Abstract
The Pd-catalyzed addition of organozinc reagents to unsaturated carbonyls in the presence of carbon monoxide provides 1,4-diketones in good yield. The reaction was studied with a number of substituted cyclic and acyclic ketones as well as α,β-unsaturated aldehydes.
The catalytic conjugate addition of organometallic nucleophiles to unsaturated carbonyls is an important method for organic synthesis. In this regard, successful catalytic addition reactions have been registered for a broad range of nucleophiles, be they aryl, vinyl, alkyl, or acetylide-based reagents.1 The conjugate addition of acyl anions represents a special class of transformations since these nucleophiles are often unstable and are therefore generated in situ during the course of a reaction.2 The Stetter3 and silyl-Stetter4 reactions are two important strategies to accomplish the disonant bond connection that conjugate addition of an acyl anion to an unsaturated carbonyl achieves. While remarkable advances have been documented in both of these acyl anion addition strategies, complementary strategies that expand the substrate scope would prove valuable. In this regard, we have begun to study the catalytic carbonylative addition of simple organometallic reagents to enones and this report describes our initial findings.5,6
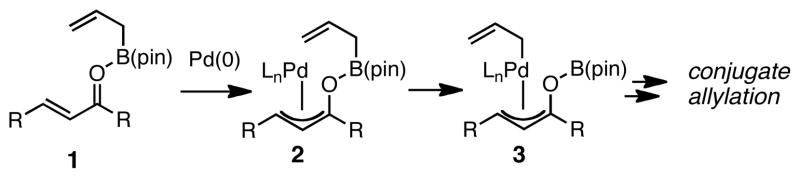
Recent studies in our laboratory have focused on the catalytic enantioselective addition of allylboron reagents to unsaturated carbonyls.7 These reactions are postulated to proceed by oxidative addition of Pd(0) or Ni(0) to a Lewis acid-activated enone (1 to 2, Scheme 1) followed by transmetallation (2 to 3) and reductive elimination. The intermediacy of Pd π-allyl complexes in this catalytic cycle, provides an opportunity for bond formation that is not often observed in catalytic conjugate addition reactions.8 Indeed, Orito and co-workers reported that the carbonylative conjugate addition of benzylzinc chloride to enones occurs in the presence of Pd catalysts and they have suggested that the reaction may proceed by a CO insertion that occurs with an intermediate similar to 3.9 Unfortunately, this process is restricted to unsubstituted enones; for those possessing a β substituent, significantly diminished reaction yields were observed. In this report, we document our studies on this reaction and describe improvements that render it operative with many unsaturated carbonyl substrates.
Scheme 1.
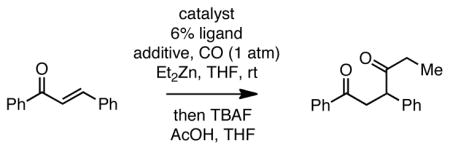
Our initial investigations focused on the addition of simple alkyl metal reagents to enones under carbonylative conditions. With conditions similar to those laid out by Orito (catalytic Pd(PPh3)4 in the presence of TMSCl and LiCl) but employing Et2Zn in place of benzylzinc chloride, we found that the carbonylative conjugate addition of chalcone proceeds efficiently, even though the enone bears a substituent at the β-carbon (Table 1, entry 1). Notably, the reaction yield improved when LiCl was omitted, and it could be improved even further by employing Pd2(dba)3/PCy3 or Pd2(dba)3/PPh3 as the catalyst. Also of note, Ni(cod)2/PCy3 and Pt(dba)3/PCy3 were ineffective at the carbonylative conjugate addition with the former not delivering any product at all, and the later only yielding the direct conjugate addition product. Similarly, a survey of other Lewis acids (BF3·OEt2, Sc(OTf)3, TiCl2(iOPr)2) was unproductive with these promoting the direct non-carbonylative conjugate addition of the ethyl group to the enone.10 In the absence of any Lewis acid at all, the reaction did not occur (Table 1, entry 3). Surprisingly, the quantity of organometallic reagent could be reduced with 0.65 equivalents of Et2Zn being sufficient for effective reaction (entry 8). Lastly, the quantity of the catalyst could be reduced with as little as 0.25 mol% Pd2(dba)3 furnishing excellent yields of carbonylative conjugate addition product (entry 6 and 7).
Table 1.
Effect of Reaction Conditions on Carbonylative Conjugate Additions to Chalconea
 | ||||
|---|---|---|---|---|
| entry | catalyst | ligand | additive | % yield |
| 1 | 5% Pd(PPh3)4 | - | TMSCl+LiCl | 70 |
| 2 | 5% Pd(PPh3)4 | - | TMSCl | 82 |
| 3 | 5% Pd(PPh3)4 | - | - | N.R. |
| 4 | 2.5% Pd2(dba)3 | PPh3 | TMSCl | 85 |
| 5 | 2.5% Pd2(dba)3 | PCy3 | TMSCl | 82 |
| 6 | 0.25% Pd2(dba)3 | PPh3 | TMSCl | 86 |
| 7 | 0.25% Pd2(dba)3 | PCy3 | TMSCl | 86 |
| 8 | 2.5% Pd2(dba)3 | PCy3 | TMSCl | 83b |
| 9 | 5% Ni(cod)2 | PCy3 | TMSCl | N.R. |
| 10 | 5% Pt(dba)3 | PCy3 | TMSCl | 0 |
Reaction with 1.3 equiv Et2Zn, 2.3 equiv TMSCl, 1 atm CO, for 4 h. Subsequent to reaction, the mixture was quenched at 0 °C with acetic acid (1.5 equiv) and TBAF (1.5 equiv) for 10 minutes and then treated with saturated Na2CO3.
Reaction with 0.65 equivalents of Et2Zn.
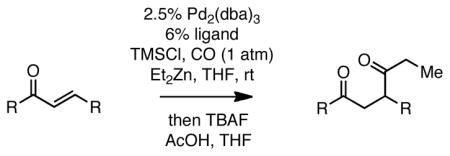
With efficient conditions established, the influence of the enone substitution was examined. Both Pd2(dba)3/PCy3 and Pd2(dba)3/PPh3 were studied as catalysts and it was found that, in addition to chalcone, other aryl substituted enones also undergo efficient carbonylative conjugate addition (Entry 1 and 2). While the data in entries 4 and 5 suggest that electron donating and electron withdrawing groups are both tolerated and ultimately lead to similar reaction yields, a significant rate difference can be observed if conversions are measured at one hour (data not shown) with the 4-methoxy substituted enone affording 28% conversion and the 4-carbomethoxy derivative achieving 97% conversion.11 Notably, aryl substitution is not a requirement as 2-penten-3-one reacted in excellent yield (entry 8). Importantly, cyclic substrates were also found to be effective. The example with cyclohexenone (entry 10) is particularly noteworthy; Orito reported that cyclohexenone undergoes carbonylative conjugate addition in 22% yield with benzylzinc chloride7; however, with the improved conditions described above, good yields of carbonylative addition product are obtained. Lastly, a sterically encumbered 4,4-disubstituted enone also afforded the desired product, although a higher catalyst loading was required for the reaction to be completed in less than 24 hours (Table 2, entry 11). Note that in entry 11 an increased amount of Et2Zn was employed to account for the fact that carbonylative addition to dba is generally observed and was expected to be more problematic at higher catalyst loadings.
Table 2.
Carbonylative Conjugate Addition of Et2Zn To α,β-Unsaturated Ketones.a
 | |||
|---|---|---|---|
| entry | substrate | % yield | |
| L=PPh3 | L=PCy3 | ||
| 1 |  |
82 | 80 |
| 2 |  |
81 | 81 |
| 3 | 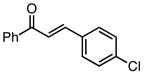 |
84 | 82 |
| 4 | 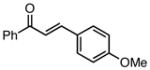 |
79 | 80 |
| 5 | 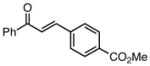 |
86 | 87 |
| 6 | 52 | 82 | |
| 7 | 67 | 66b | |
| 8 | 85 | 86 | |
| 9 | 56 | 48 | |
| 10 | 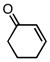 |
68 | 67 |
| 11 | 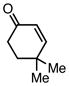 |
72 | 55c |
Reaction with 1.3 equiv Et2Zn, 2.3 equiv TMSCl, 1 atm CO, for 4 h (7 h for entry 4; 24 h for entry 11). Subsequent to reaction, the mixture was quenched at 0 °C with acetic acid (1.5 equiv) and TBAF (1.5 equiv) for 10 minutes and then treated with saturated Na2CO3.
Yield adjusted to account from unremovable impurity (ca. 10%).
10% Pd2(dba)3, 24% ligand, and 1.95 equiv Et2Zn employed.
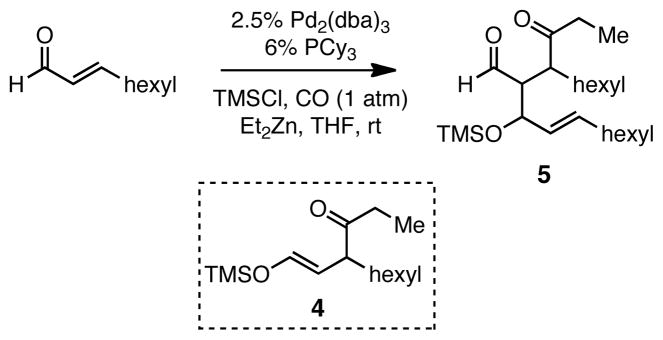
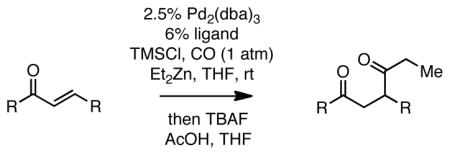
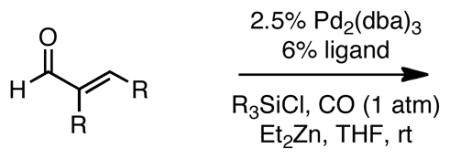
To further broaden the substrate scope, α,β-unsaturated aldehydes were subjected to the reaction. With the optimized reaction conditions described above, the carbonylative conjugate addition product derived from nonenal was not produced, but rather a compound tentatively assigned as aldol adduct 5 (Scheme 2). It was considered that 5 might arise from a pathway involving conversion of nonenal to 4 and that this trimethylsilyl enol ether might be reactive enough to undergo a Mukaiyama-aldol reaction12 with unreacted nonenal in the presence of zinc salts. To minimize this side reaction, TESCl was used as the additive in the carbonylative addition. This strategy afforded the 1,4-dicarbonyl compounds in good yields (Table 3).
Scheme 2.
Table 3.
Catalytic Carbonylative Conjugate Addition of Et2Zn to α,β-Unsaturated Aldehydes.a
 | |||||
|---|---|---|---|---|---|
| entry | substrate | product | R3SiCl | % yield | |
| L:PPh3 | L:PCy3 | ||||
| 1 | 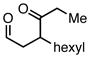 |
Et3SiCl | 78 | 69 | |
| 2 | 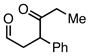 |
Et3SiCl | 41 | 39 | |
| 3 | 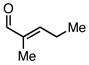 |
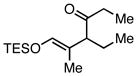 |
Et3SiCl | 90b | 72b |
| 4 | 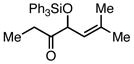 |
Ph3SiCl | 63 | 60 | |
| 5 | 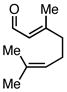 |
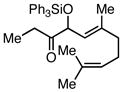 |
Ph3SiCl | 68c | |
| 6 | 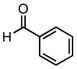 |
Et3SiCl | N.R. | ||
Reaction with 1.3 equiv Et2Zn, 2.3 equiv TMSCl, 1 atm CO; 4 h for entries 1 and 2; 20 h for entries 3 and 4; 24 h for entries 5 and 6. Subseqeunt to reaction, the mixture was quenched at 0 °C with acetic acid (1.5 equiv) and TBAF (1.5 equiv) for 10 minutes and then treated with saturated Na2CO3.
Quench (footnote a) was omitted from this reaction.
A 2:1 mixture of olefin isomers was obtained.
Employing TESCl instead of TMSCl as the additive, α,β-unsaturated aldehydes were examined in the carbonylative conjugate addition. As shown in Table 3, β-substituted enals are converted to the 1,4-addition product in good yields. With substitution at the α-carbon, protodesilylative work-up furnished a 1:1 mixture of stereoisomeric compounds. However, with a neutral workup (entry 3) the derived enol silyl ether could be obtained as a single stereoisomer and was found to be in the E configuration by NOE analysis. Interestingly, with two substituents at the β-carbon of the enal, the product of 1,2-carbonylative addition was generated in preference to the 1,4-product. With TESCl, the product was isolated in lower yields (41% for entry 4) but this was easily remedied; use of more Lewis acidic Ph3SiCl provided the carbonylative addition product in excellent yield, but for entry 5 as a mixture of olefin stereoisomers. Lastly, benzaldehyde was examined and found to be unreactive, an observation that suggests a role for α,β unsaturation even in transformations that furnish the 1,2 addition product.13
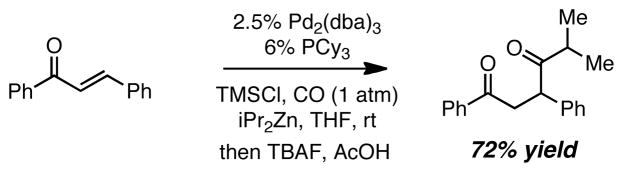
To ascertain whether the carbonylative conjugate addition reaction is limited to Et2Zn, we examined the reaction with i-Pr2Zn (Scheme 3). This reaction provided comparable yields as the reaction with Et2Zn, without requiring increased catalyst loading. Similar to the reaction with Et2Zn, the use of 0.65 equivalents of the diisopropylzinc sufficed for an effective reaction (72% yield), a feature that may be significant when more precious reagents would be required.
Scheme 3.
In conclusion, the Pd(0)-catalyzed carbonylative conjugate addition of organozinc reagents to α,β-unsaturated carbonyl compounds is an efficient reaction and accomplishes a transformation that can otherwise require multiple synthesis steps. Further efforts to broaden the scope of this reaction are currently on going and will be reported in due course.
Supplementary Material
Acknowledgments
This work was supported by the NIH (GM 64451).
Footnotes
Supporting Information Available. Complete experimental procedures and characterization data (1H and 13C NMR, IR, and mass spectrometry). This material is free of charge via the internet at http://pubs.acs.org.
References
- 1.(a) Alexakis A, Bäckval JE, Krause N, Pàmies O, Diéguez M. Chem Rev. 2008;108:2796. doi: 10.1021/cr0683515. [DOI] [PubMed] [Google Scholar]; (b) Harutyunyan SR, Hartog T, Geurts K, Minnaard AJ, Feringa BL. Chem Rev. 2008;108:2824. doi: 10.1021/cr068424k. [DOI] [PubMed] [Google Scholar]; (c) Tomioka K, Nagaoka Y. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Vol. 3. Springer-Verlag; Berlin: 1999. p. 1105. [Google Scholar]; (b) Krause N, Hoffmann-Röder A. Synthesis. 2001:171. [Google Scholar]; Hayashi T, Yamasaki K. Chem Rev. 2003;103:2829. doi: 10.1021/cr020022z. [DOI] [PubMed] [Google Scholar]; (c) Feringa BL, Naasz R, Imbos R, Arnold LA. In: Modern Organocopper Chemistry. Krause N, editor. Wiley-VCH; Weinham: 2002. p. 224. [Google Scholar]
- 2.(a) Corey EJ, Hegedus LS. J Am Chem Soc. 1969;91:4926. [Google Scholar]; (e) Lipshutz BH, Elworthy TR. Tetrahedron Lett. 1990;31:477. [Google Scholar]; (f) Thomas SE. J Chem Soc, Chem Commun. 1987:266. [Google Scholar]; (g) Hegedus LS, Perry RJ. J Org Chem. 1985;50:4955. [Google Scholar]; (h) Cooke MP, Jr, Parlman RM. J Am Chem Soc. 1977;99:5222. [Google Scholar]; (i) Seyferth D, Hui RC. J Am Chem Soc. 1985;107:4551. [Google Scholar]
- 3.Stetter H. Angew Chem Int Ed. 1976;15:639. [Google Scholar]
- 4.(a) Degl’Innocenti A, Ricci A, Mardini A, Reginato G, Colotta V. Gazz Chim Ital. 1987;117:645. [Google Scholar]; (b) Mattson AE, Bharadwaj AR, Scheidt KA. J Am Chem Soc. 2004;126:2314. doi: 10.1021/ja0318380. [DOI] [PubMed] [Google Scholar]; (c) Nahm MR, Linghu X, Potnick JR, Yates CM, White PS, Johnson JS. Angew Chem Int Ed. 2005;44:2377. doi: 10.1002/anie.200462795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For recent metal-catalyzed carbonylative conjugate additions, see: Chochois H, Sauthier M, Maerten E, Castanet Y, Mortreux A. Tetrahedron. 2006;62:11740.Sauthier M, Castanet Y, Mortreux A. Chem Commun. 2004:1520. doi: 10.1039/b403415e.Hanzawa Y, Tabuchi N, Taguchi T. Tetrahedron Lett. 1998;39:8141.Shirakawa E, Yamamoto Y, Nakao Y, Tsuchimoto T, Hiyama T. Chem Commun. 2001:1926. doi: 10.1039/b105832k.
- 6.For other recent metal-catalyzed carbonylative C-C bond forming reactions, see: Dheur J, Sauthier M, Castanet Y, Mortreux A. Adv Synth Catal. 2007;349:2499.Menard F, Weise CF, Lautens M. Org Lett. 2007;9:5365. doi: 10.1021/ol7022054.Aksin O, Dege N, Artok L, Türkmen H, Çetinkaya B. Chem Commun. 2006:3187. doi: 10.1039/b604742d.
- 7.(a) Sieber JD, Liu S, Morken JP. J Am Chem Soc. 2007;129:224. doi: 10.1021/ja067878w. [DOI] [PubMed] [Google Scholar]; (b) Sieber JD, Morken JP. J Am Chem Soc. 2008;130:4978. doi: 10.1021/ja710922h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For mechanistically-related Pd and Ni-catalyzed conjugate additions, see: Grisso BA, Johnson JR, Mackenzie PB. J Am Chem Soc. 1992;14:5160.Ogoshi S, Yoshida T, Nishida T, Morita M, Kurosawa H. J Am Chem Soc. 2001;123:1944. doi: 10.1021/ja0036099.Marshall JA, Herold M, Eidam HS, Eidam P. Org Lett. 2006;8:5505. doi: 10.1021/ol062154a.Hirano K, Yorimitsu H, Oshima K. Org Lett. 2007:1541. doi: 10.1021/ol070288y.
- 9.Yuguchi M, Tokuda M, Orito K. J Org Chem. 2004;69:908. doi: 10.1021/jo035468+. [DOI] [PubMed] [Google Scholar]
- 10.For Lewis acid promoted direct addition of organozincs to aldehydes, see: Ochiai H, Nishihara T, Tamaru Y, Yoshida Z. J Org Chem. 1988;53:1343.
- 11.This reactivity pattern finds parallel in rates of ligand substitution at Pd(0), see: Stahl SS, Thorman JL, de Silva N, Guzei IA, Clark RW. J Am Chem Soc. 2003;125:12. doi: 10.1021/ja028738z.
- 12.Mukaiyama T, Narasaka K, Banno K. Chem Lett. 1973:1011. [Google Scholar]
- 13.Ogoshi S, Yoshida T, Nishida T, Morita M, Kurosawa H. J Am Chem Soc. 2001;123:1944. doi: 10.1021/ja0036099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.