Abstract
Background
Bcl-2 is an essential regulator of programmed cell death (PCD). Overexpression of Bcl-2 is common in pancreatic cancer; the high levels have been shown to correlate with resistance to PCD. This resistance is mediated by binding of Bcl-2 via its BH-3 domain to diverse proteins including the Bax/Bak family members, various protein kinases and Beclin 1, which is involved in regulation of autophagy (type II PCD). Small molecule inhibitors of BH-3-mediated binding of Bcl-2 have been recently developed although no investigation has been conducted in pancreatic cancer, a malignancy characterized by extreme resistance to PCD.
Methods
The effect of the Bcl-2 binding inhibitor A-779024 on PCD was assessed by fluorescence activated cell sorting; the effect on Bcl-2 and other PCD-related proteins was analyzed by immunoblotting. Induction of autophagy was determined by fluorescence microscopy using a stably transfected GFP-LC3 construct to visualize autophagosome formation. Co-localization of Bcl-2 with binding partners regulating PCD was examined by immunoprecipitation and confocal immunofluorescent microscopy.
Results
A-779024 induced PCD in a dose- and time-dependent fashion. No change was seen in the protein levels of Bcl-2, Bax, Bcl-XL, or Mcl-1. Contrary to prediction, A-779024 was ineffective at inducing autophagy in these cells. Co-localization studies demonstrated that Bcl-2 was not bound to Beclin 1 and therefore treatment with A-779024 could not induce release of Beclin 1 and initiation of autophagy.
Conclusions
Disruption of Bcl-2 activity using the small molecule inhibitor A-779024 induces apoptotic but not autophagic PCD. This approach may be a novel therapy, either alone or in combination with other treatment such as chemotherapy or autophagy modulating agents in pancreatic cancer.
Keywords: autophagy, Bcl-2, BH-3, apoptosis, pancreatic cancer, Beclin 1
Introduction
Bcl-2 is an essential regulator of apoptotic and autophagic programmed cell death (PCD). Overexpression of Bcl-2 is common in pancreatic cancer; the high levels have been shown to correlate with resistance to PCD1–5. The regulation of programmed cell death by Bcl-2 involves protein:protein interactions involved in mitochondrial membrane function (apoptotic) as well as lysosome-mediated organelle destruction (autophagic) that appear to be independent. The mechanism of both of these effects is thought to be mediated by binding of Bcl-2 via its BH-3 domain to Bax/Bak family members for regulation of apoptosis or Beclin 1 for regulation of autophagy6, 7. Small molecule inhibitors of BH-3-mediated binding of Bcl-2 have been recently developed that disrupt these protein:protein interactions, although no investigation has been conducted in pancreatic cancer, a malignancy characterized by extreme resistance to chemotherapy-induced PCD.
Therapeutic targeting of Bcl-2 is rapidly emerging as a novel strategy whether by small molecule inhibitors of BH-3 binding or antisense methodology8, 9. The majority of this investigation has focused on the altered levels and/or binding of Bcl-2 family members in regulating mitochondrial membrane potential and initiation of traditional apoptotic signaling. However, Akar et al. recently reported that silencing of Bcl-2 by siRNA induces a non-apoptotic cell death in MCF-7 breast cancer cells associated with the induction of autophagy10. Traditional cancer therapy has targeted pathways involved in cell growth to induce apoptotic cell death. However, there are other mechanisms of cell death that may be effective in cancer when appropriately triggered. Autophagy is a eukaryotic, evolutionarily conserved homeostatic process in which organelles and bulk proteins are turned over by lysosomal activity11–13. In periods of metabolic stress, autophagy provides ATP and other macromolecules as energy sources to allow for cell survival14 15; however, if the intensity or duration of metabolic stress is sufficient, cells may progress to an autophagic programmed cell death that is distinct from apoptosis16, 17. The most distinctive feature of autophagy is the formation of the autophagosome, a double membrane vesicle that sequesters proteins and organelles for lysosomal delivery18. Nucleation of the autophagosome requires a complex formed by Vps34 (a class III phosphatidylinsoital-3-kinase) and Beclin119–21. Under nutrient-rich conditions, Beclin1 is bound to the anti-apoptotic factor Bcl-2; under conditions of metabolic stress, Beclin1 is released from Bcl-2 to initiate autophagosome assembly22. Therefore, overexpression of Bcl-2 may not only confer resistance to apoptosis, but also to autophagic PCD through sequestration of Beclin1.
We therefore sought to determine whether a small molecule inhibitor of BH-3 mediated Bcl-2 binding induces PCD in pancreatic cancer and to further characterize whether apoptosis or autophagy is initiated following alteration of Bcl-2 binding.
Methods
Reagents and cell lines
All chemical reagents were purchased from Sigma-Aldrich Corporation (St. Louis, MO.) unless otherwise specified. The experimental, small-molecule, Bcl-2 BH-3 domain inhibitor A-779024 was provided by Abbott Laboratories (Abbott Park, IL). The pan-Caspase inhibitor ZVAD-FMK was purchased from MBL International (Woburn, MA). Monoclonal antibodies to Akt, phospho-Akt, Beclin 1, Mcl-1, and LC-3 were purchased from Cell Signaling (Beverly, MA). Bax and Bcl-2 antibodies were purchased from BD Pharmingen (San Diego, CA). Caspase 3 antibody was purchased from Biosource (Camarillo, CA); polyclonal antibodies to Bcl-xL and actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).The MiaPaca-2 cell line was obtained from the American Type Culture Collection (Rockville, MD) and cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, sodium pyruvate, nonessential amino acids, L-glutamine, vitamins, penicillin, and streptomycin. Cells were maintained at 37°C in a humidified incubator containing 5% CO2.
Cell Cycle Analysis with Flow Cytometry
1 × 106 cells per treatment were plated in 100mm plates and allowed to recover overnight. Following treatments, cells were harvested with trypsin, washed in PBS, and resuspended in 150uL of FACSMAX solution (Genlantis, San Diego, CA) and incubated 10 minutes on ice to maximally disperse the cells. They were then fixed by adding ice cold 70% ethanol dropwise and kept at +4 degrees Celsius 1–7 days. Prior to analysis, cells were washed with PBS, then incubated with Ribonuclease A (200 mcg/ml) for 30 minutes at 37 degrees Celsius, and then stained with propridium iodide (10 mcg/ml). The cells were then analyzed using a Becton Dickenson FACscan (BD Biosciences, San Jose, CA) to determine the DNA fractions and the Sub-G0 (Apoptotic) cell population was quantified using Flowjo software (Treestar, inc., Ashland, OR).
Live Cell Fluorescence Microscopy for Autophagy
A MiaPaca-2 clonal population overexpressing eGFP-LC-3 was generated as previously described. These cells were seeded in Ibidi 8-chamber ultra-thin culture slides (Integrated BioDiagnostics; Munich, Germany) and allowed to recover for 48 hours under standard culture conditions. Culture medium was then changed to include either A-779024 2 µM or Rapamycin 2µM and the cells were incubated for 1 or 6 hours before they were imaged using a IX-71 Delta Vision (Applied Precision; Issaquah, Washington) inverted fluorescent microscope with a 60× 1.40 NA oil objective and FITC filter (excitation, 480 nm; emission, 535 nm). During imaging the cells were kept at 37 degrees Celsius with an ASI 400 air stream incubator (Nevtek; Williamsville, VA).
Immunocytochemistry
MiaPaca-2 cells were cultured and treated with specified treatments in 4-well LAB-TEK® chamber slides coated with poly-L lysine (Nalge Nunc International, Naperville, IL), then fixed first in 4% paraformaldehyde (1 min at room temperature) and then 100% ice-cold Methanol (10 minutes). Cells were washed in Phosphate buffered saline (PBS) and the cell membranes solubilized by incubation in PBS with 0.5% Triton X-100 for 20 min at room temperature. All incubations were done in a humidity chamber. Blocking of nonspecific binding was achieved by incubation in 0.1% Triton X-100 with 2% bovine serum albumin for 30 minutes at room temperature. Immunostaining consisted of incubation with monoclonal antibodies to BCL-2 (Pharmingen, 1:1000 dilution) and Beclin 1 (Cell Signaling, 1:500 dilution) overnight at 4 C. After 3 washes with 0.1% Triton X-100 in PBS slides were incubated with species-specific secondary antibodies (Alexa FluorR 488 anti-rabbit for Beclin 1, Alexa FluorR 647 anti-hamster for BCL-2; [Invitrogen, Carlsbad, CA]) for 1 hour at room temperature. The plastic chambers were then removed and coverslips mounted using Slowfade Gold® mounting medium (Invitrogen, Carlsbad, CA) containing 4,6-diamidino-2-phenylindole dihydrochloride (DAPI) for nuclear staining. Slides were visualized with a IX-71 Delta Vision inverted fluorescent microscope equipped with a 60× 1.40 NA oil objective (Olympus, Melville, NY) using DAPI, FITC, and CY-5 filters. The signal intensities through each filter were matched at the time of imaging, and the images through each filter were individually optimized for brightness and background prior to generating the final composite images.
Western Blotting
Following treatments, cells were harvested with trypsin, pelleted by centrifugation, washed with PBS once, and then resuspended in lysis buffer (Cell Signaling #9803). Total lysate protein was quantified for equal gel loading using the BioRad protein assay (BioRad Labs, Hercules, CA) Lysates were mixed with a reducing loading buffer and heated to 100 degrees Celsius for 10 minutes, resolved by SDS-PAGE, and separated proteins were then electrophoretically transferred to 0.2-mm nitrocellulose membranes (Schleicher & Schuell; Keene, NH). The blots were probed overnight with primary antibodies, and developed using species-specific secondary antibodies conjugated to horse-radish peroxidase. Immunoreactivity was detected by the enhanced chemiluminescence technique (Amersham; Piscataway, NJ).
Immunoprecipitation
MiaPaca-2 cells were cultured under standard conditions to near-confluence, then harvested with Trypsin, pelleted by centrifugation, and washed with phosphate buffered saline. The cell pellet was lysed in an immunoprecipitation buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% NP40) on ice for 5 min and clarified by centrifugation (10,000 g, 10 min). One mg of total protein used to immunoprecipitate Bcl-2, employing an immobilized monoclonal antibody to Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA), which was incubated with the lysate for 45 min at 25°C and isolated using Protein A-agarose. Proteins were then immunoblotted as described above.
Results
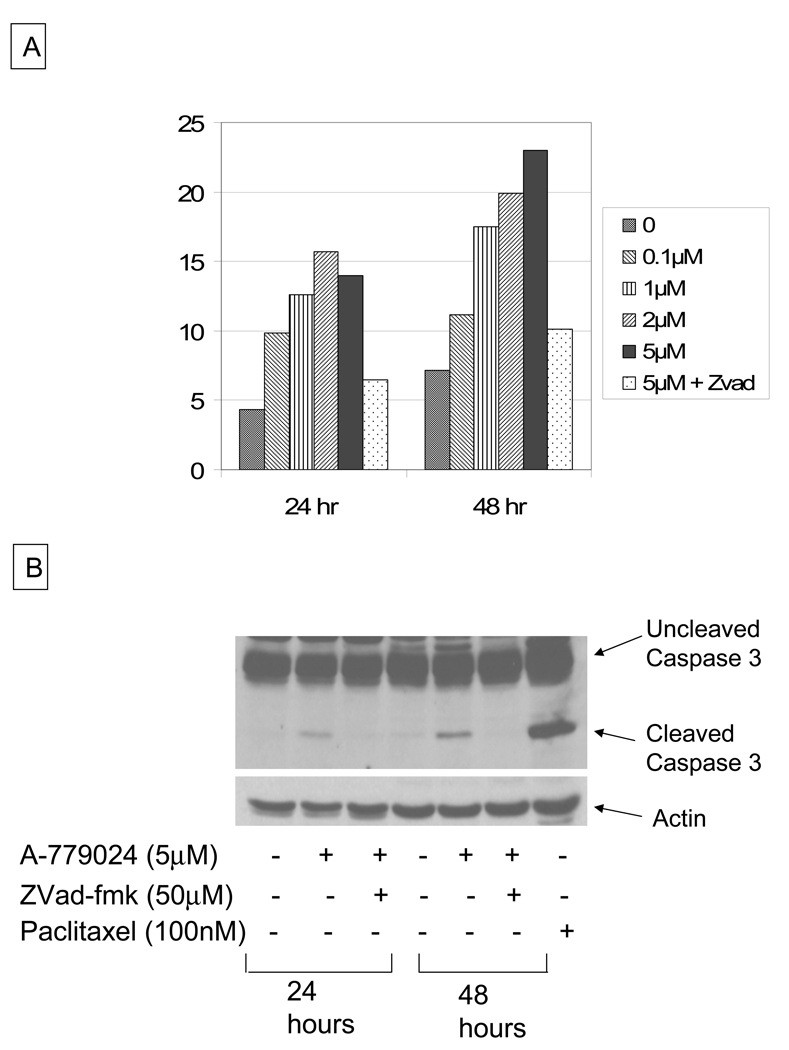
Upon treatment of MiaPaca-2 cells with increasing doses of A-779024, we observed a dose and time-dependent increase in the sub-G0 apoptotic fraction by propidium iodide flow cytometry (Figure 1A). The dose effect plateaued at 5 µM. This cell death was caspase-dependent, as evidenced by cleavage of caspase 3 to its 17kDa form by western blot with treatment (Figure 1B). To determine whether the cell death was a caspase-dependent event, cells were co-treated with the pan-caspase inhibitor ZVad-FMK. Inhibition of caspase activity in combination with the maximal dose of A-779024 reduced the cell death fraction back to the level of the control cells (Figure 1A). Inhibition of the caspase-3 cleavage was confirmed by immunoblotting (Figure 1B).
Figure 1.
A) Effect of A-779024 treatment on cell death in MIA-PaCa-2 cells at 24 and 48 hours of therapy over a broad dose-range, with effect of caspase inhibition by ZVad-fmk co-treatment at 48 hrs (5 µM A-779024 treatment), and B) effect of A-779024 treatment on caspase 3 cleavage in MIA-PaCa-2 cells at 24 and 48 hours, with effect of caspase inhibition by ZVad-fmk co-treatment. Paclitaxel is used as a positive control for caspase 3 cleavage.
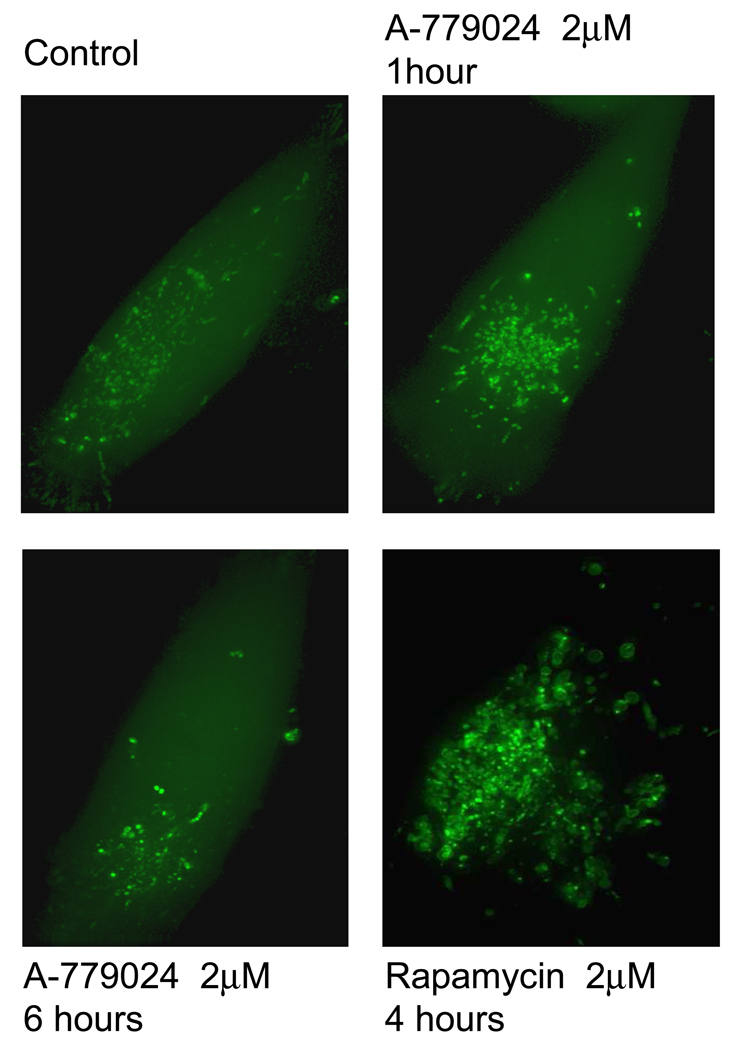
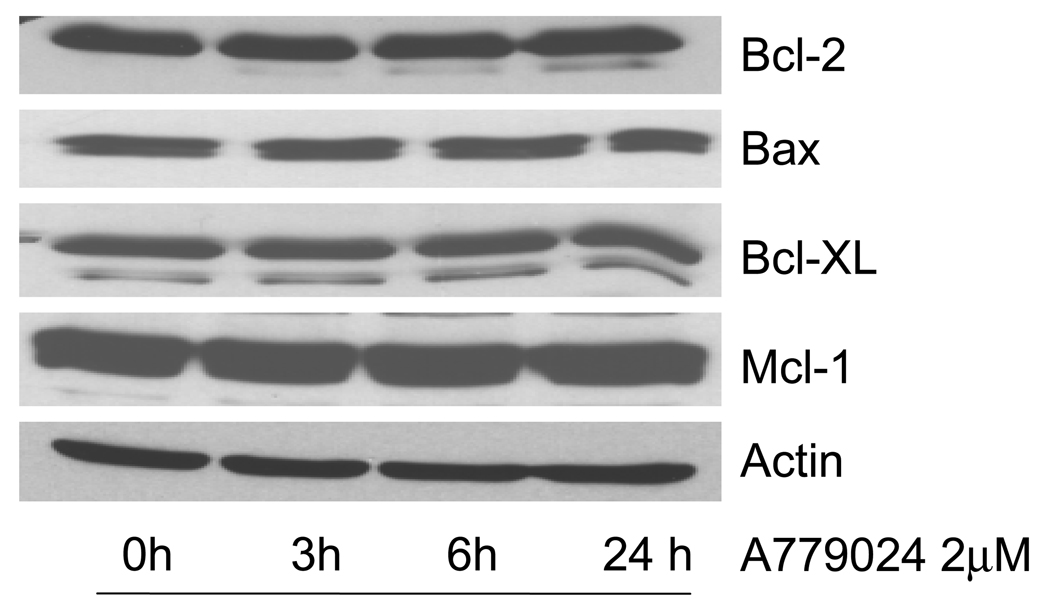
Bcl-2 has been proposed to bind Beclin 1 via its BH-3 domain, preventing Beclin 1 from initiating autophagy. We hypothesized that by inhibiting that BH-3 binding with A-779024, we should increase the pool of free Beclin 1 and therefore initiate autophagy. We treated MiaPaca-2 cells over-expressing the eGFP-LC3 plasmid with A-779024 and looked for fluorescent autophagosome formation at 1 and 6 hours. No significant increase from the baseline level of autophagy was observed at these time-points (Figure 2). Similar cells were treated with the mTOR inhibitor, rapamycin, to demonstrate the induction of autophagy as a positive control. It has been reported that BH-3 mimetics can cause decreases in Bcl-2 family protein levels, presumably by preventing homo- and hetero-dimerization and therefore directing the monomers to proteasome-mediated degradation. We therefore assessed levels of Bcl-2 and several other related proteins (Bax, Bcl-XL, and Mcl-1) over 24 hours of treatment with 2 µM A-779024. Levels of these proteins were not altered following treatment with A-779024 (Figure 3).
Figure 2.
Effect of A-779024 treatment on autophagosome formation in MIA-PaCa-2 cells stably transfected to overexpress GFP-labeled LC-3. The diffuse fluorescent haze and minimal punctae formation is indicative of baseline cells not undergoing autophagy, as seen in the control panel (top left) and after 1 and 6 hours of treatment with A-779024 at 2µM. The bottom right panel displays the effect of rapamycin as a positive control for autophagy, demonstrating loss of the diffuse fluorescent haze and formation of numerous enlarging autophagosomes.
Figure 3.
Immunoblots showing no change in the expression levels of four different Bcl-2 family proteins in MIA-PaCa-2 cells over a 24-hour time-course of 2 µM A-779024 treatment. Actin is shown as a protein loading control.
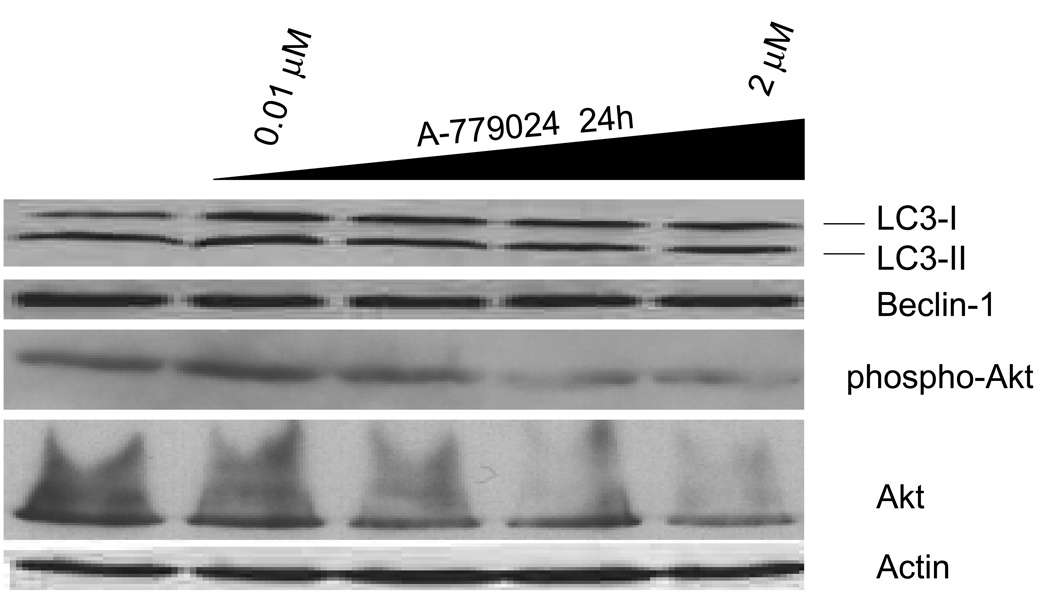
Another mechanism to evaluate the induction of autophagy is by immunoblotting for LC3, which is processed from LC3-I to LC3-II during the initiation of autophagy. MIA-PaCa-2 cells were treated with escalating doses of A-779024 for 24 hours and immunoblotting performed to detect changes in LC3 processing. No changes from baseline were seen in the relative fractions of LC3-I and LC3-II, thus confirming the absence of autophagy induction by A-779024 (Figure 4). Once released from Bcl-2, Beclin 1 will participate in the assembly of the autophagosome, but then will be degraded upon fusion of the autophagosome with the lysosome and therefore levels will decrease during autophagy. Levels of Beclin 1 were unchanged over the 24 hours of treatment with A-779024. Lastly, we examined the degree of activation of the Akt kinase, which we have previously shown is activated by binding to Bcl-2. Phosphorylated, activated Akt levels declined slightly with increasing dose of A-779024 (Figure 4).
Figure 4.
Immunoblots showing the effect of 24 hours of A-779024 treatment over a broad dose range on MIA-PaCa-2 cells’ expression of several autophagy-related proteins. The relative fractions of LC3-I and LC3-II show no change with A-779024 treatment, indicating no induction of autophagy. Beclin 1 levels remained constant as well; Akt and Phospho-Akt show a slight trend toward inhibition with increasing dose of A779024. Actin is shown as a protein loading control.
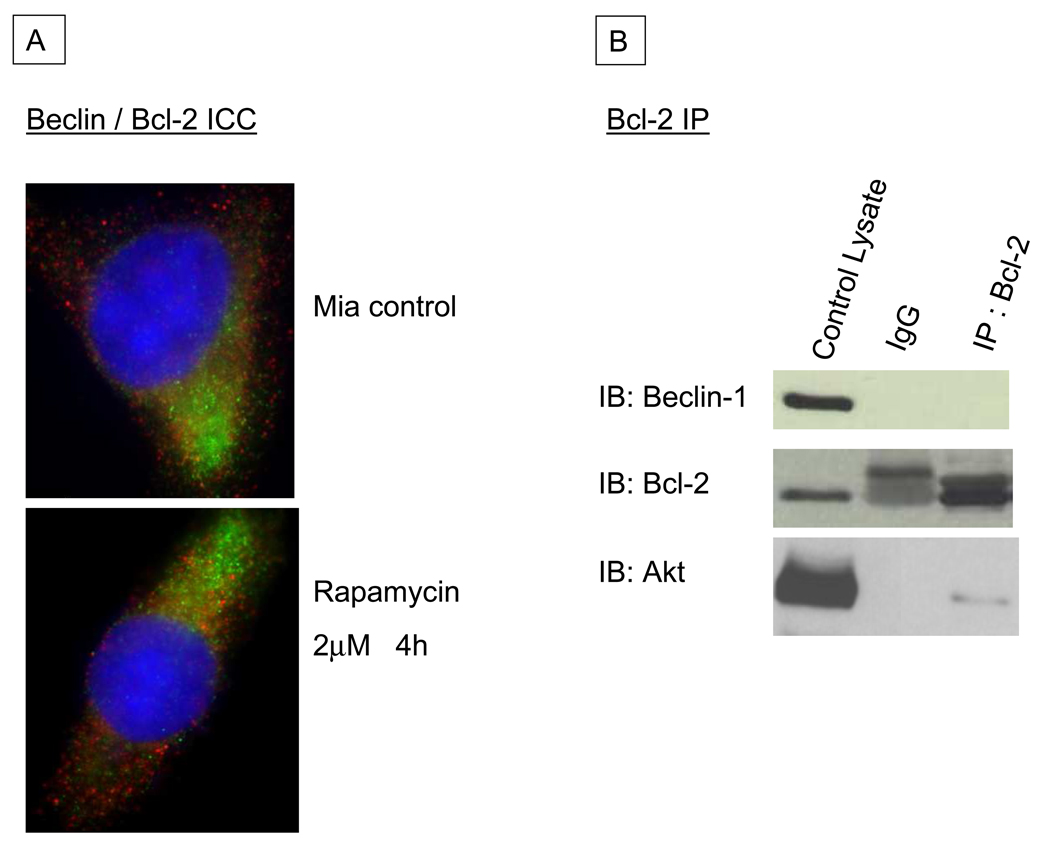
Having seen no indication that inhibition of BH-3 mediated binding of Bcl-2 altered cellular autophagy, we attempted to verify the purported constitutive binding of Bcl-2 and Beclin 1. We have previously shown that even in cells stably transfected to over-express Bcl-2, there was no change in their ability to undergo autophagy (unpublished data). As Beclin 1 has been convincingly demonstrated to be an essential part of autophagy’s machinery7, 23, we evaluated whether Bcl-2 binds to Beclin 1 in pancreatic cancer cells. To address this, we performed co-localization immunocytochemistry with fixed MiaPaca-2 cells, labeling both Beclin 1 and Bcl-2 with green and red spectra fluorescent antibodies, respectively. We saw minimal overlap between the two different signals, both under basal conditions and with autophagy induction using Rapamycin, implying that the two proteins were not bound to each other (Figure 5A). We complimented this experiment with physical evaluation for Bcl-2/Beclin 1 binding using immunoprepitation (IP) methods. Following IP of Bcl-2 in MiaPaca-2 whole cell lysate under basal conditions, no Beclin 1 was seen in the IP protein fraction by western blotting. Akt was detected in the IP fraction as we have previously reported and consistent with the decrease of Akt activation following A-77924 treatment. Both these studies imply a lack of protein:protein interaction between Bcl-2 and Beclin 1 in MiaPaca-2 cells and coupled with the evaluation of autophagy, indicate that Bcl-2 does not play a major role in the regulation of autophagy in pancreatic cancer.
Figure 5.
A: Immunocytochemistry of normal MIA-PaCa-2 cells (top) and those treated with Rapamycin to induce autophagy (bottom), labeling Beclin 1 (green) and Bcl-2 (red). Minimal to no co-locolization of the signals is observed. Nuclei are stained blue with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI). B: Immunoblots of normal MIA-PaCa-2 cell lysates following immunoprecipitation (IP) with a monoclonal Bcl-2 antibody. The left-hand lane contains lysate prior to IP, the middle lane is a control for non-specific binding showing IP with a non-specific IgG, and the right-hand lane contains the Bcl-2 IP fraction. No Beclin 1 is seen in the Bcl-2 immunoprecipitated fraction, but Akt is detected as a positive control for the IP assay.
Discussion
In this study, we demonstrate that the small molecule Bcl-2 inhibitor A-779024 induces a dose- and time-dependent apoptotic PCD in a human pancreatic cancer cell line in vitro. Given the role of Bcl-2 preventing autophagic cell death through binding to Beclin 17, 22, 24–26, we hypothesized that we would also observe autophagic cell death with a dissociation of Bcl-2 and Beclin 1 but in fact were unable to identify the induction of autophagy following A-779024 treatment. This may be due to the lack of interaction of Bcl-2 and Beclin 1 in these cells, as no discernible binding of these proteins was observed in the immunoprecipitation or confocal microscopy studies. These latter findings are relevant in understanding the mechanism of resistance to PCD in pancreatic cancer as it would appear that Bcl-2 is involved only in apoptotic but not autophagic PCD.
While Bcl-2 has been recognized as a common and significant mechanism to the apoptotic effect of chemotherapy, appropriate approaches to specifically target as an effective cancer therapy have been limited.27 The most well accepted approach has been to use antisense to decrease Bcl-2 levels, though the optimal method of vehicle delivery, maintenance of protein downregulation, and side effects on non-targeted tissues have limited the enthusiasm for human therapy.8 Small molecule BH-3 mimetics have been recently developed that disrupt binding among proteins that interact through this domain.9, 28 This approach has been hypothesized to restore apoptotic sensitivity to an external stimulus by preventing heterodimers of Bcl-2 with pro-apoptotic proteins such as Bak or Bax. However, we observed a caspase-dependent apoptosis following A-779024 treatment in the absence of any other external, apoptosis-inducing stimuli. These data suggest that there is an underlying trigger for apoptotic PCD in these cells that is prevented through overexpression of Bcl-2, and directed therapy may be sufficient as a single agent therapy. Conversely, when administered in combination with chemotherapy, the efficacy of combination may be synergistic; such studies are the ongoing focus of our laboratory.
As targeted therapy in cancer has been developed, an essential aspect is the appropriate patient selection for therapy based on individual molecular genetics of the tumor. The identification of these predictive markers of response are critical to avoid ineffective therapy in tumors unlikely to respond. Goldsmith et al. examined Bcl-2 family expression in a variety of neuroblastoma cell lines to identify predictive markers of response to Bcl-2 family antagonists. Mcl-1 expression correlated with sensitivity to the BH-3 mimetic AT-101 while Bcl-XL levels predicted response to ABT-737, a BH-3 mimetic very similar to A-77902429. Deng et al. similarly characterized a panel of lymphoma cell lines to identify a predictive marker of response to ABT-737; Bcl-2 levels were superior in predicting response when compared to Mcl-1 or other Bcl-2 family members30. These data imply that various tumors may have specific dominant anti-apoptotic proteins conferring resistance and that each tumor type will have to be characterized to identify the most appropriate predictive marker of response to BH-3 mimetics, namely whether it is Bcl-2, Mcl-1, Bcl-XL, etc that is the dominant anti-apoptotic protein in that specific cancer. Goldsmith et al.’s data implies likelihood that the different BH-3 mimetics undergoing development may have preferential binding to the BH-3 domains of different proteins, such that there is limited cross-sensitivity across this class of targeted agents in cancer therapy.
The observation that Bcl-2 does not appear to participate in the regulation of autophagy in pancreatic cancer is critical given the frequent overexpression of Bcl-2 in this disease. Agents that regulate autophagy are emerging as potential therapies in cancer, and inhibition of the mTOR pathway has been recognized as a potent inducer of autophagic cell death31, 32. Therefore, Bcl-2 overexpression, while conferring resistance to apoptotic PCD, may not similarly confer resistance to autophagic PCD and this approach warrants further investigation. In our laboratory, we have demonstrated that a subset of pancreatic cancer cells is sensitive to autophagic PCD induced by metabolic stress. While A-779024 was not capable of inducing autophagy in these cells, this result furthers our understanding of autophagy regulation in pancreatic cancer and may aid in developing new therapies. Combining BH-3 mimetics targeting apoptosis and agents that modulate autophagy may enhance their respective therapeutic effects due to the lack of Bcl-2-mediated mechanisms of resistance between these signaling pathways in pancreatic cancer.
Acknowledgements
We are grateful to the support and the reagents provided by Vincent Giranda (Abbott Oncology Laboratories). This work was supported by NIH 1RO3 CA123004 and the Isabelle J. McDonald Endowment (to RJB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sun CY, Wang BL, Hu CQ, et al. Expression of the bcl-2 gene and its significance in human pancreatic carcinoma. Hepatobiliary Pancreat Dis Int. 2002 May;1(2):306–308. [PubMed] [Google Scholar]
- 2.Ghaneh P, Kawesha A, Evans JD, Neoptolemos JP. Molecular prognostic markers in pancreatic cancer. J Hepatobiliary Pancreat Surg. 2002;9(1):1–11. doi: 10.1007/s005340200000. [DOI] [PubMed] [Google Scholar]
- 3.Fahy BN, Schlieman MG, Mortenson MM, Virudachalam S, Bold RJ. Targeting BCL-2 overexpression in various human malignancies through NF-kappaB inhibition by the proteasome inhibitor bortezomib. Cancer Chemother Pharmacol. 2005 Jul;56(1):46–54. doi: 10.1007/s00280-004-0944-5. [DOI] [PubMed] [Google Scholar]
- 4.Mortenson MM, Galante JG, Gilad O, et al. BCL-2 functions as an activator of the AKT signaling pathway in pancreatic cancer. J Cell Biochem. 2007 Dec 1;102(5):1171–1179. doi: 10.1002/jcb.21343. [DOI] [PubMed] [Google Scholar]
- 5.Bold RJ, Hess KR, Pearson AS, et al. Prognostic factors in resectable pancreatic cancer: p53 and bcl-2. J Gastrointest Surg. 1999 May–Jun;3(3):263–277. doi: 10.1016/s1091-255x(99)80068-7. [DOI] [PubMed] [Google Scholar]
- 6.Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene. 2008 Dec;27 Suppl 1:S2–S19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene. 2008 Dec;27 Suppl 1:S137–S148. doi: 10.1038/onc.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolcher AW. Targeting Bcl-2 protein expression in solid tumors and hematologic malignancies with antisense oligonucleotides. Clin Adv Hematol Oncol. 2005 Aug;3(8):635–642. 662. [PubMed] [Google Scholar]
- 9.Goldsmith KC, Hogarty MD. Small-molecule BH3 mimetics to antagonize Bcl-2- homolog survival functions in cancer. Curr Opin Investig Drugs. 2009 Jun;10(6):559–571. [PubMed] [Google Scholar]
- 10.Akar U, Chaves-Reyez A, Barria M, et al. Silencing of Bcl-2 expression by small interfering RNA induces autophagic cell death in MCF-7 breast cancer cells. Autophagy. 2008 Jul 1;4(5):669–679. doi: 10.4161/auto.6083. [DOI] [PubMed] [Google Scholar]
- 11.Levine B. Cell biology: autophagy and cancer. Nature. 2007 Apr 12;446(7137):745–747. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- 12.Moreau K, Luo S, Rubinsztein DC. Cytoprotective roles for autophagy. Curr Opin Cell Biol. 2009 Dec 30; doi: 10.1016/j.ceb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Todde V, Veenhuis M, van der Klei IJ. Autophagy: principles and significance in health and disease. Biochim Biophys Acta. 2009 Jan;1792(1):3–13. doi: 10.1016/j.bbadis.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005 Nov;12 Suppl 2:1528–1534. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- 15.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004 Apr 12;23(16):2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 16.Bursch W, Ellinger A, Gerner C, Frohwein U, Schulte-Hermann R. Programmed cell death (PCD). Apoptosis, autophagic PCD, or others? Ann N Y Acad Sci. 2000;926:1–12. doi: 10.1111/j.1749-6632.2000.tb05594.x. [DOI] [PubMed] [Google Scholar]
- 17.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000 Dec 1;290(5497):1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klionsky DJ. The correct way to monitor autophagy in higher eukaryotes. Autophagy. 2005 Jul;1(2):65. doi: 10.4161/auto.1.2.1899. [DOI] [PubMed] [Google Scholar]
- 19.Furuya N, Yu J, Byfield M, Pattingre S, Levine B. The evolutionarily conserved domain of Beclin 1 is required for Vps34 binding, autophagy and tumor suppressor function. Autophagy. 2005 Apr;1(1):46–52. doi: 10.4161/auto.1.1.1542. [DOI] [PubMed] [Google Scholar]
- 20.Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000 Nov 1;19(21):5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanida I, Sou YS, Ezaki J, Minematsu-Ikeguchi N, Ueno T, Kominami E. HsAtg4B/HsApg4B/autophagin-1 cleaves the carboxyl termini of three human Atg8 homologues and delipidates microtubule-associated protein light chain 3- and GABAA receptor-associated protein-phospholipid conjugates. J Biol Chem. 2004 Aug 27;279(35):36268–36276. doi: 10.1074/jbc.M401461200. [DOI] [PubMed] [Google Scholar]
- 22.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1- dependent autophagy. Cell. 2005 Sep 23;122(6):927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004 Apr;6(4):463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 24.Zalckvar E, Berissi H, Eisenstein M, Kimchi A. Phosphorylation of Beclin 1 by DAP-kinase promotes autophagy by weakening its interactions with Bcl-2 and Bcl-XL. Autophagy. 2009 Jul;5(5):720–722. doi: 10.4161/auto.5.5.8625. [DOI] [PubMed] [Google Scholar]
- 25.Vazquez CL, Colombo MI. Beclin 1 modulates the anti-apoptotic activity of Bcl-2: Insights from a pathogen infection system. Autophagy. Jan 25;6(1) doi: 10.4161/auto.6.1.10743. [DOI] [PubMed] [Google Scholar]
- 26.Ciechomska IA, Goemans CG, Tolkovsky AM. Why doesn't Beclin 1, a BH3-only protein, suppress the anti-apoptotic function of Bcl-2? Autophagy. 2009 Aug;5(6):880–881. doi: 10.4161/auto.9096. [DOI] [PubMed] [Google Scholar]
- 27.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009 Feb 15;15(4):1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008 Dec;27 Suppl 1:S149–S157. doi: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldsmith KC, Lestini BJ, Gross M, et al. BH3 response profiles from neuroblastoma mitochondria predict activity of small molecule Bcl-2 family antagonists. Cell Death Differ. 2009 Nov 6; doi: 10.1038/cdd.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT- 737 and conventional chemotherapeutic agents. Cancer Cell. 2007 Aug;12(2):171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Sudarsanam S, Johnson DE. Functional consequences of mTOR inhibition. Curr Opin Drug Discov Devel. Jan;13(1):31–40. [PubMed] [Google Scholar]
- 32.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2009 Dec 22; doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]