Abstract
Background
Ethanol exposure during early life has been shown to permanently alter the circadian expression of clock regulatory genes and the β-endorphin precursor proopiomelanocortin (POMC) gene in the hypothalamus. Ethanol also alters the stress- and immune-regulatory functions of β-endorphin neurons in laboratory rodents. Our aim was to determine whether the circadian clock regulatory Per2 gene modulates the action of ethanol on β-endorphin neurons in mice.
Methods
Per2 mutant (mPer2Brdml) and wild type (C57BL/6J) mice were used to determine the effect of Per2 mutation on ethanol-regulated β-endorphin neuronal activity during neonatal period using an in vitro mediobasal hypothalamic (MBH) cell culture model and an in vivo milk formula feeding animal model. The β-endorphin neuronal activity following acute and chronic ethanol treatments, was evaluated by measuring the peptide released from cultured cells or peptide levels in the MBH tissues, using enzyme-linked immunosorbent assay (ELISA).
Results
Per2 mutant mice showed a higher basal level of β-endorphin release from cultured MBH cells and a moderate increase in the peptide content in the MBH in comparison to control mice. However, unlike wild type mice, Per2 mutant mice showed no stimulatory or inhibitory β-endorphin secretory responses to acute and chronic ethanol challenges in vitro. Furthermore, Per2 mutant mice, but not wild type mice, failed to show the stimulatory and inhibitory responses of MBH β-endorphin levels to acute and chronic ethanol challenges in vivo.
Conclusions
These results suggest for the first time that the Per2 gene may be critically involved in regulating β-endorphin neuronal function. Furthermore, the data revealed an involvement of the Per2 gene in regulating β-endorphin neuronal responses to ethanol.
There are clinical reports uncovering a circadian rhythmicity of alcohol intoxication and overdose (Raymond et al., 1992). There also appears to be a relationship between alcohol drinking behavior and altered body circadian functions. In line with these views, alcohol intake has been shown to alter circadian blood pressure (Nakashita et al., 2009; Ohkubo et al., 2009), core body temperature (Devaney et al., 2003), hormone release in humans (Röjdmark et al., 2001), circadian phase shifting and free running period in mice (Seggio et al., 2009), circadian phase resetting and nocturnal activity patters in the hamster (Ruby, 2009), and rhythmicity in hypothalamic proopiomelanocortin (POMC) neurons in rats (Chen et al., 2006). Maternal alcohol drinking profoundly and permanently affects circadian function in the offspring. It is known that prenatal ethanol exposure alters core body temperature, corticosterone rhythms (Handa et al., 2007), NK cell function rhythms (Arjona et al., 2006a), phase shifting ability (Sakata-Haga et. al, 2006), and rhythmic pituitary-adrenal function in rats (Taylor et al., 1982), as well as circadian blood pressure and the heart rate of adolescent humans (Alvarez et al., 1995).
Why is the body’s circadian system so vulnerable to alcohol toxicity? One logical explanation for the circadian system vulnerability, may point to a possible alcohol specific target for the gene(s) that regulate circadian functions. The circadian clock-work involves the interaction of the so-called clock genes such as the Period (Per1, Per2, Per3), Clock, Bmal1 and Cryptochrome (Cry1, Cry2) genes into two tightly coupled transcriptional and translational feedback loops, thereby self-sustaining a circadian period of activity of the cell and modulating the expression of clock-controlled genes (Ko and Takahashi, 2006). Of these genes, the Per2 gene appears to be a putative target for alcohol. Support for this view comes from evidence showing that chronic alcohol exposure alters the circadian expression of Period genes in the hypothalamus of adult rats (Chen et al., 2004). Similarly, prenatal alcohol feeding alters circadian expression of Period genes in the hypothalamus and peripheral tissues (Chen et al., 2006; Arjona et al, 2006b; Farnell et al, 2008). Additionally, Per2 gene mutant (Per2Brdm1) mice display enhanced alcohol consumption and preference (Spanagel et al., 2005a), whereas Per1Brdm1 mutant mice do not show such an enhancement in alcohol drinking behavior (Zghoul et al., 2007). Evidence has shown, alcoholics with a specific set of polymorphisms in the Per2 gene consume less alcohol than alcoholics without the polymorphisms (Spanagel et al., 2005a, b). Hence, Per2 appears to be a targeted gene where alcohol may act on to alter circadian functions. In order to more closely elucidate the role of the circadian clock in ethanol’s effects on the brain, we examined the effect of the Per2 mutation on hypothalamic β-endorphin neuronal sensitivity to ethanol.
MATERIALS AND METHODS
Animal use
Per2 mutant (mPer2Brdml) and wild type male and female mice of the same genetic background (C57BL/6J) were obtained from The Jackson Laboratory (Bar Harbor, ME). mPer2Brdml mice carry a mutant mPer2 gene with a deletion in the PAS dimerization domain, which is critical for the interaction with other clock proteins (Zheng et al., 1999), thus rendering a non-functional PER2 protein. Although the Per2Brdml mutant mice have an albino phenotype, because it was engineered with a tyrosinase gene deficiency that affects the ability to make melanin, the Per2Brdml mutant mice has the same C57BL/6 genetic background with only a difference of the aforementioned gene deficiency. It has been shown that the albino mice strains 129/J, RF/J, SWR/J, AKR/J, A/J, and BALB/cByJ have a tyrosinase gene deficiency with no significant relationship between albinism and mean τDD, the endogenous (free-running) period of the circadian pacemaker measured in constant environmental darkness (Schwartz and Zimmerman, 1990). This suggests that the albino phenotype trait will have no implications on any circadian related studies. Similarly, gradual ethanol exposure has been shown to produce an increase in alcohol preference in both C57BL/6 mice with normal tyrosinase activity and in BALB/cByJ mice with tyrosinase deficiency (Blizard et al., 2004; Middaugh et al., 1999). Furthermore, these two mouse strains (C57BL/6By and BALB/cByJ) showed no differences in their hypothermic response or the brain level of cGMP to the same ethanol dose (Church and Feller, 1979). These data support that the tyrosinase deficiency will have very little consequences in alcohol-response studies. Mice were maintained under constant environmental conditions on a 12 h light/12 h dark cycle (lighting period from 7:00 a.m. to 7:00 p.m. with ad libitum food and water. The mPer2 Brdml mutant mice were routinely genotyped to verify the Per2 gene mutation. The primers used for detecting the Per2 gene were the following: forward1-cttgggtggagaggctattc, forward2-cattgggaggcacaagtcag, reverse1-aggtgagatgacaggagatc; reverse2-gagctgcgaacacatcctca. Male and female mPer2Brdml mice or male and female C57BL/6J mice were bred to produce neonates for MBH cell cultures or in vivo studies.
In vivo studies
In the in vivo study, postnatal day-2 (PD2) old C57BL/6 pups and Per2 Brdml mutant pups (both sexes) were fed by intubation with milk formula containing either alcohol (alcohol-fed) or an isocaloric volume of maltose dextrin (pair-fed) as originally described by Goodlet et al. (1998) and modified by Sarkar et al. (2007), or pups were undisturbed (ad libitum-fed). The alcohol–fed groups were given a milk formula containing ethanol (11.34%; vol/vol; 0.1–0.2 ml/animal; during a period of 1 minute), yielding a total daily ethanol dose of 2.5g/kg. The feeding was conducted at 1000 and 1200 h at PD7 and animals were sacrificed at 1300 h (3 h after the initiation of alcohol feeding) for acute ethanol treatment or conducted daily for 5 days between PD\ for chronic ethanol administration. Tissue samples were collected from all groups at 1300 h on PD7. The amount fed to the animals equaled 33% of their mean body weight (milliliters per gram). After feeding, the pups were immediately returned to the litter. One hour after the last feeding, six of each ad libitum-fed (AD), pair-fed (PF) or alcohol-fed (AF) neonates from six separate liters were sacrificed, their brains dissected, mediobasal hypothalami (MBH) obtained and immediately frozen for β-endorphin measurements. We have previously shown that feeding of ethanol-containing milk formula in laboratory rats increases blood level of alcohol (alcohol content was 205.15 ± 22.5 and 242.75 ± 23.75 mg/dl; N = 5) 1 and 2 h after the last feeding and undetectable 4 h after the last feeding. Animal care and treatment were performed in accordance with institutional guidelines and complied with the National Institutes of Health policy. The animal protocol used was approved by the Rutgers Animal Care and Facilities Committee.
Mediobasal hypothalamic cell cultures
The method for mouse MBH cell cultures was adopted from the previously published methods of our laboratory (Boyadjieva et al., 1994) for rat MBH cells. In brief, C57BL/6 and Per2 mutant neonates (both sexes) were sacrificed at day 1 of age, and the fetuses were removed by aseptic surgical procedure. Brains from the fetuses were immediately removed and the MBHs were separated and placed in ice-cold Hanks’ Balanced Salt Solution (HBSS) containing antibiotic solution (100 U/ml penicillin,100 μg/ml streptomycin, and 250 ng/ml amphotericin B),0.1% bovine serum albumin, and 200 μM ascorbic acid (all from Sigma-Aldrich, St. Louis, MO). The block of the MBH extended approximately 1 mm rostral to the optic chiasma and just caudal to the mammillary bodies, laterally to the hypothalamic sulci, and dorsally to ~2 mm deep. This part of the hypothalamus is known to contain glial cells and neuroendocrine neurons, produces β-endorphin, dopamine, thyrotropin-releasing hormones, and growth hormone-releasing hormone as well as glial cells (Brown, 1998). We have previously characterized the similar MBH culture system in regards to β-endorphin neuronal responses to ethanol. In MBH cultures, ethanol acutely stimulates β-endorphin secretion and POMC mRNA levels while chronically inhibiting β-endorphin secretion and POMC mRNA levels (Boyadjieva et al., 2004; Pastorcic et al., 1994). The β-endorphin neuronal responses to acute and chronic ethanol treatments in MBH cell cultures are similar to those seen in hypothalamic tissues of adult rodent brains (Boyadjieva et al., 2001; Rasmussen e al.,1998, 2000). Also in MBH cell cultures, like in the adult hypothalamus, ethanol action on β-endorphin neurons is mediated by catalase activated production of acetaldehyde (Reddy et al., 1995; Pastor and Aragon, 2008). These data support that the MBH cell culture model is useful in determining cellular mechanisms of ethanol actions on β-endorphin neurons.
MBH cells were then sedimented at 400 g for 10 min. Pellets were resuspended in HEPES-buffered Dulbecco’s Modified Eagle’s Medium (HDMEM, 4.5 g/l glucose; Sigma, St. Louis, MO). Cells were cultured into 25-cm2 polyornithine coated tissue culture flasks (2.5 million cells/flask) in HDMEM containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. On day 3, the culture medium was replaced with HDMEM containing serum supplement (30 nM selenium, 20 nM progesterone, 1 mM iron-free human transferrin, 5 mM insulin, and 100 mM putrescin) and 1% penicillin/streptomycin. This medium was changed every 3 days until the release experiment.
Release studies in vitro
These studies were conducted after maintaining the MBH neurons in cultures for 8–9 days. The cultures were treated with 2 ml of serum-free chemically defined medium containing various doses of ethanol (25, 50, 100 mM) or no ethanol. In order to evaluate the changes of β-endorphin release (during a 3 h period) in the media following acute and chronic ethanol treatments, cultures were fed with fresh medium containing ethanol at 0, 3, 6, 21, 24 and 45 h. The conditioned media from each culture was collected at 3, 6, 24 and 48 h after the initiation of the treatment. The conditioned medium samples were collected in 12 × 75 mm glass tubes and stored frozen at −20°C until use for measurements of β-endorphin levels. At the end of the treatment, the cells from each culture were extracted and measured for the total cellular protein level.
Protein measurement
The release of β-endorphin from the cultured MBH neurons was measured by determining the concentration of the peptide in the medium by enzyme immunoassay (EIA) using a kit purchased from Peninsula Laboratories, LLC (Torrance, CA). The assay was conducted according to the manufacturer’s protocol. The peptide release rate during a 3 h period of each culture was calculated using the media β-endorphin concentration (pg/ml) per μg of total cell protein, which was determined using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA).
Statistics
The mean ± SEM of the data were determined and presented in the text. Data obtained in the studies dealing with ethanol time effects on β-endorphin release of each strain were compared using one-way ANOVA followed by Newman Keuls post-hoc test. Ethanol time effects on β-endorphin release between Per2 mutant and wild type mice were statistically evaluated using two-way ANOVA followed by the Bonferroni t test. A value of p <0.05 was considered significant.
RESULTS
Comparison of the effects of ethanol on β-endorphin release from MBH cells of control and Per2 mutant mice in cultures
It has been shown previously that β-endorphin release from MBH cells is elevated after acute (1–12 hr) ethanol treatment, but decreased following chronic (24–48 hr) ethanol treatment (Sarkar and Minami, 1990: Boyadjieva et al., 1997; Poplawski, et al., 2005). To evaluate the effect of Per2 gene deletion on β-endorphin neuronal response to ethanol, the opioid secretory responses to various doses of ethanol at different time points were determined in cultured MBH cells of C57BL/6 and Per2 mutant mice.
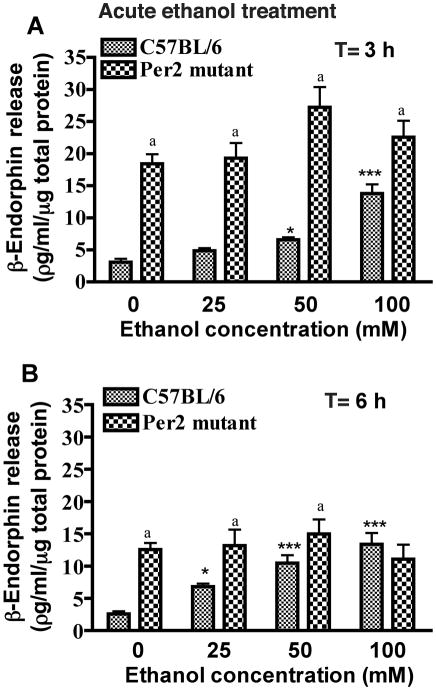
As anticipated, MBH cells of control mice demonstrated significant β-endorphin-secretory response to acute ethanol treatments for a period of 3 and 6 h in a concentration-dependent-manner (Fig. 1). The effective doses of ethanol that were able to significantly elevate β-endorphin-release were within the range of 50 and 100 mM. However, MBH cells of Per2 mice failed to show the β-endorphin-secretory response to acute treatment with either of the ethanol doses (25–100 mM) studied. Interestingly, the basal release rate of β-endorphin from MBH cells of Per2 mice was significantly higher (2–5-fold) than MBH cells of control mice (Figs. 1 and 2).
Fig. 1. Effects of acute ethanol exposure on β-endorphin release from mediobasal hypothalamic cells of C57BL/6 mice and Per2 mutant mice in primary cultures.
MBH cell cultures were treated with (25, 50, 100 mM) or without ethanol for 3 hrs (A) or 6 hrs (B). T = time of treatment. Data are mean ± SEM of six independent observations. *P <0.05, ** P <0.01, ***P <0.001, significantly different from controls of the same strain. a P< 0.05, significantly different between two strains at the same dose.
Fig. 2. Effects of chronic ethanol exposure on β-endorphin release in mediobasal hypothalamic cells of C57BL/6 mice and Per2 mutant mice.
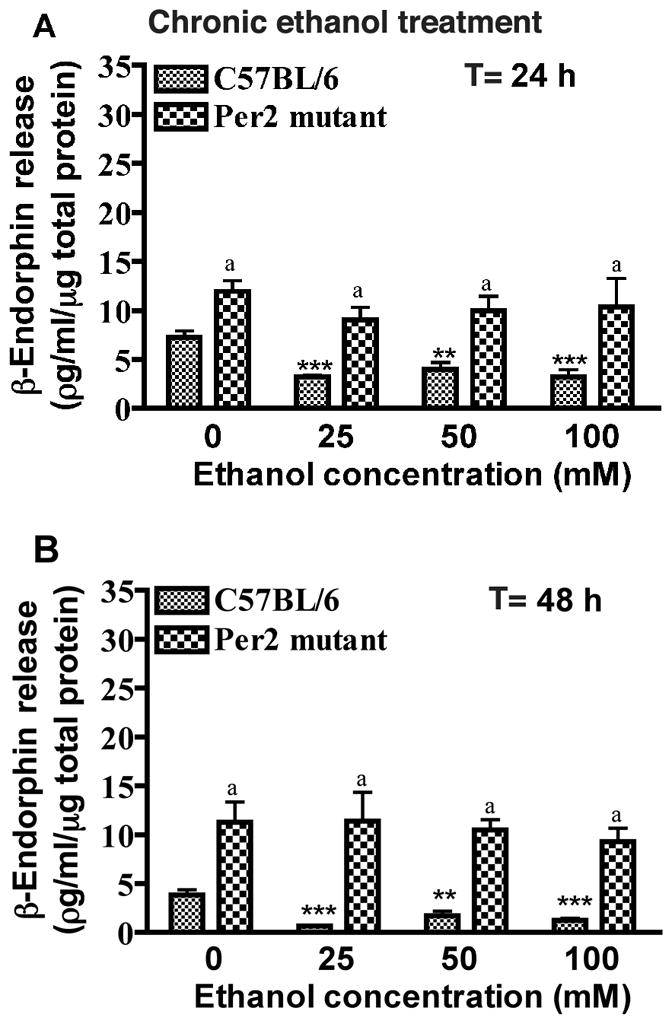
MBH cell cultures were treated with (25, 50, 100 mM) or without ethanol for 24 (A) to 48 hrs (B). T = time of treatment. Data are mean ± SEM of six independent observations. *P <0.05, ** P <0.01, ***P <0.001, significantly different from controls of the same strain. a P< 0.01, significantly different between two strains at the same dose.
When the effect of chronic ethanol treatment on β-endorphin-release from MBH cells of control mice was examined, the study revealed a significant inhibition of β-endorphin-release at 24 and 48 h by all doses (25, 50 and 100 mM) of ethanol. Chronic treatment with these ethanol doses failed to change β-endorphin-release from MBH cells of Per2 mice. This data suggests that Per2 deletion increases the basal release of β-endorphin neurons, but markedly alters the ability of these neurons to respond to ethanol.
Comparison of the effects of ethanol exposure on the MBH level of β-endorphin in control and Per2 mutant mice
The in vitro analyses of the effects of ethanol exposure on β-endorphin release from MBH cells of C57BL/6 and Per2 mutant mice clearly identified a role for this clock-regulating gene in regards to ethanol action. In the in vitro study, isolated MBH cells in culture were devoid of the influence of the rest of the central and peripheral nervous systems. Hence, ethanol effects on β-endorphin neuron were also determined in vivo.
Using milk formula, alcohol was administered acutely (3 h) or chronically (5 days) in neonatal mice. We used a 3 h treatment period for acute alcohol treatment, since this time period produced stimulatory effects on β-endorphin release in culture. We used a 5-day treatment period for chronic ethanol treatment. Although a 48 h ethanol treatment period was able to reduce β-endorphin release from MBH cells in culture, we treated the animal with ethanol for 5 days in order to establish the chronic ethanol effect on β-endorphin neurons during the equivalent of the third trimester of human pregnancy. When the MBH tissue levels of β-endorphin were compared between AD and PF groups after 3 h of feeding, no significant differences in the protein levels were found between these two treatment groups in C57BL/6 or in Per2 mutant mice (Fig. 3), suggesting that the animal handling or the formula feeding protocols did not produce any significant effect on this protein levels. However, the β-endorphin level in MBH tissue was higher in acute AF group than in AD and PF groups in C57BL/6 mice but not in Per2 mutant mice (Fig. 3). At day 5 of milk formula feeding, the β-endorphin level in MBH of PF group did not significantly differ from AD groups of C57BL/6 mice, however alcohol fed (AF) C57BL/6 mice showed a significant reduction in the peptide level in MBH tissue (Fig. 4). In Per2 mutant mice, the β-endorphin levels in MBH tissues were similar in all groups. These results suggest that, like in vitro, acute ethanol treatment increases while chronic ethanol treatment reduces the β-endorphin levels in MBH of C57BL/6 mice but not in Per2 mutant mice.
Figure 3. Effects of acute ethanol administration on β-endorphin levels in mediobasal hypothalami of C57BL/6 and Per2 mutant mice.
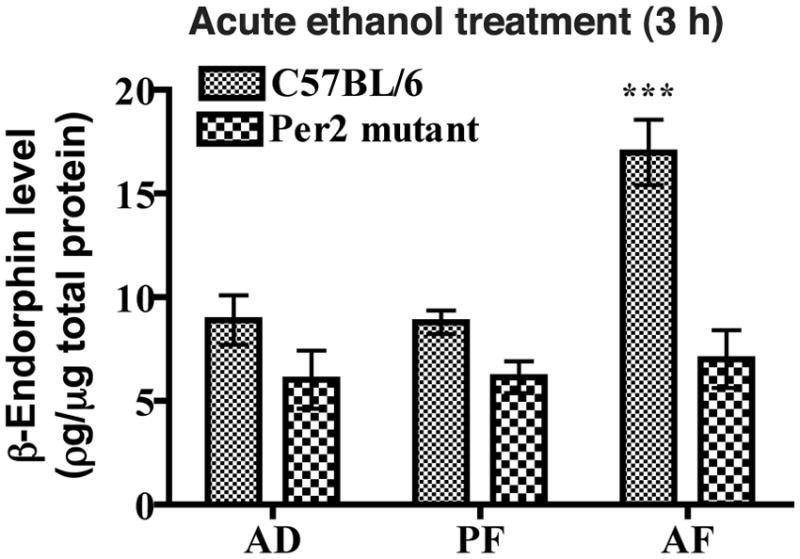
Postnatal rats were fed milk formula containing ethanol (AF) or no ethanol (PF) or left in the litter (AD) for 1 day. T = time of treatment. Data are mean ± SEM of six independent observations. *P <0.05, ** P <0.01, ***P <0.001, significantly different from controls of the same strain. a P< 0.01, significantly different between two strains at the same dose.
Figure 4. Effects of chronic ethanol administration on β-endorphin levels in mediobasal hypothalami of C57BL/6 and Per2 mutant mice.
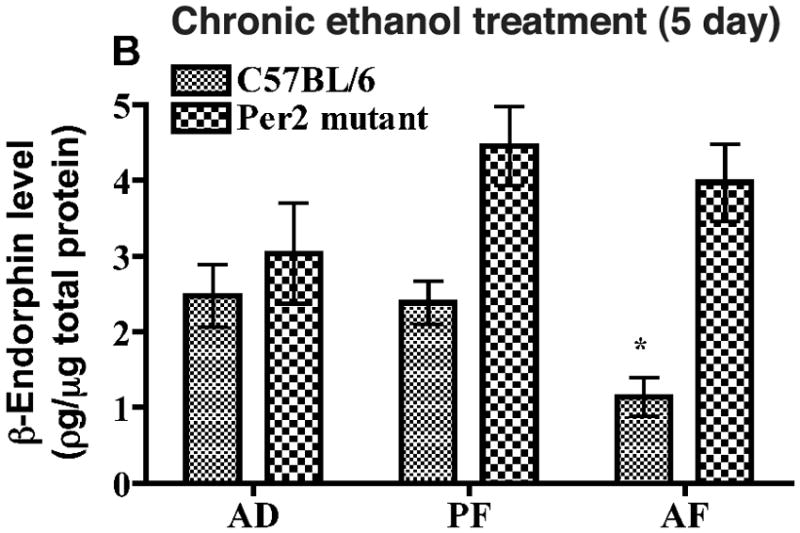
Postnatal rats were fed milk formula containing ethanol (AF) or no ethanol (PF) or left in the litter (AD) for 5 days. T = time of treatment. Data are mean ± SEM of six independent observations. *P <0.05, ** P <0.01, ***P <0.001, significantly different from controls of the same strain. a P< 0.01, significantly different between two strains at the same dose.
DISCUSSION
In this paper, we report for the first time the involvement of the Per2 gene in the β-endorphin neuronal response to ethanol. We showed here that the Per2 mutation prevents the β-endorphin stimulatory response to acute ethanol, and the β-endorphin inhibitory response to chronic ethanol treatments. Because the effect was observed not only in vivo but also in vitro cell culture systems where β-endorphin neurons may lack the influence of the central clock due to lack of the integrated neurocircutry required for SCN control mechanism, it could be suggested that the Per2 gene regulates the internal clock to control β-endorphin neuronal response to ethanol. In support of this view is the data that prenatal ethanol alters Per2 and POMC mRNA levels in the arcuate nucleus of the adult rat hypothalamus (Chen et al., 2006). Additionally, Per2 gene is identified in laser captured microdissected β-endorphin neurons (Chen et al., 2006).
We also demonstrated an increase in the basal release rate of β-endorphin in cells of MBH from Per2 mutants suggesting that the clock gene might have an inhibitory control over β-endorphin production and release. However, basal β-endorphin levels in the MBH tissue was similar in both C57BL/6 and in Per2 mutant mice, suggesting that clock gene inhibitory influence over β-endorphin production and release may be overcome by the stimulatory inputs from other CNS areas under the in vivo condition.
A core clock mechanism in the mouse SCN appears to involve a transcriptional feedback loop in which CLOCK and BMAL1 are positive regulators and three mPeriod (mPer) genes are involved in negative feedback. Clock gene products BMAL1 and CLOCK have been shown to regulate circadian expression of several genes through their binding to E-boxes (Jin et al., 1999). Thus, it is possible that the molecular circadian clock directly regulates β-endorphin producing POMC gene expression since there are putative E-box binding sites in the mouse POMC promoter (Therrien, 1993).
Our data indicated a direct action of Per2 gene in regulation of POMC gene response to ethanol. This view is in agreement of the finding by Spanagel et al, who showed increased ethanol preference following Per2 mutation possibly due to altered excitatory neurotransmission and that the increase is not related to circadian timing (Spanagel et al., 2005a). Additional support for the involvement of the Per2 gene in regulating ethanol sensitivity comes from the indirect evidence that ethanol-induced suppression of immune function is correlated with the altered expression of the Per2 gene in splenocytes (Arjona et al., 2004) and mPer2 mutation disrupts circadian expression of cytolytic factors in splenocytes (Arjona and Sarkar, 2006).
Another key question is the pathophysiological relevance of the circadian regulation of β-endorphin neurons, especially in the context of fetal alcohol spectrum disorders. β-Endorphin peptide and its precursor protein POMC, control a wide variety of physiological functions, including stress control, feeding, immune functions, tumor control, positive reinforcement and motivational properties of alcohol (De Wied et al., 1982; Gianoulakis, 1996; Konturek et al., 2005; Sarkar et al., 2008; Boyadjieva et al., 2009). Studies in laboratory rodents indicated that prenatal ethanol exposure produces significant alteration in circadian expression of Per2 and POMC genes in the hypothalamus (Chen et al., 2006), reduces immune function (Arjona et al., 2006), increases the incidence of cancers (Hilakivi-Clarke et al., 2004), and increases alcohol-drinking behavior (Chotro et al., 2003). Mutations in Per genes in mice also have been shown to produce similar effects on β-endorphin neurons (Figs 1–4), the immune system (Arjona and Sarkar, 2006), cancer development (Lee, 2006) and alcohol drinking behavior (Spanagel et al., 2005). Furthermore, the prenatal ethanol-induced stress axis dysfunction (Weinberg et al., 2008; Sarkar et al., 2007) is associated with abnormal expression of the Per2 gene in β-endorphin neurons (Arjona and Sarkar, 2006), which participate in stress control mechanisms (Sarkar et al., 2007; Boyadjieva et al., 2009). Together these data provide support for the notion that the abnormality in Per2 gene function may participate in induction of many prenatal ethanol-induced pathophysiologies. Finally, much research is needed in order to further characterize the interaction between ethanol and Per2 gene, as well as the implications of this interaction for health.
Acknowledgments
This work was supported by National Institute of Health Grants R01 AA015718 and R37 AA08757.
References
- Alvarez M, Pita S, Costas E, Cupeiro A, Chaves OF, García M, Sola A, Goyanes V. Long term effects of ritodrine on blood pressure and heart rate of adolescents exposed during the prenatal stage. Eur J Obstet Gynecol Reprod Biol. 1995;59:137–141. doi: 10.1016/0028-2243(95)02045-t. [DOI] [PubMed] [Google Scholar]
- Arjona A, Boyadjieva N, Sarkar DK. Circadian rhythms of granzyme B, perforin, IFN-gamma, and NK cell cytolytic activity in the spleen: effects of chronic ethanol. J Immunol. 2004;172:2811–2817. doi: 10.4049/jimmunol.172.5.2811. [DOI] [PubMed] [Google Scholar]
- Arjona A, Boyadjieva N, Kuhn P, Sarkar DK. Fetal ethanol exposure disrupts the daily rhythms of splenic granzyme B, IFN-gamma, and NK cell cytotoxicity in adulthood. Alcohol Clin Exp Res. 2006a;30:1039–1044. doi: 10.1111/j.1530-0277.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- Arjona A, Sarkar DK. The circadian gene mPer2 regulates the daily rhythm of IFN-γ. J Interferon Cytokine Res. 2006b;26:645–649. doi: 10.1089/jir.2006.26.645. [DOI] [PubMed] [Google Scholar]
- Blizard DA, Vandenbergh DJ, Jefferson AL, Chatlos CD, Vogler GP, McClearn GE. Effects of periadolescent ethanol exposure on alcohol preference in two BALB substrains. Alcohol. 2004;34:177–185. doi: 10.1016/j.alcohol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Boyadjieva NI, Sarkar DK. Effects of chronic alcohol on immunoreactive β-endorphin secretion from hypothalamic neurons in primary cultures:evidence for alcohol tolerance, withdrawal, and sensitization responses. Alcohol Clin Exp Res. 1994;18:1497–1501. doi: 10.1111/j.1530-0277.1994.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Boyadjieva N, Sarkar DK. The role of cAMP in ethanol-regulated beta-endorphin release from hypothalamic neurons. Alcohol Clin Exp Res. 1997;21:728–731. [PubMed] [Google Scholar]
- Boyadjieva N, Meadows GG, Sarkar DK. Chronic ethanol inhibits natural killer cell cytolytic activity: role of opioid peptide beta-endorphin. J Immunol. 2001;167:5645–5652. doi: 10.4049/jimmunol.167.10.5645. [DOI] [PubMed] [Google Scholar]
- Boyadjieva NI, Ortigüela M, Arjona A, Cheng X, Sarkar DK. β-endorphin neuronal cell transplant reduces corticotropin releasing hormone hyperresponse to lipopolysaccharide and eliminates natural killer cell functional deficiencies in fetal alcohol exposed rats. Alcohol Clin Exp Res. 2009;33:931–937. doi: 10.1111/j.1530-0277.2009.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE. An Introduction to Neuroendocrinology. Cambridge University Press; New York, NY: 1998. pp. 41–43. [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of proopiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem. 2004;88:1547–54. doi: 10.1046/j.1471-4159.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK. Prenatal ethanol exposure alters the expression of period genes governing the circadian function of β-endorphin. J Neurochem. 2006;97:1026–1033. doi: 10.1111/j.1471-4159.2006.03839.x. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C. Prenatal exposure to ethanol increases ethanol consumption: a conditioned response? Alcohol. 2003;30:19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Church AC, Feller D. The influence of mouse genotype on the changes in brain cyclic nucleotide levels induced by acute alcohol administration. Pharmacol Biochem Behav. 1979;10:335–338. doi: 10.1016/0091-3057(79)90193-x. [DOI] [PubMed] [Google Scholar]
- De Wied D, Jolles J. Neuropeptides derived from pro-opiocortin: behavioral, physiological, and neurochemical effects. Physiol Rev. 1982;62:976–1059. doi: 10.1152/physrev.1982.62.3.976. [DOI] [PubMed] [Google Scholar]
- Devaney M, Graham D, Greeley J. Circadian variation of the acute and delayed response to alcohol: investigation of core body temperature variations in humans. Pharmacol Biochem Behav. 2003;75(4):881–887. doi: 10.1016/s0091-3057(03)00170-9. [DOI] [PubMed] [Google Scholar]
- Farnell YZ, Allen GC, Nahm SS, Neuendorff N, West JR, Chen WJ, Earnest DJ. Neonatal alcohol exposure differentially alters clock gene oscillations within the suprachiasmatic nucleus, cerebellum, and liver of adult rats. Alcohol Clin Exp Res. 2008;32:544–552. doi: 10.1111/j.1530-0277.2007.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C. Implications of endogenous opioids and dopamine in alcoholism: human and basic science studies. Alcohol Alcohol (Oxford, Oxfordshire) 1996;31:33–42. [PubMed] [Google Scholar]
- Goodlett CR, Pearlman AD, Lundahl KR. Binge neonatal alcohol intubations induce dose-dependent loss of Purkinje cells. Neurotoxicol Teratol. 1998;20:285–292. doi: 10.1016/s0892-0362(97)00102-5. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Zuloaga DG, McGivern RF. Prenatal ethanol exposure alters core body temperature and corticosterone rhythms in adult male rats. Alcohol. 2007;41:567–575. doi: 10.1016/j.alcohol.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Cabanes A, de Assis S, Wang M, Khan G, Shoemaker WJ, Stevens RG. In utero alcohol exposure increases mammary tumorigenesis in rats. Br J Cancer. 2004;90:2225–2231. doi: 10.1038/sj.bjc.6601793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Konturek PC, Konturek JW, Cześnikiewicz-Guzik M, Brzozowski T, Sito E, Konturek SJ. Neuro-hormonal control of food intake: basic mechanisms and clinical implications. J Physiol Pharmacol. 2005;56(Suppl 6):5–25. [PubMed] [Google Scholar]
- Lee CC. Tumor suppression by the mammalian Period genes. Cancer Causes Control. 2006;17:525–530. doi: 10.1007/s10552-005-9003-8. [DOI] [PubMed] [Google Scholar]
- Nakashita M, Ohkubo T, Hara A, Metoki H, Kikuya M, Hirose T, Tsubota-Utsugi M, Asayama K, Inoue R, Kanno A, Obara T, Hoshi H, Totsune K, Satoh H, Imai Y. Influence of alcohol intake on circadian blood pressure variation in Japanese men: the Ohasama study. Am J Hypertens. 2009;22(11):1171–1176. doi: 10.1038/ajh.2009.160. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Metoki H, Imai Y. Alcohol intake, circadian blood pressure variation, and stroke. Hypertension. 2009;53:4–5. doi: 10.1161/HYPERTENSIONAHA.108.123018. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy AL, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Pastor R, Aragon CM. Ethanol injected into the hypothalamic arcuate nucleus induces behavioral stimulation in rats: an effect prevented by catalase inhibition and naltrexone. Behav Pharmacol. 2008;19:698–705. doi: 10.1097/FBP.0b013e328315ecd7. [DOI] [PubMed] [Google Scholar]
- Pastorcic M, Boyadjieva N, Sarkar DK. Comparison of the effects of alcohol and acetaldehyde on proopiomelanocortin mRNA expression and beta-endorphin secretion from hypothalamic neurons in primary cultures. Mol Cell Neurosci. 1994;5:580–586. doi: 10.1006/mcne.1994.1071. [DOI] [PubMed] [Google Scholar]
- Poplawski M, Boyadjieva N, Sarkar DK. Vasoactive intestinal peptide and corticotropin-releasing hormone increase β-endorphin release and POMC mRNA levels in primary cultures of hypothalamic cells: effects of acute and chronic ethanol. Alcohol Clin Exp Res. 2005;29:648–55. doi: 10.1097/01.alc.0000158834.11252.2e. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Bryant CA, Boldt BM, Colasurdo EA, Levin N, Wilkinson CW. Acute alcohol effects on opiomelanocortinergic regulation. Alcohol Clin Exp Res. 1998;22:789–801. [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res. 2000;24:1836–49. [PubMed] [Google Scholar]
- Raymond RC, Warren M, Morris RW, Leikin JB. Periodicity of presentations of drugs of abuse and overdose in an emergency department. J Toxicol Clin Toxicol. 1992;30:467–478. doi: 10.3109/15563659209021561. [DOI] [PubMed] [Google Scholar]
- Reddy BV, Boyadjieva N, Sarkar DK. Effect of ethanol, proponol, butanol and catalase enzyme blockers on beta-endorphin secretion from primary cultures of hypothalamic neurons: evidence for a mediatory role of acetaldehyde in ethanol stimulation of beta-endorphin release. Alcohol Clin Exp Res. 1995;19:339–344. doi: 10.1111/j.1530-0277.1995.tb01512.x. [DOI] [PubMed] [Google Scholar]
- Röjdmark S, Calissendorff J, Brismar K. Alcohol ingestion decreases both diurnal and nocturnal secretion of leptin in healthy individuals. Clin Endocrinol (Oxf) 2001;55:639–647. doi: 10.1046/j.1365-2265.2001.01401.x. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. Am J Physiol Regul Integr Comp Physiol. 2009;297:R729–37. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata-Haga H, Dominguez HD, Sei H, Fukui Y, Riley EP, Thomas JD. Alterations in circadian rhythm phase shifting ability in rats following ethanol exposure during the third trimester brain growth spurt. Alcohol Clin Exp Res. 2006;30:899–907. doi: 10.1111/j.1530-0277.2006.00105.x. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Minami S. Effect of acute ethanol on beta-endorphin secretion from rat fetal hypothalamic neurons in primary cultures. Life Sci. 1990;47:PL31–36. doi: 10.1016/0024-3205(90)90557-8. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Kuhn P, Marano J, Chen C, Boyadjieva N. Alcohol exposure during the developmental period induces beta-endorphin neuronal death and causes alteration in the opioid control of stress axis function. Endocrinology. 2007;148:2828–2834. doi: 10.1210/en.2006-1606. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Boyadjieva NI, Chen CP, Ortigüela M, Reuhl K, Clement KM, Kuhn P, Marano J. Cyclic adenosine monophosphate differentiated beta-endorphin neurons promote immune function and prevent prostate cancer growth. Proc Nat Acad Sci (USA) 2008;105:9105–9110. doi: 10.1073/pnas.0800289105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz WJ, Zimmerman P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J Neurocience. 1990;10:3685–3694. doi: 10.1523/JNEUROSCI.10-11-03685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggio JA, Fixaris MC, Reed JD, Logan RW, Rosenwasser AM. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. J Biol Rhythms. 2009;24:304–312. doi: 10.1177/0748730409338449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005a;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol consumption and the body’s biological clock. Alcohol Clin Exp Res. 2005b;29:1550–1557. doi: 10.1097/01.alc.0000175074.70807.fd. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Cooley-Matthews B, Poland RE. Effects of maternal ethanol consumption in rats on basal and rhythmic pituitary-adrenal function in neonatal offspring. Psychoneuroendocrinology. 1982;7:49–58. doi: 10.1016/0306-4530(82)90054-3. [DOI] [PubMed] [Google Scholar]
- Therrien M, Drouin J. Molecular determinants for cell specificity and glucocorticoid repression of the proopiomelanocortin gene. Ann N Y Acad Sci. 1993;31:680, 663–71. doi: 10.1111/j.1749-6632.1993.tb19768.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zghoul T, Abarca C, Sanchis-Segura C, Albrecht U, Schumann G, Spanagel R. Ethanol self-administration and reinstatement of ethanol-seeking behavior in Per1(Brdm1) mutant mice. Psychopharmacology (Berl) 2007;190:13–19. doi: 10.1007/s00213-006-0592-z. [DOI] [PubMed] [Google Scholar]
- Zheng B, Larkin D, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]