Abstract
Background
To study withdrawal, ethanol is usually administered chronically without interruption. However, interest has recurred in models of episodic exposure. Increasing evidence suggests that chronic intermittent exposure to ethanol leads to a sensitization effect in both withdrawal severity and in ethanol consumption. The goal of the present study was to examine mouse inbred strain differences in withdrawal severity following chronic intermittent exposure using the handling induced convulsion as the behavioral endpoint. We also sought to compare the withdrawal responses of inbred strains across acute, chronic continuous, and chronic intermittent exposure regimens.
Methods
Male mice from 15 standard inbred strains were exposed to ethanol vapor for 16 hours each day for 3 days and removed to an air chamber during the intervening 8 hours. Mice in the control groups were handled the same, except that they were exposed only to air. Daily blood ethanol concentrations were averaged for each mouse to estimate total dose of ethanol experienced.
Results
Across strains, mice had an average daily blood ethanol concentration (BEC) of 1.45 ± 0.02 mg/ml and we restricted the range of this value to 1.00 to 2.00 mg/ml. To evaluate strain differences, we divided data into two dose groups based on BEC, Low Dose (1.29 ± 0.1 mg/ml) and High Dose (1.71 ± 0.02 mg/ml). After the third inhalation exposure, ethanol- and air-exposed groups were tested hourly for handling-induced convulsions for 10 hr and at hr 24 and 25. Strains differed markedly in the severity of withdrawal (after subtraction of air control values) in both dose groups.
Conclusion
The chronic intermittent exposure paradigm is sufficient to elicit differential withdrawal responses across nearly all strains. Data from the High Dose groups correlated well with withdrawal data derived from prior acute (single high dose) and chronic continuous (for 72 hrs) ethanol withdrawal studies, supporting the influence of common genes on all three responses.
Keywords: Chronic intermittent ethanol exposure, inbred mice, alcoholism, ethanol dependence and withdrawal, convulsions, genetics
Introduction
Withdrawal from chronic administration of ethanol is characterized by hyperexcitability symptoms that wax and wane, including tremor, autonomic nervous system overactivity, and, in extreme cases, convulsions, which can be lethal. Withdrawal convulsions occur in all mammalian species studied including humans (Friedman, 1980) and demonstrate a preexisting state of physical dependence on the drug. In the early 1970’s, Dr. Dora Goldstein induced ethanol dependence in mice by administering ethanol vapor continuously to subjects confined in an inhalation chamber, and characterized a handling-induced convulsion (HIC) displayed by mice undergoing withdrawal when they are picked up by the tail (Goldstein & Pal, 1971). The HIC is a very sensitive behavioral index that has also been used to characterize and quantify the mild dependence induced by a single intraperitoneal injection of 4 g/kg ethanol in mice (Crabbe et al., 1991). This acute withdrawal reaction was first noted several hours after withdrawal in mice using threshold sensitivity to pentylenetetrazol-induced seizures as the behavioral end point (McQuarrie & Fingl, 1958). It is also seen after barbiturates, benzodiazepines, and many other central nervous system depressant drugs (Crabbe et al., 1991). HIC severity following chronic continuous ethanol inhalation depends upon dose, exposure, duration, and genotype (Goldstein, 1972; Goldstein, 1973). In subsequent studies using a version of this method, we found that inbred strains of mice differed substantially in withdrawal HIC severity (Crabbe et al., 1983) that was elevated above HIC scores observed in experimentally naive animals (Crabbe et al., 1980; Metten & Crabbe, 2005).
Interest has recurred in the use of episodic exposure to and withdrawal from ethanol to induce alcohol dependence and it has been employed in particular to investigate withdrawal-associated drinking, characterized by some as a model of relapse (Spanagel & Hölter, 1999; O’Dell et al., 2004). It has been hypothesized that repeated, intermittent exposure to ethanol, ie, gross intoxication with intervening periods of sobriety, results in a sensitized withdrawal response in humans (Ballenger & Post, 1978, Booth & Blow, 1993) and there have been reports of this in animal models as well (Becker & Hale, 1993; Becker et al., 1997a, b). Several groups have found increased voluntary consumption of ethanol by C57BL/6 mice following a chronic, intermittent exposure vapor inhalation procedure (Becker & Lopez, 2004; Finn et al., 2007). However, to the best of our knowledge, the only two standard inbred mouse strains for which data have been published following chronic intermittent vapor exposure remain C3H/He and C57BL/6; the former to study sensitization of withdrawal HICs, and the latter to study withdrawal-associated drinking.
In the present study, our first goal was to determine whether chronic intermittent ethanol vapor exposure was sufficient to produce withdrawal in a variety of inbred strains and second, to establish the magnitude of strain differences in withdrawal. We next sought to compare the strains’ responses across three dosing regimens: acute (Metten & Crabbe, 1994), chronic continuous (Metten & Crabbe, 2005), and chronic intermittent (present data). The correlation of inbred strain mean values on two traits serves as a rough index of the importance of influence of a common set of genes, as the resulting r2 indicates the proportion of shared genetic variance (Hegmann & Possidente, 1981). Because of the genetic stability of inbred strains, data on the correlated traits can be collected in different animals, as well as at different times and in different laboratories. We have successfully used this method to ascertain patterns of shared genetic influences among ethanol responses. For example, in several studies, a substantial negative genetic correlation has been reported between severity of withdrawal HICs after either chronic continuous or acute ethanol exposure and voluntary consumption of 10% ethanol by naive mice (Belknap et al., 1993, 2008; Crabbe et al., 1983; Metten & Crabbe, 1994, 2005; Metten et al., 1998b; Rhodes et al., 2007). This finding implies that at least some of the genes predisposing strains to severe ethanol withdrawal HICs also exert protective effects by inhibiting voluntary ethanol intake in naïve animals.
Materials and Methods
Animals and husbandry
Adult male mice of the following inbred strains were obtained at 5-6 weeks of age from The Jackson Laboratory (JAX; Bar Harbor, ME): 129S1/SvImJ (129), AKR/J (AKR), BALB/cByJ (BALB), BTBR T+tf/J (BTBR), C3H/HeJ (C3H), C57BL/6J (B6), C57L/J (C57L), CBA/J (CBA), DBA/1J (DBA/1), DBA/2J (DBA/2 or D2), FVB/NJ (FVB), PL/J (PL), SJL/J (SJL). Breeding stock of C57BR/cdJ (C57BR) and CE/J (CE) were purchased from JAX and bred in our facility at the Portland VA Medical Center as the mice were difficult to obtain in large enough numbers to test. A total of 289 mice (11 - 53/strain) were exposed to ethanol, while 141 mice (5 - 28/strain) constituted the control groups (See Table 1). Mice were housed 2-4 per polycarbonate or polysulfone cage with others of the same strain. Food (Purina 5001) and water were available ad libitum, and lights were on from 0600 - 1800 hr in colony rooms maintained at 22 ± 2 °C. All procedures were approved by the Portland VA Institutional Animal Care and Use Committee in accordance with USDA and USPHS guidelines. Mice were tested between 53 and 98 days old over a period spanning more than 18 months in one of six cohorts (20 - 109 mice/cohort), each representing several inbred strains. Because we were breeding them, C57BR/cdJ and CE/J were available in larger numbers than the other strains, so we included some mice of these strains in several cohorts. Although the ultimate sample sizes for these strains were large (particularly for C57BR/cdJ), there was no difference in conclusions using a randomly chosen subset, and no statistical advantage to excluding some mice, so we report all of the data here.
TABLE 1.
Sample Sizes and Treatment Conditions
| Strain | Control | Ethanol-Exposed | ||
|---|---|---|---|---|
| n (N) | n (N) | Reason for removal from | ||
| ethanol-exposed group (# removed)* | [Vapor] (mg/l) | |||
| 129S1/SvImJ | 8 | 15 (16) | other(1) | 6.5 |
| AKR/J | 8 | 11 (16) | BEC (3), other(2) | 6.5 |
| BALB/cByJ | 8 | 15 (16) | other(1) | 6.8 |
| BTBR T+tf/J | 8 | 14 (15) | BEC(1) | 6.9 |
| C3H/HeJ | 6 | 5 (15) | BEC(8), other(2) | 6.5 |
| C57BL/6J | 8 | 13 (16) | BEC(3) | 8.9 |
| C57BR/cdJ | 27 (28) | 48 (53) | BEC(1), other(4) | 7.7 |
| C57L/J | 5 | 9 (11) | BEC(2) | 9.3 |
| CBA/J | 8 | 16 | 6.3 | |
| CE/J | 10 (17) | 20 (36) | BEC(7), other(9) | 6.9 |
| DBA/1J | 8 | 15 | 5.8 | |
| DBA/2J | 7 | 11 (16) | BEC(5) | 6.3 |
| FVB/NJ | 6 | 14 (16) | BEC(1), other(1) | 8.0 |
| PL/J | 8 | 15 (16) | other(1) | 6.9 |
| SJL/J | 7 (8) | 11 (16) | BEC(4), other(1) | 6.9 |
|
|
||||
| Totals | 132 (141) | 232 (289) | BEC(35), other(22) | |
Control mice were injected with pyrazole and exposed only to air.
(N) signifies the original sample size at the beginning of the experiment, if different from final n. See text for explanation of differences within the Control (air) groups.
Some ethanol exposed mice were excluded from analyses for different reasons: BEC : blood ethanol concentration outside the allow able daily range (0.50 – 2.75 mg/ml) or average overall allowable daily BEC range (1.00 – 2.00 mg/ml); “other”: includes mice excluded because there were insufficient mice/strain in either the High or Low Dose group (as discussed in text), death, illness, or experimental error.
[Vapor]: average concentration of ethanol vapor over the three days.
Two mice in the air group and one in the ethanol group were eliminated due to experimental error in treatment. Six CE/J mice from the air treatment group were eliminated from the final data set due to excessive weight loss (20 – 22%) over the 4 days. This was an unusual occurrence as no other air- or ethanol-treated mice of any other strain were eliminated for this reason, including those in the same cohort. However, across three cohorts, nine CE/J mice from the ethanol treatment group either died or were euthanized for illness during this experiment. Thirteen additional mice (one air, twelve ethanol), across 7 other strains, either died or were euthanized for illness, leaving a total of 132 mice in the air groups and 267 mice in the ethanol groups. Column 3 of Table 1 provides a strain-wise summary of ethanol-group mice deleted for these reasons (“other”).
Ethanol dependence induction
Details of the basic inhalation exposure method have been published (Terdal & Crabbe, 1994). In the current study, we followed the method of Becker et al. (1997a,b) to achieve chronic intermittent ethanol exposure; however, we used a single exposure pattern for all mice. Mice were administered ethanol vapor (or air, for control animals) for sixteen hours each day for three days, with 8 hours of exposure to air separating periods of exposure to ethanol vapor. To insure equivalent blood ethanol concentrations (BECs) across strains, it was necessary to expose different strains to different vapor concentrations. Our experience in performing chronic continuous vapor inhalation exposure for 72 hours in standard and recombinant inbred mouse strains gave us vapor concentration settings that allowed us to minimize the number of mice needed (Metten & Crabbe, 2005; Crabbe, 1998). Mice from at least six cages from each strain were used, and animals within a cage were randomly assigned to either the air or ethanol condition.
Strains were tested in groups based on availability and on our knowledge of their sensitivity to ethanol, so that strains with similar sensitivity could be combined in a single inhalation chamber and exposed to the same ethanol vapor concentration. Mice in the ethanol groups were initially injected intraperitoneally with ethanol (Pharmco-Aaper, 1.5 g/kg, 20%v/v in saline) to raise BECs to about 1.50 mg ethanol/ml blood. An alcohol dehydrogenase inhibitor, pyrazole HCl (Sigma-Aldrich, 68.1 mg/kg, in saline, ip), was given each day to inhibit ethanol metabolism and stabilize BECs. The tested strains differ in ethanol elimination rates (Crabbe et al., 2003), so different ethanol vapor concentrations were selected (range = 5.5 – 9.5 mg ethanol/liter air; Table 1) based on Metten & Crabbe (2005) on a strain-specific basis to achieve approximately equal blood ethanol levels in all strains. For each strain, BEC measurements from the morning (see next section) were used to adjust vapor levels, if needed, as we attempted to keep BECs constant across days. Mice in the Control groups were injected with saline on Day 1, daily with pyrazole, and were placed in identical inhalation chambers where they were exposed only to air. At the time of removal of the mice from ethanol vapor each day, the air groups were moved to a second identical air chamber, while the ethanol groups were moved to the first air chamber. This was done to control for amount of disturbance and handling between groups.
Blood ethanol assessment
After each daily 16 hr exposure, all mice were removed from the chambers in small groups, gently restrained, and a 20 μl blood sample was drawn from the end of the nicked tail with a capillary tube for BEC determinations to assess the actual alcohol doses experienced by each strain during the course of vapor inhalation. They were then placed in the air chamber for 8 hr. On Days 2 and 3, they were again given 1.5 g/kg ethanol and 68.1 mg/kg pyrazole in a single injection and placed back in the ethanol vapor inhalation chambers. At the time of final withdrawal from the chambers (after three 16 hr periods of chronic intermittent ethanol inhalation and 16 hr after the last pyrazole injection), all mice again had a blood sample drawn. Assay of BECs was performed using a previously published gas chromatographic method (Rustay & Crabbe, 2004). Control mice were handled identically, injected with 68.1 mg/kg pyrazole before each 16-hour period, and had their tails nicked at the end of the 16-hour period, but blood samples were not collected.
Withdrawal testing
Immediately upon removal from the third and final ethanol vapor inhalation period, all mice were scored for HIC severity, weighed, had a blood sample taken, and were placed in their home cage. The HIC scoring was repeated each hour for 10 hr and at hrs 24 and 25. Using our modification of Goldstein’s original scale (Crabbe et al., 1991; Metten & Crabbe, 2005), handling-induced convulsions ranged in this study from no response (score = 0) to severe tonic-clinic convulsions (scores of 4, 5, and occasionally, 6). As a general index of overall withdrawal severity, the area under the entire 25-hr HIC curve was computed (AREA 25). A peak value was defined as the highest running average of three consecutive scores containing the maximum value. If there was a tie, the earliest occurrence was considered “peak.” Latency to peak was defined as the withdrawal hour of the first maximum score within those used to calculate peak. For maximum scores occurring at hour 24 or 25, peak was defined as the average of scores at hours 24 and 25.
Genetic correlations
If inbred strains have been maintained and tested in very similar environments, the extent to which a common set of genes influences two traits can be estimated by correlating the inbred strain means (Hegmann & Possidente, 1981). With few genotypes, it is somewhat difficult to achieve the usual levels of statistical significance (with only eight strains, a correlation of ∣r∣ ≥ .71 is required to achieve P<.05, and with 15 strains, this critical value is ∣r∣ ≥ .52). Given the modest power of the correlational analyses, a single outlier strain can have a marked effect on the value of a correlation, even if it is not an extreme responder for either trait. Thus, there is danger while interpreting such analyses of succumbing to either false positive or false negative results. We did not want to overlook potentially interesting relationships, so we examined scatterplots for all correlations. If a single strain appeared to be an outlier, we recomputed the correlation without it, and sometimes chose to interpret correlational relationships with the outlier deleted (see Results).
Results
Ethanol Dose
Eleven mice had a BEC on one or more days that was deemed to be too low (<0.50 mg/ml) or too high (>2.75 mg/ml) to be reasonably called equivalent exposure. Previous studies had shown that strain mean BEC at withdrawal correlates very significantly with BEC at different times during 72 hr chronic continuous inhalation exposure, and thus serves as a reasonable index of strain-specific “dose” of ethanol administered (Crabbe et al., 1983; Metten & Crabbe, 2005). In the current studies, we hypothesized that intermittency could affect the utility of the BEC at withdrawal as an index of overall exposure levels. Therefore, the overall “dose” of ethanol for each mouse was estimated as the average of their three daily BECs. For the purposes of analyses, data from seventeen mice also were eliminated from this experiment (1-7 mice each from 5 strains) for average daily BECs greater than 2.00 mg/ml or lower than 1.00 mg/ml despite having BECs each day that fell within the reasonable daily range (see Table 1).
Using the remaining 239 ethanol treated mice, average daily BEC (1.46 ± 0.02 mg/ml) was analyzed with a one-way ANOVA. Even after having removed outliers as described above, this yielded significant effects of Strain (F14, 224 = 8.35, p < 0.0001), accounting for 34% (R squared) of individual differences in BEC. Because our goal was to compare withdrawal severity across strains equated for exposure (as we had done for chronic continuous inhalation; Metten & Crabbe, 2005), we did not consider this an acceptable level of exposure matching across strains. We therefore assigned mice retrospectively into one of two groups based on their average daily BEC. Using this method, we were better able to match strains for exposure levels. The “Low Dose” group had average daily BECs of 1.29 ± 0.01 mg/ml (range: 1.00 – 1.50) and the “High Dose” group had average daily BECs of 1.71 ± 0.02 m/ml (range: 1.51 – 2.00). Those strains having at least 3 mice in a dose group were included in final analyses. Using these criteria, data from a further 7 mice (1/strain from 7 strains) were eliminated as they were single representatives within the dose group. Using all remaining mice, the C3H/HeJ and DBA/2J strains did not contain enough animals to qualify for inclusion in the Low Dose group, but were represented in the High Dose group. The following strains did not provide enough animals to qualify for the High Dose group, but were represented in the Low Dose group: AKR/J, BALB/cByJ, BTBR T+tf/J, C57BL/6J, and C57L/J. The final count for the ethanol groups was 232 mice. Ninety mice of 10 strains remained in the High Dose group, while the Low Dose group comprised 142 mice of 13 strains. Tables 2 and 3 show the daily and average daily BECs of the Low and High Dose groups, respectively.
TABLE 2.
Blood ethanol concentrations in Low Dose groups
| Strain | BEC (mg/ml) | ||||
|---|---|---|---|---|---|
| N | Day 1 | Day 2 | Day 3 | Average | |
| BEC | |||||
| 129S1/SvImJ | 7 | 1.53 ± 0.05 | 1.10 ± 0.06 | 1.52 ± 0.10 | 1.38 ± 0.05 |
| AKR/J | 11 | 1.69 ± 0.04 | 1.24 ± 0.05 | 0.85 ± 0.07 | 1.26 ± 0.05 |
| BALB/cByJ | 15 | 1.63 ± 0.06 | 1.21 ± 0.05 | 0.86 ± 0.07 | 1.23 ± 0.03 |
| BTBR T+tf/J | 13 | 1.70 ± 0.06 | 1.16 ± 0.07 | 0.90 ± 0.04 | 1.25 ± 0.04 |
| C3H/HeJ | 0 | N/A | |||
| C57BL/6J | 13 | 1.18 ± 0.07 | 1.40 ± 0.06 | 1.32 ± 0.06 | 1.30 ± 0.05 |
| C57BR/cdJ | 27 | 1.52 ± 0.05 | 1.28 ± 0.05 | 0.92 ± 0.05 | 1.24 ± 0.04 |
| C57L/J | 9 | 1.57 ± 0.08 | 1.43 ± 0.08 | 1.07 ± 0.18 | 1.35 ± 0.06 |
| CBA/J | 7 | 1.81 ± 0.07 | 1.31 ± 0.04 | 0.96 ± 0.07 | 1.36 ± 0.04 |
| CE/J | 5 | 1.54 ± 0.07 | 1.38 ± 0.06 | 1.00 ± 0.11 | 1.31 ± 0.06 |
| DBA/1J | 12 | 2.16 ± 0.09 | 0.89 ± 0.05 | 0.90 ± 0.06 | 1.32 ± 0.04 |
| DBA/2J | 0 | N/A | |||
| FVB/NJ | 5 | 1.66 ± 0.07 | 1.06 ± 0.07 | 1.16 ± 0.14 | 1.30 ± 0.07 |
| PL/J | 10 | 1.81 ± 0.05 | 1.30 ± 0.05 | 0.88 ± 0.08 | 1.33 ± 0.05 |
| SJL/J | 7 | 2.11 ± 0.12 | 0.84 ± 0.10 | 0.84 ± 0.07 | 1.26 ± 0.08 |
|
| |||||
| MEANS | 142 | 1.66 ± 0.03 | 1.21 ± 0.02 | 0.99 ± 0.02 | 1.29 ± 0.01 |
TABLE 3.
Blood ethanol concentrations in High Dose groups
| Strain | BEC (mg/ml) | ||||
|---|---|---|---|---|---|
| N | Day 1 | Day 2 | Day 3 | Average BEC | |
| 129S 1/SvImJ | 8 | 1.69 ± 0.06 | 1.38 ± 0.08 | 1.99 ± 0.12 | 1.69 ± 0.05 |
| AKR/J | 0 | N/A | |||
| BALB/cByJ | 0 | N/A | |||
| BTBR T+tf/J | 0 | N/A | |||
| C3H/HeJ | 5 | 2.12 ± 0.11 | 1.97 ± 0.09 | 1.63 ± 0.06 | 1.91 ± 0.04 |
| C57BL/6J | 0 | N/A | |||
| C57BR/cdJ | 21 | 1.96 ± 0.05 | 1.77 ± 0.03 | 1.34 ± 0.07 | 1.70 ± 0.03 |
| C57L/J | 0 | N/A | |||
| CBA/J | 9 | 2.14 ± 0.05 | 1.50 ± 0.04 | 1.19 ± 0.08 | 1.61 ± 0.04 |
| CE/J | 15 | 2.11 ± 0.07 | 1.57 ± 0.03 | 1.40 ± 0.10 | 1.69 ± 0.04 |
| DBA/1J | 3 | 2.33 ± 0.20 | 1.30 ± 0.09 | 1.14 ± 0.20 | 1.59 ± 0.03 |
| DBA/2J | 11 | 2.08 ± 0.08 | 1.89 ± 0.06 | 1.53 ± 0.07 | 1.83 ± 0.05 |
| FVB/NJ | 9 | 1.91 ± 0.10 | 1.48 ± 0.05 | 1.91 ± 0.11 | 1.76 ± 0.05 |
| PL/J | 5 | 1.94 ± 0.10 | 1.53 ± 0.08 | 1.35 ± 0.11 | 1.61 ± 0.05 |
| SJL/J | 4 | 2.62 ± 0.06 | 1.36 ± 0.05 | 1.09 ± 0.14 | 1.69 ± 0.06 |
|
| |||||
| MEANS | 90 | 2.04 ± 0.03 | 1.63 ± 0.03 | 1.47 ± 0.04 | 1.71 ± 0.02 |
We first report BEC values and the time course of withdrawal HIC scores in the Low and High Dose groups. We then report the computed indices of withdrawal severity.
BEC in the Low Dose group
Average daily BEC of the Low Dose group did not differ significantly among strains (F12,129 = 1.59, p = 0.10; Table 2). Using a repeated measures ANOVA, daily BECs differed significantly (F2,254 = 272.5, p < 0.0001) and a significant interaction of strain and day (F24,254 = 13.75, p < 0.0001) in the absence of a main effect of strain prompted us to look for strain effects for each daily value. Daily BECs differed among strains (all p < 0.0001), leading to our decision to use the averaged daily BECs as our index of total ethanol dose experienced. As expected from earlier studies, there was a significant but modest genetic correlation between BEC at the end of treatment (Day 3 BEC) and the average BEC during treatment within the Low Dose group (r = 0.59, p < .05).
Withdrawal time course in the Low Dose group
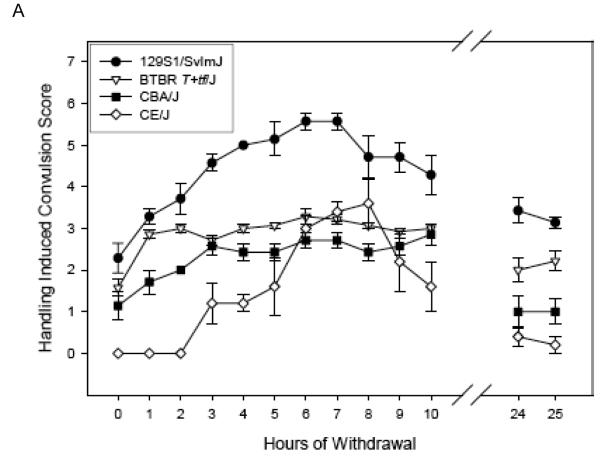
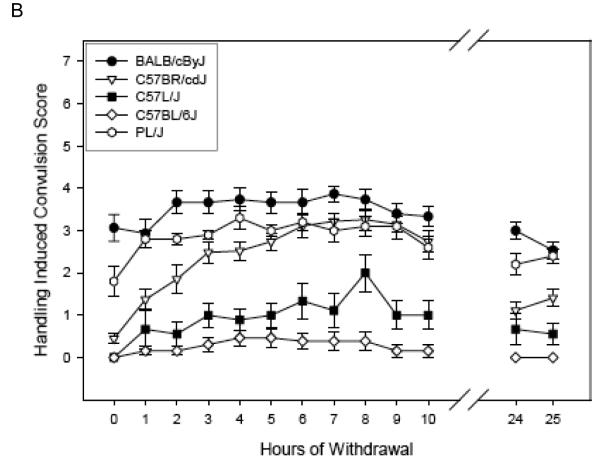
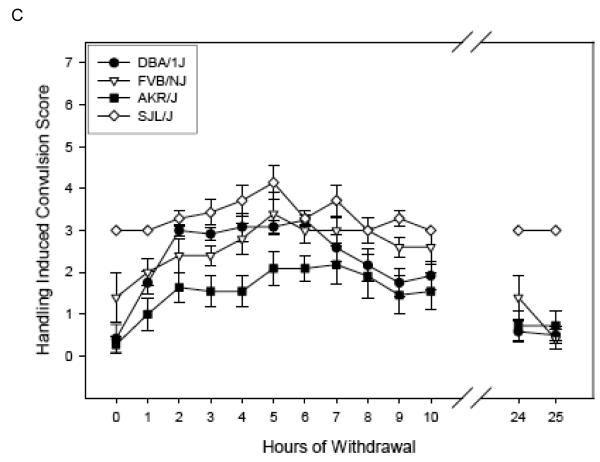
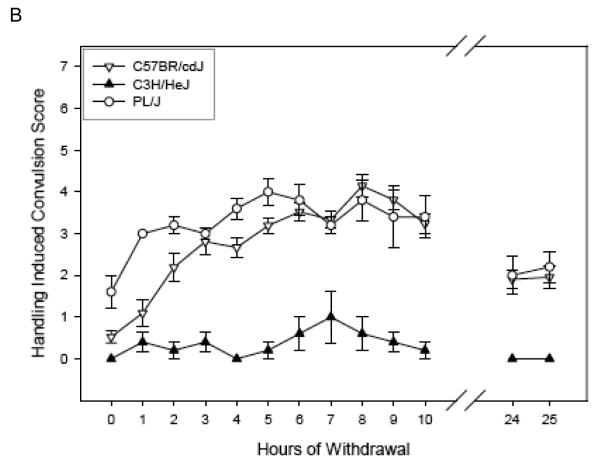
Figure 1 (Panels A-C) shows the ethanol withdrawal HIC scores during the 25 hr withdrawal test for the 13 strains in the Low Dose group. These values waxed and waned over time, increasing as ethanol was eliminated, which requires about 4 hr [see (Terdal & Crabbe, 1994)]. Withdrawal reached peak values at 2.69 to 6.40 hours (overall mean latency to peak = 4.44 ± 0.22 hr), depending upon strain (see Table 4), and tended to decline for most strains by 24 - 25 hr later (Figures 1 A-C). In contrast, HIC scores for the air-treated groups (Table 4) tended not to vary systematically over time, or to decline slightly (data not shown).
Figure 1.
(Panels A-C). Mean ± SE hourly HIC scores during withdrawal for Low Dose ethanol-treated groups. Number of mice/strain is given in Table 2. Data from air-treated groups are presented in Table 4. The panel in which a strain is depicted is arbitrary.
TABLE 4.
Handling-Induced Convulsion Severity in Control and Ethanol Withdrawing Mice
| Strain | Control* | Ethanol-exposed | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low Dose groups | High Dose groups | ||||||||
| AREA 25 | AREA 25 | ΔAREA 25 | ΔPEAK | Latency to Peak (hr)* |
AREA 25 | ΔAREA 25 | ΔPEAK | Latency to Peak (hr)* |
|
| 129S1 | 66.94 ± 2.49 | 99.43 ± 6.45 | 32.49 ± 6.45 | 2.69 ± 0.20 | 5.29 ± 0.29 | 102.00 ± 3.09 | 35.06 ± 3.09 | 2.67 ± 0.13 | 6.25 ± 0.70 |
| AKR | 2.00 ± 1.15 | 32.27 ± 7.85 | 30.27 ± 7.85 | 2.17 ± 0.31 | 4.91 ± 0.69 | ||||
| BALB | 54.13 ± 1.40 | 79.63 ± 4.72 | 25.51 ± 4.72 | 1.42 ± 0.18 | 3.13 ± 0.76 | ||||
| BTBR | 9.94 ± 3.82 | 64.54 ± 2.07 | 54.60 ± 2.07 | 2.27 ± 0.11 | 2.86 ± 0.63 | ||||
| C3H | 20.42 ± 10.14 | 5.30 ± 2.93 | 0 ** | 0.09 ± 0.40 | 3.40 ± 1.36 | ||||
| B6 | 0.00 ± 0.00 | 4.00 ± 1.77 | 4.00 ± 1.77 | 0.64 ± 0.20 | 2.69 ± 0.75 | ||||
| C57BR | 15.48 ± 2.48 | 52.41 ± 4.18 | 36.93 ± 4.18 | 2.78 ± 0.18 | 5.96 ± 0.52 | 64.67 ± 3.36 | 49.19 ± 3.36 | 3.41 ± 0.12 | 7.05 ± 0.50 |
| C57L | 6.60 ± 4.12 | 21.67 ± 6.70 | 15.07 ± 6.70 | 1.50 ± 0.27 | 5.44 ± 0.85 | ||||
| CBA | 32.56 ± 2.98 | 50.57 ± 3.16 | 18.01 ± 3.16 | 0.98 ± 0.07 | 6.14 ± 1.01 | 47.28 ± 3.31 | 14.72 ± 3.31 | 0.87 ± 0.11 | 5.78 ± 0.57 |
| CE | 0.40 ± 0.22 | 30.90 ± 3.59 | 30.50 ± 3.59 | 3.23 ± 0.33 | 6.40 ± 0.51 | 51.27 ± 6.13 | 50.87 ± 6.13 | 3.49 ± 0.48 | 10.13 ± 1.98 |
| D1 | 1.75 ± 1.05 | 42.21 ± 4.78 | 40.46 ± 4.78 | 2.88 ± 0.14 | 3.08 ± 0.42 | 63.17 ± 4.42 | 61.42 ± 4.42 | 3.29 ± 0.00 | 5.33 ± 1.20 |
| D2 | 41.00 ± 11.86 | 81.50 ± 6.71 | 40.50 ± 6.71 | 2.54 ± 0.16 | 6.64 ± 0.34 | ||||
| FVB | 17.33 ± 7.43 | 54.10 ± 8.12 | 36.77 ± 8.12 | 2.22 ± 0.32 | 4.20 ± 1.07 | 53.94 ± 4.34 | 36.61 ± 4.34 | 1.96 ± 0.09 | 5.57 ± 0.76 |
| PL | 37.44 ± 4.63 | 63.10 ± 4.41 | 25.66 ± 4.41 | 1.19 ± 0.19 | 4.00 ± 0.99 | 71.40 ± 7.42 | 33.96 ± 7.42 | 1.96 ± 0.24 | 6.60 ± 0.87 |
| SJL | 72.71 ± 0.55 | 75.86 ± 0.59 | 3.14 ± 0.59 | 1.00 ± 0.16 | 4.86 ± 0.51 | 87.75 ± 5.58 | 15.04 ± 5.58 | 1.57 ± 0.33 | 5.00 ± 1.08 |
| Means | 23.60 ± 2.21 | 51.12 ± 2.42 | 29.00 ± 1.81 | 1.97 ± 0.09 | 4.44 ± 0.22 | 64.05 ± 2.79 | 36.92 ± 2.50 | 2.50 ± 0.14 | 6.80 ± 0.41 |
For description of variables and their com putation, see text.
A “Peak” value is not shown for Control animals as HIC scores do not wax and wane in these groups. Nor is “Latency to Peak” calculated.
Withdrawal ΔAREA25 was slightly less than Control scores in the C3H/HeJ strain.
BEC in the High Dose group
In contrast to the finding in the Low Dose group, strains in the High Dose group still differed significantly in average daily BEC after our attempts at matching (F9,80 = 3.52, p = 0.001; Table 3). Examination of pairwise comparisons by Tukey’s HSD test indicated that most of the significant differences between strains were between C3H/HeJ and other strains (e.g., CBA/J and PL/J). However, elimination of the C3H/HeJ strain did not eliminate the significant effect of strain (F8,76 = 2.50, p < 0.02) on average daily BEC. Individual daily BEC values were also analyzed and the effects of Strain (F9,78 = 3.70, p < 0.001), Day, (F2, 156 =126.64, p < 0.0001) and their interaction (F18,156 = 10.28, p < 0.0001) were significant. Separate analyses of daily BECs also showed significant strain effects (all p < 0.0001). There was a positive genetic correlation between BEC at the end of treatment (Day 3 BEC) and the average BEC during treatment within the High Dose group that did not reach significance (r = 0.49, p = 0.15).
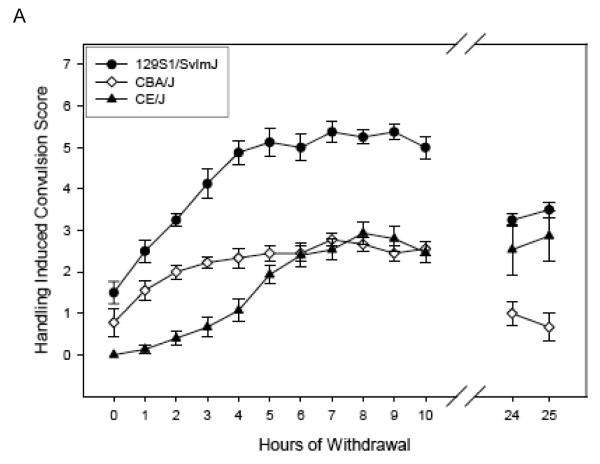
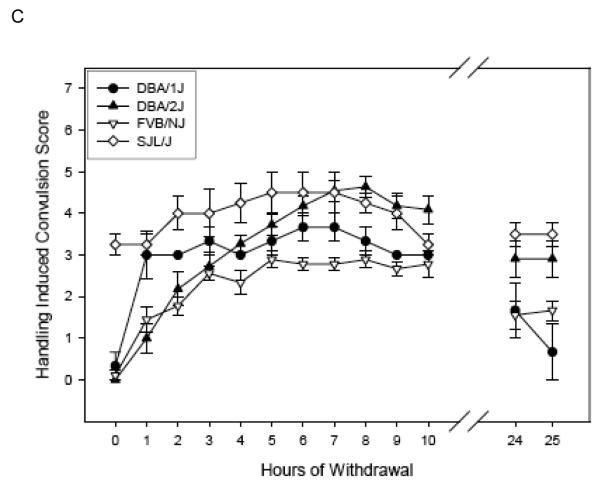
Withdrawal time course in the High Dose group
Figure 2 (Panels A-C) shows the ethanol withdrawal HIC scores during the 25 hr withdrawal test for the 10 strains in the High Dose group. For ease of comparison, the panels of Figures 1 & 2 show the same strains together (e.g., panel A of Figure 1 shows Low Dose 129S1/SvImJ, BTBR T+tf/J, CBA/J, and CE/J mice, and 129S1/SvImJ, CBA/J, and CE/J mice of the High Dose are represented in panel A of Figure 2). Withdrawal reached peak values at 3.40 to 10.13 hours (overall mean latency to peak = 6.80 ± 0.41 hr), depending upon strain, (Table 4) and tended to decline for most strains by 24 - 25 hr later (Figures 2 A-C). Three CE/J mice in the High Dose group exhibited very high HIC scores (6-7) at 24 or 25 hours, leading to long latency to peak scores overall in this group. Elimination of the CE/J strain from this measure left a range of latencies from 3.40 to 7.05 hours (mean = 6.13 ± 0.25).
Figure 2.
(Panels A-C). Mean ± SE hourly HIC scores during withdrawal for High Dose ethanol-treated groups. Number of mice/strain is given in Table 2. Data from air-treated groups are presented in Table 4. Panel – strain combinations are the same as in Figure 1.
Indexing withdrawal severity
The usual measure of withdrawal severity is to calculate the area under the total HIC withdrawal curve time course. Table 4 shows these AREA25 scores for both ethanol dose groups and the Control group for each strain. Missing HIC scores (between hrs 11-23) are inferred to compute these areas. Among the 8 strains represented in both Low and High Dose groups, the effects on AREA25 scores by strains (F7,212 = 47.13, p < 0.0001), treatments (F2,212 = 106.43, p < 0.0001), and their interaction (F14,212 = 3.12, p < 0.001) were all significant. As expected, within-dose effects of strain were significant as well (Low AREA25, F12,129 = 23.52, p < 0.0001; High AREA25, F9,80 = 16.34, p < 0.0001; Control AREA25, F14, 117 = 29.96, p < 0.0001). Strain mean Control scores for these groups were correlated with withdrawal scores in the ethanol dose groups (Control with High: r = 0.64, p = 0.052; Control with Low: r = 0.84, p < 0.001). Our previous studies examining withdrawal after 72hr chronic continuous inhalation in these same standard inbreds revealed a similar pattern [r = 0.80; (Metten & Crabbe, 2005)], as did another study with B6 and D2 inbreds and their 25 BXD RI strains [r = 0.47; (Crabbe, 1998)].
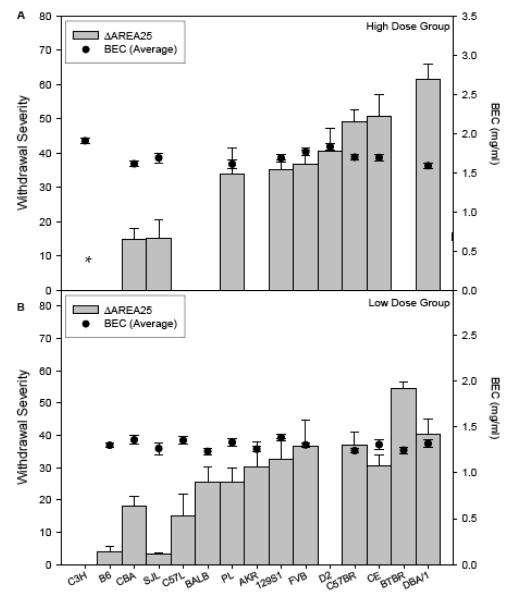
To create an index of ethanol withdrawal severity that was independent of untreated HIC scores in our study of BXD RI strains, we explored several possible methods, including computing difference scores for each individual ethanol-withdrawing animal from its strain’s Control group mean, and computing individual residual scores from linear and nonlinear regression of ethanol scores on Control group scores (Crabbe, 1998). We found these and other measures to be highly genetically correlated, as they are in the current study (data not shown), so we report here only one index (see Figure 3). To estimate the difference between each ethanol dose group’s and Control group’s AREA25 scores, we followed the same practice used in our earlier studies (Crabbe, 1998; Metten & Crabbe, 2005). We subtracted the mean value for each strain’s Control group AREA25 from each individual ethanol-treated animal’s AREA25 score. These values, termed “ΔAREA25,” are also shown in Table 4. One-way ANOVAs showed that strains differed in ΔAREA25 (Low: F12,129 = 8.35, p < 0.0001; High: F9,80 = 11.30, p < 0.0001), and the strain differences in this measure accounted for 44% and 56% of the variance in individual differences, respectively. Assignment of mice to dose group using average daily BEC or the BEC on the final day produced the same strain rank orders in ΔAREA25 scores (Spearman’s r ≥ 0.96). All strains except C3H/HeJ showed significant withdrawal at either the High or Low Dose exposure (ΔAREA25 > 0 by one-sample t-tests, p ≤ 0.05; Figure 3). Neither dose group showed a genetic correlation of Control group AREA25 scores with ΔAREA25 scores (−0.46 ≤ r ≤ −0.29, NS), indicating that the difference score did not over-correct for the control condition. Withdrawal severity between High and Low Dose groups was significantly genetically correlated (r = 0.85, p < 0.01, n = 8 strains).
Figure 3.
Strain mean withdrawal severity after chronic intermittent ethanol exposure at two exposure levels. Significant withdrawal was shown by all strains except for C3H/HeJ, as discussed in the text. Note that not all strains were included in each dose group. For computation of ΔAREA25, see results.
Panel A: High Dose group strain mean ± SE withdrawal severity scores (ΔAREA25) are shown (Left ordinate). Also shown is the mean BEC ± SE, averaged over the three daily values (Right ordinate).
* The ΔAREA25 score for C3H/HeJ was corrected to zero as shown in Table 4.
Panel B: Low Dose group strain mean ± SE withdrawal severity scores (ΔAREA25) are shown (Left ordinate). Also shown is the mean BEC ± SE, averaged over the three daily values (Right ordinate).
Table 4 also shows the peak withdrawal severity (ΔPEAK) scores for the ethanol treated groups, which was corrected by subtraction of mean Control group scores at the time that each strain showed its ethanol peak response. The latency to reach peak withdrawal (Goldstein, 1972) in the ethanol groups is also shown. These variables sometimes serve as useful indices for withdrawal scores among genotypes treated with rapidly acting drugs (Metten et al., 1998a, 2007). The ΔPeak and latency measures differed among strains (Low Dose: F12,129= 14.23, p < 0.0001, F12,129= 3.42, p < 0.001, respectively; High Dose: F9,80= 12.73, p < 0.0001, F8,90= 2.17, p < 0.05, respectively). The ΔPeak and latency measures were only significantly positively correlated within the High Dose group (High Dose: r = 0.72, p < 0.02; Low Dose: r = 0.27, p = 0.38). As expected, strain mean ΔPEAK scores were positively correlated with ΔAREA25 (High Dose: r = 0.95, p < 0.01; Low Dose: r = 0.75, p = 0.06).
In an early study with 18 inbred strains, we found a positive genetic correlation of r = 0.57 between BEC (at the time of withdrawal from 72 hr, chronic continuous ethanol exposure) and area under the 25 hr HIC curve, suggesting that about 1/3 of the variance in strain differences in withdrawal severity could be accounted for by BEC differences (Crabbe et al., 1983). In that study, all strains were treated in a single vapor concentration, and BECs varied markedly across strains. We corrected for strain differences in ethanol dose by regressing ΔAREA25 scores on BEC at withdrawal and then used the residual from that regression to index strain-specific withdrawal severity (Crabbe et al., 1983). In the present study, ΔAREA25 and average daily BEC within dose groups are shown in Figure 3. Average daily BEC and ΔAREA25 were not genetically correlated within the Low Dose group (r = −0.20, p =0.51), where strains did not differ significantly in BEC. An apparent, but modest, genetic correlation within the High Dose group (r = −0.51, p = 0.13), upon examination of the scatterplot, proved to be entirely dependent on the C3H/HeJ strain, and when this strain was removed the correlation became r = 0.01 (p = 0.85).
Genetic Correlations of Severity of Chronic Intermittent Withdrawal with Acute and Chronic Continuous Withdrawal
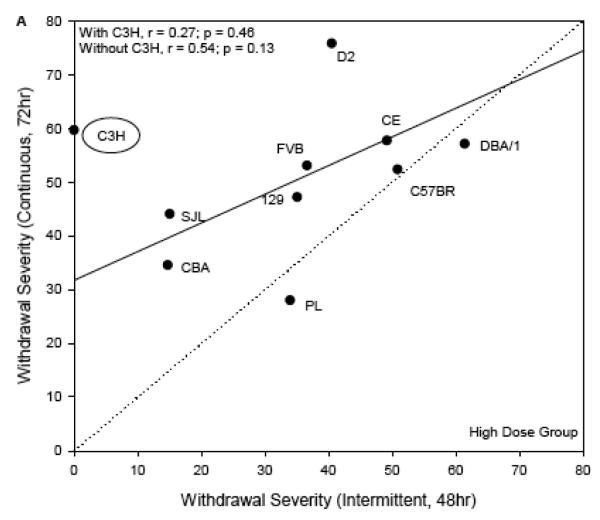
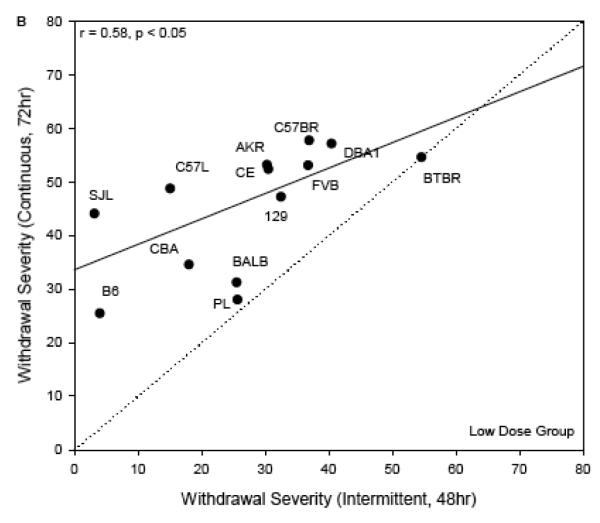
To compare withdrawal severity across dosing regimens (acute, chronic continuous, and chronic intermittent), we used ΔAREA25 from the two dose groups independently. Figure 4 shows the scatterplots of strain means for severity of chronic continuous withdrawal (y axis, both panels) with chronic intermittent withdrawal (High Dose group: x axis, panel A; Low Dose group: x axis, panel B). The genetic correlation between these withdrawal scores was significant for strains in the Low Dose group (r = 0.58, p < 0.05; Figure 4B). The one-to-one lines are shown, which represent the hypotheses that withdrawal severity was the same following either continuous or intermittent exposure. Withdrawal severity for nearly all strains appeared to be more severe when vapor inhalation was continuous over 72 hrs than when broken up into intermittent episodes of 3 × 16 hr periods. This can be seen where the strain responses deviate from the one-to-one line in the upward direction. For the strains in the High Dose group, the analogous genetic correlation did not appear at first to be significant (r = 0.27, p = 0.46; Figure 4A). The notable outlier strain from this relationship was C3H/HeJ, which had much milder withdrawal in the current experiment than after 72 hrs of continuous vapor inhalation. When this strain was removed, the correlation of chronic continuous and intermittent withdrawal became r = 0.54, p = 0.13. Similar to the Low Dose group comparison, withdrawal appeared to be more severe after continuous than after intermittent inhalation in most strains in the High Dose group.
Figure 4.
Withdrawal severity in the current experiment (ΔAREA25, abscissa), plotted versus comparable data following chronic continuous vapor inhalation for 72 hr (ordinate; Metten & Crabbe, 2005). Each point represents a strain mean value. The regression lines (solid line) and one-to-one lines (dotted line) are shown. Panel A: High Dose chronic intermittent exposure. The regression line shown was computed without the C3H/HeJ strain as discussed in the text. Panel B: Low Dose chronic intermittent exposure.
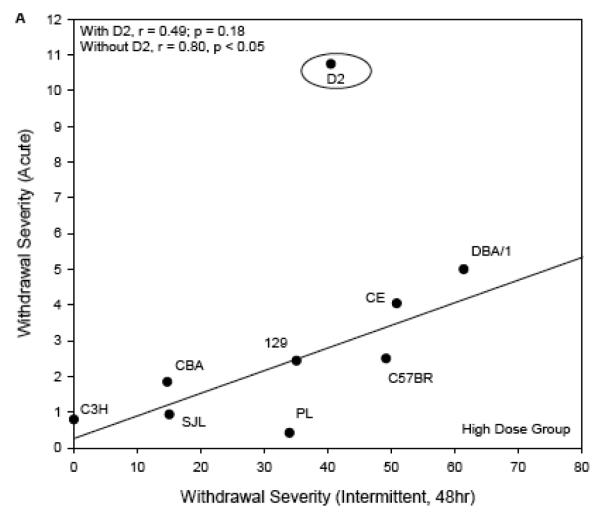
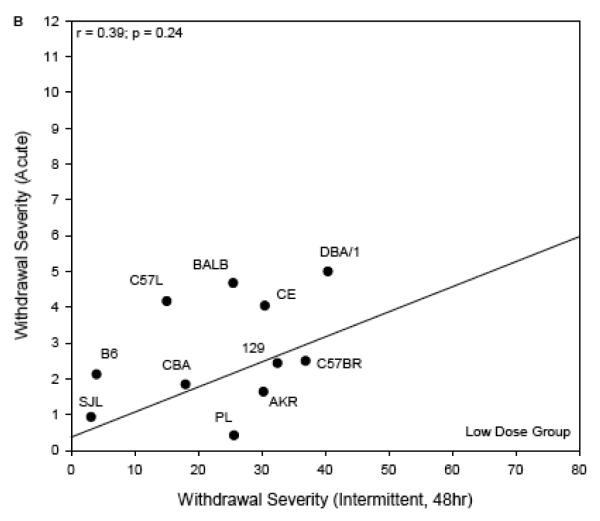
For the comparison with acute withdrawal (Figure 5), the regression lines are shown rather than the one-to-one lines for the scatterplots of High Dose data (panel A) and Low Dose (panel B) vs published acute withdrawal severity scores (Metten & Crabbe, 1994). The one-to-one line was not an appropriate guide because the time courses of withdrawal in these two experiments were very different. Using ΔAREA25 for the present data, and the most directly comparable index for acute withdrawal severity (area under the curve, corrected for pre-drug baseline differences in HIC), the genetic correlations of the traits were r = 0.39, p = 0.24 (Low Dose groups) and r = 0.49, p = 0.18 (High Dose groups). There was a notable outlier to the correlation in the High Dose groups. DBA/2J mice had much more severe acute withdrawal than any other strain (Metten & Crabbe, 1994), but only moderate withdrawal after chronic intermittent exposure. Exclusion of this strain resulted in a significant genetic correlation between withdrawal from chronic intermittent ethanol and acute withdrawal (High Dose: r = 0.80, p < 0.05, Figure 4A). The DBA/2J strain was not represented in the Low Dose groups.
Figure 5.
Withdrawal severity in the current experiment (ΔAREA25, abscissa), plotted versus comparable data following a single 4 g/kg ip injection of ethanol (ordinate; Metten & Crabbe, 1994). Each point represents a strain mean value. The regression lines are shown. One-to-one lines are not shown because the time course for acute withdrawal testing differs from that following chronic intermittent exposure.
Panel A: High Dose chronic intermittent exposure. The regression line shown was computed without the DBA/2J strain as discussed in the text.
Panel B: Low Dose chronic intermittent exposure.
Discussion
The results of this study show that strains are differentially sensitive to withdrawal from ethanol delivered by chronic, intermittent vapor inhalation. The results also show that three 16-hour periods of vapor inhalation are sufficient to produce significant withdrawal in 14 of the 15 inbred strains tested here. Both High and Low Dose groups showed significant strain differences in withdrawal severity. Of the 8 strains we were able to include in both dose groups, all showed significant withdrawal at both doses. Only C3H/HeJ did not show significant withdrawal severity (Table 4, Figure 3A). Severity of withdrawal after chronic intermittent ethanol exposure at the High Dose was genetically correlated with withdrawal severity after 72 hr chronic continuous ethanol exposure (Figure 4A; Metten & Crabbe, 2005) and with acute withdrawal severity (Figure 5A; Metten & Crabbe, 1994). Withdrawal after Low Dose chronic intermittent exposure was also correlated with 72 hr chronic continuous withdrawal, but not with acute withdrawal (Figures 4B & 5B). This suggests that many of the same genes influence ethanol withdrawal HIC severity across all three administration paradigms, although there are clearly also substantial independent genetic contributions to withdrawal that depend on the exposure paradigm.
We found that withdrawal severity for most strains was less than found previously in our chronic continuous exposure experiment (Metten & Crabbe, 2005). This is likely to be partly because the total duration of vapor inhalation was less, 48 vs 72 hrs (Goldstein, 1972; Becker et al., 1997b). An additional potential difference between the two experiments was the total BEC achieved. For the chronic continuous exposure experiment (Metten & Crabbe, 2005), the average BEC across strains was comparable to that in the High Dose group in the present experiment (average daily doses, 1.71 ± 0.02 mg/ml vs. 1.71 ± 0.02 mg/ml, respectively). In a series of experiments using Swiss Webster outbred mice administered ethanol by chronic continuous exposure, Goldstein (1972) showed that the severity of ensuing HICs was a joint function of BEC × duration of chronic, continuous vapor exposure. Thus, for our Low Dose data, animals were less severely exposed on both counts, while for the High Dose groups, dose was similar but duration shorter.
BEC proved difficult to equate among strains, and even within some strains. However, dividing our sample into High and Low Dose groups allowed us to match strains for relatively low BEC values, and greatly improved the imperfect match at higher BECs (Figure 3). Strain rank orders of withdrawal scores were nearly identical whether the mice were assigned to dose group using the average daily BEC or the BEC on the final day. The presence of pyrazole during vapor inhalation likely further attenuates the possible significant physiologic consequences of BECs within this restricted range.
To our knowledge, there are no systematic, published data regarding the effect on withdrawal severity of temporal patterning of BECs over time in mice during chronic continuous exposures or across multiple intermittent exposures. It has been shown in rats that increasing BECs over approximately 2 weeks or increasing the product of BEC × duration of chronic continuous vapor inhalation increases withdrawal symptoms (LeBourhis & Aufrere, 1983; Karanian et al., 1986). Few studies report the pattern of BECs across time. Where such sampling is performed, it is usually reported as having been used to maintain BECs within a given limited range, much as we have done here. Usually, such sampling is based on a relatively small number of animals, precluding any ability to relate the pattern of BECs to withdrawal severity score, either across treatment groups or across individual animals. We have heard anecdotal conjectures that the speed at which alcohol is eliminated is related to the severity of withdrawal. The reference is usually in the context of acute doses administered intravenously. With adjunct pyrazole administration, as in the current studies, the elimination rate for ethanol is slowed markedly (Becker, 1994). One study has measured elimination rates in mice starting at equivalent BECs after either just 16 hours or multiple-16 hour periods of vapor inhalation and found no difference in elimination rate (Becker, 1994). Likewise, BECs measured 8 hours after each vapor inhalation period did not differ between exposure groups (both groups showed daily BECs of ~0.15 mg/ml); withdrawal severity, however, differed markedly (Becker, 1994). We know of no other systematic studies of withdrawal severity after vapor inhalation that relate elimination rate to either dose, BEC, or duration of exposure. In the current data, BECs declined across days despite our best efforts to maintain them at a fixed level. We systematically increased the vapor concentration each day, but to a degree that was evidently insufficient to maintain stable levels. How much to increase vapor concentrations depends on the genotype and sex of the animals, and with large numbers of animals of different genotypes, maintaining the stability of BECs is a significant technical challenge (Terdal et al, 1994; Crabbe, 1998; Metten & Crabbe, 2005; H. Becker, personal communication). Our data might have led to conclusions that depend somewhat on this declining pattern.
Withdrawal HIC severity is clearly positively related to ethanol dose. This is true for acute withdrawal from a single intraperitoneal dose (doses of 3.5 – 5.0 g/kg in Swiss Webster mice; Metten & Crabbe, 1994), and for fixed-duration chronic continuous exposure to vapor (Crabbe, 1998; Goldstein, 1972). As dose is usually estimated from the final BEC taken at the end of the exposure (e.g., Crabbe et al 1983; Metten & Crabbe, 2005), our current results may also be somewhat idiosyncratic to the extent that the final (Day 3) BECs were lower than those reported in many studies, particularly in our Low Dose group. For example, Griffin et al. (2009a) recently analyzed retrospective data from many experiments in their laboratory where C57BL/6J mice were given 4 days of chronic intermittent exposure to ethanol vapor. Preference drinking was established before the vapor exposures, and was found to be elevated after withdrawal, but only in those animals whose average daily BECs exceeded 1.75 mg/ml. In other words, the phenomenon of withdrawal-associated drinking seemed to require exceeding a threshold level of exposure that would clearly be below that seen by the animals in our Low Dose groups, and by most of our High Dose group animals as well (see Tables 2 and 3). Dr. Becker’s group has also shown progressively increased withdrawal HIC in C3H/HeNCrl mice after two to nine vs one 16-hr intermittent ethanol vapor exposure periods (Becker et al., 1997b, Becker 1994; Becker & Veatch, 2002). In another study, male C3H/HeNCrl mice showed significantly greater withdrawal HIC scores than females after three or four (but not one or two) 16-hour ethanol exposures (Veatch et al., 2007). How the inbred strains we studied here would respond to other doses, exposure durations, patterns of ethanol exposure, and multiple vs single withdrawal episodes (i.e., scored after each 16 hour period) remains unknown without further studies.
It is interesting that the C3H/HeJ strain showed very low withdrawal in the current experiment (Figures 2 & 3). They appeared to be a very sensitive strain in the sense that they reached the highest average BEC of any strain; indeed, we could not obtain enough animals to include in our Low Dose group. Yet, they showed no measurable withdrawal after chronic intermittent exposure. Their HIC scores following air exposure averaged just less than 1 each hour (Table 4). In an early study of C3H/HeN mice tested for HIC with and without pyrazole, animals in both groups also averaged HIC scores of less than 1 each hour (Crabbe et al., 1983). Thus, the failure of withdrawing mice to show elevated HICs in the current study was not due to overly high HIC scores in the Control group. Their low withdrawal was similar to their response in our previously published acute withdrawal experiment (Figure 5A; Metten & Crabbe, 1994). In marked contrast, this strain showed very severe withdrawal following 72 hr chronic continuous vapor inhalation (Figure 4A; Metten & Crabbe, 2005). Although it is difficult to compare the magnitude of withdrawal HICs across laboratories, the very low withdrawal score reported here is hard to reconcile with previously published data from Dr. Becker’s laboratory (Becker & Hale, 1993; Becker et al., 1997b), where C3H/HeNCrl mice have substantial withdrawal after 3 or 4 intermittent daily exposures.
In attempting to understand this discrepancy, we compared our chronic continuous data from our 2005 study with published reports from Dr. Becker’s laboratory. In one study (Becker & Hale, 1993), C3H/HeNCrl mice were given either 48 continuous hours of vapor inhalation (BEC at withdrawal = 2.19 ± 0.11 mg/ml) or 16 hours × 3 days, with intermittent periods of 8 hours in air (BEC at withdrawal = 1.61 ± 0.48 mg/ml). These groups were also compared with air controls or 16 hour controls. Importantly, mice in this study given intermittent exposure to ethanol had withdrawal severity scores that were nearly twice those in the continuous group (approximately 23 vs 12; Figure 3 in Becker & Hale, 1993), despite having experienced lower BECs. The HIC scale used was very similar (modified from our published scale). In a second study (Becker et al., 1997a), mice were given either 48 continuous hours or 16 × 3 intermittent hours as above in ethanol vapor. BEC at the end of this vapor phase was about 1.60 mg/ml in both groups. Two other groups were given air during this period, but otherwise treated identically. After 8 hours in air, the continuous, intermittent, and one of the air groups were administered 40 continuous hours in ethanol vapor (BECs at withdrawal were somewhat lower, 1.12 – 1.35 mg/ml, respectively). Despite being equated for duration of ethanol vapor exposure, the group experiencing intermittent ethanol in the first 48 hours had greater withdrawal severity scores compared to the continuously exposed group (approximately 40 vs. 17, respectively). Both groups had higher withdrawal scores than either control group (Becker et al., 1997a).
In our study of chronic continuous vapor inhalation (Metten & Crabbe, 2005), C3H/HeJ mice were administered 72 hours of continuous ethanol (BEC at withdrawal = 2.00 ± 0.11 mg/ml). Withdrawal severity scores of C3H/HeJ mice averaged 59.67 ± 7.19 (after subtraction of control values, 1.17 ± 0.53). The comparison of withdrawal severity scores across these studies is problematic, but argues that our chronic continuous withdrawal results in the C3H/HeJ strain are somewhat similar to those Dr. Becker’s laboratory obtains in C3H/HeNCrl mice, and that it is the chronic intermittent results we report here that are more dissimilar.
One explanation that seems likely to us is that this is due to a substrain difference. All of the studies from Dr. Becker’s laboratory used C3H/HeNCrl (called C3H/He by Becker’s group and C3H/Hecr by Veatch et al., 2007) mice from Charles River Laboratories (Portage, MI), while we used C3H/HeJ mice from The Jackson Laboratory (Bar Harbor, ME). If C3H/HeJ mice are less sensitive to acute withdrawal and to withdrawal after chronic intermittent vapor exposure than C3H/HeNCrl mice, but not to withdrawal from chronic continuous exposure, a substrain difference could explain the apparent discrepancy.
Alternatively, or in addition, it is possible that a procedural difference between the laboratories accounts for the very low withdrawal in C3H/HeJ we saw here. Both laboratories used adult, male mice, and the handling-induced convulsion scoring scales used, while not identical, were very similar. Food, water, air, humidity, and many other variables including experimenter were different, and could possibly have affected results differentially in one or the other laboratory (Crabbe et al., 1999; Wahlsten et al., 2003; Chesler et al., 2002), although we think this unlikely. Besides our use of only 3 days of chronic intermittent exposure (vs 4 in most of Dr. Becker’s studies), other differences include: use of 200 proof grain ethanol vs 95% ethanol; preparation of ethanol as 20% v/v vs 8% w/v; and 1.5 g/kg vs 1.6 g/kg ethanol loading doses (Crabbe vs Becker laboratories, respectively). These differences seem unlikely to explain substantial differences in withdrawal severity. Another possible difference we note is that mice in their studies achieved BECs of 1.48 mg/ml after one 16 hr, and 1 – 1.75 mg/ml after the third 16 hr inhalation period (e.g., Becker et al., 1997b), while in our study, mice were exposed to higher BECs daily (Table 3; from 2.12 to 1.97 to 1.63 mg/ml). However, it seems unlikely to us that somewhat higher initial ethanol exposure should produce less severe withdrawal unless the animals were moribund, which was not the case in the present experiment. Although substrains are among the closest possible genetic relatives across mouse genotypes, substrain differences in behavior are well-known, including in their propensity to drink alcohol solutions (Blizard et al., 2004; Khisti et al., 2006), and we believe this to be the most likely explanation, although the biological mechanism remains obscure.
One reason for the present study was to explore genetic contributions to ethanol withdrawal after an exposure paradigm that is being used to study the increase in preference drinking known as “withdrawal-associated drinking” (e.g., Griffin et al., 2009a,b; Finn et al., 2007; Dhaher et al., 2009; Lopez & Becker, 2005). To date, the only published data of which we are aware are from the C57BL/6J inbred strain. In one study, as few as three 16-hour exposures to ethanol vapor inhalation have been shown to increase drinking relative to mice receiving air treatment (Finn et al., 2007). The C57BL/6J strain is well known to drink ethanol preferentially (Wahlsten et al., 2006) and to experience mild withdrawal HICs after acute or chronic continuous ethanol exposure (Metten & Crabbe, 1994, 2005). Their relatively low withdrawal is supported by the present data, where the strain showed nearly the least severe withdrawal (Table 4, Figure 3 bottom panel). The present results also demonstrate two chronic intermittent exposure conditions that were sufficient to produce significant withdrawal HICs in most inbred strains, which may be helpful in further studies examining withdrawal-associated drinking in other strains. A recent retrospective study from the Becker laboratory has indicated that withdrawal induced drinking is enhanced in C57BL/6J mice when using relatively high BECs during four 16 hr intermittent vapor inhalation exposures, and that four repetitions of 16-hr ethanol/8 hr air cycles with a week of drinking between each produced greater withdrawal induced drinking than did two cycles (Griffin et al., 2009a; Lopez & Becker, 2005).
Prior analyses have established a negative genetic correlation between ethanol drinking and withdrawal HIC severity (Metten et al., 1998b). The relationship obtains across multiple genetic populations, suggesting that there is a real (but unspecified) functional relationship. The populations include the BXD RI recombinant inbred strains (derived from the F2 intercross of C57BL/6J and DBA/2J inbreds), lines selectively bred from the F2 cross for either drinking or acute withdrawal, F1 crosses derived from the BXD RI strains, standard inbred strains, and WSP vs WSR lines of mice (WSR, selected for low withdrawal HICs after chronic, continuous exposure, drink more than WSP, selected for severe withdrawal HICs; Kosobud & Crabbe, 1988). In each of these populations, drinking data derived from a 10% two-bottle choice paradigm (Phillips et al., 1994; Rodriguez et al., 1995; Metten et al., 1998b; Belknap et al., 1993; Kosobud & Crabbe, 1988) were compared with withdrawal following chronic continuous ethanol vapor exposure (Crabbe, 1998; Crabbe et al., 1983; Metten et al., 1998b) or following a single injection (Metten et al., 1998a,b; Buck et al., 1997; Metten & Crabbe, 1994). The correlation was not significant in every experiment, and its magnitude where present depended on the population and the exact data sets employed to compute it (for discussion of interpretation of genetic correlations from such data, see Crabbe et al., 1990). Most relevant to the current experiment, across 13 standard inbred strains, including many of the ones used here, the correlations of drinking with withdrawal from chronic continuous exposure were r = −0.65 (p = .016) and with acute withdrawal, r = −0.20 (15 strains, NS; see Metten et al, 1998b).
We were, therefore, interested in whether the current chronic, intermittent withdrawal data would negatively correlate with drinking. There were 11 strains in the High Dose group and 9 strains in the Low Dose group for which we had drinking data (Belknap et al., 1993). The relationship was in the predicted direction for both the High and Low Dose groups, although the correlations were small (r = −0.15, NS; r = −0.29, NS, respectively). When scatterplots for the correlations were examined, it became clear that there was one outlier strain in each correlation, C57BR/cdJ. These animals showed very high withdrawal scores in both groups, but abundant data suggests that these mice also show very high preference drinking (Wahlsten et al., 2006), a tendency shared with all other strains of the C57/C58 lineage. That C57BR/cdJ have high withdrawal scores was not an aberration, as they also show relatively high withdrawal after either acute (Figure 5) or chronic continuous ethanol exposure (Figure 4). When we recomputed the correlations without this strain, correlations with drinking improved for both the High and Low Dose data (r = −0.52, p = 0.18; r = −0.51, p = 0.14, respectively). We conclude that the negative genetic relationship between drinking and withdrawal severity extends to chronic intermittent paradigms. This suggests that some genes that lead mice to drinking ethanol solutions in preference to water may also exert a protective effect against severe withdrawal HICs when the same genotypes are exposed to ethanol, regardless of the exposure paradigm.
ACKNOWLEDGMENTS
Supported by NIAAA Grants AA10760, AA12714, AA06243, AA13519, and a Merit Review Grant from the Department of Veterans Affairs. Thanks to: Amanda Barkley-Levenson, Lauren Lyon Brown, John Harkness, Alex M. Henry, Katie Mordarski, Scott Philibin for expert technical assistance, and to Stephanie E. Spence for checking the references.
Reference List
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Becker HC. Positive relationship between the number of prior ethanol withdrawal episodes and the severity of subsequent withdrawal seizures. Psychopharmacology. 1994;116:26–32. doi: 10.1007/BF02244867. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Hale RL. Exacerbation of ethanol withdrawal seizures in mice with a history of multiple withdrawal experience. Pharmacology Biochemistry and Behavior. 1997a;57(1/2):179–183. doi: 10.1016/s0091-3057(96)00303-6. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Weathersby RT. Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol. 1997b;14(4):319–326. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: An animal model of alcohol withdrawal “kindling.”. Alcohol Clin.Exp.Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin.Exp.Res. 2004;28(12):1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Becker HC, Veatch LM. Effects of Lorazepam treatment for multiple ethanol withdrawals in mice. Alcohol Clin.Exp.Res. 2002;26(3):371–380. [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Metten P, Beckley EH, Crabbe JC. Multivariate analyses reveal common and drug-specific genetic influences on responses to four drugs of abuse. Trends Pharmacol. Sci. 2008;29(11):537–543. doi: 10.1016/j.tips.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA, Vandenbergh DJ, Jefferson AL, Chatlos CD, Vogler GP, McClearn GE. Effects of periadolescent ethanol exposure on alcohol preference in two BALB substrains. Alcohol. 2004;34(2-3):177–185. doi: 10.1016/j.alcohol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Booth BM, Blow FC. The kindling hypothesis: Further evidence from a U. S. national study of alcoholic men. Alcohol & Alcoholism. 1993;28(5):593–598. [PubMed] [Google Scholar]
- Buck KJ, Metten P, Belknap JK, Crabbe JC. Quantitative trait loci involved in genetic predisposition to acute alcohol withdrawal in mice. J Neurosci. 1997;17(10):3946–3955. doi: 10.1523/JNEUROSCI.17-10-03946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci. Biobehav. Rev. 2002;26:907–923. doi: 10.1016/s0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Provisional mapping of quantitative trait loci for chronic ethanol withdrawal severity in BXD recombinant inbred mice. J Pharmacol Exp Ther. 1998;286:263–271. [PubMed] [Google Scholar]
- Crabbe JC, Cotnam CJ, Cameron AJ, Schlumbohm JP, Rhodes JS, Metten P, Wahlsten D. Strain differences in three measures of ethanol intoxication in mice, the screen, dowel and grip strength tests. Genes, Brain and Behavior. 2003;2:201–213. doi: 10.1034/j.1601-183x.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284(5420):1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Janowsky JS, Young ER, Rigter H. Handling induced convulsions in twenty inbred strains of mice. Substance and Alcohol Actions/Misuse. 1980;1:159–163. [PubMed] [Google Scholar]
- Crabbe JC, Merrill CD, Belknap JK. Acute dependence on depressant drugs is determined by common genes in mice. Journal of Pharmacology and Experimental Therapeutics. 1991;257(2):663–667. [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcoholism: Clin. Exp. Res. 1990;14(2):141–51. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Young ER, Kosobud A. Genetic correlations with ethanol withdrawal severity. Pharmacology Biochemistry & Behavior. 1983;18(Suppl.1):541–547. doi: 10.1016/0091-3057(83)90233-2. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Finn DA, Oberbeck DL, Yoneyama N, Snelling CC, Wu W, Hitzemann RJ. Electrolytic lesions of the medial nucleus accumbens shell selectively decrease ethanol consumption without altering preference in a limited access procedure in C57BL/6J mice. Pharmacol. Biochem. Behav. 2009;92(2):335–342. doi: 10.1016/j.pbb.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcoholism: Clinical and Experimental Research. 2007;31(6):939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Friedman HJ. Assessment of physical dependence on and withdrawal from ethanol in animals. In: Rigter H, Crabbe JC, editors. Alcohol Tolerance and Dependence. Elsevier/North-Holland Biomedical Press; Amsterdam: 1980. pp. 93–121. [Google Scholar]
- Goldstein DB. Relationship of alcohol dose to intensity of withdrawal signs in mice. J.Pharmacol.Exp.Ther. 1972;180:203–215. [PubMed] [Google Scholar]
- Goldstein DB. Inherited differences in intensity of alcohol withdrawal reactions in mice. Nature. 1973;245:154–156. doi: 10.1038/245154a0. [DOI] [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin. Exp. Res. 2009a;33(11):1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl.) 2009b;201(4):569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegmann JP, Possidente B. Estimating genetic correlations from inbred strains. Behav.Genet. 1981;11:103–114. doi: 10.1007/BF01065621. [DOI] [PubMed] [Google Scholar]
- Karanian J, Yergey J, Lister R, D’Souza N, Linniola M, Salem N., Jr. Characterization of an automated apparatus for precise control of inhalation chamber ethanol vapor and blood ethanol concentrations. Alcoholism: Clinical and Experimental Research. 1986;10(4):443–447. doi: 10.1111/j.1530-0277.1986.tb05121.x. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Wolstenholme J, Shelton KL, Miles MF. Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice. Alcohol. 2006;40(2):119–126. doi: 10.1016/j.alcohol.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosobud A, Bodor AS, Crabbe JC. Voluntary consumption of ethanol in WSP, WSC, and WSR selectively bred mouse lines. Pharmacol. Biochem. Behav. 1988;29:601–607. doi: 10.1016/0091-3057(88)90026-3. [DOI] [PubMed] [Google Scholar]
- Le Bourhis B, Aufrere G. Alcohol exposure pattern and physical dependence. Pharmacol. Biochem. Behav. 1983;18(Suppl. 1):511–514. doi: 10.1016/0091-3057(83)90227-7. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl.) 2005;181(4):688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- McQuarrie DG, Fingl E. Effects of single doses and chronic administration of ethanol on experimental seizures in mice. J Pharmacol.Exp.Ther. 1958;124:264–271. [PubMed] [Google Scholar]
- Metten P, Belknap JK, Crabbe JC. Drug withdrawal convulsions and susceptibility to convulsants after short-term selective breeding for acute ethanol withdrawal. Behav Brain Res. 1998a;95:113–122. doi: 10.1016/s0166-4328(97)00216-7. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Common genetic determinants of severity of acute withdrawal from ethanol, pentobarbital and diazepam in inbred mice. Behavioural Pharmacology. 1994;5:533–547. doi: 10.1097/00008877-199408000-00014. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behavioural Neuroscience. 2005;119(4):911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Metten P, Buck KJ, Merrill CM, Roberts AJ, Yu C-H, Crabbe JC. Use of a novel mouse genotype to model acute benzodiazepine withdrawal. Behav Genet. 2007;37(1):160–170. doi: 10.1007/s10519-006-9094-3. [DOI] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998b;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin. Exp. Res. 2004;28(11):1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol Clin. Exp. Res. 1994;18:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu C-H, Brown LL, Finn DA, Garland T, Jr., Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes, Brain and Behavior. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez LA, Plomin R, Blizard DA, Jones BC, McClearn GE. Alcohol acceptance, preference, and sensitivity in mice. II. Quantitative trait loci analysis using BXD recombinant inbred strains. Alcohol Clin. Exp. Res. 1995;19:367–373. doi: 10.1111/j.1530-0277.1995.tb01517.x. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Crabbe JC. Genetic analysis of rapid tolerance to ethanol’s incoordinating effects in mice: Inbred strains and artificial selection. Behavior Genetics. 2004;34(4):441–451. doi: 10.1023/B:BEGE.0000023649.60539.dd. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Hölter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol. 1999;34(2):231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Terdal ES, Crabbe JC. Indexing withdrawal in mice: matching genotypes for exposure in studies using ethanol vapor inhalation. Alcohol Clin.Exp.Res. 1994;18:542–547. doi: 10.1111/j.1530-0277.1994.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Veatch LM, Wright TM, Randall CL. Only male mice show sensitization of handling-induced convulsions across repeated ethanol withdrawal cycles. Alcohol Clin.Exp.Res. 2007;31(3):477–485. doi: 10.1111/j.1530-0277.2006.00328.x. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc. Natl. Acad. Sci., U.S.A. 2006;103(44):16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Hendricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003;54(1):283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]