Abstract
EGFR is a tyrosine kinase that participates in the regulation of cellular homeostasis. Following ligand binding, EGFR stimulates downstream cell signaling cascades that influence cell proliferation, apoptosis, migration, survival and complex processes, including angiogenesis and tumorigenesis. EGFR has been strongly implicated in the biology of human epithelial malignancies, with therapeutic applications in cancers of the colon, head and neck, lung, and pancreas. Accordingly, targeting EGFR has been intensely pursued, with the development of a series of promising molecular inhibitors for use in clinical oncology. As is common in cancer therapy, challenges with respect to treatment resistance emerge over time. This situation is certainly true of EGFR inhibitor therapies, where intrinsic and acquired resistance is now well recognized. In this Review, we provide a brief overview regarding the biology of EGFR biology, preclinical and clinical development of EGFR inhibitors, and molecular mechanisms that underlie the development of treatment resistance. A greater understanding of the mechanisms that lead to EGFR resistance may provide valuable insights to help design new strategies that will enhance the impact of this promising class of inhibitors for the treatment of cancer.
Introduction
In 1962, Stanley Cohen isolated and characterized a salivary gland protein that induced eye-lid opening and tooth eruption in newborn mice.1 Further experimentation showed that this protein could stimulate the proliferation of epithelial cells and was thus named epidermal growth factor (EGF).2 It was not until a decade later, when Graham Carpenter performed experiments using 125iodine-labeled EGF, that the presence of specific binding receptors for EGF on target cells were identified.3 Subsequently, Carpenter and coworkers identified the epidermal growth factor receptor (EGFR) as a 170 kilodalton membrane protein that increased the incorporation of 32phosphorus into EGFR in response to EGF treatment of A431 epidermoid carcinoma cells.4 A group of collaborators isolated, cloned and characterized the sequence of human EGFR from normal placental cells and A431 tumor cells in 1984.5 Over the same time period, it was discovered that modification of proteins by phosphorylation on tyrosine residues might be a critical step in tumorigenesis.6,7 Shortly after these discoveries, EGFR was recognized as a receptor tyrosine kinase (RTK). This effort over two decades led to the identification of the prototypical RTK and its ligand. The identification of EGFR as an RTK contributed to pivotal studies that advanced our understanding of RTK activation and phosphorylation, and resulted in the elucidation of EGFR regulation of downstream signaling via PLC/PKC and RAS/RAF/MEK/ERK pathways.8,9
During the 1980s, several reports described the overexpression of EGFR in a variety of epithelial tumors, which supported the hypothesis that dysregulated EGFR expression and signaling may have a critical role in the etiology of human cancers.5,10–14 These findings led to investigations to target the receptor with an antibody directed against the extracellular domain of EGFR.15 Mendelsohn and colleagues developed a series of anti-EGFR monoclonal antibodies, including mAb225 (C225) and mAb528. The mAb225 showed promising antitumor activity in culture and in mouse xenograft models, which subsequently led to its development as a clinical agent.15,16 FDA approval was given in 2004 for its use in colorectal cancer. In parallel, the rational design of anti-EGFR small-molecule tyrosine kinase inhibitors (TKIs) came to the fore. The development of these agents was further supported by findings that mutations in the EGFR tyrosine kinase domain led to decreased tyrosine function and downstream signaling.17–19 The inhibitory action of quinazolines was reported in 1994,20,21 which was soon followed by the development of gefitinib, the first small-molecule inhibitor targeting EGFR.22 Gefitinib was approved by the FDA in 2003 for use in non-small-cell lung cancer (NSCLC). EGFR inhibitors have shown highly promising activity in the clinic,23–30 which has led to EGFR being one of the most studied molecular targets in clinical oncology. Coincident with this interest in targeting EGFR was the identification of intrinsic and acquired resistance to EGFR inhibitors. Indeed, the first report calling for a uniform clinical definition of acquired resistance to EGFR inhibitors was published in January 2010.31 In this Review, we focus on what is known about resistance to EGFR inhibitors in the preclinical and clinical setting. We also discuss potential methods to overcome resistance to EGFR inhibitors and future strategies to optimize successful integration of EGFR-targeting therapies in oncology.
EGFR biology
Aberrant expression or activity of EGFR has been identified as an important factor in many human epithelial cancers, including head and neck squamous-cell carcinoma (HNSCC), NSCLC, colorectal cancer (CRC), breast cancer, pancreatic cancer and brain cancer. EGFR is a member of the EGFR tyrosine kinase family, which consists of EGFR (ErbB1/HER1), HER2/neu (ErbB2), HER3 (ErbB3) and HER4 (ErbB4). All family members contain an extracellular ligand-binding domain (domains I, II, III, IV), a single membrane-spanning region, a juxtamembrane nuclear localization signal, and a cytoplasmic tyrosine kinase domain. HER receptors are ubiquitously expressed in various cell types, but primarily in those of epithelial, mesenchymal and neuronal origin. Under homeostatic conditions, receptor activation is tightly regulated by the availability of ligands, which collectively form the EGF family.8 This family is divided into three distinct groups. The first includes EGF, transforming growth factor alpha (TGF-α) and amphiregulin, which all bind specifically to EGFR. The second group includes betacellulin, heparin-binding EGF and epiregulin, which bind to both EGFR and HER4. The third group is composed of the neuregulins (NRG1–4), which is further subdivided based on their ability to bind HER3 and HER4 (NRG1 and NRG2), or only to HER4 (NRG3 and NRG4).32 HER2 has no known ligand.33 Ligand binding to domains I and III of the RTK induces major conformational changes that lead to the dimerization loop in domain II of the receptor being exposed.34 This exposure of the dimerization loop allows receptor homodimerization or heterodimerization at the plasma membrane. This interaction activates the RTK, which causes autophosphorylation of the cytoplasmic tails of each dimer pair. HER3 is the only family member that lacks intrinsic kinase activity;35 however, downstream signaling is readily achieved through heterodimerization.36 Phosphorylated cytoplasmic tails serve as docking sites for numerous proteins that contain Src homology and phosphotyrosine binding domains.
EGFR activation stimulates many complex intracellular signaling pathways that are tightly regulated by the presence and identity of the ligand, heterodimer composition, and the availability of phosphotyrosine-binding proteins. The two primary signaling pathways activated by EGFR include the RAS/RAF/MEK/ERK and the PI3K/AKT axes; however, Src tyrosine kinases, PLCγ, PKC, and STAT activation and downstream signaling have also been well documented (Figure 1).8.9 Tumor cell proliferation, survival, invasion and angiogenesis can be promoted through activation of these pathways. In addition to traditional cytoplasmic signaling, EGFR also acts as a membrane-bound chaperone protein for the sodium/glucose cotransporter, SGLT1.37 These results point to a new kinase-independent role for EGFR in promoting metabolic homeostasis in cancer cells.
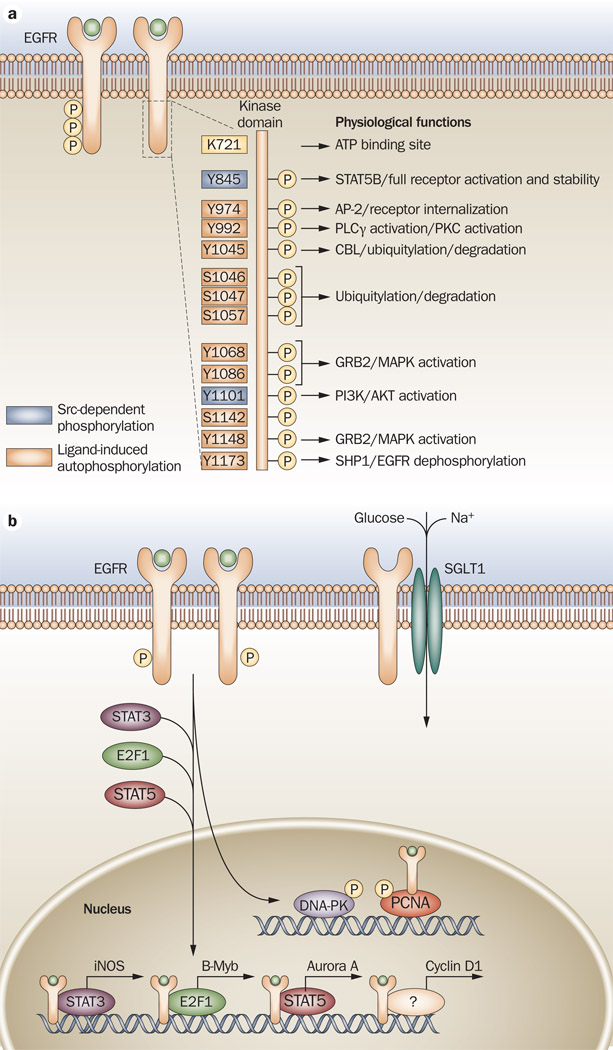
Figure 1.
EGFR biology. a | Ligand binding to EGFR causes receptor homodimerization or heterodimerization, which leads to transphosphorylation of the cytoplasmic tail tyrosine residues. Lysine 721 (K721) is the critical site for ATP-binding and kinase activity of EGFR (shown in yellow). Mutation of this amino acid causes the receptor to become inactive.153,154 Tyrosine phosphorylation in the C-terminus includes Y974, Y992, Y1045, Y1068, Y1086, Y1148 and Y1173 (shown in orange), or SFKs can phosphorylate Y845 and Y1101 (shown in purple). Reported biological effects of phosphorylation of each tyrosine are noted.155–158 b | EGFR has been consistently detected in the nuclei of cancer cells, primary tumor specimens and highly proliferative tissues.38–42 EGFR binds to STAT3 to increase expression of iNOS,47 E2F1 to increase expression of B-Myb,46 and with STAT5 to increase expression of Aurora A.52 It also increases the expression of cyclin D1.40 EGFR has kinase-dependent activity within the nucleus of proliferating cells, which includes the phosphorylation of PCNA leading to its stability and enhancing cell proliferation,53 and translocation and activation of DNA-PK.159 Abbreviations: AP-2, transcription factor AP-2; B-Myb, Myb-related protein B; CBL, E3 ubiquitin-protein ligase CBL; DNA-PK, DNA-dependent protein kinase catalytic subunit; E2F1, transcription factor E2F1; EGFR, epidermal growth factor receptor; GRB2, growth factor receptor-bound protein 2; iNOS, inducible nitric oxide synthase; MAPK, mitogen-activated protein kinase; P, phosphorylation; PCNA, proliferating cell nuclear antigen; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; PLCγ, 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase gamma-1; SFK, Src family kinase; SGLT1, sodium/glucose cotransporter 1; SHP1, tyrosine-protein phosphatase non-receptor type 6; SRC, proto-oncogene tyrosine-protein kinase Src; STAT, signal transducer and activator of transcription.
EGFR has been consistently detected in the nuclei of cancer cells from primary tumor specimens and highly proliferative tissues.38–42 Increased nuclear EGFR localization correlates with poor clinical outcome in patients with breast cancer,43 oropharyngeal HNSCC,44 and ovarian cancer.45 Nuclear localization of EGFR is associated with increased expression of cyclin D1,40 B-Myb,46 inducible nitric oxide synthase47 and COX-2,48 all of which increase G1/S progression of the cell cycle and proliferation of cancer cells. A novel nuclear localization sequence for EGFR and its family members has been reported.49 Furthermore, mechanisms of transport of EGFR to the nucleus have been reported.50 These mechanisms involve interactions with dynamin, importins, Sec1, and exportin-1.50,51 More importantly, reports have indicated a mechanism of EGFR-mediated kinase-independent gene regulation in the nucleus, which involves direct interaction with the transcription factors STAT3, STAT5 and E2F1.46,47,52 In addition, nuclear EGFR functions as a tyrosine kinase in the nucleus, phosphorylating and stabilizing proliferating cell nuclear antigen and thus enhancing the proliferative potential of cancer cells.53 As data accrues implicating the functional impact of nuclear EGFR, it becomes valuable to understand the extent to which this protein may contribute to cancer growth and progression, but also to the therapeutic response to EGFR-targeted therapies.
EGFR inhibitors
Monoclonal antibodies
Cetuximab (C225, Erbitux® [Bristol-Myers Squibb, New York, NY]) is an immunoglobulin G1 chimeric mouse–human monoclonal antibody that specifically targets the extracellular domain of EGFR (Table 1). It has a mean half-life of approximately 112 hours in humans (63–230 hours).15,16,54 Cetuximab functions by blocking endogenous ligand binding to the extracellular domain of the EGFR and enhances receptor internalization and degradation. Cetuximab can also induce antibody-dependent cell-mediated cytotoxicity (Table 2).55 Cetuximab has exhibited promising antitumor activity in clinical trials as monotherapy and when used in combination with chemotherapy and/or radiation, particularly in the settings of metastatic CRC27,56–59 and HNSCC.23,60,61 In 2004, the FDA approved cetuximab for use in combination with irinotecan in the treatment of patients with EGFR-expressing metastatic CRC refractory to irinotecan-based chemotherapy. In addition, cetuximab was approved for use as a single agent in metastatic CRC patients who cannot tolerate irinotecan-based therapies. In 2006, the FDA approved the use of cetuximab in combination with radiation for the treatment of locoregionally advanced HNSCC. In addition, cetuximab was approved as a single agent for the treatment of patients with recurrent or metastatic HNSCC for whom platinum-based therapy had failed.
Table 1.
EGFR inhibitors
| Inhibitor | Manufacturer | Class | Specificity | FDA indication and year of approval |
|---|---|---|---|---|
| Cetuximab C225, Erbitux® |
ImClone Systems | Mouse-human chimeric antibody |
EGFR | EGFR-expressing mCRC in patients refractory to irinotecan-based chemotherapy (2004) Locally or regionally advanced HNSCC in combination with radiotherapy (2006) |
| Matuzumab EMD72000 |
EMD Pharmaceuticals | Mouse-human chimeric antibody |
EGFR | Not yet approved |
| Nimotuzumab h-R3, TheraCIM® |
YM BioSciences | Human antibody | EGFR | Not yet approved |
| Panitumumab ABX-EGF, Vectibix® |
Amgen | Human antibody | EGFR | EGFR-expressing mCRC with progression on or following chemotherapy regimens containing fluoropyrimidine, oxaliplatin, and irinotecan (2006) |
| Zalutumumab HuMax-EGFr |
Genmab | Human antibody | EGFR | Not yet approved |
| Erlotinib OSI-774, Tarceva® |
Genentech | Anilinoquinazoline- based reversible inhibitor |
EGFR | NSCLC as a monotherapy after failure of at least one prior chemotherapy (2004) Advanced pancreatic cancer in combination with gemcitabine for patients who have not received previous chemotherapy (2005) |
| Gefitinib ZD1839, Iressa® |
AstraZeneca | Anilinoquinazoline- based reversible inhibitor |
EGFR | Locally advanced metastatic NSCLC cancer after failure of both platinum- based and docetaxel chemotherapies (2003) |
| Vandetanib ZD6474, Zactima® |
AstraZeneca | Anilinoquinazoline- based inhibitor |
EGFR VEGFR2 RET- tyrosine kinase |
NSCLC Submitted for approval in June 2009, but application withdrawn in October 2009 in NSCLC setting with chemotherapy |
| Lapatinib GW572016, Tykerb® |
GlaxoSmithKline | Thiazolylquinazoline- based reversible inhibitor |
EGFR HER2 |
Metastatic breast cancer in combination with capecitabine whose tumors overexpress HER2 and have received prior therapy, including an anthracycline, a taxane, and trastuzumab (2006) |
| Pelitinib EKB-569 |
Wyeth | Cyanoquinoline-based irreversible inhibitor |
EGFR HER2 |
Not yet approved |
Abbreviations: HNSCC, head and neck squamous cell carcinoma; mCRC, metastatic colorectal cancer; NSCLC, non-small cell lung cancer;
Table 2.
Proposed mechanisms of action for EGFR inhibitors
| Molecular mechanism | Mechanism of action |
|---|---|
| Inhibits ligand binding | Prevents activation of the tyrosine kinase domain and further prevents downstream activation of prosurvival pathways162,163 |
| Inhibits cell cycle progression | Cetuximab causes cell arrest in the G1 gap phase of the cell cycle via an increase in the cell cycle inhibitor p27kip1 as well as an inhibition of PCNA164 |
| ADCC | Cetuximab treatment to patients with lung cancer causes an enhanced activity in ADCC by interleukin-2 through activation of natural killer cells165,166 |
| Enhances apoptosis | Treatment with cetuximab or other similar anti-EGFR antibodies alters the balance of Bcl-2 (antiapoptotic) and Bax (proapoptotic) proteins to promote more apoptosis167,168 |
| Suppresses DNA-PK activity | Cetuximab induces radiosensitization in A549 cells and eliminates DNA-PK repair activity (via increased H2AX)169 |
| Enhances antitumor effects of radiation | Tumors are more radiosensitive with treatment of cetuximab due to the promotion to a more susceptible phase of the cell cycle. Radiation- induced nuclear transport of EGFR is inhibited by treatment with cetuximab169,170 |
| Inhibition of tyrosine kinase domain | Inhibits signal transduction of EGFR, preventing downstream signaling |
| Nonspecific HER family inhibition | Gefitinib has been suggested to inhibit both HER2 and HER3 receptors. Treatment with gefitinib induces a conformational change in EGFR that changes its ability to form dimers with other HER family receptor Erlotinib directly inhibits HER2 kinase activation and downstream signaling events171–175 |
| Decreased ligand binding | Treatment with TKIs decreases the affinity of the EGFR to its ligand176 |
Abbreviations: ADCC, antibody dependent cellular cytotoxicity; DNA-PK, DNA-dependent protein kinase; EGFR, epidermal growth factor receptor; PCNA, proliferating cell nuclear antigen; TKI, tyrosine kinase inhibitor.
Panitumumab (ABX-EGF, Vectibix® [Amgen, Thousand Oaks, CA]) is a fully humanized immunoglobulin G2 monoclonal antibody with high affinity for EGFR and a mean half-life of approximately 7.5 days in humans (range 3.6–10.9 days; Table 1).62 Panitumumab functions by blocking EGF and TGF-α binding to EGFR, and also leads to receptor internalization and degradation (Table 2).63 Panitumumab has exhibited promising antitumor activity in several clinical trials, and in 2006 gained FDA approval for the treatment of patients with EGFR-expressing metastatic CRC with disease progression following chemotherapy regimens containing fluoropyrimidine, oxaliplatin, and irinotecan.64–66
Tyrosine kinase inhibitors
TKIs under active clinical investigation are mostly derived from quinazoline, which are low molecular weight synthetic molecules that block the magnesium-ATP-binding pocket of the intracellular tyrosine kinase domain (Table 1). Several drugs, such as gefitinib and erlotinib, are specific for EGFR, while others (lapatinib, vandetanib, AEE788 [Novartis, Basel, Switzerland]) inhibit more than one receptor in addition to EGFR, such as HER2 and VEGFR2. TKIs block ligand-induced receptor autophosphorylation by binding to the tyrosine kinase domain and disrupting tyrosine-kinase activity, thereby abrogating intracellular downstream signaling (Table 2). The FDA approved gefitinib through a new accelerated process in May 2003 as monotherapy for the treatment of patients with locally advanced or metastatic NSCLC after failure of both platinum-based and docetaxel chemotherapies. As a condition of accelerated approval, the FDA required demonstration of a survival benefit in a subsequent clinical trial. After three large, prospective studies (INTACT 1, INTACT 2 and ISEL) showed no improvement in overall survival, the original FDA approval was modified in 2005, limiting the indication to cancer patients who, in the opinion of their treating physician, are currently benefiting or have previously benefited from gefitinib treatment. Erlotinib was originally approved in November 2004 as monotherapy for the treatment of NSCLC patients who did not respond to at least one prior chemotherapy. In November 2005, erlotinib was approved in combination with gemcitabine for advanced pancreatic cancer patients who have not received previous chemotherapy.
EGFR inhibitors—from bench to clinic
EGFR has been linked to the growth of many human epithelial malignancies, including NSCLC, metastatic CRC, HNSCC, and pancreatic cancer. Systematic laboratory and clinical research have facilitated the translation of EGFR inhibitors into common use in clinical oncology. Table 3 provides details of selected clinical trials that have promoted these efforts. For each EGFR inhibitor, a complex series of preclinical and clinical milestones predate FDA approval. A common theme in some examples of EGFR drug development is the beneficial impact of information gained from basic, translational and clinical research studies over time. In each anatomic area described, there have been key elements of the progress that were derived from applying laboratory findings to the clinical arena, and clinical findings that have helped advances in the laboratory setting. Indeed, this is the essence of translational cancer research, and is beautifully reflected by the emerging story of EGFR drug development.
Table 3.
Selected clinical trials of EGFR inhibitors
| Trial | Drug | Cancer | Clinical design | Median overall survival (months) |
Response rate (%) |
Median progression- free survival (months) |
|---|---|---|---|---|---|---|
| Cunningham et al.59 BOND |
Cetuximab | mCRC | Cetuximab | 8.6 | 22.9 | 4.1 |
| Cetuximab + irinotecan |
6.9 (P=0.48) |
10.8 (P=0.007) |
1.5 | |||
| Van Cutsem et al.56 CRYSTAL |
Cetuximab | mCRC | FOLFIRI | 18.6 | 38.7 | 8.0 |
| FOLFIRI + cetuximab |
19.9 (P=0.31) |
46.9 (P=0.004) |
8.9 (P=0.048) |
|||
| Bokemeyer et al.95 OPUS |
Cetuximab | mCRC | FOLFOX | NR | 36 | 7.2 |
| FOLFOX + cetuximab |
NR | 46 | 7.2 | |||
| Borner et al.26 SAKK |
Cetuximab | mCRC | CAPOX | 16.5 | 14 | 5.8 |
| CAPOX + cetuximab |
20.5 | 41 | 7.2 | |||
| Sobrero et al.27 EPIC |
Cetuximab | mCRC | Irinotecan | 100 | 4.2 | 2.6 |
| Irinotecan + cetuximab |
10.7 (P=0.71) |
16.4 (P<0.0001) |
4.0 (P<0.0001) |
|||
| Jonker et al.58 NICI |
Cetuximab | mCRC | Supportive care |
4.6 |
0 | NR |
| Supportive care + cetuximab |
6.1 (P=0.32) |
8.0 (P<0.001) |
NR | |||
| Baselga et al.117 | Cetuximab | HNSCC | Platinum CT + cetuximab |
6.1 | 11 | NR |
| Herbst et al.178 | Cetuximab | HNSCC | Cisplatin + cetuximab PD group 1 |
6.1 | 20 | 3.0 |
| Cisplatin + cetuximab PD group 2 |
4.3 | 6 | 2.0 | |||
| Cisplatin + cetuximab SD group |
11.7 | 18 | 4.9 | |||
| Vermorken et al.60 |
Cetuximab | HNSCC | Platinum CT + FU | 7.4 | 20 | 3.3 |
| Platinum CT + FU + cetuximab |
10.1 (P=0.04) |
36 (P<0.001) |
5.6 (P<0.001) |
|||
| Bonner et al.23 | Cetuximab | HNSCC | Radiotherapy | 29.3 | 64 | 12.4 |
| Radiotherapy + cetuximab |
49.0 (P=0.03) |
74 (P=0.02) |
17.1 (P=0.006) |
|||
| Van Cutsem et al.66 |
Panitumumab | mCRC | BSC | NR | 0 | 1.8 |
| BSC + panitumumab |
NR (P=0.81) |
10 (P<0.001) |
2.0 (P<0.0001) |
|||
| Shepherd et al.72 | Erlotinib | NSCLC | Placebo | 4.7 | <1 | 1.8 |
| Erlotinib | 6.7 (P<0.001) |
8.9 (P<0.001)) |
2.2 (P<0.001) |
|||
| Herbst et al.70 Tribute |
Erlotinib | NSCLC | Platinum CT + paclitaxel + placebo |
10.5 | 19.3 | NR |
| Platinum CT + paclitaxel + erlotinib |
10.6 (P=0.95) |
21.5 (P=0.36) |
NR | |||
| Gatzemeier et al.71 Talent |
Erlotinib | NSCLC | Gemcitabine + platinum CT + placebo |
10.75 | 29.9 | NR |
| Gemcitabine + platinum CT + erlotinib |
11.02 (P=0.49) |
31.5 | NR | |||
| Moore et al.28,29 | Erlotinib | Pancreatic | Gemcitabine + placebo |
5.91 | 8.0 | 3.55 |
| Gemcitabine + erlotinib |
6.24 (P=0.038) |
8.6 (P=0.07) |
3.75 (P=0.004) |
|||
| Kris et al.67 IDEAL 2 |
Gefitinib | NSCLC | 250mg gefitinib |
7 | 12.0 (P=0.005) |
– |
| 500mg gefitinib | 6 (P=0.40) |
9.0 (P=0.06) |
– | |||
| Fukuoka et al.68 IDEAL 1 |
Gefitinib | NSCLC | 250mg gefitinib | 7.6 | 18.4 | 2.7 |
| 500mg gefitinib | 8.0 | 19.0 | 2.8 | |||
| Giaccone et al.69 INTACT1 |
Gefitinib | NSCLC | CT + placebo | 10.9 | 44.8 | NR |
| CT + 250mg gefitinib |
9.9 |
50.3 |
NR |
|||
| CT + 500 mg gefitinib |
9.9 (P=0.4560) |
49.7 | NR | |||
| Herbst et al.24 INTACT2 |
Gefitinib | NSCLC | Platinum CT + placebo |
8.7 | 28.7 | NR |
| Platinum CT + 250mg gefitinib |
9.8 | 30.4 | NR | |||
| Platinum CT + 500mg gefitinib |
9.9 (P=0.6385) |
30.0 | NR | |||
| Thatcher et al.25 ISEL |
Gefitinib | NSCLC | BSC + placebo | 5.1 | 1.3 | NR |
| BSC + gefitinib | 5.6 (P=0.087) |
8.0 (P < 0.001) |
NR | |||
| Geyer et al.179 | Lapatinib | mBC | Capecitabine | 8.0 | 14 | 4.1 |
| Capecitabine + lapatinib |
10.4 | 22 (P=0.09) |
8.4 (P<0.001) |
|||
| Di Leo et al.30 | Lapatinib | mBC | Paclitaxel + placebo |
21.75 | 25.3 | 5.73 |
| Paclitaxel + lapatinib |
24.78 (P=0.216) |
35.1 (P<0.008) |
7.25 (P=0.142) |
|||
Abbreviations: BSC, best supportive care; CAPOX, capecitabine, oxaliplatin; CT, chemotherapy; FOLFIRI, folinic acid (leucovorin), fluorouracil, irinotecan; FOLFOX, folinic acid (leucovorin), fluorouracil, oxaliplatin; FU, fluorouracil; HNSCC, head and neck squamous cell carcinoma; mBC, metastatic breast cancer; mCRC, metastatic colorectal cancer; NSCLC, non-small cell lung cancer; PD, progressive disease; SD, stable disease.
Gefitinib and erlotinib in clinical trials
Phase II trials showed a promising response and symptom improvement rate with gefitinib as monotherapy in patients with advanced stage NSCLC, thereby contributing to FDA approval of this agent in 2003 as second-line or third-line treatment.67,68 However, subsequent phase III trials, INTACT 1 and INTACT 2, which tested gefitinib in the first-line setting with concurrent doublet chemotherapy, did not identify improvement in overall survival or time to progression (TTP).24,69 The FDA recommended, therefore, to limit the indications for gefitinib to patients currently or previously being treated or enrolled on approved clinical trials. Erlotinib also faced challenges in clinical advancement for NSCLC with two major phase III clinical trials, TALENT and TRIBUTE (similar design to the INTACT trials), which showed no improvement in overall survival or TTP.70,71 It was not until the BR 21 trial, which compared erlotinib with placebo in the second-line or third-line setting for advanced NSCLC patients, that erlotinib established a survival benefit prompting FDA approval.72 The mixed results of these clinical trials initiated further investigations aimed at identifying population subsets that may be more likely to benefit from EGFR TKIs. Analyses of biospecimens from clinical trials identified a unique subpopulation of patients (Asian, female, never-smokers, adenocarcinoma histology) who were most likely to respond to EGFR TKIs.25,72,73 The landmark identification of a subset of lung cancers harboring mutations in the EGFR tyrosine kinase domain stimulated tremendous research activity and improved understanding of methods to enrich the selection of patients for lung cancer trials who are most likely to derive benefit from EGFR TKI therapy approaches.74–76 This work also stimulated the discovery of an EGFR resistance mutation (T790M) in lung cancer patients receiving chronic gefitinib treatment.77–79
A series of clinical trials have specifically selected patients with documented EGFR mutations to enrich the population of subjects who are most likely to benefit from first-line treatment with EGFR TKI therapy.80–86 These studies have uniformly demonstrated impressive response rates in the range of 50–70% with excellent progression-free survival (PFS) and overall survival rates. These trials also exhibit notably improved treatment tolerance compared with conventional platinum-based doublet chemotherapy regimens, despite the inclusion in some studies of elderly patients with poor performance status. Indeed, gefitinib gained approval in Europe in 2009 for adults with locally advanced or metastatic NSCLC with EGFR mutations in all lines of therapy.
Cetuximab in clinical trials
Cetuximab has also undergone active clinical evaluation in advanced NSCLC. A series of phase II trials suggested activity of cetuximab in combination with platinum doublets in the first-line treatment setting.87–91 Two phase III trials have been reported, including the FLEX and BMS099 trials.92,93 The FLEX trial demonstrated an improvement in overall survival with the addition of cetuximab to first-line cisplatin and vinorelbine. The BMS099 trial evaluated the addition of cetuximab to carboplatin/taxane in the first-line setting and identified an improvement of overall response rate, but not a statistically significant improvement in PFS (the primary end point of the study).
A phase I trial of HNSCC in 1997–1998 enrolled 16 patients with locoregionally advanced tumors and provided the first clinical signal that adding cetuximab to radiation may improve tumor response and disease control.94 Despite the absence of true phase II data, a phase III trial that enrolled 424 patients was carried out between 1999–2002 that confirmed a 10% overall survival advantage for patients receiving cetuximab in combination with curative radiation for advanced HNSCC compared with radiotherapy alone.23 The influence of radiation and EGFR inhibition on proliferation, apoptosis, cell repopulation, angiogenesis, and DNA damage repair needs to be clarified. Interestingly, this result was in contrast to the trials in NSCLC where concurrent administration of EGFR inhibitors with cytotoxic chemotherapy did not prove advantageous. This finding may reflect the different tumor types (HNSCC versus NSCLC), different classes of EGFR inhibitors (monoclonal antibodies versus TKIs), or the distinction between radiation and chemotherapy as the cytotoxic treatment modality.
EGFR inhibitors in CRC
The advancement of EGFR inhibitors in CRC also reveals a complex story with continuing stepwise improvements in our understanding of tumor biology and patient selection. The emergence of KRAS mutation status as a valuable predictor of response to cetuximab therapy for patients with metastatic CRC is a reminder that examination of molecular signatures for each individual tumor can provide powerful information to guide optimal therapy selection.56,95–102 Panitumumab affords another valuable approach for metastatic CRC patients with chemorefractory disease.66 Despite promising preclinical and early clinical data that suggested the potential value of combining EGFR inhibitors with VEGF inhibitors in CRC, clinical trial data did not demonstrate improved efficacy and showed increased toxicity in this setting.103
Resistance to EGFR antibodies
EGFR expression as a predictor of response
In light of the high specificity of anti-EGFR monoclonal antibodies for the extracellular domain of the EGFR, the initial assumption was that these agents would be most effective in tumors with robust overexpression of EGFR. Indeed, it was anticipated that expression levels of EGFR would serve as a predictive biomarker for the likelihood of response to cetuximab therapy, which would parallel the clinical paradigm in breast cancer, where women with breast cancer who have high HER2 receptor expression are more likely to respond to trastuzumab anti-HER2 therapy. However, early clinical studies did not confirm a correlation between EGFR expression level by immunohistochemistry and likelihood of response to EGFR inhibitor therapy.104 In fact, Chung et al.104 confirmed that several CRC patients who received cetuximab exhibited a major objective response despite the absence of measureable EGFR. Collectively, these studies suggest that immunohistochemistry-based assays measuring EGFR expression does not serve as a robust predictor for response to cetuximab therapy.
EGFR copy number as a predictor of response
Studies analyzing EGFR copy number have suggested that it may provide some predictive and prognostic value in CRC. Lièvre et al.96 reported that increased EGFR copy number assessed by chromogenic in situ hybridization, was significantly associated with objective tumor response to cetuximab therapy (P = 0.04). When EGFR copy number was assessed by PCR, it was found that increased EGFR copy number was significantly associated with prolonged survival, indicating a potential prognostic value of EGFR copy number (P = 0.03).105 Moroni et al.106 analyzed EGFR copy number by fluorescence in situ hybridization (FISH) and found a significant association between high EGFR copy number and response to both cetuximab and panitumumab (P = 0.01).107
KRAS mutation as a predictor of response
KRAS mutation status in CRC has emerged as an important predictive biomarker that enables improved identification of patients more likely to respond to EGFR inhibitors. Lievre et al.96 reported in 2006 that KRAS with mutations at codon 12 or 13 might be predictive of resistance to cetuximab therapy. In this report, they analyzed 30 patients with metastatic CRC treated with cetuximab for KRAS, BRAF and PIK3CA mutations. KRAS mutations were found in 43% of tumors (13 tumors), and was significantly associated with resistance to cetuximab therapy (P = 0.002).96 To confirm these findings, Di Fiore et al.97 studied 59 patients with chemorefractory metastatic CRC treated with cetuximab plus chemotherapy. Direct sequencing SNaPshot® (Applied Biosystems, Foster City, CA, USA) and PCR-ligase assays determined KRAS mutations. KRAS mutations were highly predictive of resistance to cetuximab plus chemotherapy.97 A larger study was performed to measure the KRAS mutation status in 113 patients with irinotecan-refractory metastatic CRC treated with cetuximab. The authors reported that KRAS wildtype is a strong predictor of significant increase in overall survival (P <0.001) in this cohort of patients.98
In a seminal clinical report investigating KRAS mutational status, Van Cutsem et al.58 investigated the efficacy of cetuximab plus irinotecan, fluorouracil, and leucovorin (FOLFIRI) as first-line treatment for metastatic CRC and sought associations between the mutation status of KRAS and clinical response to cetuximab. In this study, 599 patients received cetuximab plus FOLFIRI, and 599 received FOLFIRI alone. First-line treatment with cetuximab plus FOLFIRI reduced the risk of disease progression compared with FOLFIRI alone, and the benefit of cetuximab was limited to patients with KRAS wildtype tumors.56 Since the publication of these studies, several additional clinical trials have further strengthened these findings.99–102,108 This collective body of work has led to a Provisional Clinical Opinion from ASCO in 2009 stating that all patients with metastatic CRC who are candidates for anti-EGFR antibody therapy should have their tumor tested for KRAS mutations in a CLIA (clinical laboratory improvement amendments)-accredited laboratory. If codons 12 or 13 of KRAS are mutated, patients with metastatic CRC should not receive anti-EGFR antibody therapy as part of their treatment.109
In patients with metastatic CRC and wildtype KRAS, the expression of EGFR ligands has also shown promise as a predictor of response to cetuximab therapy. The first report showed that patients with increased expression of epiregulin and amphiregulin exhibited disease control.110 Jacobs et al.111 extended these findings and found that expression profiling of epiregulin and amphiregulin may predict both PFS and overall survival in those with KRAS wildtype metastatic CRC who are treated with cetuximab and irinotecan.
Mechanisms of EGFR antibody resistance
EGFR mutations
In 2004, a series of landmark papers identified EGFR mutations in the tyrosine kinase domain in NSCLC patients that predicted response to the TKIs erlotinib and gefitinib.74–76 These mutations included in-frame deletion of amino acids 746–750 in exon 19, and a point mutation in exon 21 (L858R). More importantly, these mutations led to gain-of-function and conferred dependence of the tumor cell on the mutated kinase. These mutations in EGFR rendered tumors dramatically more sensitive to the effects of erlotinib and gefitinib than tumors without these mutations. This important finding has stimulated a prolific body of preclinical and clinical research that has substantially advanced our understanding of EGFR mutations and their role in governing response to small-molecule TKIs directed against EGFR. However, no mutations in EGFR have been identified to date that are reliably predictive for response to antibody-based EGFR therapies.112 This finding suggests that other molecular mechanisms may exist that modulate intrinsic (primary) or acquired (secondary) resistance to EGFR antibody-based therapies (Figure 2).
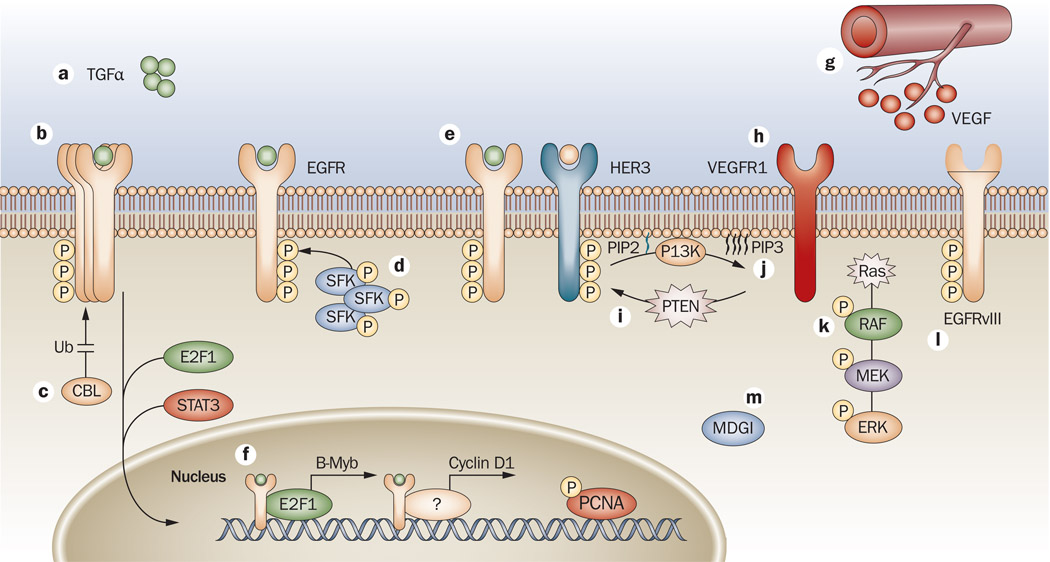
Figure 2.
Mechanisms of resistance to EGFR antibodies. a | One mechanism of resistance to cetuximab is overexpression of the EGFR ligand TGFα.160 b | Overexpression of EGFR has also been implicated in the development of acquired resistance.117 c | Ubiquitylation is important for mechanisms of escape to cetuximab therapy.117,122 d | Modulation of EGFR by SFKs, and increased activity of SFKs in cetuximab-resistant lines have been reported.122,123 e | The binding and activation of EGFR or HER2 to HER3 has been reported, which allows prolonged signals to the PI3K/AKT pathway.117,123 f | Translocation of EGFR to the nucleus has a role in resistance to cetuximab.125 g | Increased VEGF production leads to altered angiogenesis and enhanced escape from cetuximab therapy.115,116 h | VEGFR1 also contributes to resistance to cetuximab.120 i–j | Mutations in both PTEN and Ras have been implicated in impaired response to cetuximab therapy.96 k | Mutations in KRAS keep it in a constant GTP-bound, active state, allowing it to send signals downstream independently from RTK activation. l | EGFRvIII a truncated form of EGFR that is constitutively phosphorylated in a ligand-independent manner.121,161 m | MDGI alters trafficking of EGFR, leading to resistance to cetuximab therapy.126 Abbreviations: B-Myb, Myb-related protein B; CBL, E3 ubiquitin-protein ligase CBL; E2F1, transcription factor E2F1; EGFR, epidermal growth factor receptor; ERK, mitogen-activated protein kinase 3; MDGI, mammary derived growth inhibitor; P, phosphorylation; PCNA, proliferating cell nuclear antigen; PI3K, phosphatidylinositol 3-kinase; PIP3, phosphatidylinositol 3,4,5-trisphosphate PTEN, phosphatase and tensin homolog; RTK, receptor tyrosine kinase; SFK, Src family kinase; STAT3, signal transducer and activator of transcription 3; TGFα, transforming growth factor alpha; Ub, ubiquitylation; VEGF, vascular endothelial growth factor; VEGFR1, vascular endothelial growth factor receptor 1.
Altered VEGF/VEGFR expression
EGFR signaling can contribute to the production of several proangiogenic factors in tumors, including VEGF and basic fibroblast growth factor.113,114 To investigate whether altered angiogenesis could serve as a potential mechanism of resistance to cetuximab therapy, Viloria-Petit et al.115 examined the high EGFR-expressing A431 cell line in mouse xenografts. Tumor xenografts were treated with three different EGFR-blocking antibodies (mR3, hR3 or cetuximab). Tumors treated with these three anti-EGFR antibodies led to prompt regression of the tumor followed by a long latency period. Once the tumors reappeared, they were refractory to a second round of antibody therapy. Several established cell lines from hR3 and mR3-treated tumors (cells from cetuximab-treated tumors could not be obtained) retained their sensitivity to these antibodies, whereas some variants exhibited accelerated growth rate and attenuated response to hR3 and mR3 in subsequent testing.115 Owing to the reported suppressive effects of EGFR inhibitors on VEGF production, the researchers hypothesized that these anti-EGFR antibodies inhibited EGFR-mediated VEGF production, thereby decreasing angiogenesis and leading to decreased tumor growth. They further postulated that escaping this angiogenic inhibition might have contributed to anti-EGFR antibody resistance. Indeed, five of six resistant variants exhibited increased VEGF expression. Furthermore, A431 parental cells transfected with VEGF resulted in resistance to anti-EGFR antibodies in vivo. This report indicated that resistance could emerge in tumors that increase their VEGF production.
In 2004, Ciardiello et al.116 reported that ZD6474, a dual EGFR/VEGFR2 TKI, could overcome resistance to cetuximab. In this study, the investigators developed cetuximab-resistant GEO CRC cell lines in vivo by prolonged exposure to cetuximab. This treatment led to tumor control for 80–90 days followed by tumor growth, despite continuation of cetuximab therapy. Discontinuation of cetuximab and treatment of these resistant tumors with ZD6474 resulted in efficient tumor growth inhibition for up to an additional 150 days. Cell lines derived from this work showed a dramatic increase in phosphorylated MAPK, increased COX-2 and VEGF protein expression compared with parental controls. The authors concluded that inhibition of VEGFR signaling in cetuximab-resistant tumor cells offered a potential anticancer strategy. In addition to cetuximab resistant clones, Ciardiello and colleagues also generated gefitinib-resistant GEO colon cancer cells.116 Resistant clones resulting from these experiments also exhibited an increase in protein expression of COX-2 and VEGF. Collectively, these data suggest that challenge with both classes of EGFR inhibitors can alter VEGF production, and highlights neoangiogenesis as a potential shared mechanism of EGFR inhibitor escape. Although this work has indicated that ZD6474 may be a viable treatment for tumors that manifest resistance to prolonged cetuximab therapy, ZD6474 itself demonstrates anti-EGFR activity. Tumors with resistance to anti-EGFR antibody therapy may retain sensitivity to EGFR-based TKI therapies. Similar findings have since been reported.117–119 Work from Bianco et al.120 has further implicated the VEGF and VEGFR system in resistance to cetuximab. Here they reported that VEGFR1 was overexpressed in cells with resistance to cetuximab. Experiments silencing VEGFR1 in cetuximab-resistant cells restored sensitivity to cetuximab, whereas exogenous overexpression of VEGFR1 in cetuximab-sensitive cells conferred resistance to cetuximab. A similar analysis of cells with resistance to gefitinib also exhibited increased expression of VEGFR1.120
EGFRvIII
Although no point mutations are known to be associated with resistance to cetuximab or panitumumab, preclinical models analyzing the EGFR variant III (EGFRvIII), which lacks the ligand-binding domain, have provided new information.121 In a study analyzing HNSCC tumors, 42% of tumors expressed EGFRvIII, and this correlated with increased proliferation in vitro and increased tumor growth in vivo.121 To determine if this variant could contribute to cetuximab resistance, HNSCC cells were engineered to overexpress EGFRvIII. These tumors showed decreased proliferation in response to cetuximab treatment compared with vector-only controls. These findings suggest that a percentage of HNSCC tumors may express EGFRvIII and this protein contributes to cetuximab resistance.121
Ubiquitination of EGFR
Two papers have identified the potential role of EGFR ubiquitination as a mechanism of acquired resistance to cetuximab.117,122 Wheeler et al.123 developed cells with acquired resistance to cetuximab in vitro by prolonging and escalating dose exposure to cetuximab. Several resistant clones were derived from this work that had increased EGFR expression compared with parental controls, which was associated with dysregulation of EGFR internalization or degradation. This altered processing of EGFR led to sustained signaling from EGFR, which led to activation of HER3. Lu et al.122 took a similar approach and found that EGFR had an increased association with the ubiquitin ligase CBL, leading to increased ubiquitination and downregulation of EGFR. Although lower levels of EGFR were expressed in these cetuximab-resistant cells, EGFR retained strong activity that seemed to be associated with cooperation with Src family kinases (SFKs). Similar to these findings, it was reported in 2008 that EGFR and SFKs cooperate in acquired resistance to cetuximab.123 In this work, cells with acquired resistance to cetuximab exhibited robust expression of active SFKs, and this activity enhanced EGFR activation of HER3 and the PI3K/AKT pathway, leading to enhanced survival. Blockade of SFK activity using dasatinib could, therefore, resensitize tumors to cetuximab therapy.
Cellular localization of EGFR
EGFR has been reported to function in the nucleus as a transcription factor as well as a tyrosine kinase that enhances cell proliferation.53,124 Furthermore, nuclear EGFR is a prognostic factor in human disease.43–45 Subcellular distribution of EGFR to the nucleus might have a role in resistance to cetuximab therapy.125 Clones with acquired resistance to cetuximab expressed nuclear EGFR, which regulated the expression of several genes involved in G1/S progression. The authors reported that nuclear translocation of EGFR was mediated by SFKs and that abrogation of SFK activity led to loss of nuclear EGFR, increased membrane EGFR, and resensitization to cetuximab.125
Nevo et al.126 investigated the role of mammary-derived growth factor inhibitor (MDGI) in conferring resistance to cetuximab. The authors reported that MDGI, a small cytosolic protein involved in fatty-acid binding, leads to the intracellular accumulation of EGFR where it remains active, and cannot be targeted by cetuximab therapy.126 These data suggest that the subcellular distribution of EGFR may be an effective escape from cetuximab therapy.
Epithelial-mesenchymal transition
Epithelial-mesenchymal transition has also been implicated in the resistance to both cetuximab and EGFR small-molecule inhibitors. Fuchs et al.127 reported that in a series of 12 hepatocellular carcinoma cells classified as epithelial or mesenchymal (based on E-cadherin and vimentin expression), the cells exhibited variable sensitivity to EGFR inhibitors. Cells that were identified as epithelial had increased sensitivity to erlotinib, gefitinib and cetuximab compared with cells that were defined as mesenchymal. The authors further reported that mesenchymal cells had increased AKT and STAT3 activation associated with elevated expression of the integrin-linked kinase ILK, which led to resistance.127
Resistance to EGFR TKIs
Despite excellent clinical response to EGFR TKIs in NSCLC patients harboring mutations in the catalytic domain, acquired resistance following initial response often manifests within 6–12 months of therapy.31 Pao et al.77 reported that molecular analysis of EGFR in patients with acquired resistance to gefitinib or erlotinib contain a secondary mutation in exon 20, which leads to substitution of methionine for threonine at position 790 (T790M) in the kinase domain.77,79 T790M of EGFR is considered to be the ‘gatekeeper’ residue, which is an important determinant of inhibitor specificity in the ATP-binding pocket of EGFR. Substitution of this residue in EGFR with a bulky methionine may cause resistance by steric interference with binding of TKIs, including gefitinib and erlotinib.77–79 However, further research on this mutation has shown that it may cause resistance to these agents by increasing the affinity for ATP.128 Since these reports were published, several studies have shown that the T790M mutation is actually present before the patient commences initial therapy.129 This finding suggests that this mutation may confer a survival advantage to the tumor and is probably selected for while the patient is receiving anti-EGFR TKI treatment.129–133 The identification of the EGFR T790M mutation has led to preclinical and clinical development of irreversible EGFR TKIs to effectively target this mechanism of resistance.78
An activating mutation of KRAS is present in 15–30% of NSCLC.134,135 Unlike the somatic mutations that arise in EGFR in non-smokers, KRAS mutations are highly prevalent in smoking-associated tumors.136,137 These mutations in KRAS may be a marker of primary resistance to both gefitinib and erlotinib.138
Mechanisms of resistance to EGFR TKIs
Several mechanisms of resistance to erlotinib and gefitinib have been described in laboratory-based models (Figure 3).
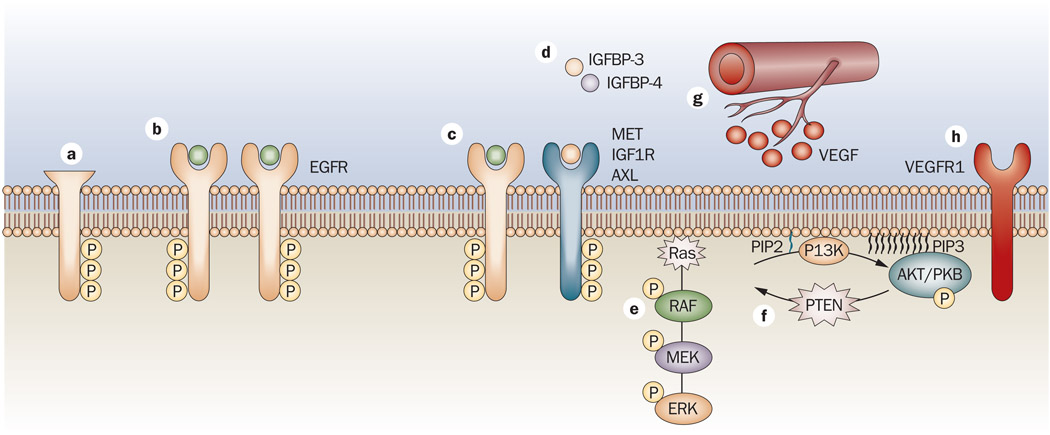
Figure 3.
Mechanisms of resistance to EGFR TKIs. a | A mutant form of EGFR termed EGFRvIII has an in-frame deletion mutation that produces a truncated 150 kDa protein, which is constitutively phosphorylated in a ligand-independent manner.161 b | EGFR-dependent tumors that are initially sensitive to EGFR TKIs acquire a mutation at threonine 790. Substitution of this residue in EGFR with a bulky methionine may cause resistance by steric interference with binding of TKIs, including gefitinib and erlotinib.77–79 c | Tumors can become resistant when individual tumor cells undergo an oncogenic shift, which has been noted with several other RTKs, including HGF receptor,148 AXL and IGF1R. d | In addition to IGF1R as a mechanism of escape, downregulation of the IGF- binding proteins IGFBP3 and IGFBP4, have been implicated in resistance to TKIs. These proteins are crucial for regulating the levels of IGF1R ligands, and loss leads to overactivation of the receptor.152 e–f | Mutations in both PTEN and Ras have been implicated in impaired response to TKI therapy.134,135 g | Cells that developed acquired resistance to gefitinib in vivo were shown to have increased VEGF production leading to altered angiogenesis and enhanced escape from cetuximab therapy.116 h | VEGFR1 has also been implicated in the contribution to resistance to EGFR TKIs.120 Abbreviations: AXL, tyrosine-protein kinase receptor UFO (AXL oncogene); EGFR, epidermal growth factor receptor; HGF, hepatocyte growth factor; IGF1R, insulin-like growth factor 1 receptor; IGFBP, insulin-like growth factor-binding protein; kDa, kilodalton; PTEN, phosphatase and tensin homolog; RTK, receptor tyrosine kinase; TKI, tyrosine kinase inhibitor, VEGF, vascular endothelial growth factor; VEGFR1, vascular endothelial growth factor receptor 1.
EGFRvIII
One of these involves the mutant, constitutively active form of EGFR termed EGFRvIII, which is commonly found in glioblastoma multiforme (GBM). GBM is a highly malignant primary brain tumor that accounts for >50% of all brain cancers diagnosed. EGFR is amplified in 50% of all GBM cases, and 40% of these cases express EGFRvIII. GBM cell lines expressing EGFRvIII are resistant to gefitinib and, therefore, require higher drug doses and prolonged exposure to decrease the activity of EGFRvIII.139 Studies analyzing the cell cycle in EGFR-expressing lines versus EGFRvIII lines revealed that DNA synthesis in EGFR lines is inhibited by gefitinib in a dose-dependent manner whereas it is unchanged in EGFRvIII lines. In addition, cells expressing EGFRvIII have higher activation of AKT, which is not affected by gefitinib treatment.
Role of oncogenic shift
One of the prevalent biological themes underlying intrinsic or acquired resistance involves ‘oncogenic shift’, which occurs when other membrane-bound RTK signaling pathways are involved in resistance. For example, HER2 and HER3 have been linked to gefitinib resistance. Erjala et al.140 investigated molecular predictors of gefitinib response in HNSCC and measured key proteins in the EGFR signaling pathway. They reported an association between EGFR copy number and gefitinib sensitivity. Gefitinib-resistant cells had increased expression levels of HER2 and total HER3 protein. To determine if this increased activity of HER2 could contribute to gefitinib resistance, gefitinib was combined with pertuzumab, an antibody that targets HER2 heterodimerization.140 This study resulted in an additive growth-inhibitory effect over gefitinib alone in gefitinib-resistant HNSCC cell lines. The authors concluded that EGFR amplification may predict sensitivity to gefitinib and that HER2 and HER3 may contribute to gefitinib resistance. Other studies of acquired resistance to gefitinib or erlotinib have suggested that ADAM17 (disintegrin and metalloproteinase domain-containing protein 17) can mediate the release of heregulin, leading to autocrine loop activation of HER2 and HER3 and thus provide a mechanism of escape from gefitinib.141 HER2 and HER3 may serve as potential predictive markers and as therapeutic targets for combination therapy in the treatment of HNSCC with gefitinib.140
Activation of the AKT/mTOR pathway
Another established finding in EGFR inhibitor resistance is the activation of the AKT/mTOR pathway leading to enhanced cell survival. PI3K phosphorylates phosphatidylinositol (4,5)-disphosphate (PIP2) to phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which serves as a docking site for AKT where it is activated by PDK1 and PDK2. Phosphatase and tensin homolog (PTEN) dephosphorylates PIP3 back to PIP2. Mutations in or loss of PTEN expression142,143 may serve as a marker of primary resistance to gefitinib and erlotinib.144,145 However, in a cohort of gefitinib-treated NSCLC, no correlation between PTEN and response to gefitinib treatment was observed, and researchers have questioned the role of these proteins in mediating primary insensitivity to gefitinib.146 Engelman et al.147 observed that gefitinib reduced AKT only in NSCLC cell lines where it inhibits growth. To better understand this observation, immunoprecipitates of PI3K from gefitinib-resistant and sensitive lines were analyzed. PI3K was exclusively associated with HER3 in gefitinib-sensitive cell lines, and gefitinib could dissociate this activity. These results suggested that HER3 couples EGFR to the PI3K/AKT pathway in gefitinib-sensitive NSCLC cell lines, but not gefitinib-resistant lines.147 Further studies implicating HER3 have centered on gefitinib-resistant EGFR-mutant lung cancer lines. These cells lines displayed amplification of MET, which prolonged activation of the HER3/PI3K/AKT axis. Abrogation of MET activity restored sensitivity to gefitinib. To assess the clinical relevance of MET as a mechanism of resistance, Engelman et al.148 also examined whether MET amplification could be detected in NSCLCs with mutant EGFR that had become resistant to gefitinib. MET amplification was detected in 4 out of 18 (22%) gefitinib/erlotinib-resistant tumor specimens.148 This study was one of the first to suggest that oncogenic shift, beyond the HER family, may contribute to resistance to EGFR-targeted therapies.148 In addition to MET amplification, overexpression of hepatocyte growth factor (HGF), which is a ligand of MET, has been implicated in the development of acquired resistance to gefitinib. Yano et al.149 reported that lung adenocarcinoma patients harboring EGFR-activating mutations, but no T790M mutation or MET amplification, showed a dramatic increase in HGF and activation of MET. This finding suggests that increased production of HGF represents a novel mechanism of gefitinib resistance in lung adenocarcinoma with EGFR-activating mutations.149
Role of IGF-1R in resistance to EGFR inhibitors
The IGF-1 receptor (IGF-1R) is ubiquitously expressed in cancer cells. This membrane-bound RTK has a role in tumor cell proliferation, differentiation, apoptosis, and metastasis.150 It has also been strongly implicated in mediating resistance to the EGFR inhibitor AG1478 (A.G. Scientific, San Diego, CA). Analyzing two primary GBM cell lines with equal EGFR protein expression levels, but with distinct sensitivities to the TKI AG1478, indicated an upregulation of IGF-1R that resulted in sustained signaling to PI3K/AKT and ribosomal protein S6 kinase.151 In addition to IGF-1R being implicated in resistance to gefitinib, IGF-binding proteins enhance acquired resistance to gefitinib. In these studies, A431 squamous-cell carcinoma lines were used to develop acquired resistance to gefitinib. Gefitinib-resistant clones exhibited hyperphosphorylation of IGF-1R and constitutive association of insulin-receptor substrate-1 with PI3K. Blockade of IGF-1R signaling disrupted this complex and restored gefitinib ability to downregulate PI3K/AKT signaling and cell growth. Gene profiling of the gefitinib-resistant clones indicated that resistant cells had markedly decreased expression of IGFBP3 and IGFBP4. These proteins are crucial for modulating the levels of the IGF-1R ligands, IGF-1 and IGF-2. Loss of these two regulatory proteins led to an increased availability of IGF-1 and IGF-2 and thus constitutive activation of IGF-1R. Experiments using recombinant IGFBP-3 restored sensitivity of resistant cells to gefitinib.152 Despite these reports in laboratory-based models of the role of IGF-1R and its associated regulatory elements, clinical analysis of IGF-1R expression in tumors from NSCLC patients indicated a lack of association with resistance to gefitinib.146
Future directions and conclusions
The advancement of EGFR inhibitors for cancer therapy has moved rapidly in the broad context of oncology therapeutics. The fact that four new EGFR inhibitors (gefitinib, cetuximab, erlotinib, panitumumab) received FDA approval for use in oncology over a period of less than 4 years is a remarkable testament to the functional role of EGFR as a molecular target that regulates tumor cell behavior and response to treatment. Indeed, additional promising drugs with multitarget activity (including anti-EGFR action) are in active development or have received FDA approval in oncology, such as lapatinib. These new agents will enable the systematic evaluation of multitarget inhibition strategies, which include EGFR blockade, to affect tumor response in human cancers.
Despite rapid advances in EGFR oncology therapeutics over the past decade, substantial room for progress remains. Most cancer patients do not respond to EGFR inhibitor therapy, which implies intrinsic resistance. Even in those patients who do achieve a clear tumor response to EGFR inhibitors, the majority will eventually manifest disease progression, which implies acquired resistance. Improving our ability to identify the tumors that rely on EGFR signaling for their growth is critical to the optimal selection of patients for therapy. This concept is beautifully borne out by the EGFR mutation studies first reported in 2004 that identified activating mutations in EGFR that confer a high likelihood of response to the anti-EGFR TKIs.74–76
Alternative RTK pathways that are activated following EGFR inhibition is another area for investigation. These alternative pathways may bypass or evade inhibition of EGFR signaling, thereby enabling combinations of agents to simultaneously attack multiple molecular targets for cancer growth inhibition. Finally, the capacity of cancer cells to adapt to treatment suggests that additional mechanisms of resistance to EGFR inhibitors may have a key role in regulating tumor response, such as the induction of tumor/stromal interactions (angiogenesis), translocation of surface receptors to the nucleus, altered DNA damage response, and as yet undiscovered mutations. Advancing our knowledge of specific cellular and molecular mechanisms of resistance to EGFR inhibitor therapies will illuminate new strategies to improve this promising class of agents.
Key points.
EGFR has been the most comprehensively studied molecular target in oncology therapeutics over the past decade
Four primary EGFR inhibitors—gefitinib, cetuximab, erlotinib, and panitumumab—received FDA approval in oncology over a period of less than 4 years (2003–2006)
Current FDA-approved indications for primary EGFR inhibitors include selected patients with colorectal cancer, head and neck cancer, lung cancer and pancreatic cancer
EGFR mutations in a cohort of lung cancer patients and KRAS mutations in a cohort of colorectal cancer patients are powerful predictors of response to specific EGFR inhibitors
Intrinsic and acquired resistance to EGFR inhibitors is increasingly well recognized
A greater understanding of molecular mechanisms of resistance to EGFR inhibitors is stimulating new treatment strategies to enhance the impact of these promising agents
Acknowledgments
The laboratory of P. M. Harari is supported in part by the National Institutes of Health/National Cancer Institute Grant R01 CA 113,448. E. F. Dunn is supported by the National Institutes of Health T32 grants (Grant CA009614-17 Physician Scientist Training in Cancer Medicine).
Footnotes
Competing interests
D. L. Wheeler declares an association with the following company: Bristol-Myers Squibb. P. M. Harari declares an association with the following companies: Amgen, AstraZeneca, Genentech and ImClone. See the article online for full details of the relationships. E. F. Dunn declares no competing interests.
Review criteria
The data for this Review were obtained by searching the PubMed database. The search terms included “cetuximab”, “erlotinib”, “gefitinib”, “EGFR and resistance”, “HER3”, “HER family ligands”, “TGF alpha”, “epidermal growth factor”, “amphiregulin”, and “Src family kinases” with no publication date limitations. Full articles of relevant abstracts were obtained and references were checked for additional material when appropriate. In addition, proceedings from conferences of ASCO, AACR, and ASTRO between 1950 and 2010 were searched for relevant abstracts using the terms “cetuximab”, “gefitinib”, “erlotinib”, “EGFR” and “resistance”.
Contributor Information
Deric L. Wheeler, Department of Human Oncology, University of Wisconsin Comprehensive Cancer Center, 1111 Highland Avenue Madison, WI 53705, USA
Emily F. Dunn, Department of Human Oncology, University of Wisconsin Comprehensive Cancer Center, 1111 Highland Avenue Madison, WI 53705, USA
Paul M. Harari, Department of Human Oncology, University of Wisconsin Comprehensive Cancer Center, 600 Highland Avenue, Madison, WI 53792, USA
References
- 1.Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J. Biol. Chem. 1962;237:1555–1562. [PubMed] [Google Scholar]
- 2.Cohen S. The stimulation of epidermal proliferation by a specific protein (EGF) Dev. Biol. 1965;12:394–407. doi: 10.1016/0012-1606(65)90005-9. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter G, Lembach KJ, Morrison MM, Cohen S. Characterization of the binding of 125-I-labeled epidermal growth factor to human fibroblasts. J. Biol. Chem. 1975;250:4297–4304. [PubMed] [Google Scholar]
- 4.Carpenter G, King L, Jr, Cohen S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature. 1978;276:409–410. doi: 10.1038/276409a0. [DOI] [PubMed] [Google Scholar]
- 5.Ullrich A, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- 6.Eckhart W, Hutchinson MA, Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979;18:925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- 7.Hunter T, Sefton BM. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc. Natl Acad. Sci. USA. 1980;77:1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 9.Marmor MD, Skaria KB, Yarden Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:903–913. doi: 10.1016/j.ijrobp.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Libermann TA, et al. Expression of epidermal growth factor receptors in human brain tumors. Cancer Res. 1984;44:753–760. [PubMed] [Google Scholar]
- 11.Libermann TA, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 12.Libermann TA, et al. Amplification and overexpression of the EGF receptor gene in primary human glioblastomas. J. Cell Sci. Suppl. 1985;3:161–172. doi: 10.1242/jcs.1985.supplement_3.16. [DOI] [PubMed] [Google Scholar]
- 13.Veale D, Ashcroft T, Marsh C, Gibson GJ, Harris AL. Epidermal growth factor receptors in non-small cell lung cancer. Br. J. Cancer. 1987;55:513–516. doi: 10.1038/bjc.1987.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weichselbaum RR, et al. Epidermal growth factor receptor gene amplification and expression in head and neck cancer cell lines. Head Neck. 1989;11:437–442. doi: 10.1002/hed.2880110510. [DOI] [PubMed] [Google Scholar]
- 15.Sato JD, et al. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol. Biol. Med. 1983;1:511–529. [PubMed] [Google Scholar]
- 16.Kawamoto T, et al. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc. Natl Acad. Sci. USA. 1983;80:1337–1341. doi: 10.1073/pnas.80.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honegger AM, et al. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987;51:199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- 18.Honegger AM, et al. A mutant epidermal growth factor receptor with defective protein tyrosine kinase is unable to stimulate proto-oncogene expression and DNA synthesis. Mol. Cell. Biol. 1987;7:4568–4571. doi: 10.1128/mcb.7.12.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redemann N, et al. Anti-oncogenic activity of signalling-defective epidermal growth factor receptor mutants. Mol. Cell. Biol. 1992;12:491–498. doi: 10.1128/mcb.12.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fry DW, et al. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science. 1994;265:1093–1095. doi: 10.1126/science.8066447. [DOI] [PubMed] [Google Scholar]
- 21.Osherov N, Levitzki A. Epidermal-growth-factor-dependent activation of the src-family kinases. Eur. J. Biochem. 1994;225:1047–1053. doi: 10.1111/j.1432-1033.1994.1047b.x. [DOI] [PubMed] [Google Scholar]
- 22.Wakeling AE, et al. Specific inhibition of epidermal growth factor receptor tyrosine kinase by 4-anilinoquinazolines. Breast Cancer Res. Treat. 1996;38:67–73. doi: 10.1007/BF01803785. [DOI] [PubMed] [Google Scholar]
- 23.Bonner JA, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 24.Herbst RS, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 2. J. Clin. Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 25.Thatcher N, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 26.Borner M, et al. Adding cetuximab to capecitabine plus oxaliplatin (XELOX) in first-line treatment of metastatic colorectal cancer: a randomized phase II trial of the Swiss Group for Clinical Cancer Research SAKK. Ann. Oncol. 2008;19:1288–1292. doi: 10.1093/annonc/mdn058. [DOI] [PubMed] [Google Scholar]
- 27.Sobrero AF, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:2311–2319. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 28.Moore MJ, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 29.Senderowicz AM, et al. Erlotinib/gemcitabine for first-line treatment of locally advanced or metastatic adenocarcinoma of the pancreas. Oncology (Williston Park) 2007;21:1696–1706. [PubMed] [Google Scholar]
- 30.Di Leo A, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J. Clin. Oncol. 2008;26:5544–5552. doi: 10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackman D, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J. Clin. Oncol. 2010;28:357–360. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 33.Klapper LN, et al. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc. Natl Acad. Sci. USA. 1999;96:4995–5000. doi: 10.1073/pnas.96.9.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 35.Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL., 3rd Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc. Natl Acad. Sci. USA. 1994;91:8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallasch C, et al. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J. 1995;14:4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weihua Z, et al. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell. 2008;13:385–393. doi: 10.1016/j.ccr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marti U, et al. Localization of epidermal growth factor receptor in hepatocyte nuclei. Hepatology. 1991;13:15–20. [PubMed] [Google Scholar]
- 39.Cao H, Lei ZM, Bian L, Rao CV. Functional nuclear epidermal growth factor receptors in human choriocarcinoma JEG-3 cells and normal human placenta. Endocrinology. 1995;136:3163–3172. doi: 10.1210/endo.136.7.7540549. [DOI] [PubMed] [Google Scholar]
- 40.Lin SY, et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat. Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 41.Lo HW, Hsu SC, Hung MC. EGFR signaling pathway in breast cancers: from traditional signal transduction to direct nuclear translocalization. Breast Cancer Res. Treat. 2006;95:211–218. doi: 10.1007/s10549-005-9011-0. [DOI] [PubMed] [Google Scholar]
- 42.Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br. J. Cancer. 2006;94:184–188. doi: 10.1038/sj.bjc.6602941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo HW, et al. Novel prognostic value of nuclear epidermal growth factor receptor in breast cancer. Cancer Res. 2005;65:338–348. [PubMed] [Google Scholar]
- 44.Psyrri A, et al. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin. Cancer Res. 2005;11:5856–5862. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- 45.Xia W, et al. Nuclear expression of epidermal growth factor receptor is a novel prognostic value in patients with ovarian cancer. Mol. Carcinog. 2009;48:610–617. doi: 10.1002/mc.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanada N, et al. Co-regulation of B-Myb expression by E2F1 and EGF receptor. Mol. Carcinog. 2006;45:10–17. doi: 10.1002/mc.20147. [DOI] [PubMed] [Google Scholar]
- 47.Lo HW, et al. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–589. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Lo HW, Cao X, Zhu H, Ali-Osman F. Cyclooxygenase-2 is a novel transcriptional target of the nuclear EGFR-STAT3 and EGFRvIII-STAT3 signaling axes. Mol. Cancer Res. 2010;8:232–245. doi: 10.1158/1541-7786.MCR-09-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsu SC, Hung MC. Characterization of a novel tripartite nuclear localization sequence in the EGFR family. J. Biol. Chem. 2007;282:10432–10440. doi: 10.1074/jbc.M610014200. [DOI] [PubMed] [Google Scholar]
- 50.Lo HW, et al. Nuclear-cytoplasmic transport of EGFR involves receptor endocytosis, importin beta1 and CRM1. J. Cell. Biochem. 2006;98:1570–1583. doi: 10.1002/jcb.20876. [DOI] [PubMed] [Google Scholar]
- 51.Liao HJ, Carpenter G. Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol. Biol. Cell. 2007;18:1064–1072. doi: 10.1091/mbc.E06-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hung LY, et al. Nuclear epidermal growth factor receptor (EGFR) interacts with signal transducer and activator of transcription 5 (STAT5) in activating Aurora-A gene expression. Nucleic Acids Res. 2008;36:4337–4351. doi: 10.1093/nar/gkn417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang SC, et al. Tyrosine phosphorylation controls PCNA function through protein stability. Nat. Cell Biol. 2006;8:1359–1368. doi: 10.1038/ncb1501. [DOI] [PubMed] [Google Scholar]
- 54.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin. Cancer Res. 1995;1:1311–1318. [PubMed] [Google Scholar]
- 55.Kimura H, et al. Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci. 2007;98:1275–1280. doi: 10.1111/j.1349-7006.2007.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Cutsem E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 57.Saltz LB, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J. Clin. Oncol. 2007;25:4557–4561. doi: 10.1200/JCO.2007.12.0949. [DOI] [PubMed] [Google Scholar]
- 58.Jonker DJ, et al. Cetuximab for the treatment of colorectal cancer. N. Engl. J. Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 59.Cunningham D, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 60.Vermorken JB, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 61.Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J. Clin. Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 62.Yang XD, Jia XC, Corvalan JR, Wang P, Davis CG. Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit. Rev. Oncol. Hematol. 2001;38:17–23. doi: 10.1016/s1040-8428(00)00134-7. [DOI] [PubMed] [Google Scholar]
- 63.Yang XD, et al. Eradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant chemotherapy. Cancer Res. 1999;59:1236–1243. [PubMed] [Google Scholar]
- 64.Hecht JR, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J. Clin. Oncol. 2009;27:672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 65.Giusti RM, et al. U.S. Food and Drug Administration approval: panitumumab for epidermal growth factor receptor-expressing metastatic colorectal carcinoma with progression following fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy regimens. Clin. Cancer Res. 2008;14:1296–1302. doi: 10.1158/1078-0432.CCR-07-1354. [DOI] [PubMed] [Google Scholar]
- 66.Van Cutsem E, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J. Clin. Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 67.Kris MG, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 68.Fukuoka M, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) J. Clin. Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 69.Giaccone G, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 1. J. Clin. Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Herbst RS, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J. Clin. Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 71.Gatzemeier U, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J. Clin. Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 72.Shepherd FA, et al. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 73.Tsao MS, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N. Engl. J. Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 74.Paez JG, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 75.Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 76.Pao W, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl Acad. Sci. USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pao W, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwak EL, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc. Natl Acad. Sci. USA. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobayashi S, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 80.Asahina H, et al. A phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutations. Br. J. Cancer. 2006;95:998–1004. doi: 10.1038/sj.bjc.6603393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Inoue A, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J. Clin. Oncol. 2009;27:1394–1400. doi: 10.1200/JCO.2008.18.7658. [DOI] [PubMed] [Google Scholar]
- 82.Inoue A, et al. Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J. Clin. Oncol. 2006;24:3340–3346. doi: 10.1200/JCO.2005.05.4692. [DOI] [PubMed] [Google Scholar]
- 83.Rosell R, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 84.Sequist LV, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J. Clin. Oncol. 2008;26:2442–2449. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 85.van Zandwijk N, et al. EGFR and KRAS mutations as criteria for treatment with tyrosine kinase inhibitors: retro- and prospective observations in non-small-cell lung cancer. Ann. Oncol. 2007;18:99–103. doi: 10.1093/annonc/mdl323. [DOI] [PubMed] [Google Scholar]
- 86.Yoshida K, et al. Prospective validation for prediction of gefitinib sensitivity by epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer. J. Thorac. Oncol. 2007;2:22–28. [PubMed] [Google Scholar]
- 87.Butts CA, et al. Randomized phase II study of gemcitabine plus cisplatin or carboplatin [corrected], with or without cetuximab, as first-line therapy for patients with advanced or metastatic non small-cell lung cancer. J. Clin. Oncol. 2007;25:5777–5784. doi: 10.1200/JCO.2007.13.0856. [DOI] [PubMed] [Google Scholar]
- 88.Herbst RS, et al. A phase II randomized selection trial evaluating concurrent chemotherapy plus cetuximab or chemotherapy followed by cetuximab in patients with advanced non-small cell lung cancer (NSCLC): Final report of SWOG 0342 [abstract] J. Clin. Oncol. 2007;25:7545. [Google Scholar]
- 89.Robert F, et al. Phase I/IIa study of cetuximab with gemcitabine plus carboplatin in patients with chemotherapy-naive advanced non-small-cell lung cancer. J. Clin. Oncol. 2005;23:9089–9096. doi: 10.1200/JCO.2004.00.1438. [DOI] [PubMed] [Google Scholar]
- 90.Rosell R, et al. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancer. Ann. Oncol. 2008;19:362–369. doi: 10.1093/annonc/mdm474. [DOI] [PubMed] [Google Scholar]
- 91.Thienelt CD, et al. Multicenter phase I/II study of cetuximab with paclitaxel and carboplatin in untreated patients with stage IV non-small-cell lung cancer. J. Clin. Oncol. 2005;23:8786–8793. doi: 10.1200/JCO.2005.03.1997. [DOI] [PubMed] [Google Scholar]
- 92.Pirker R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 93.Lynch TJ, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J. Clin. Oncol. 2010;28:911–917. doi: 10.1200/JCO.2009.21.9618. [DOI] [PubMed] [Google Scholar]
- 94.Robert F, et al. Phase I study of anti-epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J. Clin. Oncol. 2001;19:3234–3243. doi: 10.1200/JCO.2001.19.13.3234. [DOI] [PubMed] [Google Scholar]
- 95.Bokemeyer C, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 96.Lièvre A, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 97.Di Fiore F, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br. J. Cancer. 2007;96:1166–1169. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Roock W, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann. Oncol. 2008;19:508–515. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 99.Amado RG, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 100.Karapetis CS, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 101.Bokemeyer C, et al. KRAS status and efficacy of first-line treatment of patients with metastatic colorectal cancer (mCRC) with FOLFOX with or without cetuximab: The OPUS experience [abstract] J. Clin. Oncol. 2008;26:4000. [Google Scholar]
- 102.Van Cutsem E, et al. KRAS status and efficacy in the first-line treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLFIRI with or without cetuximab: The CRYSTAL experience [abstract] J. Clin. Oncol. 2008;26:2. [Google Scholar]
- 103.Punt CJ, Tol J. More is less—combining targeted therapies in metastatic colorectal cancer. Nat. Rev. Clin. Oncol. 2009;6:731–733. doi: 10.1038/nrclinonc.2009.168. [DOI] [PubMed] [Google Scholar]
- 104.Chung KY, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J. Clin. Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 105.Lenz HJ, et al. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J. Clin. Oncol. 2006;24:4914–4921. doi: 10.1200/JCO.2006.06.7595. [DOI] [PubMed] [Google Scholar]
- 106.Moroni M, et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol. 2005;6:279–286. doi: 10.1016/S1470-2045(05)70102-9. [DOI] [PubMed] [Google Scholar]
- 107.Sartore-Bianchi A, et al. Epidermal growth factor receptor gene copy number and clinical outcome of metastatic colorectal cancer treated with panitumumab. J. Clin. Oncol. 2007;25:3238–3245. doi: 10.1200/JCO.2007.11.5956. [DOI] [PubMed] [Google Scholar]
- 108.Punt CJ, et al. Randomized phase III study of capecitabine, oxaliplatin, and bevacizumab with or without cetuximab in advanced colorectal cancer (ACC), the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG) [abstract] J. Clin. Oncol. 2008;26 doi: 10.1093/annonc/mdm607. LBA4011. [DOI] [PubMed] [Google Scholar]
- 109.Allegra CJ, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J. Clin. Oncol. 2009;27:2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 110.Khambata-Ford S, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J. Clin. Oncol. 2007;25:3230–3237. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 111.Jacobs B, et al. Amphiregulin and epiregulin mRNA expression in primary tumors predicts outcome in metastatic colorectal cancer treated with cetuximab. J. Clin. Oncol. 2009;27:5068–5074. doi: 10.1200/JCO.2008.21.3744. [DOI] [PubMed] [Google Scholar]
- 112.Mukohara T, et al. Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations. J. Natl Cancer Inst. 2005;97:1185–1194. doi: 10.1093/jnci/dji238. [DOI] [PubMed] [Google Scholar]
- 113.Goldman CK, et al. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol. Biol. Cell. 1993;4:121–133. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ciardiello F, et al. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin. Cancer Res. 2001;7:1459–1465. [PubMed] [Google Scholar]
- 115.Viloria-Petit A, et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res. 2001;61:5090–5101. [PubMed] [Google Scholar]
- 116.Ciardiello F, et al. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor therapy. Clin. Cancer Res. 2004;10:784–793. doi: 10.1158/1078-0432.ccr-1100-03. [DOI] [PubMed] [Google Scholar]
- 117.Wheeler DL, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang S, Armstrong EA, Benavente S, Chinnaiyan P, Harari PM. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004;64:5355–5362. doi: 10.1158/0008-5472.CAN-04-0562. [DOI] [PubMed] [Google Scholar]
- 119.Matar P, et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin. Cancer Res. 2004;10:6487–6501. doi: 10.1158/1078-0432.CCR-04-0870. [DOI] [PubMed] [Google Scholar]
- 120.Bianco R, et al. Vascular endothelial growth factor receptor-1 contributes to resistance to anti-epidermal growth factor receptor drugs in human cancer cells. Clin. Cancer Res. 2008;14:5069–5080. doi: 10.1158/1078-0432.CCR-07-4905. [DOI] [PubMed] [Google Scholar]
- 121.Sok JC, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin. Cancer Res. 2006;12:5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 122.Lu Y, et al. Epidermal growth factor receptor (EGFR) ubiquitination as a mechanism of acquired resistance escaping treatment by the anti-EGFR monoclonal antibody cetuximab. Cancer Res. 2007;67:8240–8247. doi: 10.1158/0008-5472.CAN-07-0589. [DOI] [PubMed] [Google Scholar]
- 123.Wheeler DL, et al. Epidermal growth factor receptor cooperates with Src family kinases in acquired resistance to cetuximab. Cancer Biol. Ther. 2009;8:696–703. doi: 10.4161/cbt.8.8.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang SC, Hung MC. Nuclear transloaction of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin. Cancer Res. 2009;15:6484–6489. doi: 10.1158/1078-0432.CCR-08-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li C, Iida M, Dunn EF, Ghia AJ, Wheeler DL. Nuclear EGFR contributes to acquired resistance to cetuximab. Oncogene. 2009;28:3801–3813. doi: 10.1038/onc.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nevo J, et al. Mammary-derived growth inhibitor alters traffic of EGFR and induces a novel form of cetuximab resistance. Clin. Cancer Res. 2009;15:6570–6581. doi: 10.1158/1078-0432.CCR-09-0773. [DOI] [PubMed] [Google Scholar]
- 127.Fuchs BC, et al. Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res. 2008;68:2391–2399. doi: 10.1158/0008-5472.CAN-07-2460. [DOI] [PubMed] [Google Scholar]
- 128.Yun CH, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl Acad. Sci. USA. 2008;105:2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kosaka T, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 130.Gow CH, Shih JY, Chang YL, Yu CJ. Acquired gefitinib-resistant mutation of EGFR in a chemonaive lung adenocarcinoma harboring gefitinib-sensitive mutation L858R. PLoS Med. 2005;2:e269. doi: 10.1371/journal.pmed.0020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kwak EL, et al. Epidermal growth factor receptor kinase domain mutations in esophageal and pancreatic adenocarcinomas. Clin. Cancer Res. 2006;12:4283–4287. doi: 10.1158/1078-0432.CCR-06-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bell DW, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat. Genet. 2005;37:1315–1316. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- 133.Shih JY, Gow CH, Yang PC. EGFR mutation conferring primary resistance to gefitinib in non-small-cell lung cancer. N. Engl. J. Med. 2005;353:207–208. doi: 10.1056/NEJM200507143530217. [DOI] [PubMed] [Google Scholar]
- 134.Rodenhuis S, et al. Mutational activation of the K-ras oncogene. A possible pathogenetic factor in adenocarcinoma of the lung. N. Engl. J. Med. 1987;317:929–935. doi: 10.1056/NEJM198710083171504. [DOI] [PubMed] [Google Scholar]
- 135.Mitsudomi T, et al. ras gene mutations in non-small cell lung cancers are associated with shortened survival irrespective of treatment intent. Cancer Res. 1991;51:4999–5002. [PubMed] [Google Scholar]
- 136.Husgafvel-Pursiainen K, et al. K-ras mutations in human adenocarcinoma of the lung: association with smoking and occupational exposure to asbestos. Int. J. Cancer. 1993;53:250–256. doi: 10.1002/ijc.2910530213. [DOI] [PubMed] [Google Scholar]
- 137.Ahrendt SA, et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001;92:1525–1530. doi: 10.1002/1097-0142(20010915)92:6<1525::aid-cncr1478>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 138.Pao W, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Learn CA, et al. Resistance to tyrosine kinase inhibition by mutant epidermal growth factor receptor variant III contributes to the neoplastic phenotype of glioblastoma multiforme. Clin. Cancer Res. 2004;10:3216–3224. doi: 10.1158/1078-0432.ccr-03-0521. [DOI] [PubMed] [Google Scholar]
- 140.Erjala K, et al. Signaling via ErbB2 and ErbB3 associates with resistance and epidermal growth factor receptor (EGFR) amplification with sensitivity to EGFR inhibitor gefitinib in head and neck squamous cell carcinoma cells. Clin. Cancer Res. 2006;12:4103–4111. doi: 10.1158/1078-0432.CCR-05-2404. [DOI] [PubMed] [Google Scholar]
- 141.Zhou BB, et al. Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in non-small cell lung cancer. Cancer Cell. 2006;10:39–50. doi: 10.1016/j.ccr.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kohno T, Takahashi M, Manda R, Yokota J. Inactivation of the PTEN/MMAC1/TEP1 gene in human lung cancers. Genes Chromosomes Cancer. 1998;22:152–156. doi: 10.1002/(sici)1098-2264(199806)22:2<152::aid-gcc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 143.Soria JC, et al. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin. Cancer Res. 2002;8:1178–1184. [PubMed] [Google Scholar]
- 144.She QB, Solit D, Basso A, Moasser MM. Resistance to gefitinib in PTEN-null HER-overexpressing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3'-kinase/Akt pathway signaling. Clin. Cancer Res. 2003;9:4340–4346. [PubMed] [Google Scholar]
- 145.Bianco R, et al. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene. 2003;22:2812–2822. doi: 10.1038/sj.onc.1206388. [DOI] [PubMed] [Google Scholar]
- 146.Cappuzzo F, et al. Insulin-like growth factor receptor 1 (IGFR-1) is significantly associated with longer survival in non-small-cell lung cancer patients treated with gefitinib. Ann. Oncol. 2006;17:1120–1127. doi: 10.1093/annonc/mdl077. [DOI] [PubMed] [Google Scholar]
- 147.Engelman JA, et al. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc. Natl Acad. Sci. USA. 2005;102:3788–3793. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Engelman JA, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 149.Yano S, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–9487. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 150.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 151.Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 2002;62:200–207. [PubMed] [Google Scholar]
- 152.Guix M, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J. Clin. Invest. 2008;118:2609–2619. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Russo MW, Lukas TJ, Cohen S, Staros JV. Identification of residues in the nucleotide binding site of the epidermal growth factor receptor/kinase. J. Biol. Chem. 1985;260:5205–5208. [PubMed] [Google Scholar]
- 154.Gullick WJ, Downward J, Foulkes JG, Waterfield MD. Antibodies to the ATP-binding site of the human epidermal growth factor (EGF) receptor as specific inhibitors of EGF-stimulated protein-tyrosine kinase activity. Eur. J. Biochem. 1986;158:245–253. doi: 10.1111/j.1432-1033.1986.tb09744.x. [DOI] [PubMed] [Google Scholar]
- 155.Biscardi JS, et al. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J. Biol. Chem. 1999;274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 156.Grøvdal LM, Stang E, Sorkin A, Madshus IH. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp. Cell Res. 2004;300:388–395. doi: 10.1016/j.yexcr.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 157.Buday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73:611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- 158.Keilhack H, et al. Phosphotyrosine 1173 mediates binding of the protein-tyrosine phosphatase SHP-1 to the epidermal growth factor receptor and attenuation of receptor signaling. J. Biol. Chem. 1998;273:24839–24846. doi: 10.1074/jbc.273.38.24839. [DOI] [PubMed] [Google Scholar]
- 159.Dittmann K, Mayer C, Kehlbach R, Rodemann HP. Radiation-induced caveolin-1 associated EGFR internalization is linked with nuclear EGFR transport and activation of DNA-PK. Mol. Cancer. 2008;7:69. doi: 10.1186/1476-4598-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rajput A, et al. A novel mechanism of resistance to epidermal growth factor receptor antagonism in vivo. Cancer Res. 2007;67:665–673. doi: 10.1158/0008-5472.CAN-06-2773. [DOI] [PubMed] [Google Scholar]
- 161.Batra SK, et al. Epidermal growth factor ligand-independent, unregulated, cell-transforming potential of a naturally occurring human mutant EGFRvIII gene. Cell Growth Differ. 1995;6:1251–1259. [PubMed] [Google Scholar]
- 162.Wong SF. Cetuximab: an epidermal growth factor receptor monoclonal antibody for the treatment of colorectal cancer. Clin. Ther. 2005;27:684–694. doi: 10.1016/j.clinthera.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 163.Li S, et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 164.Baselga J. The EGFR as a target for anticancer therapy—focus on cetuximab. Eur. J. Cancer. 2001;37:S16–S22. doi: 10.1016/s0959-8049(01)00233-7. [DOI] [PubMed] [Google Scholar]
- 165.Kurai J, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin. Cancer Res. 2007;13:1552–1561. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 166.Martinelli E, De Palma R, Orditura M, De Vita F, Ciardiello F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin. Exp. Immunol. 2009;158:1–9. doi: 10.1111/j.1365-2249.2009.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mandal M, Adam L, Mendelsohn J, Kumar R. Nuclear targeting of Bax during apoptosis in human colorectal cancer cells. Oncogene. 1998;17:999–1007. doi: 10.1038/sj.onc.1202020. [DOI] [PubMed] [Google Scholar]
- 168.Liu B, et al. Induction of apoptosis and activation of the caspase cascade by anti-EGF receptor monoclonal antibodies in DiFi human colon cancer cells do not involve the c-jun N-terminal kinase activity. Br. J. Cancer. 2000;82:1991–1999. doi: 10.1054/bjoc.2000.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dittmann K, Mayer C, Rodemann H. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiother. Oncol. 2005;76:157–161. doi: 10.1016/j.radonc.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 170.Huang S, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–1940. [PubMed] [Google Scholar]
- 171.Campiglio M, et al. Inhibition of proliferation and induction of apoptosis in breast cancer cells by the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor ZD1839 ('Iressa') is independent of EGFR expression level. J. Cell. Physiol. 2004;198:259–268. doi: 10.1002/jcp.10411. [DOI] [PubMed] [Google Scholar]
- 172.Moasser MM, Basso A, Averbuch SD, Rosen N. The tyrosine kinase inhibitor ZD1839 ("Iressa") inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001;61:7184–7188. [PubMed] [Google Scholar]
- 173.Moulder SL, et al. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61:8887–8895. [PubMed] [Google Scholar]
- 174.Normanno N, et al. Cooperative inhibitory effect of ZD1839 (Iressa) in combination with trastuzumab (Herceptin) on human breast cancer cell growth. Ann. Oncol. 2002;13:65–72. doi: 10.1093/annonc/mdf020. [DOI] [PubMed] [Google Scholar]
- 175.Schaefer G, Shao L, Totpal K, Akita RW. Erlotinib directly inhibits HER2 kinase activation and downstream signaling events in intact cells lacking epidermal growth factor receptor expression. Cancer Res. 2007;67:1228–1238. doi: 10.1158/0008-5472.CAN-06-3493. [DOI] [PubMed] [Google Scholar]
- 176.Lichtner RB, Menrad A, Sommer A, Klar U, Schneider MR. Signaling-inactive epidermal growth factor receptor/ligand complexes in intact carcinoma cells by quinazoline tyrosine kinase inhibitors. Cancer Res. 2001;61:5790–5795. [PubMed] [Google Scholar]
- 177.Baselga J, et al. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J. Clin. Oncol. 2005;23:5568–5577. doi: 10.1200/JCO.2005.07.119. [DOI] [PubMed] [Google Scholar]
- 178.Herbst RS, et al. Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J. Clin. Oncol. 2005;23:5578–5587. doi: 10.1200/JCO.2005.07.120. [DOI] [PubMed] [Google Scholar]
- 179.Geyer CE, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]