee extension work rates in young and older men and women, we modeled VO2 max (an estimate of cardiorespiratory fitness) as a covariate in the statistical analyses (Parker et al. 2008). Interestingly, aerobic fitness (VO2 max) differentiated the leg hemodynamic responses to exercise in all subject groups except older women, such that higher aerobic fitness was associated with greater leg hemodynamic responses to knee extensor exercise in young and older men and young women (Fig. 4). For example, fitness was a significant covariate in the leg blood flow or vascular conductance versus workrate relation in young women (p < 0.01 for both comparisons) and a marginal covariate in older (p = 0.09 for both comparisons) and younger (p = 0.02 and 0.08, respectively) men. In contrast, aerobic fitness was a highly insignificant covariate in both analyses in older women (p = 0.94 and 0.87, respectively). These findings are in agreement with our previously published work regarding the effect of age on peak calf vasodilatory capacity in men versus women, in which we investigated the influence of fitness on observed age differences in peak calf conductance (evoked by limb ischemia) by comparing outcomes in young and older men (Proctor et al. 2005) and women (Ridout et al. 2005) matched for VO2peak. In men, matching older versus young men for fitness abolished the age-associated decline in peak calf conductance, whereas in women, the reduction in peak calf conductance with age persisted despite matching older and younger subjects for fitness. It should be noted, though, that Martin et al. (1991) found that maximal leg vasodilatory capacity (evoked by fatiguing ischemic exercise rather than ischemia alone, as used in our studies assessing peak vascular conductance described previously) was higher in both older trained men and women relative to their sedentary counterparts. Again, observed sex differences in the modulatory effect of fitness and/or physical activity on the aging cardiovascular system in humans appear not to be universal but rather unique to specific vascular outcomes, vasodilator pathways or arterial regions.
Fig. 4.
Representation of age- and sex- specific cardiorespiratory fitness effect on leg vascular conductance response to knee extensor exercise: femoral vascular conductance expressed as group means ± SD at absolute knee extensor exercise work rates in young women (upper left), older women (upper right), young men (lower left) and older men (lower right) from Parker et al. (2008). For each age and sex group, ten subjects were separated into groups by higher and lower maximal oxygen uptake (VO2 max) scores (i.e., five most vs. five least fit in study population). Quadriceps muscle mass (estimated anthropometrically as described in Parker et al. 2008) was not significantly different between higher versus lower fit subjects in any age–sex group. Asterisk indicates significant (p < 0.05) difference between higher versus lower fit subjects at each work rate
Vascular responses to non-exercise stimuli
Finally, there is very recent evidence indicating that vascular responses to non-exercise stimuli are influenced by chronic exercise differentially in older men versus women. Black et al. (2009) investigated conduit artery vasodilation (with brachial artery flow-mediated dilation, or FMD) in response to 5 min forearm ischemia in both sedentary and fit older men and women relative to young controls. Interestingly, fitness did not distinguish between conduit dilator responses in men, whereas older sedentary women exhibited significantly lower brachial artery dilator function than older fit women or young women. Moreover, exercise training resulted in a beneficial training adaptation (i.e., improved brachial vasodilation) in older sedentary women only. Older sedentary men did not respond to exercise training with improved FMD. It should be noted that these findings are in contrast to published data from very young adults and older men only. Pahkala et al. (2008) found that the chronic physical activity was directly associated with brachial artery flow-mediated dilation in Finnish adolescent boys but not girls, but attributed the lack of association in girls to the lower chronic physical activity levels of the female study population rather than to a true sex difference per se. Similarly, Wray et al. found that FMD is augmented by exercise training in older men when there is a baseline deficit in dilation (Wray et al. 2006); similar results were observed with respect to endothelium-dependent dilation in response to acetylcho-line infusions in older men before and after exercise training (DeSouza et al. 2000). These heterogenous results raise the possibility that the effect of fitness on vascular responses to non-exercise stimuli are dependent on both sex and the magnitude of the initial (i.e., baseline) vasodilatory response in older adults.
Possible explanations for sex-dependent adaptive responses to physical activity with advancing age
Differences in cardiovascular regulation between the sexes
There are fundamental sex differences in vascular responsiveness and cardiovascular regulation, which could explain some of the heterogeneous adaptive responses to physical activity and exercise training between women and men. For example, young women exhibit augmented brachial artery flow-mediated dilation (Levenson et al. 2001; Sarabi et al. 1999) and beta-adrenergic mediated forearm vasodilation (Kneale et al. 2000) relative to young men. Moreover, the forearm vasodilatory response to acetylcholine (Dietz 1999) as well as peak calf reactive hyperemia (Proctor et al. 2005; Ridout et al. 2005) tend to be higher in women, while the forearm vasoconstrictor response to norepinephrine (Bowyer et al. 2001; Kneale et al. 2000) and calf vasoconstrictor response to cold pressor and isometric handgrip maneuvers (Hogarth et al. 2007) are blunted in women relative to men. It has also been reported that plasma catecholamine responses to small muscle isometric exercise are greater in men (Gustafson and Kalkhoff 1982), as are metaboreflex responses (i.e., increases in muscle sympathetic nerve activity) to handgrip exercise (Ettinger et al. 1996). Sex differences in adrenergic/vasodilator receptor density, sympathetic vascular transduction, and downstream signaling in the vasculature (Keys et al. 2005; Kneale et al. 2000) could underlie attenuated vaoconstrictor responses in women when compared with men. Young women also exhibit less effective baroreflex buffering of blood pressure than men (Christou et al. 2005) as well as reduced sympathetic nerve activity responses to head-up tilt (Shoemaker et al. 2001). With such widespread evidence of sex differences in vasoregulatory and autonomic balance, as well as arterial compliance (Debasso et al. 2004; van der Heijden-Spek et al. 2000), it is not surprising that the integrative mechanisms by which systemic blood pressure is regulated, both at rest (Hart et al. 2009) and during acute whole body exercise (Adams et al. 1987; Astrand and Rodahl 1974; Ridout et al. 2010; Wiebe et al. 1998), differ between women and men.
Collectively, this large body of evidence supports the hypothesis that sex differences in a host of cardiovascular variables at rest and in response to acute exercise evoke a differential balance of regulatory challenges for the cardiovascular system of aging women compared to men; over the course of a training period (short- or long-term) this could contribute to the heterogeneous effects of fitness and/ or physical activity on cardiovascular outcomes in older adults. In addition, the sex differences in cardiovascular aging presented earlier in this review (e.g., marked increase in sympathetic activation, reduction in diastolic filling and autonomic dysfunction observed in older women) could also interact with the acute response to exercise to further exaggerate sex-specificity of short- and long-term responses to physical activity on cardiovascular aging in older adults.
Influence of sex hormones
The differential exposure to male and female sex hormones could also contribute to the potential sex-specificity of both cardiovascular aging and its modulation by acute and chronic physical activity. Certainly, the influence of female sex hormones in young women and the relatively abrupt cessation of their production at menopause have been shown to have a significant influence on both systemic and local indicators of cardiovascular function. For example, carotid artery stiffness, brachial-ankle pulse wave velocity, blood pressure, and cholesterol increase following meno-pause (Izumi et al. 2006; Peters et al. 1999; Takahashi et al. 2005). In addition, myriad studies have provided evidence that estrogen and progesterone contribute to a cardioprotective phenotype in young women (Vitale et al. 2009). Similarly, testosterone contributes beneficially to cardiovascular function in men (Maggio and Basaria 2009) and the reduction in testosterone associated with age has been correlated to diminished myocardial perfusion and augmented vascular stiffness (Webb et al. 2008). Interestingly, there is an interaction between recreational physical activity and steroid hormones in postmenopausal women such that higher physical activity is associated with lower levels of steroid hormone levels (both testosterone and estradiol) in postmenopausal women (Bertone-Johnson et al. 2009; Chan et al. 2007; McTiernan et al. 2006). In contrast, in men, higher levels of physical activity are positively associated with total and free testosterone (Shiels et al. 2009). Therefore, it is possible that the modulation of the cardiovascular system by physical activity in older adults is in part affected by endogenous levels of sex hormones that differ between men and women.
Sex-specific dose–response of exercise and cardiovascular adaptation
An alternative explanation for sex-specific adaptations of the cardiovascular system to fitness and physical activity is that different acute and/or chronic doses of exercise are required to evoke certain cardiovascular adaptations in men versus women. For example, a recent review by Milne and Noble (2008) presented the hypothesis that the acutely cardioprotective phenotype of young women may in fact necessitate bouts of higher-intensity exercise to evoke chronic myocardial benefits with respect to the heat shock protein hsp70. Moreover, an investigation of the common femoral artery shear rate response to exercise from our laboratory found that both young and older women exhibited a greater rise in shear rate across increasing knee extensor work rates than young or older men (Gonzales et al. 2009). We postulated that this sex difference could have implications for the vascular adaptability to exercise training in men and women. Similarly, Proctor et al. (2001) showed a significant effect of gender on several adaptations to a 9–12 week aerobic exercise training protocol in young adults. Specifically, young men did not exhibit changes in resting or exercising cardiac output following training, whereas women demonstrated reduced cardiac outputs both at rest and during exercise. Conversely, exercise-induced reductions in splanchnic blood flow with training were significantly less following training in men but not women. Collectively, these data raise the possibility that the dose of either acute or chronic physical activity required to affect specific cardiovascular parameters or cellular adaptive pathways may differ in men and women, and could in part underlie sex differences in exercise-induced cardiovascular plasticity.
Implications: sex-specific modulation of chronic disease by physical activity and inactivity
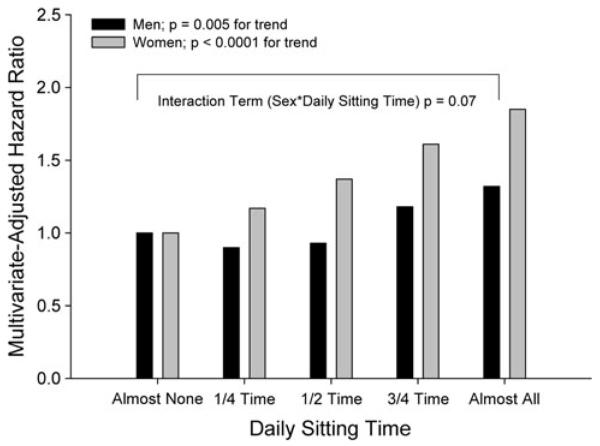
On an epidemiological basis, there is accumulating evidence of sex-specific protection against disease risk associated with fitness and physical activity. Specifically, chronic physical activity mitigates several pathological disease states and/or risk factors more substantially in men than women. For example, Valentine et al. (2009) reported that neither physical activity (assessed with questionnaire) nor cardiorespiratory fitness (assessed with a maximal oxygen consumption treadmill test) were associated with circulating C-reactive protein in older women; in contrast, peak oxygen uptake was inversely associated with C-reactive protein in older men. Moreover, Solbu et al. (2008) reported that hard physical activity was protective against an increase in urinary albumin-excretion (a cardiovascular risk factor that clusters with metabolic syndrome) in men but not women. Similarly, physical activity reduces the risk of metabolic syndrome in young men more substantially than in young women (Remsberg et al. 2007). Interestingly, there is also evidence that physical inactivity may exert a more harmful influence in women, as several studies have suggested stronger associations between the amount of sedentary activity and metabolic profiles in women versus men. For example, Dunstan et al. (2007) found a strong deleterious relationship between physical inactivity (as indicated by hours of television-viewing time) and glycemic measures in Australian women but not men. More recently, the authors extended that finding to metabolic variables, finding that television-viewing time was more strongly associated with a number of negative metabolic risk variables in women than men in over 4,000 Australian adults (Healy et al. 2008). The stronger effect of inactivity on health risk factors in women even extends to mortality risk, as the effect of both daily sitting time and low fitness on mortality also appears to be at least marginally sex-specific. For example, results from a recent investigation by Katzmarzyk et al. (2009) indicate substantially higher hazard ratios for all-cause (Fig. 5) and cardiovascular disease mortality in women than men with increasing daily sitting time, although the effect of sex on the multivariate-adjusted model for daily sitting time on mortality risk was not statistically significant at p = 0.07 and 0.08, respectively. Moreover, the relative risk of mortality associated with low fitness is approximately 40% higher in women than men (i.e., adjusted relative mortality risk of 2.10 vs. 1.52; Blair et al. 1996). Collectively, these studies suggest that the modulation of cardiovascular aging by both sex and physical activity/fitness is also apparent in epidemiological associations of chronic disease risk, with greater disease protection conferred to men (vs. women) in many studies investigating the influence of chronic physical activity and inactivity on outcomes. Whether or not the sex-specific modulation of disease risk is related to or independent of the age–sex interaction of habitual physical activity on cardiovascular adaptations as described above must be elucidated by further research.
Fig. 5.
The effect of daily sitting time on all-cause mortality in 17,013 male and female Canadians 18–90 year of age. The amount of time participants spent sitting during work, school and housework was obtained from the 1981 Canada Fitness Survey and categorized as (1) almost none of the time, (2) approximately one-fourth of the time, (3) approximately half of the time, (4) approximately three-fourths of the time, or (5) almost all of the time. Mortality was assessed from Canadian Mortality Database records for the years 1981–1993 and represented as multivariate hazard ratios adjusted for age, smoking, alcohol consumption, leisure time physical activity and Physical Activity Readiness Questionnaire. Graph created from data contained in Table 3, Katzmarzyk et al. (2009)
Implications: exercise prescription
Regular physical activity is widely recommended as a nonpharmacological means of combating age-related changes in body composition, losses of functional capacity, and reducing the risk or delaying the development of chronic degenerative conditions. Exercise guidelines for improving cardiovascular health and reducing the risk of cardiovascular events in previously sedentary middle-aged and older adults generally emphasize moderately intense, large muscle mass activities such as regular walking and/or leg cycling at a brisk pace. Such aerobic exercise prescriptions have proven effective in favorably modifying certain cardiovascular parameters (resting systolic blood pressure, central artery compliance, peak exercise capacity and O2 uptake) (Chodzko-Zajko et al. 2009; DeSouza et al. 2000; Tanaka et al. 2000). However, sex differences in the magnitude of certain cardiovascular adaptations to exercise may make the standard exercise prescription less effective for optimizing cardiovascular health uniformly in men and women. For example, maximal cardiac stroke volume and leg vasodilator capacity appear to be less modifiable in older women compared to older men and younger adults (Martin et al. 1990; Ridout et al. 2005; Spina 1999). Considering that the prevalence of aerobic exercise training program “non-responders” (i.e., no increase in VO2 max after 6 months) increases with increasing age and lower baseline fitness in women exposed to lower, more commonly prescribed exercise intensities (~50% of VO2 max; Sisson et al. 2009), one could predict that a typical aerobic walking program would likely fall short of providing the optimal therapeutic benefits in these central and peripheral responses for many older women. It may be, therefore, that in order for older women to experience equal improvements in these specific outcomes as their male counterparts, moderately higher intensities of aerobic exercise, and/or ancillary lifestyle or pharmacological treatments may be necessary (Paterson et al. 2007). Likewise, the lack of effect of exercise training on flow-mediated dilation in older men (Black et al. 2009) may indicate that alternative or complementary therapies are necessary to increase the maximal vasodilator reserve in older men in lieu of aerobic exercise training alone.
Modulation of cardiovascular aging by additional factors
There is growing recognition on part of both researchers and clinicians that the effect of aging on the cardiovascular system is sex-specific. The purpose of this review has been to present collective evidence suggesting that, additionally, the factors modifying cardiovascular aging may also be sex-specific. Specifically, we have focused on the modulatory effects of habitual physical activity and/or cardio-respiratory fitness and the resultant sex-specific cardiovascular adaptations. However, it should be noted that emerging evidence supports sex-specific modulation of the cardiovascular system by other factors, such as oxidative stress, genetics and pharmacological treatment, as well. For example, the influence of reductions in oxidative stress on resting leg blood flow (Jablonski et al. 2007; Moreau et al. 2007) is sex-dependent: acute ascorbic acid infusion fully restores the blunted resting leg blood flow observed in older men to the normal values observed in young men, whereas the same intervention only partially restores the blunted resting leg blood flow exhibited by older women (i.e., resting leg blood flow is improved but not fully restored to levels observed in young women). In contrast, carotid artery compliance is improved by reducing oxidative stress (again with acute ascorbic acid infusion) in postmenopausal women but not older men (Eskurza et al. 2004; Moreau et al. 2005). Additional examples include sex-specificity in the effect of perilipin haplotype on cardiovascular outcomes in older adults (Jenkins et al. 2009), as well as the efficacy of hypertension medications for reducing blood pressure (Gu et al. 2008).
Summary and future directions/perspectives
The current review presents emerging information regarding modulation of cardiovascular aging by habitual physical activity and cardiorespiratory fitness in men versus women. Unfortunately, data assembled in the current review do not provide a uniform and clear picture regarding the interaction between cardiovascular aging and fitness in humans. For some variables, it appears that fitness and physical activity more strongly influence the cardiovascular system in older men versus women, and for other variables the direction of the sex difference is opposite or even null. The heterogeneity in findings may be attributable to the limited nature of current investigations specifically aimed at determining sex differences in the effect of either exercise training or chronic physical activity on the cardiovascular system. Interestingly, the growing body of evidence regarding chronic disease risk and physical activity/inactivity in men and women also indicates that physical activity plays a less effective role, and physical inactivity a more substantive role, in modulating certain chronic disease risks in women. We postulate that this may be an implication of the sex-specific plasticity of the aging cardiovascular system. Mechanisms such as sex hormones, sex-specificity of acute responses to exercise, and differing dose–response relationships to cardiovascular outcomes in men and women may contribute to the observed differences regarding the influence of physical activity fitness on the cardiovascular system, disease risk, and aging. Substantially more research should be aimed at elucidating mechanisms and therapeutic benefits associated with observed cardiovascular adaptations to short- and long-term exercise training programs in men and women.
At the least, the information presented in this review should encourage investigators who study cardiovascular aging to (1) consider the inclusion of both sexes in their studies, with sufficient numbers to detect sex differences, if possible, and (2) include estimates of physical activity and fitness (with physical activity questionnaires, accelerometers or pedometers, and oxygen uptake tests) with which to then conduct covariate analyses with physical activity and fitness on outcome variables. Such investigations will further our understanding of both exercise prescription as an aging countermeasure as well the mechanisms underlying observed sex differences.
Acknowledgments
The authors would like to acknowledge the assistance of Sandra Smithmyer, Justin Pelberg, Aaron Mishkin, and Samuel Ridout in contributing to research discussed in the current article.
References
- Adams KF, Vincent LM, McAllister SM, el-Ashmawy H, Sheps DS. The influence of age and gender on left ventricular response to supine exercise in asymptomatic normal subjects. Am Heart J. 1987;113:732–742. doi: 10.1016/0002-8703(87)90714-9. [DOI] [PubMed] [Google Scholar]
- Astrand PO, Rodahl K. Textbook of work physiology: physiological bases of exercise. McGraw-Hill; New York: 1974. [Google Scholar]
- Baylis C. Sexual dimorphism in the aging kidney: differences in the nitric oxide system. Nat Rev Nephrol. 2009;5:384–396. doi: 10.1038/nrneph.2009.90. [DOI] [PubMed] [Google Scholar]
- Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- Bertone-Johnson ER, Tworoger SS, Hankinson SE. Recreational physical activity and steroid hormone levels in postmenopausal women. Am J Epidemiol. 2009;170:1095–1104. doi: 10.1093/aje/kwp254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol. 2009;297:H1109–H1116. doi: 10.1152/ajpheart.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SN, Kampert JB, Kohl HW, 3rd, Barlow CE, Macera CA, Paffenbarger RS, Jr, Gibbons LW. Influences of cardio-respiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- Bowyer L, Brown MA, Jones M. Vascular reactivity in men and women of reproductive age. Am J Obstet Gynecol. 2001;185:88–96. doi: 10.1067/mob.2001.114502. [DOI] [PubMed] [Google Scholar]
- Chan MF, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Khaw KT. Usual physical activity and endogenous sex hormones in postmenopausal women: the European prospective investigation into cancer-norfolk population study. Cancer Epidemiol Biomarkers Prev. 2007;16:900–905. doi: 10.1158/1055-9965.EPI-06-0745. [DOI] [PubMed] [Google Scholar]
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111:494–498. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- Collier SR. Sex differences in the effects of aerobic and anaerobic exercise on blood pressure and arterial stiffness. Gend Med. 2008;5:115–123. doi: 10.1016/j.genm.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Debasso R, Astrand H, Bjarnegard N, Ryden Ahlgren A, Sandgren T, Lanne T. The popliteal artery, an unusual muscular artery with wall properties similar to the aorta: implications for susceptibility to aneurysm formation? J Vasc Surg. 2004;39:836–842. doi: 10.1016/j.jvs.2003.12.005. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Dietz NM. Gender and nitric oxide-mediated vasodilation in humans. Lupus. 1999;8:402–408. doi: 10.1177/096120339900800515. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol. 2006;290:H272–H278. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- Dunstan DW, Salmon J, Healy GN, Shaw JE, Jolley D, Zimmet PZ, Owen N. Association of television viewing with fasting and 2-h postchallenge plasma glucose levels in adults without diagnosed diabetes. Diabetes Care. 2007;30:516–522. doi: 10.2337/dc06-1996. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol Heart Circ Physiol. 2004;286:H1528–H1534. doi: 10.1152/ajpheart.00879.2003. [DOI] [PubMed] [Google Scholar]
- Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G, Whisler SK, McClain JM, Smith MB, Yang QX, Sinoway LI. Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol. 1996;80:245–251. doi: 10.1152/jappl.1996.80.1.245. [DOI] [PubMed] [Google Scholar]
- Fu Q, Vongpatanasin W, Levine BD. Neural and nonneural mechanisms for sex differences in elderly hypertension: can exercise training help? Hypertension. 2008;52:787–794. doi: 10.1161/HYPERTENSIONAHA.108.118927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink DF, George KP, Chantler PD, Clements RE, Sharp L, Hodges G, Stephenson C, Reilly TP, Patwala A, Szakmany T, Tan LB, Cable NT. A study of presbycardia, with gender differences favoring ageing women. Int J Cardiol. 2009;137:236–245. doi: 10.1016/j.ijcard.2008.06.086. [DOI] [PubMed] [Google Scholar]
- Gonzales JU, Parker BA, Ridout SJ, Smithmyer SL, Proctor DN. Femoral shear rate response to knee extensor exercise: an age and sex comparison. Biorheology. 2009;46:145–154. doi: 10.3233/BIR-2009-0535. [DOI] [PubMed] [Google Scholar]
- Gu Q, Burt VL, Paulose-Ram R, Dillon CF. Gender differences in hypertension treatment, drug utilization patterns, and blood pressure control among US adults with hypertension: data from the National Health and Nutrition Examination Survey 1999–2004. Am J Hypertens. 2008;21:789–798. doi: 10.1038/ajh.2008.185. [DOI] [PubMed] [Google Scholar]
- Gustafson AB, Kalkhoff RK. Influence of sex and obesity on plasma catecholamine response to isometric exercise. J Clin Endocrinol Metab. 1982;55:703–708. doi: 10.1210/jcem-55-4-703. [DOI] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlik RJ, Phillips CL, Brock DB, Lohman K, Haskell W, Snell P, O'Toole M, Ribisl P, Vaitkevicius P, Spurgeon HA, Lakatta EG, Pullen P. Walking may be related to less vascular stiffness in the Activity Counseling Trial (ACT) Am Heart J. 2005;150:270–275. doi: 10.1016/j.ahj.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Haykowsky MJ, Ezekowitz JA, Armstrong PW. Therapeutic exercise for individuals with heart failure: special attention to older women with heart failure. J Card Fail. 2004;10:165–173. doi: 10.1016/j.cardfail.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Healy GN, Dunstan DW, Salmon J, Shaw JE, Zimmet PZ, Owen N. Television time and continuous metabolic risk in physically active adults. Med Sci Sports Exerc. 2008;40:639–645. doi: 10.1249/MSS.0b013e3181607421. [DOI] [PubMed] [Google Scholar]
- Hogarth AJ, Mackintosh AF, Mary DA. Gender-related differences in the sympathetic vasoconstrictor drive of normal subjects. Clin Sci (Lond) 2007;112:353–361. doi: 10.1042/CS20060288. [DOI] [PubMed] [Google Scholar]
- Huxley VH. Sex and the cardiovascular system: the intriguing tale of how women and men regulate cardiovascular function differently. Adv Physiol Educ. 2007;31:17–22. doi: 10.1152/advan.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Ohta T, Zhang J, Hashimoto S, Tanaka H. Influence of age and gender on exercise training-induced blood pressure reduction in systemic hypertension. Am J Cardiol. 1999;84:192–196. doi: 10.1016/s0002-9149(99)00233-7. [DOI] [PubMed] [Google Scholar]
- Izumi S, Muano T, Mori A, Kika G, Okuwaki S. Common carotid artery stiffness, cardiovascular function and lipid metabolism after menopause. Life Sci. 2006;78:1696–1701. doi: 10.1016/j.lfs.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol. 2007;103:1715–1721. doi: 10.1152/japplphysiol.00533.2007. [DOI] [PubMed] [Google Scholar]
- Jasperse JL, Seals DR, Callister R. Active forearm blood flow adjustments to handgrip exercise in young and older healthy men. J Physiol. 1994;474:353–360. doi: 10.1113/jphysiol.1994.sp020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins NT, McKenzie JA, Damcott CM, Witkowski S, Hagberg JM. Endurance exercise training effects on body fatness, VO2 max, HDL-C subfractions, and glucose tolerance are influenced by a PLIN haplotype in older Caucasians. J Appl Physiol. 2009;108:498–506. doi: 10.1152/japplphysiol.01018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc. 2009;41:998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- Keys JR, Zhou RH, Harris DM, Druckman CA, Eckhart AD. Vascular smooth muscle overexpression of G protein-coupled receptor kinase 5 elevates blood pressure, which segregates with sex and is dependent on Gi-mediated signaling. Circulation. 2005;112:1145–1153. doi: 10.1161/CIRCULATIONAHA.104.531657. [DOI] [PubMed] [Google Scholar]
- Kneale BJ, Chowienczyk PJ, Cockcroft JR, Coltart DJ, Ritter JM. Vasoconstrictor sensitivity to noradrenaline and NGmonomethyl-L-arginine in men and women. Clin Sci (Lond) 1997;93:513–518. doi: 10.1042/cs0930513. [DOI] [PubMed] [Google Scholar]
- Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- Kohrt WM, Malley MT, Coggan AR, Spina RJ, Ogawa T, Ehsani AA, Bourey RE, Martin WH, 3rd, Holloszy JO. Effects of gender, age, and fitness level on response of VO2 max to training in 60–71 yr olds. J Appl Physiol. 1991;71:2004–2011. doi: 10.1152/jappl.1991.71.5.2004. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–H1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- Levenson J, Pessana F, Gariepy J, Armentano R, Simon A. Gender differences in wall shear-mediated brachial artery vasoconstriction and vasodilation. J Am Coll Cardiol. 2001;38:1668–1674. doi: 10.1016/s0735-1097(01)01604-7. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Iloputaife I, Gagnon M, Kiely DK, Serrador JM. Enhanced vasoreactivity and its response to antihypertensive therapy in hypertensive elderly women. Hypertension. 2006;47:377–383. doi: 10.116110.1161/01.HYP.0000202595.69583.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- Maggio M, Basaria S. Welcoming low testosterone as a cardiovascular risk factor. Int J Impot Res. 2009;21:261–264. doi: 10.1038/ijir.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WH, 3rd, Kohrt WM, Malley MT, Korte E, Stoltz S. Exercise training enhances leg vasodilatory capacity of 65-yr-old men and women. J Appl Physiol. 1990;69:1804–1809. doi: 10.1152/jappl.1990.69.5.1804. [DOI] [PubMed] [Google Scholar]
- Martin WH, 3rd, Ogawa T, Kohrt WM, Malley MT, Korte E, Kieffer PS, Schechtman KB. Effects of aging, gender, and physical training on peripheral vascular function. Circulation. 1991;84:654–664. doi: 10.1161/01.cir.84.2.654. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol. 1998;275:R1600–R1604. doi: 10.1152/ajpregu.1998.275.5.R1600. [DOI] [PubMed] [Google Scholar]
- McBride SM, Flynn FW, Ren J. Cardiovascular alteration and treatment of hypertension: do men and women differ? Endocrine. 2005;28:199–207. doi: 10.1385/ENDO:28:2:199. [DOI] [PubMed] [Google Scholar]
- McGavock J, Mandic S, Lewanczuk R, Koller M, Muhll IV, Quinney A, Taylor D, Welsh R, Haykowsky M. Cardiovascular adaptations to exercise training in postmenopausal women with type 2 diabetes mellitus. Cardiovasc Diabetol. 2004;3:3. doi: 10.1186/1475-2840-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, Perri MG, Stanczyk FZ, Van Horn L, Wang CY. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring) 2006;14:1662–1677. doi: 10.1038/oby.2006.191. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Barton M. Need for research on estrogen receptor function: importance for postmenopausal hormone therapy and atherosclerosis. Gend Med. 2008;5(Suppl A):S19–S33. doi: 10.1016/j.genm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Milne KJ, Noble EG. Response of the myocardium to exercise: sex-specific regulation of hsp70. Med Sci Sports Exerc. 2008;40:655–663. doi: 10.1249/MSS.0b013e3181621311. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension. 2005;45:1107–1112. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Gavin KM, Plum AE, Seals DR. Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause. 2006a;13:951–958. doi: 10.1097/01.gme.0000243575.09065.48. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Silver AE, Dinenno FA, Seals DR. Habitual aerobic exercise is associated with smaller femoral artery intima-media thickness with age in healthy men and women. Eur J Cardiovasc Prev Rehabil. 2006b;13:805–811. doi: 10.1097/01.hjr.0000230103.55653.42. [DOI] [PubMed] [Google Scholar]
- Moreau KL, DePaulis AR, Gavin KM, Seals DR. Oxidative stress contributes to chronic leg vasoconstriction in estrogendeficient postmenopausal women. J Appl Physiol. 2007;102:890–895. doi: 10.1152/japplphysiol.00877.2006. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Spina RJ, Martin WH, 3rd, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- Okura H, Inove H, Tomon M, Nishiyama S, Yoshikawa T, Yoshida K, Yoshikawa J. Impact of Doppler-derived left ventricular diastolic performance on exercise capacity in normal individuals. Am Heart J. 2000;139:716–722. doi: 10.1016/s0002-8703(00)90054-1. [DOI] [PubMed] [Google Scholar]
- Okura H, Takada Y, Yamabe A, Kubo T, Asawa K, Ozaki T, Yamagishi H, Toda I, Yoshiyama M, Yoshikawa J, Yoshida K. Age- and gender-specific changes in the left ventricular relaxation: a Doppler echocardiographic study in healthy individuals. Circ Cardiovasc Imaging. 2009;2:41–46. doi: 10.1161/CIRCIMAGING.108.809087. [DOI] [PubMed] [Google Scholar]
- Ostadal B, Netuka I, Maly J, Besik J, Ostadalova I. Gender differences in cardiac ischemic injury and protection—experimental aspects. Exp Biol Med (Maywood) 2009;234:1011–1019. doi: 10.3181/0812-MR-362. [DOI] [PubMed] [Google Scholar]
- Pahkala K, Heinonen OJ, Lagstrom H, Hakala P, Simell O, Viikari JS, Ronnemaa T, Hernelahti M, Sillanmaki L, Raitakari OT. Vascular endothelial function and leisure-time physical activity in adolescents. Circulation. 2008;118:2353–2359. doi: 10.1161/CIRCULATIONAHA.108.791988. [DOI] [PubMed] [Google Scholar]
- Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Herr MD, Proctor DN. Sex differences in leg vasodilation during graded knee extensor exercise in young adults. J Appl Physiol. 2007;103:1583–1591. doi: 10.1152/japplphysiol.00662.2007. [DOI] [PubMed] [Google Scholar]
- Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol. 2008;104:655–664. doi: 10.1152/japplphysiol.01150.2007. [DOI] [PubMed] [Google Scholar]
- Paterson DH, Jones GR, Rice CL. Ageing and physical activity: evidence to develop exercise recommendations for older adults. Can J Public Health. 2007;98(Suppl 2):S69–S108. [PubMed] [Google Scholar]
- Peters HW, Westendorp IC, Hak AE, Grobbee DE, Stehouwer CD, Hofman A, Witteman JC. Menopausal status and risk factors for cardiovascular disease. J Intern Med. 1999;246:521–528. doi: 10.1046/j.1365-2796.1999.00547.x. [DOI] [PubMed] [Google Scholar]
- Poirier P, Garneau C, Bogaty P, Nadeau A, Marois L, Brochu C, Gingras C, Fortin C, Jobin J, Dumesnil JG. Impact of left ventricular diastolic dysfunction on maximal treadmill performance in normotensive subjects with well-controlled type 2 diabetes mellitus. Am J Cardiol. 2000;85:473–477. doi: 10.1016/s0002-9149(99)00774-2. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Miller JD, Dietz NM, Minson CT, Joyner MJ. Reduced submaximal leg blood flow after high-intensity aerobic training. J Appl Physiol. 2001;91:2619–2627. doi: 10.1152/jappl.2001.91.6.2619. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol. 2003;95:1963–1970. doi: 10.1152/japplphysiol.00472.2003. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Le KU, Ridout SJ. Age and regional specificity of peak limb vascular conductance in men. J Appl Physiol. 2005;98:193–202. doi: 10.1152/japplphysiol.00704.2004. [DOI] [PubMed] [Google Scholar]
- Remsberg KE, Rogers NL, Demerath EW, Czerwinski SA, Choh AC, Lee M, Chumlea WC, Sun SS, Towne B, Siervogel RM. Sex differences in young adulthood metabolic syndrome and physical activity: the Fels longitudinal study. Am J Hum Biol. 2007;19:544–550. doi: 10.1002/ajhb.20615. [DOI] [PubMed] [Google Scholar]
- Ridout SJ, Parker BA, Proctor DN. Age and regional specificity of peak limb vascular conductance in women. J Appl Physiol. 2005;99:2067–2074. doi: 10.1152/japplphysiol.00825.2005. [DOI] [PubMed] [Google Scholar]
- Ridout SJ, Parker BA, Smithmyer SL, Gonzales JU, Beck KC, Proctor DN. Age and sex influence the balance between maximal cardiac output and peripheral vascular reserve. J Appl Physiol. 2010;108:483–489. doi: 10.1152/japplphysiol.00985.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlic P, Steinberger J, Thom T, Wilson M, Hong Y, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Sarabi M, Millgard J, Lind L. Effects of age, gender and metabolic factors on endothelium-dependent vasodilation: a population-based study. J Intern Med. 1999;246:265–274. doi: 10.1046/j.1365-2796.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- Sevre K, Lefrandt JD, Nordby G, Os I, Mulder M, Gans RO, Rostrup M, Smit AJ. Autonomic function in hypertensive and normotensive subjects: the importance of gender. Hypertension. 2001;37:1351–1356. doi: 10.1161/01.hyp.37.6.1351. [DOI] [PubMed] [Google Scholar]
- Shiels MS, Rohrmann S, Menke A, Selvin E, Crespo CJ, Rifai N, Dobs A, Feinleib M, Guallar E, Platz EA. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes Control. 2009;20:877–886. doi: 10.1007/s10552-009-9318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol. 2001;281:H2028–H2035. doi: 10.1152/ajpheart.2001.281.5.H2028. [DOI] [PubMed] [Google Scholar]
- Sisson SB, Katzmarzyk PT, Earnest CP, Bouchard C, Blair SN, Church TS. Volume of exercise and fitness nonresponse in sedentary, postmenopausal women. Med Sci Sports Exerc. 2009;41:539–545. doi: 10.1249/MSS.0b013e3181896c4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbu MD, Kronborg J, Eriksen BO, Jenssen TG, Toft I. Cardiovascular risk-factors predict progression of urinary albumin-excretion in a general, non-diabetic population: a gender-specific follow-up study. Atherosclerosis. 2008;201:398–406. doi: 10.1016/j.atherosclerosis.2008.02.027. [DOI] [PubMed] [Google Scholar]
- Soto PF, Herrero P, Schechtman KB, Waggoner AD, Baumstark JM, Ehsani AA, Gropler RJ. Exercise training impacts the myocardial metabolism of older individuals in a gender-specific manner. Am J Physiol Heart Circ Physiol. 2008;295:H842–H850. doi: 10.1152/ajpheart.91426.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina RJ. Cardiovascular adaptations to endurance exercise training in older men and women. Exerc Sport Sci Rev. 1999;27:317–332. [PubMed] [Google Scholar]
- Spina RJ, Miller TR, Bogenhagen WH, Schechtman KB, Ehsani AA. Gender-related differences in left ventricular filling dynamics in older subjects after endurance exercise training. J Gerontol A Biol Sci Med Sci. 1996;51:B232–B237. doi: 10.1093/gerona/51a.3.b232. [DOI] [PubMed] [Google Scholar]
- Stauffer BL, Westby CM, Greiner JJ, Van Guilder GP, Desouza CA. Sex differences in endothelin-1-mediated vasoconstrictor tone in middle-aged and older adults. Am J Physiol Regul Integr Comp Physiol. 2010;298:R261–R265. doi: 10.1152/ajpregu.00626.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Miura S, Mori-Abe A, Kawagoe J, Takata K, Ohmichi M, Kurachi H. Impact of menopause on the augmentation of arterial stiffness with aging. Gynecol Obstet Invest. 2005;60:162–166. doi: 10.1159/000086570. [DOI] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18:127–132. doi: 10.1161/01.atv.18.1.127. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Terzi S, Sayar N, Bilsel T, Enc Y, Yildirim A, Ciloğlu F, Yesilcimen K. Tissue Doppler imaging adds incremental value in predicting exercise capacity in patients with congestive heart failure. Heart Vessels. 2007;22:237–244. doi: 10.1007/s00380-006-0961-x. [DOI] [PubMed] [Google Scholar]
- Valentine RJ, Vieira VJ, Woods JA, Evans EM. Stronger relationship between central adiposity and C-reactive protein in older women than men. Menopause. 2009;16:84–89. doi: 10.1097/gme.0b013e31817fcb8f. [DOI] [PubMed] [Google Scholar]
- van der Heijden-Spek JJ, Staessen JA, Fagard RH, Hoeks AP, Boudier HA, van Bortel LM. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: a population study. Hypertension. 2000;35:637–642. doi: 10.1161/01.hyp.35.2.637. [DOI] [PubMed] [Google Scholar]
- Vitale C, Mendelsohn ME, Rosano GM. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol. 2009;6:532–542. doi: 10.1038/nrcardio.2009.105. [DOI] [PubMed] [Google Scholar]
- Wahren J, Saltin B, Jorfeldt L, Pernow B. Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest. 1974;33:79–86. doi: 10.3109/00365517409114201. [DOI] [PubMed] [Google Scholar]
- Webb CM, Elkington AG, Kraidly MM, Keenan N, Pennell DJ, Collins P. Effects of oral testosterone treatment on myocardial perfusion and vascular function in men with low plasma testosterone and coronary heart disease. Am J Cardiol. 2008;101:618–624. doi: 10.1016/j.amjcard.2007.09.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EP, Spina RJ, Holloszy JO, Ehsani AA. Gender differences in the decline in aerobic capacity and its physiological determinants during the later decades of life. J Appl Physiol. 2006;101:938–944. doi: 10.1152/japplphysiol.01398.2005. [DOI] [PubMed] [Google Scholar]
- Wiebe CG, Gledhill N, Warburton DE, Jamnik VK, Ferguson S. Exercise cardiac function in endurance-trained males versus females. Clin J Sport Med. 1998;8:272–279. doi: 10.1097/00042752-199810000-00004. [DOI] [PubMed] [Google Scholar]
- Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol. 2006;290:H1271–H1277. doi: 10.1152/ajpheart.00883.2005. [DOI] [PubMed] [Google Scholar]