Abstract
Follicular lymphoma (FL) can exhibit variant histologic patterns that can lead to confusion with other B-cell lymphomas and reactive conditions. Diagnostic markers such as CD10 and BCL2 may be difficult to interpret in variant FL patterns, and are often diminished or absent in the interfollicular and diffuse components. We evaluated two recently characterized germinal center B-cell markers, HGAL and LMO2, in 127 FL patient biopsies (94 nodal, 33 extranodal), and correlated the findings with histologic pattern, cellular composition, grade and additional immunostains (CD20, CD3, CD21, CD10, BCL2 and BCL6). Architectural patterns included predominantly follicular (75%) and follicular and diffuse (25%); ten cases showed marginal zone differentiation and 3 were floral variants. Eighty-nine cases were low grade (38 grade 1; 51 grade 2) and 38 were grade 3 (29 grade 3A and 9 grade 3B). HGAL had the highest overall sensitivity of detecting FL and was superior in detecting the interfollicular and diffuse components compared with BCL2, LMO2, CD10 and BCL6. All 28 cases that lacked CD10, expressed HGAL, and the majority also expressed LMO2. Our results show that HGAL and LMO2 are sensitive markers for FL diagnosis. The addition of HGAL and LMO2 to the immunohistologic panel is beneficial in the work-up of nodal and extranodal B-cell lymphomas and the efficacy of HGAL in detecting the follicular, interfollicular and diffuse components of FL is of particular value in the setting of variant immunoarchitectural patterns.
Keywords: Follicular lymphoma, low-grade B-cell lymphoma, immunoarchitecture, immunohistochemistry
INTRODUCTION
Follicular lymphoma (FL) is a neoplasm derived from germinal center B-cells that accounts for approximately 20% of non-Hodgkin lymphomas largely occurring in North America and Western Europe.(1)Typically, in a lymph node involved by FL, the normal nodal architecture is effaced by closely packed follicles that are comprised of centroblasts and centrocytes in variable proportions, attenuated mantle zones, loss of polarization and lack of tingible body macrophages. Interfollicular infiltration of neoplastic B-cells is common. According to the World Health Organization classification, an at least partially follicular growth pattern is required for the diagnosis of FL.(27) The growth pattern can be comprised of predominantly follicular (>75% follicular), combined follicular and diffuse (25–75% follicular), or a predominantly diffuse (<25% follicular) architecture. The extent of the follicular component can be estimated by using an immunostain, such as CD21 or CD23, to highlight follicular dendritic cell (FDC) meshworks within neoplastic follicles. Areas of diffuse growth lack FDC meshworks and the overlap between focal areas of diffuse growth and an interfollicular component may be subtle.(27) In addition, there is wide variation in follicular morphology as well as the cytologic features comprising neoplastic follicles that can also confound the diagnosis of follicular lymphoma; key among these are follicular lymphoma with marginal zone differentiation and floral variants of follicular lymphoma.(28)
FL can present with variant immunoarchitectural patterns that leads to confusion with other small B-cell lymphomas, particularly marginal zone lymphoma, as well as reactive conditions. Immunohistologic markers typically used in the work-up of FL, such as CD10, BCL6 and BCL2, exhibit variable degrees of sensitivity and often lack concordance of expression. The expression of CD10 and BCL6 is usually stronger in the follicular component than in the interfollicular component, and these markers frequently fail to highlight interfollicular neoplastic B-cells altogether; they are also frequently downregulated in FL with marginal zone differentiation, in bone marrow infiltrates and in the peripheral blood.(7, 12) Interpretation of BCL6 staining is additionally confounded by its expression in subsets of T-cells and in paracortical hyperplasia.(4) BCL2 reactivity is seen in a high proportion of low grade FL (grades 1 and 2), but only 50% of grade 3 FL reliably stain for this marker.(18)BCL2 is also expressed in other B-cell lymphomas in the differential diagnosis and in T-cells, which also makes its interpretation in variant patterns difficult. Furthermore, all three markers can be absent in some cases of FL. Similar to immunohistologic markers, molecular polymerase chain reaction and fluorescence in situ hybridization studies aimed at detecting the t(14;18) translocation also show variability, particularly in FL occurring in extranodal sites. In a recent study, we reported that 54% of extranodal and 42% of nodal FL showed evidence of t(14;18) with a higher frequency of minor breakpoints in cases of extranodal FL.(29) These data indicate that molecular detection of FL can also be highly variable, further contributing to the difficulty of separating FL from other small B-cell lymphomas and reactive conditions. Therefore, additional markers that reliably detect FL are needed.
We previously characterized the protein expression profiles of two markers, Human Germinal center Associated Lymphoma (HGAL; also know as GCET2) and LIM-Only transcription factor 2 (LMO2), which were found to be expressed in germinal center B-cells and in subsets of germinal center B-cell-derived lymphomas.(22, 23) In the current study, we evaluated the utility of HGAL and LMO2, in comparison to routinely used markers CD10, BCL2 and BCL6, in the diagnosis of FL and its immunoarchitectural variants.
MATERIALS AND METHODS
Case selection
A total of 127 FL cases seen in consultation at Stanford University Department of Pathology, spanning the period between 2002 and 2009, comprise this study. Cases diagnosed as follicular lymphoma, on which there were additional unstained sections or paraffin blocks amenable for further analyses were selected for this study. The cases are enriched for variant morphologic and immunoarchitectural patterns as those cases are generally more difficult to diagnosis and more likely to lead to consultation. Morphologic and immunohistologic findings on all cases were reviewed by two pathologists (SFY and YN), and selectively reviewed by RAW. The diagnoses were confirmed and grading performed according to WHO 2008 criteria.(27) This cohort of FL cases did not overlap with our previously published papers.(22, 23, 29) Institutional Review Board approval was obtained for these studies.
Morphologic evaluation was performed on formalin-fixed, paraffin-embedded tissue sections. The overall histologic pattern, grade, presence or absence of sclerosis and vasculature were evaluated in all cases. Ninety-four cases presented in lymph nodes, the remaining 33 were extranodal. Cutaneous FL were excluded from the study because of its distinctive clinical and immunophenotypic features. Among the 127 cases, 89 (70%) were low grade (38 grade 1, 51 grade 2) and 38 (30%) were grade 3 (29 grade 3A and 9 grade 3B). Lymphomas without diffuse areas were designated “follicular only”. An area of diffuse follicular lymphoma was diagnosed if diffuse sheets of neoplastic B-cells were present without a clear-cut follicular growth pattern, irrespective of the size of the area (diffuse areas ranged from 10% to 90% in our case cohort). Simple broadening of interfollicular areas was not considered sufficient for designation as a diffuse area. Diffuse follicle center lymphomas (devoid of any follicular areas) were not included in this study.
Immunohistologic analyses were performed on 4-micron thick formalin-fixed, paraffin-embedded tissue sections using automated stainers. All primary antibodies, sources, retrieval conditions and reagents used for immunohistochemistry of markers that were the focus of this study are detailed in Table 1. Staining in follicular, interfollicular and diffuse components of the neoplastic infiltrate was scored separately and the percent of involvement of each component was tracked. CD21 staining of FDC meshworks was used in conjunction with histologic features to assess the extent of the diffuse component. The degree of BCL2 positivity was variable and interpretation of BCL2 staining was performed in careful comparison to a pan T-cell marker such as CD3 or CD5, at least one of which was always performed as part of the immunohistologic work-up. An excess of BCL2 in the presence of an appropriate architectural context was interpreted as compatible with the diagnosis of FL. Due to the difficulty of assessing BCL2 staining in interfollicular and diffuse growth areas, particular attention was paid to those cases. Reduced or partial staining of BCL2 was also encountered including those in which only a perinuclear rim of staining was present. Interpretation of BCL2 staining was improved when there was good preservation of cytologic detail.
Table 1.
Reagents and conditions used for immunohistologic studies
| Antibody | Clone | Dilution | Staining Conditions | Manufacturer |
|---|---|---|---|---|
| CD20 | L26 | 1:1000 | Ventana; Standard retrieval | Dako, Capinteria, CA |
| CD3 | Rabbit polyclonal | 1:50 | Ventana; Standard retrieval | Cell Marque, Hot Springs, AZ |
| CD5 | 4C7 | 1:200 | Ventana; Standard retrieval | Leica/Novocastra, Newcastle Upon Tyne, UK |
| CD21 | IF8 | 1:20 | Ventana; Protease retrieval | Dako |
| CD10 | 56C6 | 1:20 | Ventana; Standard retrieval | Leica |
| BCL2 | 124 | 1:20 | Dako; Citrate retrieval | Dako |
| BCL6 | GL191E/A8 | Neat | Ventana; Mild retrieval | Dako |
| HGAL | 1H1 (A7) | 1:5 | Dako-EDTA-MACH2 polymer | Natkunam et al., Blood 2005 |
| LMO2 | 1A9-1 | 1:150 | Ventana; Mild retrieval | Natkunam et al., Blood 2007 |
The consultation cases that comprise this study cohort also had other ancillary tests performed at the time of diagnosis that were not the main focus of the current paper; these are summarized as follows: IgM was performed in 71 cases (40 were positive); PCR for IgH or t(14;18) was performed in 21 cases (four positive for each test). Flow cytometry data was available for 12 cases of which all were positive for CD10 and showed immunoglobulin light chain restriction. The proliferation marker Ki-67 was assessed in 33 cases and showed a variable range of expression across grades of follicular lymphoma: grades 1 and 2 showed 10–20% growth fraction in most cases but 2 cases showed discordantly high proliferation; similarly, grade 3A and B showed a range of proliferation between 20–70% with the exception of one cases of each that showed a growth fraction of 5–10%. These additional markers are not always performed as part of a diagnostic work-up at our center and are only obtained if necessary to confirm the diagnosis or to further assess for a diffuse component.
Statistical Methodology
To assess the ability of each marker to identify cases of follicular lymphoma, we computed the sensitivity of each marker for identifying lymphoma. The sensitivity is defined as , which in our study was the proportion of lymphoma cases correctly identified as positive. The 95% confidence interval for the sensitivity was computed as , where N is the sample size. We computed the sensitivity and 95% confidence intervals for all cases together, as well as for the interfollicular component and diffuse components separately.
RESULTS
Histologic findings
Four main architectural patterns were designated: follicular only, follicular and diffuse, marginal zone differentiation and floral variant (Figure 1). Overall, 95 cases (75%) showed a follicular only growth pattern and 32 (25%) showed a combined follicular and diffuse pattern. In all 95 cases with a follicular pattern, the normal nodal architecture was fully effaced by the follicular growth. Follicles varied in size, and although most cases (60 of 127, 47%) showed crowding of follicles, others did not. Fourty-four of 127 cases (35%) showed extension of neoplastic follicles into or beyond the lymph node capsule. Additionally, 20 of 127 (16%) showed variation in the shape and size of follicles; typical examples of irregular, coalescent, serpiginous, confluent and serrated follicles are illustrated (Figure 2). These morphologies were seen in cases with a follicular or a follicular and diffuse growth pattern.
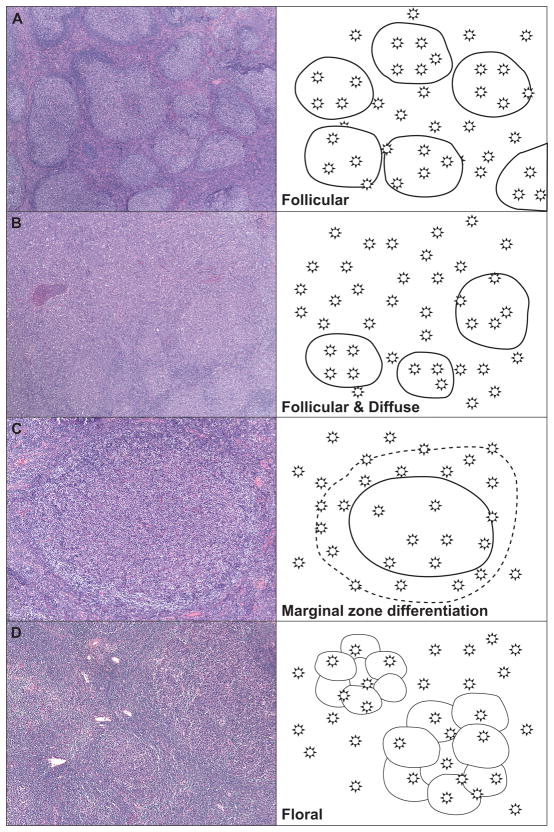
Figure 1. Architectural Patterns in Follicular Lymphoma.
Architectural pattern of follicular lymphoma and representative cartoons showing the distribution of neoplastic B-cells. These patterns include: (A) Follicular pattern, characterized by complete effacement of normal architecture by variably sized neoplastic follicles containing a variable mixture of centrocytes and centroblasts; (B) Follicular and diffuse architecture, characterized by at least a partial follicular pattern associated with between 10–90% diffuse areas with sheets of neoplastic cells; (C) Marginal zone differentiation, characterized by monocytoid cells with moderate to abundant pale cytoplasm and rounded centrally placed nuclei were seen in clusters, or more commonly, surrounding neoplastic follicles; (D) Floral variant with invaginations of mantle zone lymphocytes into neoplastic follicles distorting the follicular architecture.
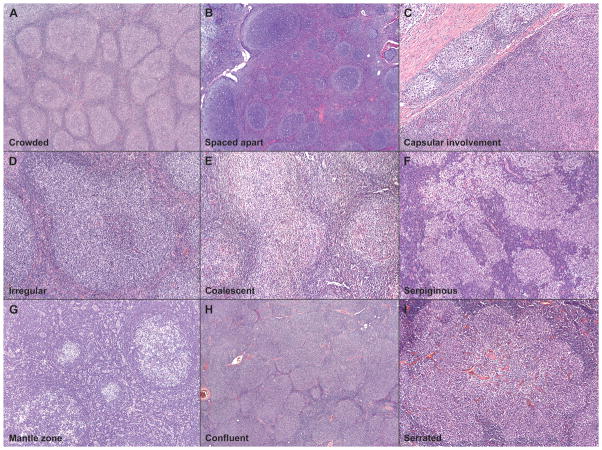
Figure 2. Spectrum of Follicle Morphology.
Neoplastic follicles in follicular lymphoma showed either crowded (A) or spaced-apart (B) follicles, and many also showed involvement of the capsule (C). In addition, the follicles also showed wide variation in the shape and size: typical examples of irregular (D), coalesced (E), serpiginous (F), mantle zone differentiation (G), confluent (H), and serrated (I) follicles are illustrated.
Of the 32 of 127 (25%) cases with a follicular and diffuse growth pattern, follicles merged into diffuse areas completely lacking follicular architecture. Five of these cases showed cytologic features compatible with a grade 3 FL in the diffuse areas, and were therefore, given the diagnosis of diffuse large B-cell lymphoma (DLBCL) in addition to FL.(27)
Marginal zone differentiation was evident in 10 of 127 cases (8%), and occurred in both types of patterns (6 in follicular only and 4 in follicular and diffuse). Monocytoid cells with moderate to abundant pale cytoplasm and rounded centrally placed nuclei were seen in clusters, or more commonly, surrounding neoplastic follicles. Three of 127 (2%) were floral variants of follicular lymphoma; two cases showed partial invaginations of mantle zone lymphocytes into neoplastic follicles distorting their architecture, whereas the third case showed massive mantle zone cell influx into neoplastic follicles resulting in a “microfollicular” pattern and exhibiting near-complete disruption of the follicles. Sclerosis was observed in 49 of 127 (39%) cases and ranged from thin interstitial sclerosis in 28, thick compartmentalizing sclerosis in 12 and extensive areas of sclerosis in 9 cases. Prominent vasculature was seen in 18 of 127 (16%) cases.
The cellular composition and cytologic features of the neoplastic infiltrate showed variation and some typical examples are illustrated in Figure 3. The vast majority of cases contained an admixture of centrocytes and centroblasts arranged in a disorderly fashion within neoplastic follicles as well as in diffuse areas. Large cells with cleaved morphology were present in 22 of 127 (17%) cases. An interfollicular (IF) component of neoplastic cells was almost always present (123 of 127; 97%); this component showed a predominance of centrocytes, although these IF cells were typically smaller and less cleaved than centrocytes present within follicles. Two of 127 (1.5%) cases showed tingible body macrophages within neoplastic follicles. The neoplastic cells in 4 of 127 (3%) cases showed signet ring features; 2 were clear cell variants with nuclei displaced by clear vacuoles, while the other 2 cases showed nuclei pushed aside by eosinophilic Russell body-like material. An additional case showed Reed-Sternberg-like cells within follicles.
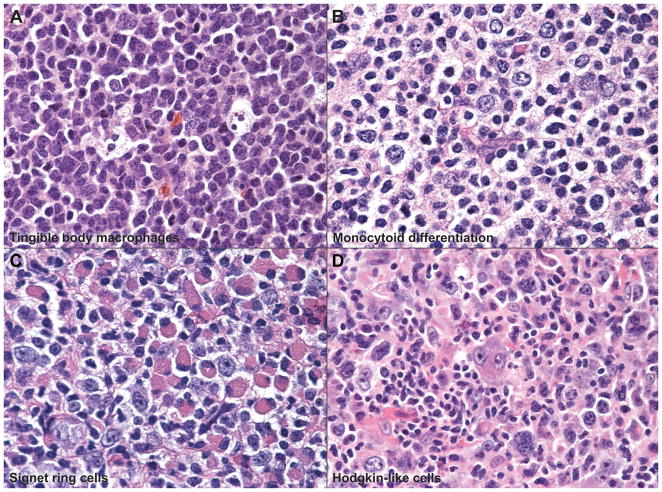
Figure 3. Spectrum of Cytologic Features.
The cellular composition and cytologic features of the neoplastic follicles showed variation and include: (A) follicles with tingible body macrophages, (B) monocytoid differentiation, (C) signet-ring like features with eccentric nuclei and abundant cytoplasm or cytoplasmic inclusions, and (D) a rare example of FL with Hodgkin/Reed-Sternberg-like cells within follicles.
Immunohistologic findings
The immunohistologic findings are summarized in Table 2. CD20 was positive in all cases in the follicular, interfollicular and diffuse components. CD21 highlighted intact FDC meshworks in 50 of 52 (96%) cases tested. Among the five markers, 28 of 120 (23%) cases lacked CD10 and 26 of 120 (22%) lacked BCL2. Nine cases lacked both CD10 and BCL2 (7.5%); 8 of them were stained for HGAL and all were positive (100%), whereas 9 were stained for LMO2 and 5 were positive (56%). Two cases lacked both HGAL and LMO2; both were positive for CD10, whereas one was positive for BCL2.
Table 2.
Summary of Immunohistologic Findings
| Marker | Number Tested | Number Positive | Follicular Component (All cases) | Diffuse*** Component (32 cases) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Grade 1 | Grade 2 | Grade 3A | Grade 3B | F only* | F + IFC** | |||
| CD10 | 120 | 92/120 77% [69–84] |
26/37 70% [56–85] |
43/47 91% [84–99] |
19/27 70% [54–88] |
4/9 44% [12–77] |
27/120 23% [15–30] |
65/120 54% [45–63] |
21/32 66% [49–82] |
| BCL2 | 120 | 94/120 78% [71–86] |
34/37 92% [83–100] |
38/49 78% [65–89] |
17/26 65% [47–84] |
5/8 62% [29–96] |
6/120 5% [1–9] |
88/120 73% [65–81] |
23/31 74% [59–90] |
| BCL6 | 43 | 20/43 47% [32–61] |
6/14 43% [17–69] |
10/15 67% [43–91] |
3/9 33% [2–64] |
½ 50% [0–1] |
6/43 14% [4–24] |
14/43 33% [19–47] |
4/6 67% [29–100] |
| HGAL | 117 | 110/117 94% [90–98] |
36/37 97% [92–100] |
43/47 91% [84–99] |
24/25 96% [88–100] |
7/8 88% [64–100] |
13/117 11.1% [5–17] |
97/117 83% [76–90] |
29/30 97% [90–100] |
| LMO2 | 118 | 83/118 70% [62–79] |
26/37 70% [56–85] |
30/44 68% [54–82] |
21/28 75% [59–91] |
6/9 67% [36–97] |
0/118 0% |
83/118 70% [62–79] |
22/29 76% [60–91] |
Staining in the follicles only;
Staining in the follicles and interfollicular components;
A diffuse component was present in 32 cases; confidence intervals are shown in square brackets.
Sensitivity analyses among the five markers (CD10, BCL6, BCL2, HGAL and LMO2) were performed to evaluate the overall sensitivity as well as the sensitivity of detection of the interfollicular and diffuse components. The sensitivity indices with 95% confidence intervals (CI) are tabulated (Table 2). Representative examples of the immunohistologic staining of all 5 markers are shown in Figure 4. Overall, HGAL performed significantly better that the other four markers in overall sensitivity across all grades of FL as well as in the interfollicular and diffuse components (the lower 95% CI for HGAL in all 3 analyses is higher than the calculated sensitivity for the next most sensitive marker, BCL2). The other markers showed a somewhat mixed performance: for overall sensitivity, BCL2 and CD10 performed similar to each other and both were more sensitive than LMO2 or BCL6; LMO2 and BCL2 were comparable in their sensitivity of detection of the interfollicular component, and were superior to CD10 and BCL6; confidence intervals were wide for the diffuse component analysis due to fewer cases in that category, but with the exception of HGAL which was superior, the other markers showed approximately equal sensitivity in detecting the diffuse component. An example of a case of FL that lacks CD10 and BCL2 but expresses HGAL and LMO2 is shown (Figure 5).
Figure 4. Immunoarchitectural Patterns of Follicualr lymphoma.
Typical examples of the immunostaining patterns of CD10 (A and B), BCL6 (C and D), BCL2 (E and F), HGAL (G and H), and LMO2 (I and J) are shown. The staining in neoplastic follicles as well as the staining in interfollicular neoplastic B-cells are illustrated. The third column includes panels at higher magnification showing details of staining in the interfollicular component.
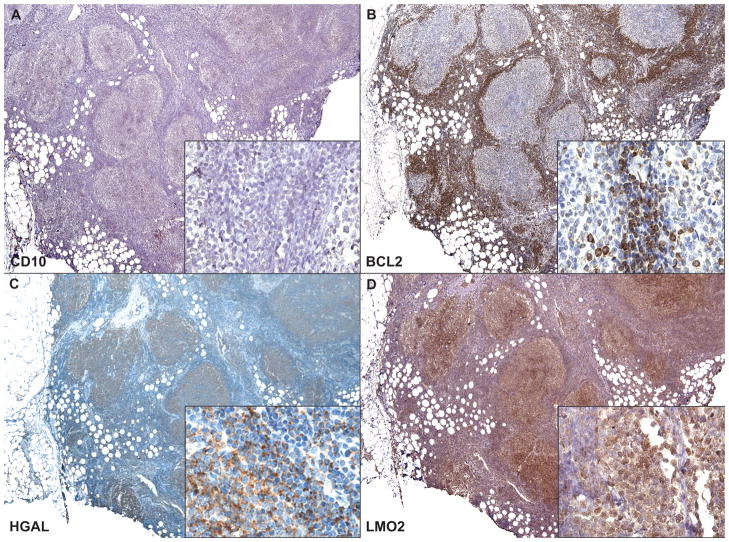
Figure 5. Case Example of Follicular Lymphoma.
A representative example of a FL case that lacked CD10 (A) and BCL2 (B) is illustrated. In this case example, both HGAL (C) and LMO2 (D) are positive in the follicular and interfollicular components, and confirm the diagnosis of follicular lymphoma. Higher magnification insets showing the interfollicular component are included in all panels.
Of FL cases with marginal zone differentiation, 6 of 9 (67%) were positive for CD10; 8 of 9 (89%) for BCL2; all for HGAL; 8 of10 (80%) for LMO2 and 4 of 6 (67%) for BCL6. An interfollicular component of CD10-positive cells outside marginal zone differentiation was found in 5 of 6 cases whereas 8 of 9 cases showed HGAL-positive cells; among these, 3 cases showed partial CD10 staining whereas one case showed partial HGAL staining. All 3 cases of floral variant FL stained for CD10, BCL2, HGAL and LMO2; CD10 and HGAL were positive in all three, whereas BCL2 was positive in one and LMO2 in two. There was no correlation with the expression of any of these markers with FL grade (Table 2).
DISCUSSION
Variant immunoarchitectural patterns of FL can be difficult to distinguish from those of other B-cell lymphomas and reactive conditions. Three markers that are routinely used in the diagnosis of FL CD10, BCL2 and BCL6 show variability of expression and are often diminished or absent in the interfollicular and diffuse components.(27) Similarly, cytogenetic and molecular studies aimed at detecting t(14;18) can also show variability, further confounding the diagnosis of FL. Therefore, additional markers that are better able to uniformly and reliably stain all components of follicular lymphoma infiltrates would improve the separation of FL from other B-cell lymphomas, and consequently, lead to more appropriate clinical management of patients. Moreover, identifying an abnormal interfollicular or diffuse distribution of cells labeling for a germinal center B-cell marker may be helpful in supporting a diagnosis of lymphoma in a difficult case. Our previous studies had shown that HGAL and LMO2 proteins are expressed with high specificity in normal germinal center B-cells and in subsets of germinal center-derived B-cell lymphomas.(22, 23) We therefore, sought to address whether they would also add sensitivity to the diagnosis of FL, particularly when variant morphologic and immunophenotypic features confound the diagnosis. Our findings show that both HGAL and LMO2 are sensitive markers for the diagnosis of FL, and that HGAL is superior to all other markers tested in this study (CD10, BCL2, BCL6 and LMO2), in detecting the interfollicular and diffuse components of FL.
Variant architectural patterns and cytologic features of neoplastic infiltrates in follicular lymphoma can result in diagnostic difficulty. Although the presence of diffuse areas in low-grade follicular lymphoma is not associated with an adverse outcome, diffuse areas together with increased large cells, are two important histologic parameters that offer information regarding possible transformation of FL. Furthermore, marginal zone differentiation of FL causes a morphologic overlap with marginal zone lymphomas that secondarily colonize normal follicles; in cases such as these, careful comparisons of the expression patterns of germinal center markers and the morphology need to be taken into consideration to separate FL from marginal zone lymphoma. The floral variant can also present with extensive disruption of the follicular architecture, resulting in morphologic overlap with other small B-cell lymphomas and reactive conditions such as progressive transformation of germinal centers. In addition, the variation in cytologic features also raises other entities in the differential diagnosis: for example, although typically tingible body macrophages are absent in neoplastic follicles with few exceptions, their presence raises a reactive follicular hyperplasia in the differential diagnosis. Similarly, signet ring features are likely to cause confusion with plasmacytoid neoplasia as well as undifferentiated carcinoma. The example of FL with Hodgkin/Reed-Sternberg-like cells raised the differential diagnosis of a lymphocyte-rich classical Hodgkin lymphoma, lymphocyte predominant Hodgkin lymphoma as well as a composite lymphoma that is composed of follicular and Hodgkin lymphoma. These variant follicular architectures and cytologic features are important to recognize as they often complicate the diagnosis of FL, particularly in small biopsies.
Foremost among markers used in FL diagnosis is CD10, which is expressed in normal and neoplastic follicles, in addition to granulocytes, B- and T-cell precursors and in follicular helper T-cells.(13) Its expression in the interfollicular neoplastic infiltrate of FL, when present, can serve as a valuable discriminatory feature.(6) In addition, CD10 is extremely helpful in the separation of FL from other small B-cell lymphomas, particularly when a follicular growth pattern is lacking or adequate tissue to evaluate follicular architecture is lacking (fine needle aspirates, needle cores, small or fragmented biopsies). It is also the most frequently used (and often the only) marker in flow cytometry panels employed to detect a lymphoma of follicle center derivation. Differentiating a marginal zone lymphoma with colonized follicles associated with residual CD10-positive follicle center cells from an FL lacking CD10 can be difficult. (6, 20) Equally problematic is a FL with a significant diffuse component or a diffuse follicle center lymphoma (not included in this study), which can morphologically mimic a marginal zone lymphoma. The lack of CD10 does not preclude FL, nor does its expression eliminate a marginal zone lymphoma, as CD10-positive marginal zone lymphomas also rarely occur.(8, 13, 15) In addition, CD10 is less frequently expressed in grade 3 FL and in FL involving the bone marrow or peripheral blood.(8) Rare cases of mantle cell lymphomas can also show partial positivity for CD10 (3 of 127 cases).(10)
In the current study, CD10 was positive in 77% of FL cases with the following breakdown among grades: grade 1 (70%), grade 2 (92%), grade 3A (70%) and 3B (44%) (p value=0.006). It was absent in the interfollicular component of 23% of our FL cohort. The overall CD10 positivity of 80% is within the range of what is reported in other studies in the literature.(2, 8, 14, 19)In the study by Eshoa and collegues, CD10 expression was detected in 70–100% of FL grades 1 and 2, whereas it was detected only in 20% of grade 3 FL.(8) Our findings are similar and suggest that CD10 expression is frequently weak to absent in grade 3 FL as well as in the interfollicular component of FL.
Virtually all B-cell neoplasms with the exception of Burkitt lymphoma express BCL2;(18) however, its down-regulation in normal germinal center B-cells can be exploited in the separation of reactive from neoplastic follicles. Given its frequent expression in most B-cell lymphomas, its presence should be interpreted with caution when follicular architecture is lacking. Additionally, normal mantle zone B-cells, primary follicles and normal T-cells express BCL2,(31) and therefore, additional stains for IgD, IgM and pan T-cell markers such as CD3 or CD5 should be used to eliminate these possibilities as well as partial expression of BCL2 (sometimes reduced to perinuclear rim of staining) in neoplastic B-cells. Furthermore, although most BCL2-negative FL lack t(14;18), a mutated BCL2 gene may also lead to a false-negative immunostaining result due to alteration of the BCL2 epitope recognized by the most commonly used antibodies.(26) Overall, up to 10% of FL lack expression of BCL2; this protein is expressed in 85–90% of grades 1 and 2 FL whereas only 50% of grade 3 FL express this protein.(18, 26) In the current study, BCL2 was positive in 78% of cases, with 92% and 78% in grades 1 and 2 FL, and 65% and 62% in grade 3A and 3B, respectively. As with CD10, these results are in agreement with previously published literature.(2, 5, 14, 17–19)
BCL6 is expressed in normal germinal center B-cells and in a significant proportion of FL, including grade 3B FL that often lack CD10 and BCL2.(3, 4, 11, 24, 25) In addition, its expression has been recently reported in CD10-negative, MUM1/IRF4-positive FL that frequently present in the elderly as high grade (3A or 3B) FL in the absence of the t(14;18) translocation.(16) BCL6 is a useful adjunct in the diagnosis of CD10 and/or BCL2-negative FL. Its expression is best interpreted in conjunction with a CD3 or CD5 stain as BCL6 is also expressed in a subset of follicular T-cells. In the current study, only a third of the cases had enough material to evaluate BCL6 staining. Similar to CD10 and BCL2, our results are in agreement with the published literature: BCL6 was expressed in 43% and 67% of grades 1 and 2 FL and in 33% and 50% of grade 3A and 3B FL; it was absent in the interfollicular component of 14% of cases tested.
Our previous studies on HGAL had showed that this protein is specifically expressed in the cytoplasm of germinal center B-cells, but was absent in mantle and marginal zone B-cells and in the interfollicular and paracortical regions in normal tonsils and lymph nodes.(22) Its high degree of specificity for germinal center B-cells make it an ideal marker for the detection of germinal center-derived B-cell lymphomas. HGAL is expressed in the majority of FL regardless of grade (97% in grades 1 and 2 FL and 95% in grade 3 FL in our prior study (22); 94% in grades 1 and 2 FL and 96% in grade 3A and 88% in grade 3B FL, in the current study). Therefore, unlike CD10, BCL2 and BCL6, HGAL is capable of staining the majority of grade 3A and 3B FL. In our prior study, HGAL expression was found in only 4% of marginal zone lymphomas and was absent in mantle cell and T-cell lymphomas.(22) In the current study, HGAL staining showed the highest degree of overall sensitivity for FL among the markers we tested. In addition, all cases that lacked CD10 and BCL2, expressed HGAL. Furthermore, HGAL was also found to be the most sensitive marker for the detection of the interfollicular as well as the diffuse components of FL. These results suggest that HGAL is a highly beneficial addition to an immunodiagnostic panel of markers in the work-up of small B-cell lymphomas, not only because of its high specificity and sensitivity in detecting FL, but also because of its efficacy in detecting the interfollicular and diffuse components of FL. Our results attest to HGAL as a superior marker in the diagnosis of problematic FL cases and for differentiating FL from other mimics with a follicular architecture.
Previously, we showed that the transcription factor LMO2 was expressed in normal germinal center B-cells and in a subset of lymphomas derived from those cells in addition to bone marrow hematopoietic precursors and endothelial cells.(9, 23) LMO2 protein expression also has an important role in the prognostication of diffuse large B-cell lymphomas in patients treated with anthracycline-based chemotherapy with and without the anti-CD20 antibody, rituximab.(21) Its important role in angiogenesis and erythropoiesis has also been elucidated previously.(30) It is weakly expressed in mantle zone B-cells but not in mantle cell or marginal zone lymphomas.(23) In the current study, LMO2 was expressed in 70% of FL cases, which is in the same range as we had previously reported in an independent series of FL cases (50% of FL regardless of grade).(23) Because LMO2, like HGAL, is capable of staining grade 3 FL at a similar frequency as low grade FL, its efficacy in detecting CD10- and BCL2-negative FL was almost comparable to that of HGAL (6 of 8, 75%, in contrast to 100% for HGAL). These data suggest that LMO2 is also a useful adjunct in the diagnosis of FL. Although its overall sensitivity is less than that of HGAL, its performance was comparable to CD10 and BCL2 and superior to BCL6. Since LMO2 appears not to be down-regulated in higher grade FL or the interfollicular and diffuse components of FL, its utility in variant immunoarchitectural patterns of FL and in cases that lack CD10 and BCL2, is similar to that of HGAL. One added advantage of LMO2 is its crisp nuclear localization that allows for easier interpretation of the stain on paraffin sections in comparison to the diffuse cytoplasmic staining pattern of the HGAL protein.
Acknowledgments
Supported in part by NIH P0I CA34233
We thank Edward Gilbert for technical assistance with immunohistochemistry, Anet James for photography and Thelma Santa-Maria for administrative support.
References
- 1.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 1998;9:717–720. doi: 10.1023/a:1008265532487. [DOI] [PubMed] [Google Scholar]
- 2.Bacon CM, Ye H, Diss TC, et al. Primary follicular lymphoma of the testis and epididymis in adults. Am J Surg Pathol. 2007;31:1050–1058. doi: 10.1097/PAS.0b013e31802ee4ab. [DOI] [PubMed] [Google Scholar]
- 3.Bosga-Bouwer AG, van den Berg A, Haralambieva E, et al. Molecular, cytogenetic, and immunophenotypic characterization of follicular lymphoma grade 3B; a separate entity or part of the spectrum of diffuse large B-cell lymphoma or follicular lymphoma? Hum Pathol. 2006;37:528–533. doi: 10.1016/j.humpath.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Cattoretti G, Chang CC, Cechova K, et al. BCL-6 protein is expressed in germinal-center B cells. Blood. 1995;86:45–53. [PubMed] [Google Scholar]
- 5.Damaj G, Verkarre V, Delmer A, et al. Primary follicular lymphoma of the gastrointestinal tract: a study of 25 cases and a literature review. Ann Oncol. 2003;14:623–629. doi: 10.1093/annonc/mdg168. [DOI] [PubMed] [Google Scholar]
- 6.Dogan A, Bagdi E, Munson P, et al. CD10 and BCL-6 expression in paraffin sections of normal lymphoid tissue and B-cell lymphomas. Am J Surg Pathol. 2000;24:846–852. doi: 10.1097/00000478-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Dogan A, Du MQ, Aiello A, et al. Follicular lymphomas contain a clonally linked but phenotypically distinct neoplastic B-cell population in the interfollicular zone. Blood. 1998;91:4708–4714. [PubMed] [Google Scholar]
- 8.Eshoa C, Perkins S, Kampalath B, et al. Decreased CD10 expression in grade III and in interfollicular infiltrates of follicular lymphomas. Am J Clin Pathol. 2001;115:862–867. doi: 10.1309/B6MK-J7NF-A6JP-X56K. [DOI] [PubMed] [Google Scholar]
- 9.Gratzinger D, Zhao S, West R, et al. The transcription factor LMO2 is a robust marker of vascular endothelium and vascular neoplasms and selected other entities. Am J Clin Pathol. 2009;131:264–278. doi: 10.1309/AJCP5FP3NAXAXRJE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gualco G, Weiss LM, Harrington WJ, Jr, et al. BCL6, MUM1, and CD10 expression in mantle cell lymphoma. Appl Immunohistochem Mol Morphol. 18:103–108. doi: 10.1097/PAI.0b013e3181bb9edf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y, Karube K, Kawano R, et al. Bcl2-negative follicular lymphomas frequently have Bcl6 translocation and/or Bcl6 or p53 expression. Pathol Int. 2007;57:148–152. doi: 10.1111/j.1440-1827.2006.02072.x. [DOI] [PubMed] [Google Scholar]
- 12.Harris NL, Nadler LM, Bhan AK. Immunohistologic characterization of two malignant lymphomas of germinal center type (centroblastic/centrocytic and centrocytic) with monoclonal antibodies. Follicular and diffuse lymphomas of small-cleaved-cell type are related but distinct entities. Am J Pathol. 1984;117:262–272. [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins RA, Blankenship JE, Kinney MC. Application of immunohistochemistry in the diagnosis of non-Hodgkin and Hodgkin lymphoma. Arch Pathol Lab Med. 2008;132:441–461. doi: 10.5858/2008-132-441-AOIITD. [DOI] [PubMed] [Google Scholar]
- 14.Huang WT, Hsu YH, Yang SF, et al. Primary gastrointestinal follicular lymphoma: a clinicopathologic study of 13 cases from Taiwan. J Clin Gastroenterol. 2008;42:997–1002. doi: 10.1097/MCG.0b013e3180f62b12. [DOI] [PubMed] [Google Scholar]
- 15.Isaacson PG. Haematopathology practice: the commonest problems encountered in a consultation practice. Histopathology. 2007;50:821–834. doi: 10.1111/j.1365-2559.2007.02707.x. [DOI] [PubMed] [Google Scholar]
- 16.Karube K, Guo Y, Suzumiya J, et al. CD10-MUM1+ follicular lymphoma lacks BCL2 gene translocation and shows characteristic biologic and clinical features. Blood. 2007;109:3076–3079. doi: 10.1182/blood-2006-09-045989. [DOI] [PubMed] [Google Scholar]
- 17.Kojima M, Yamanaka S, Yoshida T, et al. Histological variety of floral variant of follicular lymphoma. APMIS. 2006;114:626–632. doi: 10.1111/j.1600-0463.2006.apm_424.x. [DOI] [PubMed] [Google Scholar]
- 18.Lai R, Arber DA, Chang KL, et al. Frequency of bcl-2 expression in non-Hodgkin's lymphoma: a study of 778 cases with comparison of marginal zone lymphoma and monocytoid B-cell hyperplasia. Mod Pathol. 1998;11:864–869. [PubMed] [Google Scholar]
- 19.Lu D, Medeiros LJ, Eskenazi AE, et al. Primary follicular large cell lymphoma of the testis in a child. Arch Pathol Lab Med. 2001;125:551–554. doi: 10.5858/2001-125-0551-PFLCLO. [DOI] [PubMed] [Google Scholar]
- 20.Naresh KN. Nodal marginal zone B-cell lymphoma with prominent follicular colonization - difficulties in diagnosis: a study of 15 cases. Histopathology. 2008;52:331–339. doi: 10.1111/j.1365-2559.2007.02951.x. [DOI] [PubMed] [Google Scholar]
- 21.Natkunam Y, Farinha P, Hsi ED, et al. LMO2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy with and without rituximab. J Clin Oncol. 2008;26:447–454. doi: 10.1200/JCO.2007.13.0690. [DOI] [PubMed] [Google Scholar]
- 22.Natkunam Y, Lossos IS, Taidi B, et al. Expression of the human germinal center-associated lymphoma (HGAL) protein, a new marker of germinal center B-cell derivation. Blood. 2005;105:3979–3986. doi: 10.1182/blood-2004-08-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Natkunam Y, Zhao S, Mason DY, et al. The oncoprotein LMO2 is expressed in normal germinal-center B cells and in human B-cell lymphomas. Blood. 2007;109:1636–1642. doi: 10.1182/blood-2006-08-039024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ott G, Katzenberger T, Lohr A, et al. Cytomorphologic, immunohistochemical, and cytogenetic profiles of follicular lymphoma: 2 types of follicular lymphoma grade 3. Blood. 2002;99:3806–3812. doi: 10.1182/blood.v99.10.3806. [DOI] [PubMed] [Google Scholar]
- 25.Pittaluga S, Ayoubi TA, Wlodarska I, et al. BCL-6 expression in reactive lymphoid tissue and in B-cell non-Hodgkin's lymphomas. J Pathol. 1996;179:145–150. doi: 10.1002/(SICI)1096-9896(199606)179:2<145::AID-PATH565>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Schraders M, de Jong D, Kluin P, et al. Lack of Bcl-2 expression in follicular lymphoma may be caused by mutations in the BCL2 gene or by absence of the t(14;18) translocation. J Pathol. 2005;205:329–335. doi: 10.1002/path.1689. [DOI] [PubMed] [Google Scholar]
- 27.Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. [Google Scholar]
- 28.Warnke R, Weiss LM, Chan JKC, Cleary ML, Dorfman RF. Tumors of the lymph nodes and spleen. Washington, D.C: Armed forces institute of pathology; 1995. [Google Scholar]
- 29.Weinberg OK, Ma L, Seo K, et al. Low stage follicular lymphoma: biologic and clinical characterization according to nodal or extranodal primary origin. Am J Surg Pathol. 2009;33:591–598. doi: 10.1097/PAS.0b013e31818e6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada Y, Pannell R, Forster A, et al. The oncogenic LIM-only transcription factor Lmo2 regulates angiogenesis but not vasculogenesis in mice. Proc Natl Acad Sci U S A. 2000;97:320–324. doi: 10.1073/pnas.97.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zutter M, Hockenbery D, Silverman GA, et al. Immunolocalization of the Bcl-2 protein within hematopoietic neoplasms. Blood. 1991;78:1062–1068. [PubMed] [Google Scholar]