Abstract
Background
Emerging evidence suggests that taurine acts as a partial agonist at glycine receptors (GlyR) in vitro and in vivo. Ethanol acts as an allosteric modulator at the GlyR producing a leftward shift of the glycine concentration-response curve, with no enhancing effects observed at saturating glycine concentrations. However, to date no electrophysiological studies have been performed on ethanol modulation of taurine-activated GlyR.
Methods
Wildtype α1 GlyR, or those bearing a serine-267 to isoleucine replacement (S267I), were homomerically expressed in Xenopus oocytes and voltage-clamped at 70 mV. Ethanol was co-applied with varying concentrations of glycine or taurine and the enhancing effects of ethanol compared.
Results
Ethanol potentiated glycine- and taurine-activated GlyR responses in a concentration-dependent manner. It shifted taurine and glycine concentration-response curves to the left, having no effects at saturating agonist concentrations. Chelation of zinc by tricine decreased ethanol enhancement of taurine-gated GlyR function. The S267I mutation prevented ethanol enhancement of taurine-mediated responses as previously also reported for glycine.
Conclusion
Ethanol modulates taurine activation of GlyR function by a mechanism similar to that of the full agonist glycine. The lack of effect of ethanol at saturating taurine concentrations provides mechanistic information on alcohol actions at the GlyR.
Keywords: ethanol, glycine receptor, taurine, partial agonist, oocyte electrophysiology
INTRODUCTION
Many small amino acids such as glycine, taurine and β-alanine act as agonists at the glycine receptor (GlyR) and are found throughout the central nervous system (Shibanoki et al., 1993; Lynch, 2004; Albrecht and Schousboe, 2005). Although glycine is often assumed to be the endogenous ligand activating the GlyR, evidence exists for taurine also functioning as a neurotransmitter in many brain regions. For example, an amino acid uptake inhibitor for taurine increases the strychnine-sensitive current in CA3 pyramidal cells in hippocampal slice preparations (Mori et al, 2002), suggesting that taurine may regulate inhibitory tone by affecting tonic GlyR activity. Taurine has also been detected in the nucleus accumbens (nAcc) where it is hypothesized to act as the primary endogenous ligand for the GlyR (Ericson et al., 2006). In the rat hippocampus extracellular taurine is found at basal concentrations about the same as those of glycine, and NMDA receptor activation increases that 3–5 fold (Shibanoki et al., 1993).
Unlike the full agonist glycine, taurine and β-alanine act as partial agonists at the GlyR. Taurine has approximately half the efficacy of glycine on homomeric α1 GlyR expressed in Xenopus oocytes and less than 10% of the efficacy of glycine on homomeric α2 GlyR (Schmeiden et al, 1992). A recent study of GlyR kinetics by Lape et al. (2008) showed that the intra-cluster open probability (Po) of channels exposed to a saturating concentration of taurine was 54%, compared to 96% for glycine. By fitting single channel recordings obtained from α1β GlyRs in the presence of taurine or glycine the authors showed that the significant differences in Po between the two were not due to differences in the rates of the final opening and closing transitions but rather in the rates that governed transitions to and from a pre-open “flipped” state. Hence, the full agonist glycine appears to bind with much greater affinity to the flipped state compared to the resting state than does the partial agonist taurine.
Alcohols and volatile anesthetics act as allosteric modulators in enhancing glycine activation of the GlyR (Mascia et al., 1996a,b). They leftshift glycine concentration-response curves, but display no enhancing effects at saturating glycine concentrations. The mechanisms by which ethanol (EtOH) exerts these effects have recently been determined. Ethanol increases decay times of the glycinergic currents without affecting conductance (Eggers and Berger, 2004). Using a kinetic model to simulate GlyR whole-cell currents, Eggers and Berger (2004) concluded that ethanol probably acts either to decrease the dissociation (koff) or to increase the association (kon) of glycine. Recently we showed that ethanol has no effects on conductance and minimally affects open or closed dwell times and likelihoods (Welsh et al., 2009). Instead it enhances GlyR function predominantly by lengthening the durations of bursts. In the present report we examined ethanol enhancement of GlyR function, comparing receptor activation by glycine with that produced by taurine. We also hypothesized that ethanol should not affect the magnitudes of currents elicited by maximally-effective taurine concentrations if the primary actions of ethanol are mediated through its lengthening of burst durations.
MATERIALS AND METHODS
Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Xenopus laevis were obtained from Xenopus Express (Homosassa, FL).
Oocyte isolation and cDNA nuclear injection
Ooctyes were surgically removed from Xenopus laevis housed at 19C on a 12hr light/dark cycle. Stage V and VI oocytes were selected and placed in isolation media containing 108 mM NaCl, 1 mM EDTA, 2 mM KCl, and 10 mM HEPES. Forceps were used to manually remove the thecal and epithelial layers. The oocyte follicular layer was removed using a 10 minute exposure to 0.5 mg/ml Sigma type 1A collagenase in buffer containing 83 mM NaCl, 2 mM MgCl2, and 5 mM HEPES. Injection of a 30 nl sample of the glycine α1 receptor subunit cDNA (1.5 ng/30 nl) in a modified pBK-CMV vector (Mihic et al., 1997) was made into the animal poles of oocytes by the “blind” method of Colman (1984), using a digital micropipette (10 – 15 μm tip size) attached to a microdispenser. Oocytes were stored in the dark at 19C in 96-well plates containing Modified Barth’s Saline (MBS) [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 10 mM HEPES, 0.82 mM MgSO4·7H2O, 0.33 mM Ca(NO3)2, 0.91 mM CaCl2 at pH 7.5] plus 2 mM sodium pyruvate, 0.5 mM theophylline, 10 U/ml penicillin, 10 mg/l streptomycin, and 50 mg/l gentamycin, sterilized by passage through a 0.22 μm filter. Oocytes expressed GlyR within 24 hours and all electrophysiological measurements were made within 5 days of cDNA injection.
Oocyte electrophysiological recording
Two high-resistance (0.5–10 MΩ) glass electrodes filled with 3 M KCl were used to impale the animal poles of oocytes. Cells were voltage-clamped at −70 mV using a Warner Instruments OC-725C oocyte-clamp (Hamden, CT) and MBS or MBS + 2.5 mM tricine was perfused over them at a rate of 2 ml/min using a Masterflex USA peristaltic pump (Cole Parmer Instrument Co, Vernon Hills, IL) through 18-gauge polyethylene tubing. All drug solutions were prepared in either MBS or MBS + 2.5 mM tricine. Drug applications (5–90 sec) were followed by 6–15 minute washout periods as appropriate. Currents were recorded on a strip-chart recorder (Cole Parmer Co.) with a <0.5 s full-scale pen movement response time and, unless stated otherwise, peak currents were measured and used in data analysis. Currents produced in the presence of agonist plus ethanol were compared to currents generated by agonist alone.
Statistics
Experimental values are listed as the mean ± standard error. The equation (eq. 1): y = min + (max − min)/(1+(x/EC50)−Hillslope) was used to fit agonist concentration-response curves in order to determine the half-maximally-effective agonist concentrations (EC50) for each oocyte. EC50 values were log-transformed for statistical comparisons. Paired t-tests or two-way ANOVAs with Tukey’s posthoc comparisons were performed using SigmaPlot ver. 11.0 (Systat Software, San Jose, CA).
RESULTS
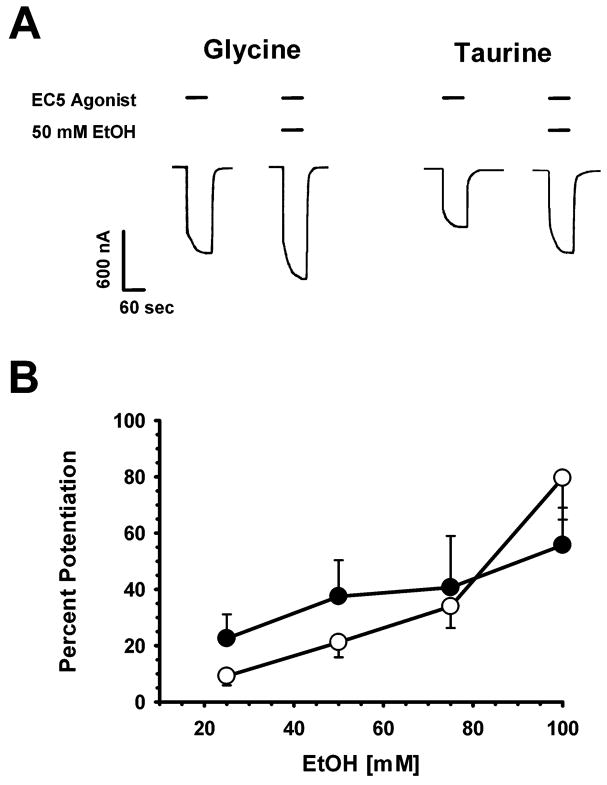
We compared the enhancing effects of ethanol on glycine- and taurine-induced currents elicited by wildtype (WT) α1 homomeric glycine receptors. In the first experiment, 25, 50, 75 or 100 mM ethanol was co-applied with a concentration of agonist producing between 5 and 10 percent of a maximally-effective agonist response (EC5). For taurine the EC5 concentration used was 5% of the effect of maximal taurine, while EC5 glycine was calculated as that same fraction of the maximal effect of glycine. Sample tracings of currents elicited by EC5 glycine (56.7 ± 4.2 μM) or taurine (376.2 ± 52.8 μM) in the absence and presence of 50 mM ethanol are shown in Fig. 1A. Ethanol was able to enhance both glycine- and taurine-mediated currents, in a concentration-dependent manner (Fig. 1B). Statistical analyses using a two-way ANOVA showed there was a significant effect of ethanol concentration [F(3,71) =4.54, p < 0.006), but no significant difference in the degree of ethanol enhancement of responses induced by glycine versus taurine [F(1,71) = 0.09, p > 0.76], and no interaction between the two factors [F(3,71) = 0.8, p > 0.49].
Figure 1.
Ethanol enhances glycine- and taurine-activated α1 GlyR in a concentration-dependent manner. A. Sample tracings of ethanol potentiation of glycine and taurine responses. Each pair of traces shows EC5 agonist plus EC5 agonist co-applied with 50 mM EtOH applied for 60 sec. The left pair of tracings show glycine activation of GlyR while taurine responses are shown on the right. Horizontal bars over tracings indicate the time of glycine or taurine ± EtOH exposure. B. Concentration-dependent potentiation of glycine and taurine responses by ethanol. The ordinate represents the percent potentiation of responses in seen the presence of EtOH compared to those produced by agonist applied alone. Open circles depict EtOH enhancement of glycine-activated α1 GlyR (n=6) and filled circles depict EtOH enhancement of EC5 taurine responses (n = 12). Data are shown as mean + standard error of the mean (S.E.M.).
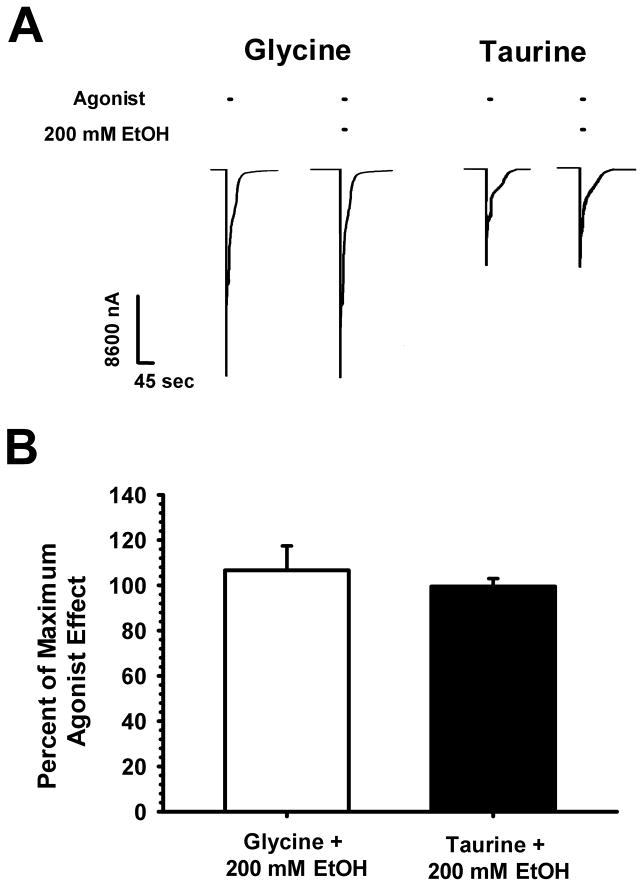
Next we compared the effects of 200 mM ethanol on GlyR responses elicited by saturating concentrations of glycine and taurine. Fig. 2A shows that taurine produced smaller maximal currents than did glycine, in keeping with its classification as a partial agonist. EtOH (200 mM) did not affect peak currents produced by either 10 mM glycine or 100 mM taurine. Average responses are shown in Fig. 2B and the effect of 200 mM EtOH on maximally-effective agonist concentrations did not differ significantly between glycine and taurine [t(10) = 0.63, p > 0.54].
Figure 2.
Ethanol does not affect maximally-effective glycine and taurine responses. A. Sample tracings of saturating concentrations of glycine or taurine with and without 200 mM ethanol. The left pair of tracings show the effects of 10 mM glycine and 10 mM glycine + 200 mM EtOH with the right pair of traces showing 100 mM taurine and 100 mM taurine + 200 mM EtOH effects. Horizontal bars over tracings indicate the time of glycine or taurine ± EtOH exposure. B. Average effects of 200 mM EtOH on glycine and taurine responses. The ordinate depicts the percent current observed in the presence of 200 mM EtOH compared to that produced by glycine (open bar) and taurine (filled). Data are shown as mean + S.E.M. of 6 oocytes.
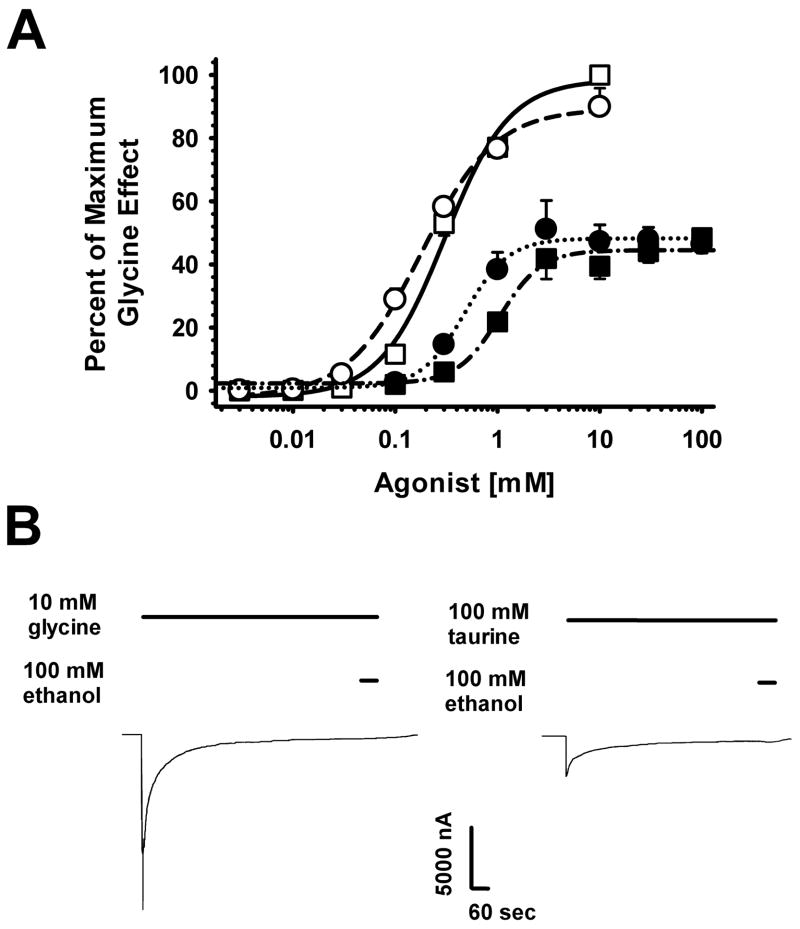
Previous studies demonstrated that ethanol acts as an allosteric modulator by leftshifting glycine concentration-response curves. We examined the effects of EtOH on currents observed in response to application of various concentrations of glycine and taurine, expressing all responses as a percentage of the current elicited by a saturating (10 mM) concentration of glycine (Fig. 3). Glycine activated the α1 GlyR in a concentration-dependent manner with EC50 of 0.31 ± 0.04 mM and a Hill coefficient, nH of 1.47 ± 0.16. Addition of 100 mM ethanol leftshifted the glycine concentration-response curve, with the EC50 decreasing by 37.6 ± 10.1%. The glycine EC50 in the presence of EtOH was 0.19 ± 0.01 mM with a Hill slope of 1.24 ± 0.14. Taurine applied alone (EC50 = 1.20 ± 0.07 mM, nH = 1.40 ± 0.15) was about 4 fold less potent than glycine and possessed 49% of its efficacy. Responses to taurine + 100 mM ethanol were leftshifted from the taurine alone curve, with the EC50 decreasing by 56.3 ± 1.6%. The taurine EC50 in the presence of EtOH was 0.52 ± 0.04 mM with a Hill slope of 1.35 ± 0.34. EtOH significantly decreased agonist EC50s for both glycine [t(6) = 3.61, p < 0.012] and taurine [t(6) = 8.82, p < 0.001]. The percent decrease in agonist EC50 produced by ethanol was not significantly different when the receptor was activated by glycine compared to taurine [t(6) = 1.87, p > 0.11). Ethanol had no effects at saturating glycine or taurine concentrations during short (5 sec) co-applications but this experiment did not test for possible effects on desensitization. When one applies a saturating concentration of agonist for a few minutes, equilibrium is reached between receptors in the open and desensitized states. If ethanol affects desensitization, it should affect the magnitudes of those currents. Fig. 3B shows sample tracings of saturating glycine or taurine concentrations applied for 12–15 min and then ethanol co-applied for 60 sec. No effects of ethanol were observed.
Figure 3.
Ethanol decreases agonist EC50 but does not affect peak currents or desensitization. A. Ethanol shifts glycine and taurine concentration-response curves to the left. Open symbols represent glycine responses and filled symbols show taurine-mediated currents. Squares are responses elicited by agonist applied alone, and circles are agonist + 100 mM EtOH. Drug applications lasted 60 sec and all responses are expressed as percentages of the effects of 10 mM glycine. Lines are logistic fits to each set of data. Data are shown as mean ± S.E.M. of 4–8 oocytes. B. Sample tracings showing the effects of application of maximally-effective concentrations of glycine (10 mM) or taurine (100 mM) to oocytes for 12–15 min, until steady currents were observed. Ethanol applied for 1 min, as indicated by horizontal bars over tracings, did not affect the equilibrium reached between open and desensitized states.
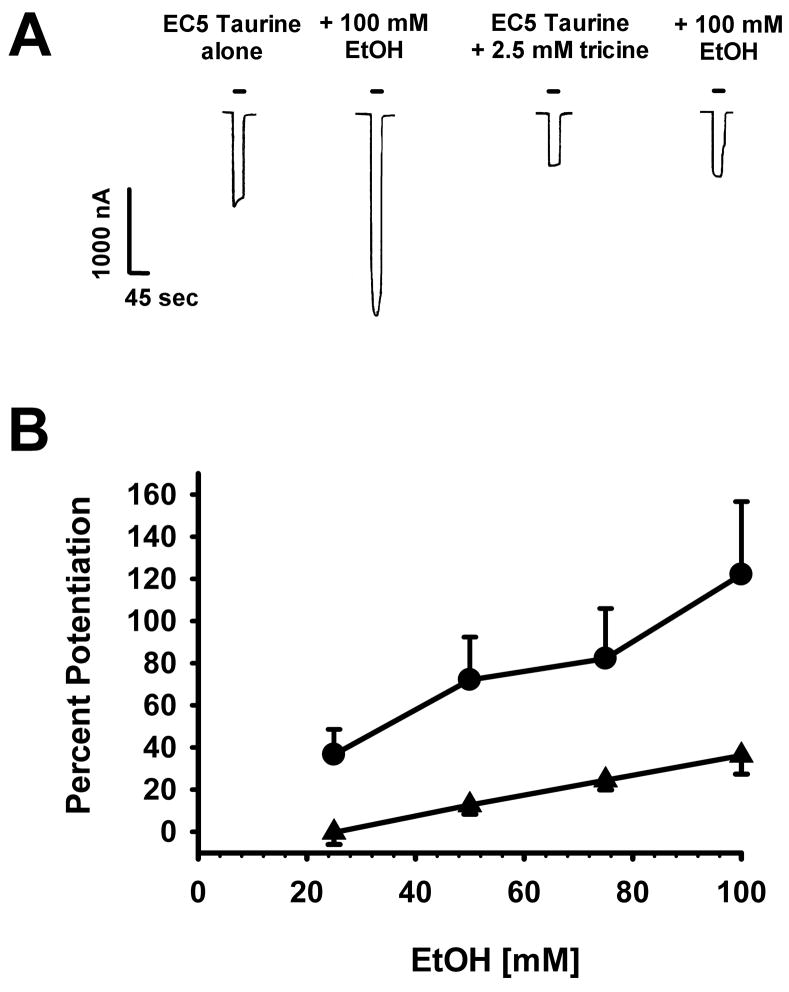
Recently we discovered that the zinc chelator tricine decreases the magnitude of EtOH potentiation of GlyR currents, suggesting that these two allosteric modulators interact in a synergistic fashion to enhance GlyR function (McCracken et al., 2010). We tested if this synergism was also seen when EC5 taurine activated the GlyR (Fig. 4). The filled circles in Fig. 4 represent the same responses to EC5 taurine plus four concentrations of ethanol as those shown in Fig. 1. The filled triangles show responses to the same concentrations of taurine + ethanol but in the presence of 2.5 mM tricine in the bath. A two-way repeated measures ANOVA demonstrates a significantly lower alcohol effect in the presence of tricine [F(1,31) = 10.5, p<0.05].
Figure 4.
The zinc chelator tricine decreases ethanol enhancement of taurine-mediated responses. A. Sample tracings showing currents elicited by EC5 taurine applied with or without 25 – 100 mM EtOH for 60 sec in the absence or presence of 2.5 mM tricine. Horizontal bars over tracings indicate the time of taurine ± EtOH exposure. B. Ethanol concentration is plotted against percent potentiation compared to EC5 taurine applied minus ethanol, with (filled triangles) or without tricine (filled circles). Data are shown as mean ± S.E.M. of 4 oocytes.
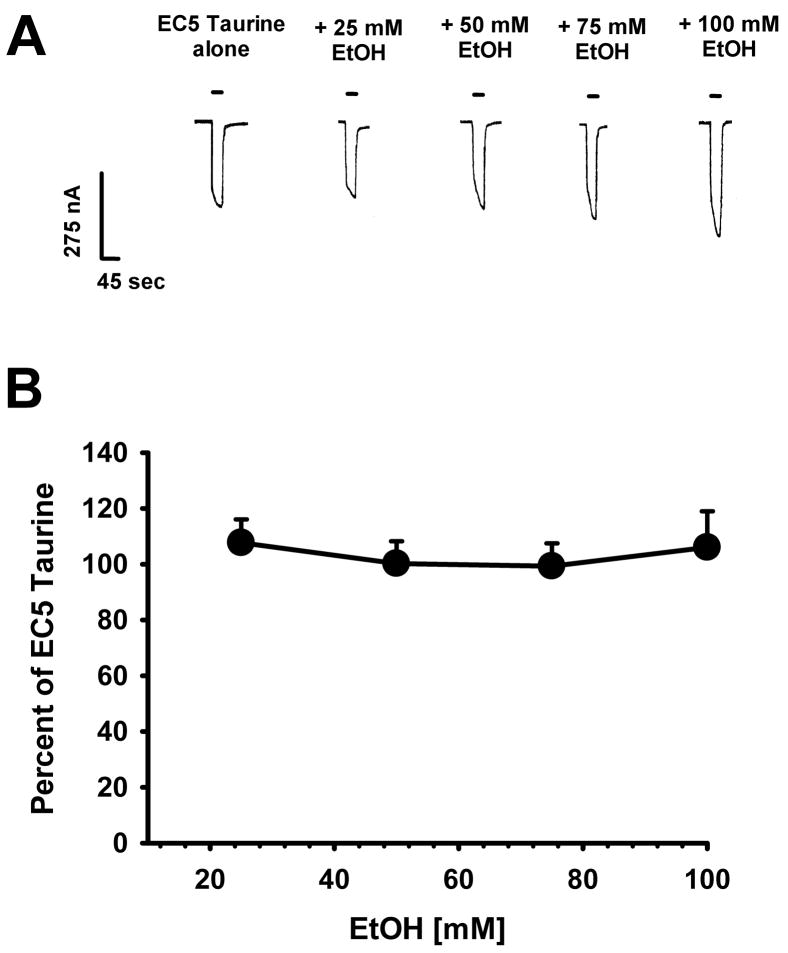
Lastly, we repeated previous experiments looking at ethanol effects on EC5 taurine responses, but this time studying a mutant of the α1 homomeric GlyR in which residue serine-267 was mutated to isoleucine (S267I) (Fig. 5). Previous work showed that the S267I mutant was insensitive to the potentiating actions of EtOH, instead displaying a weak inhibitory effect on glycine-activated S267I α1 GlyR (Mihic et al., 1997). Fig. 5A shows sample traces resulting from EC5 taurine applied alone and in the presence of 25, 50, 75, or 100 mM ethanol. Responses to application of taurine + ethanol on S267I α1 GlyR showed no effect compared to taurine applied alone.
Figure 5.
Ethanol does not enhance taurine-mediated currents in the S267I mutant. A. Sample tracings of the effects of EC5 taurine alone and taurine co-applied with 25, 50, 75 or 100 mM EtOH on current responses in S267I α1 GlyR. Horizontal bars over tracings indicate the time of taurine ± EtOH exposure. B. Ethanol concentration is plotted against the ethanol-induced percent of current produced by EC5 taurine; 100% means no effect of EtOH. Data are shown as mean ± S.E.M. of 7 oocytes.
DISCUSSION
The mesolimbic dopamine pathway of the brain, including a dopaminergic projection from the ventral tegmental area to the nAcc, is thought to mediate the rewarding effects of ethanol (Gonzales et al., 2004). At least some ethanol effects are thought to be due to its enhancement of GlyR function since microdialysis of glycine into the nAcc increases extracellular accumbal dopamine levels, and decreases alcohol consumption by alcohol-preferring Wistar rats (Molander et al., 2005). Recently it was suggested that taurine rather than glycine may be the endogenous ligand acting on accumbal GlyR, as taurine perfusion significantly increases dopamine release (Ericson et al., 2006). Since no studies had been performed on ethanol modulation of taurine-activated GlyRs, we compared the actions of ethanol onα1 homomeric GlyR activated by taurine with those activated by glycine.
Ethanol enhances GlyR currents elicited by low concentrations of either glycine or taurine, with EtOH concentrations in the 25 – 100 mM range producing similar enhancement of the effects of each agonist. Previous work demonstrated that ethanol and octanol do not potentiate currents elicited by maximally-effective glycine concentrations (Mascia et al., 1996a,b), in agreement with our present findings. Because taurine possesses lower efficacy at the α1 GlyR, we questioned whether ethanol-induced enhancement might be observed at saturating concentrations of this partial agonist. Since the Po of a saturating concentration of glycine is above 90% one could hypothesize that the lack of alcohol enhancement of maximally-effective glycine concentrations was due to a ceiling effect in Po, assuming EtOH affected Po. However, no such ceiling effect would be expected with taurine which has a Po of about 50%. However, our data show that ethanol does not enhance GlyR function in the presence of saturating concentrations of taurine. These findings suggest that ethanol enhances taurine-gated GlyR currents by a mechanism similar to its potentiation of glycine-activated GlyRs. In an earlier single channel study, we showed that ethanol enhances the effects of low concentrations of glycine by increasing burst durations and not by affecting intraburst Po or conductance (Welsh et al., 2009). Ethanol retards the rate of glycine unbinding without affecting open channel lifetimes. Our whole-cell data in the present manuscript support this mechanism for ethanol modulation of the GlyR but extend it to higher glycine concentrations where agonist unbinding is no longer a factor and where enhancement of currents is no longer seen. The same is true for saturating concentrations of taurine, suggesting that ethanol affects the taurine-activated GlyR in a similar manner.
When one applies maximal concentrations of an agonist, individual GlyR activations occur in clusters that are bounded by agonist binding and channel opening at the beginning of the cluster and desensitization at cluster termination. The use of maximal concentrations of agonist means the receptor spends almost all of it’s non-desensitized time in the fully-liganded state; thus, even if ethanol affects glycine unbinding, which we’ve shown it does (Welsh et al., 2009), still no EtOH effect would be seen because of the high agonist association rate seen in the presence of saturating agonist concentrations. Thus, at saturating agonist concentrations ethanol could conceivably increase currents only by: (1) increasing conductance; (2) increasing intra-cluster Po, or (3) antagonizing desensitization, leading to longer clusters. In the tracings shown in Fig. 3b, we exposed oocytes to saturating concentrations of glycine or taurine and observed large currents that quickly desensitized. After several minutes of agonist exposure channels would be in equilibrium, cycling between open and desensitized states. At this time 100 mM ethanol was co-applied with glycine or taurine, but no effects were seen. This means that ethanol was not antagonizing desensitization since that would increase average cluster length and thus produce an increased whole-cell current. Since taurine has a Po considerably lower than that of glycine, there could have also been a possibility of increasing the intra-cluster Po, but that also didn’t happen.
In a recent paper, Lape et al. (2008) proposed that the efficacy disparity between glycine and taurine was due to differences in the abilities of agonists to transition the receptor to an intermediate ‘flipped’ state before opening the channel. Since EtOH does not increase the efficacy of saturating taurine or glycine concentrations, this suggests that alcohol doesn’t favor transitioning of the agonist-gated receptor from closed to flipped states. Interestingly, this may be somewhat different from the mechanisms of alcohol modulation of a partial agonist on the related 5-HT3 receptor; 100 mM ethanol significantly increases the response to a saturating concentration of dopamine by more than 50% (Lovinger et al., 2000), leading the authors to conclude that ethanol potentiates 5-HT3 receptor function at least in part by increasing Po. However they could not exclude the possibility that ethanol also had effects on agonist affinity that were not measurable using their experimental paradigm.
Zinc, another allosteric modulator of the GlyR, may be an important factor to consider when studying GlyR function. Zinc is found throughout the CNS, often co-localized with the GlyR and synaptically released in areas such as the hippocampus and spinal cord (Vogt et al., 2000; Schrøder et al., 2000). At low concentrations zinc enhances the effects of both glycine and taurine on the α1 GlyR (Miller et al. 2005; Miller et al. 2008). Single channel studies of zinc enhancement of the GlyR determined that zinc’s main effect was to increase mean burst durations and kinetic modeling suggested that this was due to a decrease in the unbinding rate of glycine (Laube et al., 2000). However, zinc may potentiate taurine-activated GlyRs through a different mechanism because Zn2+ increases taurine, but not glycine, efficacy. It seems that, in addition to zinc antagonizing taurine dissociation, it also increased transition rates from closed to open channel states, thereby promoting gating (Laube et al., 2000). The investigators concluded that this constituted some evidence for a separate gating pathway for taurine.
In this study we used tricine to chelate zinc and showed a decrease in the ability of ethanol to modulate the taurine-activated receptor. There may thus be an interaction between the zinc signal and the ethanol signal during channel activation by taurine, similar to the results obtained by McCracken et al. (2010) who showed that tricine significantly reduced the degree of ethanol potentiation of glycine-activated GlyR. It may be that ethanol and zinc act in concert to increase agonist affinity and that this conclusion holds true for taurine as well. In comparing alcohol effects on glycine- versus taurine-activated α1 GlyR we also investigated a single amino acid mutation that reduces ethanol potentiation of glycine-activated currents to determine if similar effects were observed using a partial agonist. The S267 residue is proposed to play a role in the binding of alcohol and volatile anesthetics, composing part of a binding pocket for these drugs (Mihic et al., 1997). The size of the residue at position 267 inversely correlates with the degree of ethanol potentiation, with the S267I mutation eliminating the enhancing effects of ethanol (Ye et al., 1998). When the S267I mutant was tested using EC5 taurine as the agonist, no ethanol enhancement was observed. These data suggest that any differences that occur in the gating pathway between agonist binding and channel opening for glycine versus taurine converge by the time the gating signal approaches the alcohol binding pocket between transmembrane segments two and three. This is in agreement with a study showing that taurine and glycine produce similar conformational changes in this area (Han et al., 2004).
In conclusion, we compared ethanol enhancement of GlyR activated by the full agonist glycine with activation produced by the partial agonist taurine. In both cases EtOH shifted agonist concentration-response curves to the left and had no effects at saturating agonist concentrations. This is consistent with the conclusions of our previous single channel studies in which we reported that alcohol does not appear to act by increasing Po and thereby stabilizing the open state of the GlyR. In addition, EtOH enhancement of taurine function was decreased in the presence of the zinc chelator tricine and absent in the S267I mutant, which we previously also reported for glycine. These data suggest that EtOH enhances the effects of taurine by mechanisms very similar to those of glycine. Our findings illustrate that taurine activation of the GlyR can be enhanced by pharmacologically-relevant EtOH concentrations and are timely given an increasing awareness of possible taurine effects on GlyR in vivo.
Acknowledgments
Sources of support: Supported by NIAAA/NIH grants R01AA11525 (to S.J.M) and F31AA017802 (to B.T.W.)
References
- Albrecht J, Schousboe A. Taurine interaction with neurotransmitter receptors in the CNS: An update. Neurochem Res. 2005;30:1615–1621. doi: 10.1007/s11064-005-8986-6. [DOI] [PubMed] [Google Scholar]
- Colman A. Expression of exogenous DNA in Xenopus oocytes. In: Hames BD, Higgins SJ, editors. Transcription and Translation: A Practical Approach. Oxford Press; Washington, DC: 1984. pp. 49–69. [Google Scholar]
- Eggers ED, Berger AJ. Mechanisms for the modulation of native glycine receptor channels by ethanol. J Neurophysiol. 2004;91:2685–2695. doi: 10.1152/jn.00907.2003. [DOI] [PubMed] [Google Scholar]
- Ericson M, Molander A, Stomberg R, Soderpalm B. Taurine elevates dopamine levels in the rat nucleus accumbens: antagonism by strychnine. Eur J Pharmacol. 2006;23:3225–3229. doi: 10.1111/j.1460-9568.2006.04868.x. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Han NR, Clements JD, Lynch JW. Comparison of taurine- and glycine-induced conformational changes in the M2–M3 domain of the glycine receptor. J Biol Chem. 2004;279:19559–19565. doi: 10.1074/jbc.M400548200. [DOI] [PubMed] [Google Scholar]
- Lape R, Colquhoun D, Sivilotti LG. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 2008;454:722–728. doi: 10.1038/nature07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Kinetic and mutational analysis of Zn2+ modulation of recombinant human inhibitory glycine receptors. J Physiol. 2000;522:215–230. doi: 10.1111/j.1469-7793.2000.t01-1-00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Sung KW, Zhou Q. Ethanol and trichloroethanol alter gating of 5-HT3 receptor-channels in NCB-20 neuroblastoma cells. Neuropharmacology. 2000;39:561–570. doi: 10.1016/s0028-3908(99)00164-1. [DOI] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Mascia MP, Mihic SJ, Valenzuela CF, Schofield PR, Harris RA. A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol. 1996a;50:402–406. [PubMed] [Google Scholar]
- Mascia MP, Machu TK, Harris RA. Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br J Pharmacol. 1996b;119:1331–1336. doi: 10.1111/j.1476-5381.1996.tb16042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Trudell JR, Goldstein BE, Harris RA, Mihic SJ. Zinc enhances ethanol modulation of the alpha1 glycine receptor. Neuropharmacol. 2010;58:676–681. doi: 10.1016/j.neuropharm.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Miller PS, Da Silva HMA, Smart TG. Molecular basis for zinc potentiation at strychnine-sensitive glycine receptors. J Biol Chem. 2005;280:37877–37884. doi: 10.1074/jbc.M508303200. [DOI] [PubMed] [Google Scholar]
- Miller PS, Topf M, Smart TG. Mapping a molecular link between allosteric inhibition and activation of the glycine receptor. Nat Struct Mol Biol. 2008;15:1084–1093. doi: 10.1038/nsmb.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander A, Löf E, Stomberg R, Ericson M, Söderpalm B. Involvement of accumbal glycine receptors in the regulation of voluntary ethanol intake in the rat. Alcohol Clin Exp Res. 2005;29:38–45. doi: 10.1097/01.alc.0000150009.78622.e0. [DOI] [PubMed] [Google Scholar]
- Mori M, Gahwiler BH, Gerber U. β-alanine and taurine as endogenous agonists at glycine receptors in the rat hippocampus in vitro. J Physiol. 2002;539:191–200. doi: 10.1113/jphysiol.2001.013147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieden V, Kuhse J, Betz H. Agonist pharmacology of neonatal and adult glycine receptor α subunits: Identification of amino acid residues involved in taurine activation. EMBO J. 1992;11:2025–2032. doi: 10.1002/j.1460-2075.1992.tb05259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrøder HD, Danscher G, Jo SM, Su H. Zinc-enriched boutons in rat spinal cord. Brain Res. 2000;868:119–122. doi: 10.1016/s0006-8993(00)02238-1. [DOI] [PubMed] [Google Scholar]
- Shibinoki S, Kogure M, Sugahara M, Ishikawa K. Effect of systemic administration of N-methyl-D-Aspartic acid on extracellular taurine level measured by microdialysis in the hippocampal CA1 field and striatum of rats. J Neurochem. 1993;61:1698–1704. doi: 10.1111/j.1471-4159.1993.tb09806.x. [DOI] [PubMed] [Google Scholar]
- Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- Welsh BT, Goldstein BE, Mihic SJ. Single channel analysis of ethanol enhancement of glycine receptor function. J Pharmacol Exp Ther. 2009;330:198–205. doi: 10.1124/jpet.109.154344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Koltchine VV, Mihic SJ, Mascia MP, Wick MJ, Finn SE, Harrison NL, Harris RA. Enhancement of glycine receptor function by ethanol is inversely correlated with molecular volume at position α267. J Biol Chem. 1998;273:3314–3319. doi: 10.1074/jbc.273.6.3314. [DOI] [PubMed] [Google Scholar]