Abstract
Background
The serotonin transporter gene-linked polymorphic region (5-HTTLPR) has been proposed as a predictor of antidepressant response. Insertion or deletion of a 44bp long region gives rise to short 'S' and long 'L' forms of the promoter region, the 'S' form being associated with reduced serotonin transporter expression.
Methods
A systematic review and meta-analysis was performed to clarify the effect of 5-HTTLPR on antidepressant response and remission rates. Data were obtained from 28 studies with 5408 participants. Three genotype comparisons were tested - SS versus (SL or LL), (SS or SL) versus LL, and SS versus LL.
Results
There was no statistically significant effect on antidepressant response. Compared to L carriers, there was an apparent effect of the SS genotype on remission rate (RR 0.88; 95% CI 0.79 to 0.98; p=0.02). However, after trim and fill correction for missing data, the effect disappeared (RR 0.92; 95% CI 0.81 to 1.05; p=0.23) indicating that the initial significant effect was likely the result of publication bias. No significant effect on remission rate was seen for SS versus LL and SS/SL versus LL. Substantial unexplained heterogeneity of effect sizes was observed between studies, pointing to additional interacting factors contributing to an association in some cases.
Conclusions
The 5-HTTLPR biallelic short/long polymorphism by itself does not appear to usefully predict antidepressant response.
Keywords: depression, meta-analysis, 5-HTTLPR, polymorphism, treatment response, antidepressant
Introduction
Selective serotonin reuptake inhibitors (SSRI) are widely prescribed first line treatments for major depressive disorder (MDD) and act by blocking the reuptake of serotonin (5-HT) from the synaptic cleft. Despite evidence of a possible early onset of action (1), recent data show that only a third of patients respond to treatment with citalopram (2). Therefore it is tantalizing to speculate that it may be possible to a priori predict patients who are more likely to respond to an SSRI or antidepressants in general. This has been the premise of the concept of personalized medicine in the treatment of MDD (3) and polymorphisms within the serotonin transporter are an attractive candidate.
A serotonin transporter linked polymorphic region polymorphism in the promoter region of the serotonin transporter (5-HTTLPR) (4) has aroused much interest over recent years and is arguably the most well studied genetic polymorphism in clinical neuroscience. Insertion or deletion of a 44bp long region gives rise to short 'S' and long 'L' forms of the promoter region, the 'S' form being associated with lower levels of transporter expression (5). This biallelic polymorphism is proposed to modulate a number of factors including vulnerability to depression (6), some anxiety-related personality measures (7), and putative imaging phenotypes (8), although more recent data questions this link with depression vulnerability (9). As the serotonin transporter is the site of action of SSRIs, it is certainly plausible that differing levels of this protein might affect their efficacy, whether directly or via adaptive changes in other aspects of serotonergic function (10).
The first report of an effect of 5-HTTLPR genotype and antidepressant response came in the landmark study of Smeraldi and colleagues in 1998 when they found that individuals with the SS genotype (who would therefore have lower levels of transporter expression) were less likely to respond to treatment with the SSRI antidepressant, fluvoxamine (11). Many subsequent studies investigated this effect; some replicated an association of genotype with response while others did not. When data from 15 studies were combined in a meta-analysis, a highly significant association of SS with worse remission rate (<0.0001) and SS and SL with worse response rate (p 0.0002) was found (12). These data supported the potential of 5-HTTLPR genotype as a predictor of antidepressant response. If this finding were to hold true it could represent a landmark advance in our understanding of the illness and result in improved prediction of response to SSRIs.
However, in the short period since that analysis, many further studies have reported, resulting in a threefold increase over the originally reported dataset. The one study that stands out amongst the other recent studies is the STAR*D study, the single largest study of this type which in itself included data from more participants than the entire previous meta-analysis. Intriguingly, in contradistinction to the previous reports the STAR*D study did not find any effect of the 5-HTTLPR on treatment outcome phenotypes, although there was an effect on adverse event rates (13).
It is therefore appropriate to evaluate the utility of polymorphisms in the 5-HTTLPR as a biomarker or clinical predictor of outcome to antidepressant treatment. We systematically assessed this crucial issue by performing a meta-analysis of all currently available data including both published manuscripts and searching for unpublished data.
Methods and Materials
Trials in which patients with major depressive disorder received antidepressant medication and outcomes were reported by 5-HTTLPR polymorphism status were sought. Electronic databases were searched (EMBASE, MEDLINE, PsycINFO) to from 1996 to July 2009 Week 1 with search terms (5HT OR 5-HT OR 5HTT OR SERT OR transporter OR LPR OR serotonin OR SLC6A4) AND (VNTR OR variant OR polymorphism OR allele OR “tandem repeat$”) AND (antidepressant OR SSRI or SRI or “reuptake inhibitor” OR paroxetine OR citalopram OR fluoxetine OR fluvoxamine OR sertraline OR escitalopram OR mirtazapine OR nefazodone OR trazodone OR TCA OR tricyclic OR reboxetine OR nortriptyline OR lofepramine OR imipramine OR amitriptiline OR venlafaxine OR duloxetine OR dosulepin OR dothiepin OR MAOI OR RIMA OR moclobemide OR phenelzine OR tranylcyp$ OR mianserin OR milnacipran OR bupropion OR tianeptine OR agomelatin$ OR clomipramine OR doxepin OR trimipramine OR desipramine OR selegiline OR gepirone). Studies were also identified from reference lists of identified studies, earlier reviews, and personal reference collections.
Statistical analyses were performed using R version 2.5 (14) with the meta package (15) Relative risk (RR) treatment estimates were calculated for both fixed effects (Mantel-Haenszel) and random effects models (DerSimonian-Laird). Random effects estimates are reported in the text, although both are given in figures and no qualitative differences in estimates of effect were observed. There is a lack of certainty about dominance of effects of the 'S' and 'L' alleles and various studies use different methods and rationales to group these alleles. In order to study the effect of the S and L alleles in a systematic and unbiased way and to minimize the chance of a type II error three comparisons were tested - (a) SS versus (SL or LL), (b) (SS or SL) versus LL, and (c) SS versus LL. Effects on both antidepressant 'response' and 'remission' were tested, defined by Hamilton Depression Rating Scale (HDRS) score 50% reduction or endpoint score of 7 or less respectively, where data were available, or as defined by each study. Where possible, data were stratified by class of antidepressant employed. Unpublished data were sought where necessary and obtained as shown below.
Statistical heterogeneity was identified using the I2 measure (16). I2 values describe the percentage of total variation across studies that is due to heterogeneity rather than chance variation; values of 25, 50, and 75% are sometimes considered low, medium, and high heterogeneity respectively (16). Funnel plots were prepared of effect estimate against its standard error (17), and tested for evidence of asymmetry with a modified linear regression test (18). Where funnel plot asymmetry was observed, estimates of effect corrected for small study effects, such as publication bias, were generated by the trim and fill method (19). The trim and fill method uses available data to estimate the numbers and outcomes of missing (unreported) studies, and recalculates the overall effect that would be observed with their inclusion.
Sensitivity analyses were performed including only those studies where SSRI antidepressants were used, or those where participants were explicitly restricted to unipolar depression, and an influence analysis was performed to measure the effects of excluding single studies. Exploratory random effects meta-regression was performed to test potential explanatory variables (study duration, year of publication, participant age, gender, geographic location, caucasian ethnicity) leading to heterogeneity (20). Meta-regression investigates the effect of study-level characteristics on estimates of effect, and is statistically less powerful than within-study comparisons. As meta-regression can be vulnerable to false-positive findings, it is may be best considered as hypothesis-generating (21).
Results
A total of 28 trials provided suitable data for inclusion in this meta-analysis (see Table 1). The studies varied in size; the largest individual study was STAR*D, although the majority of other studies included over 100 participants. Studies were performed in a wide range of geographic locations; however participant diagnoses were made to standard internationally accepted criteria in all cases (in most cases DSM-IV was used). Some studies included a minority of participants with bipolar depression (Table 1). Study authors provided additional unpublished data in a number of cases which aided analysis (23,34,38,40,41,44,46,48).
Table 1. Characteristics of included studies.
HDRS – Hamilton Depression Rating Scale. MADRS – Montgomery Asberg Depression Rating Scale. CGI – Clinical Global Impression Scale. Response and remission criteria refer to those used in this meta-analysis, in some cases alternative criteria were employed in original study publications.
| Study | Year | Study size (n) |
Antidepressant used |
Diagnostic Criteria |
%age Bipolar |
Location | Ethnicity | Response criterion |
Remission Criterion |
Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Smeraldi | 1998 | 53 | fluvoxamine | DSM IV | 27 | Italy | Not specified | HDRS 7 or less |
(11) | |
| Kim | 2000 | 120 | fluoxetine or paroxetine |
DSM III R | 0 | USA | Korean ancestry | HDRS 50% reduction |
(22) | |
| Pollock | 2000 | 95 | paroxetine or nortriptyline |
DSM IV | 0 | USA | Not specified | HDRS <11 | (23) | |
| Zanardi | 2000 | 58 | paroxetine | DSM IV | unclear | Italy | Italian descent | HDRS 7 or less |
(24) | |
| Zanardi | 2001 | 88 | fluvoxamine | DSM IV | 31 | Italy | Italian antecedents for at least two generations |
HDRS 7 or less |
(25) | |
| Rausch | 2002 | 51 | fluoxetine | DSM IV | 0 | USA | Not specified | HDRS 50% reduction |
(26) | |
| Yoshida | 2002 | 54 | fluvoxamine | DSM IV | 0 | Japan | Japanese | MADRS 50% reduction |
(27) | |
| Yu | 2002 | 121 | fluoxetine | DSM IV | 0 | China | Ethnic Chinese | HDRS 50% reduction |
HDRS 7 or less |
(28) |
| Arias | 2003 | 131 | citalopram | DSM IV | 0 | Spain | Spanish origin | HDRS 50% reduction |
HDRS 7 or less |
(29) |
| Joyce | 2003 | 86 | fluoxetine | DSM III R | 9 (bipolar II) |
New Zealand |
95% European, largely British descent |
MADRS reduction 60% or more |
(30) | |
| Durham | 2004 | 106 | sertraline | DSM IV | 0 | USA | 95.4% Caucasian, 2.8% Black, 1.38% other, and 0.46% Asian |
HDRS 50% reduction |
(31) | |
| Lee | 2004 | 128 | various (57% SSRI) |
DSM IV | 0 | Korean | Korean | CGII 1 or 2 | (32) | |
| Serretti | 2004 | 220 | paroxetine or fluvoxamine |
DSM IV | 42 | Italy | Italian antecedents for at least two generations |
HDRS 7 or less |
(33) | |
| Kraft | 2005 | 96 | fluoxetine | DSM IV | 0 | USA | 78% Cauca- sian, 6% African American, 8% Hispanic, 5% Asian, and 3% other |
CGI I much or very much improved |
(34) | |
| Kato | 2006 | 100 | paroxetine or fluvoxamine |
DSM IV | 0 | Japan | Japanese | HDRS 50% reduction |
HDRS 7 or less |
(35) |
| Kim | 2006 | 241 | fluoxetine or sertraline |
DSM IV | unclear | Korea | Korean | HDRS 50% reduction |
(36) | |
| Ng | 2006 | 45 | sertraline | DSM-IV | 0 | Australia & Malaysia |
67% Chinese 33% Caucasian |
HDRS 50% reduction |
(37) | |
| Popp | 2006 | 109 | various | ICD 10 | 12 | Germany | 97% Caucasian | response on CGI |
(38) | |
| STAR*D | 2006 | 1659 | citalopram | DSM IV | 0 | USA | 80% White, 14% Black, 6% Other |
QIDS-C16 50% reduction |
QIDS-C <6 | (39) |
| Hong | 2006 | 224 | fluoxetine | DSM IV | 0 | Taiwan | Ethnic Chinese | HDRS 50% reduction |
HDRS 7 or less |
(40) |
| Kirchheiner | 2007 | 190 | various | DSM IV | 12 | Germany | Caucasian | HDRS 50% reduction |
HDRS 7 or less |
(41) |
| Bozina | 2007 | 130 | paroxetine | DSM-IV | 0 | Croatia | Caucasian (Croatian) | HDRS 50% reduction |
(42) | |
| Kang | 2007 | 101 | mirtazapine | DSM-IV | unclear | Korea | Korean | HDRS 50% reduction |
(43) | |
| Dogan | 2008 | 64 | sertraline | DSM-IV | 0 | Turkey | Turkish | HDRS 50% reduction |
HDRS 7 or less |
(44) |
| Wilkie | 2009 | 166 | various | DSM-IV | 0 | UK | Caucasian | HDRS 50% reduction |
HDRS 7 or less |
(45) |
| Ruhé | 2009 | 42 | paroxetine | DSM-IV | 0 | Netherlands | 69% Caucasian, 17% Creole, 14% Asian |
HDRS 50% reduction |
(46) | |
| Maron | 2009 | 135 | escitalopram | DSM-IV | 0 | Estonia | 96% Estonian | HDRS 50% reduction |
HDRS 7 or less |
(47) |
| GENDEP | 2009 | 795 | escitalopram or nortriptyline |
DSM-IV / ICD-10 |
0 | Europe | White European parentage |
HDRS 50% reduction |
HDRS 7 or less |
(48) |
Antidepressant response
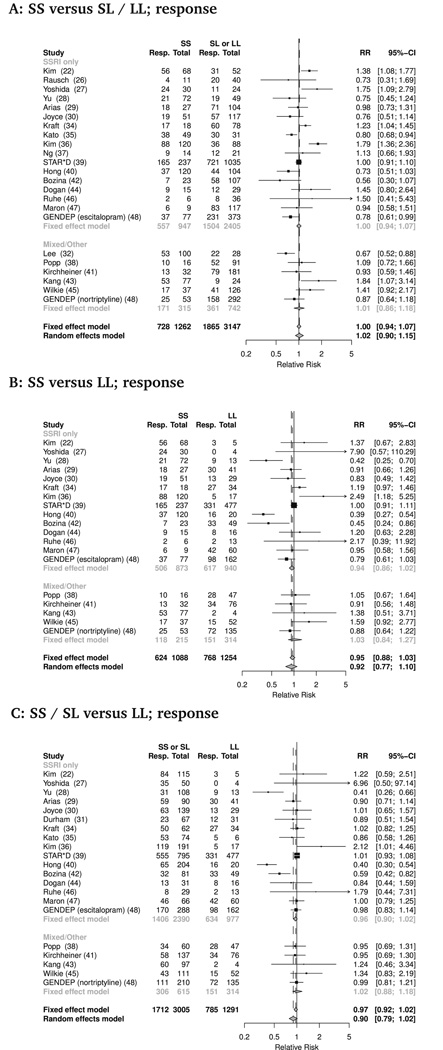
A total of 23 studies (combined n=4894) provided data on antidepressant response rates, defined in a majority of studies as a 50% reduction or more in HDRS score from baseline (Table 1). No statistically significant effect of transporter promoter length polymorphism on rates of antidepressant response was seen (Figure 1). This was true for all three genotype comparisons tested - SS versus (SL or LL): RR 1.01 (95% Confidence Interval 0.90 to 1.15, p=.78); SS versus LL: RR 0.92 (95% CI 0.77 to 1.10, p=.37); (SS or SL) versus LL: RR 0.90 (95% CI 0.79 to 1.02, p=0.09). In all three comparisons, substantial statistical heterogeneity was evident, with I2 > 70% in all cases.
Figure 1.
Serotonin transporter promoter length polymorphism and antidepressant response. Forest plots of response rates by genotype for three comparisons, (a) SS vs SL/LL, (b) SS vs LL, (c) SS/SL vs LL. Individual study and pooled estimates applying both fixed and random effects models shown. For clarity study effect estimates are grouped by those that used of SSRI alone, or those employing non-SSRI or a variety of antidepressant agents.
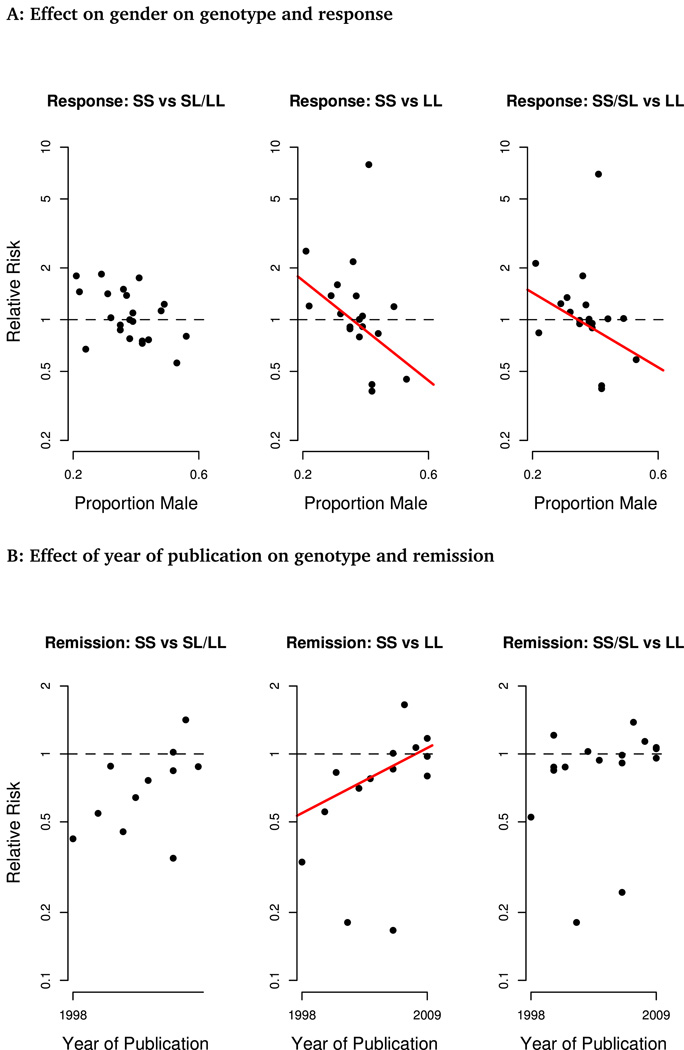
Heterogeneity remained high when only studies including exclusively unipolar depression were included, and exploratory meta-regression found no significant effect of year of publication, study duration, participant age, ethnicity or geographic location on estimates of genotype effect. For the comparisons of SS or S carriers against LL genotypes, there was an interaction of effect with proportion of male participants (SS vs LL, p=0.023; SS/SL vs LL, p=0.047), such that the LL genotype was associated with a higher chance of response in studies with more male participants and a lower chance of response in those including more females (Figure 4A). This effect was also observed when proportion male was considered as a dichotomous measure (greater or less than sample median; p<.05 in both cases). This interaction did not reach statistical significance for the SS vs SL/LL comparisons (p=.08).
Figure 4.
Meta-regression analyses finding significant effects. A. Effect of gender on association of genotype with response rates. B. Effect of year of publication on association of genotype with remission rates. Lines indicate significant effects (p<0.05).
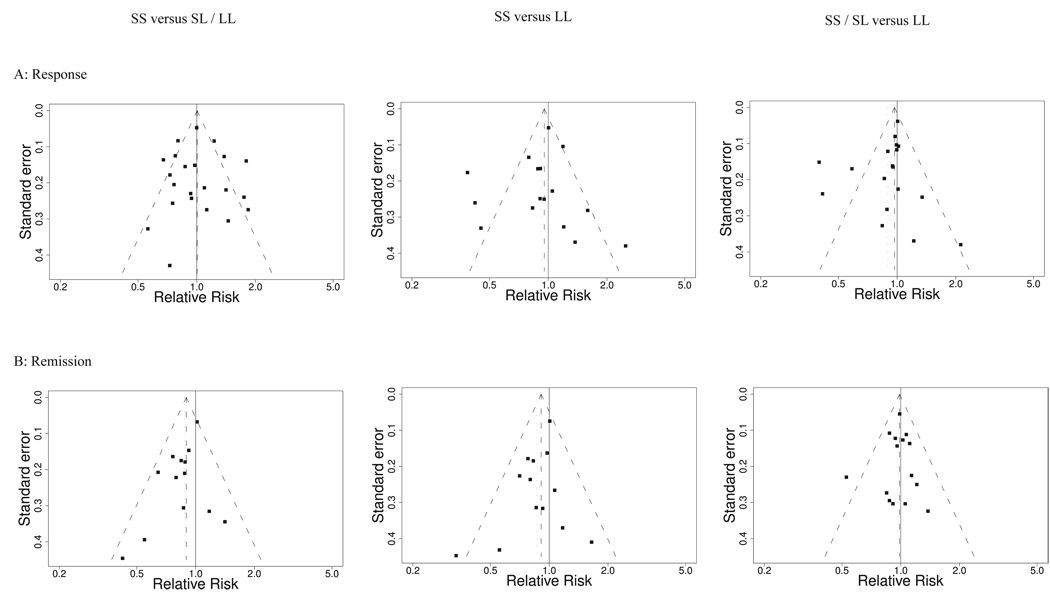
Visual funnel plot inspection for comparisons versus LL suggested estimates of effect size might be skewed by two or three outlier results, however there was no marked asymmetry of funnel plot evident on statistical testing (Figure 2A, p>0.5 in all cases). The absence of effect of the 5-HTTLPR polymorphism on antidepressant response continued to be evident when analyses were repeated restricting studies to those using SSRI antidepressants only, or without any bipolar participants. Excluding no individual study from analysis qualitatively altered the results, except for the comparison of SS/SL vs LL where exclusion of the study of Kim and colleagues (36) led to a statistically significant effect estimate with the random effects model (p=0.04), but not using fixed effects (p=0.13).
Figure 2.
Funnel plots of effect estimate as relative risk against its standard error for (a) response and (b) remission comparisons of SS versus SL/LL (left), SS versus LL (middle), and SS/SL versus LL (right) with random effects estimate and 95% CI shown. Statistically significant asymmetry for remission rate comparisons of SS versus LL (p=0.02) and SS versus SL/LL (p=0.02).
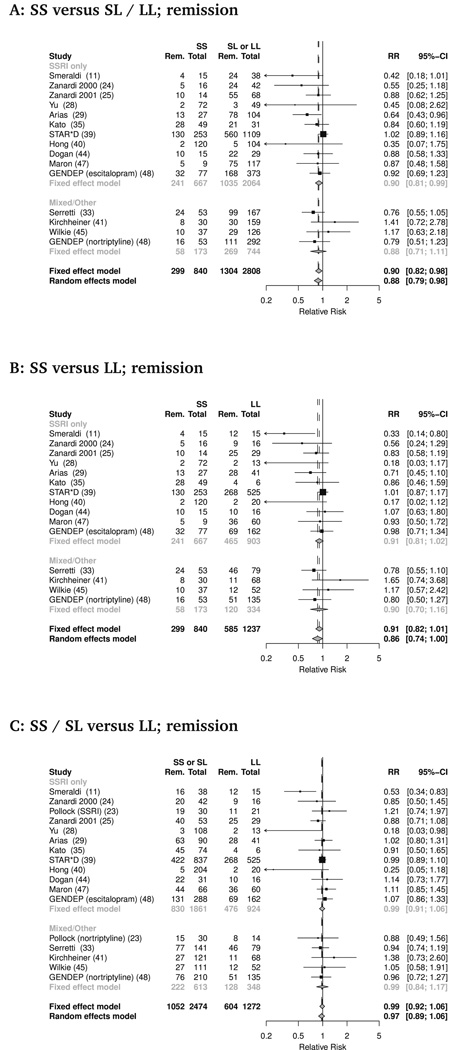
Remission
A total of 15 studies (combined n= 4099) provided data on remission rates, defined as HDRS score of 7 or less in the majority of studies (Table 1). Rates of remission with antidepressant treatment did appear to vary with transporter promoter polymorphism in one comparison of genotypes (Figure 3). Therefore participants with the SS genotype had lower rates of remission than L carriers - SS versus (SL or LL): RR 0.88 (95% CI 0.79 to 0.98, p=0.02). However, compared to LL, neither SS nor S carriers differed significantly -SS versus LL: RR 0.86 (95% CI 0.74 to 1.00, p=0.06), (SS or SL) versus LL: RR 0.97 (95% CI 0.89 to 1.06, p=0.47). This difference between SS genotype and L carriers was robust to restriction of analysis by antidepressant class but not diagnosis: remission rates were lower in participants with SS genotype versus (SL or LL) considering studies of SSRIs alone (RR 0.87; 95% CI 0.76 to 0.99, p=0.04), but not when analysis was restricted to exclusively unipolar cohorts (RR 0.94; 95% CI 0.84 to 1.05, p=0.26).
Figure 3.
Serotonin transporter promoter length polymorphism and remission rate with antidepressant treatment. Forest plots of remission rates by genotype for three comparisons, (a) SS vs SL/LL, (b) SS vs LL, (c) SS/SL vs LL. Individual study and pooled estimates applying both fixed and random effects models shown. For clarity study effect estimates are grouped by those that used of SSRI alone, or those employing non-SSRI or a variety of antidepressant agents.
Estimates of effect on remission rates were more sensitive than response to the inclusion or exclusion of individual studies. The difference between SS and L carriers was no longer statistically significant if either of two studies (29,33) were omitted, whereas the comparison of SS and LL reached statistical significance (p=0.03) if either of two studies (13,41) were omitted.
For remission comparisons, there were lower levels of statistical heterogeneity than observed for response rates (I2 between 14 and 30%) indicating that more, but not all, of the variation between study estimates for the remission comparisons could be explained by the play of chance alone (16).
These was evident asymmetry on funnel plots for the comparisons against SS genotype both on visual inspection and on formal statistical testing (p=0.016 in both cases, Figure 2B). For the SS or SL versus LL comparison, there was a trend towards asymmetry (p=0.09). When trim and fill correction for confounding by publication bias was applied, there was no longer any significant effect of genotype on rates of remission (SS versus (SL or LL): RR 0.92, 95% CI 0.81 to 1.05, p=0.23; SS versus LL: RR 0.92, 95% CI 0.77 to 1.10, p=0.37; (SS or SL) versus LL, RR 0.97, 95% CI 0.88 to 1.07, p=0.55).
Meta-regression revealed an effect of year of publication on estimate of difference between SS and LL (p=0.03; Figure 4B), with the difference decreasing with time. No significant associations with other pre-specified explanatory variables were identified, in particular no interaction of gender with genotype and remission was observed (p>0.4 for each comparison).
Discussion
We report the largest meta-analysis of the effect of 5-HTTLPR polymorphisms on response or remission to antidepressant medication in patients with MDD. Our results find no significant effect of the biallelic polymorphism on response to SSRI medication. While we see a weak effect of the polymorphism on remission, a number of factors like publication bias or lack of reporting of the remission outcome appear to explain this finding. Therefore there is little evidence to suggest continued scrutiny of this particular polymorphism, in isolation, to predict antidepressant response
The available evidence to test the association between the serotonin transporter promoter polymorphism and antidepressant response has substantially increased over recent years. A recent meta-analysis of this topic, published in 2007, included data from 15 studies with 1435 participants (12). Since then there has been an exponential increase in publications in this area. Therefore the current analysis has the advantage of the inclusion of new data from both the STAR*D study and from over 2000 participants in other studies that have also recently reported resulting in a near quadrupling of the population available to answer the question asked in this study. With the benefit of these additional data, there does not appear to be any effect of the biallelic 5-HTTLPR polymorphism on antidepressant response rate. This lack of effect is not simply explained by the inclusion of the negative STAR*D data, since the same result is found when data from that study are excluded from analysis.
There was evidence of considerable heterogeneity of effect for response rates. Thus while there may be no substantial overall effect of 5-HTTLPR genotype, there may be groups for which there remains an effect. The variability is not readily explained by clinical sample characteristics - while studies have been performed in many centres internationally, there has been substantial similarity of antidepressant agents used, and in the diagnostic criteria by which participants have been assessed. It may well be that additional and yet unknown gene × gene or gene × environment interactions may be more useful in predicting response. However, investigating such interactions does increase the risks of type I error, and robust evidence of replication may be required to avoid false positive findings (49).
An interaction with gender was observed here, such that studies with more male participants tended to observe an association of the S allele with worse response to antidepressant treatment, in contrast to an improved response in studies with more female participants. This finding must as yet be considered provisional, not least as it would not survive correction for the multiple meta-regression tests performed.
Interestingly, interactions of genotype effect with gender have recently been described using within-study comparisons, however while the effect reported here is in keeping with the findings of the GENDEP study (48), it is in the opposite direction to the findings of a recent case-control study (50). A future analysis employing individual patient data might be able to clarify the true extent of any such interaction.
Interestingly, while preclinical models demonstrate clear effects on levels of transporter expression (4,51), in human populations the link may not always be so clear cut (52). There are several possible explanations for this finding - for example it has been shown that an A>G SNP exists within the longer form of the 5HTT promoter region, and increased transporter expression may only be observed where the long ‘L’ allele contains the A form (39). Against this, the STAR*D dataset for example has been analysed using both biallelic (S and L) and triallelic (S, LA, and LG) approaches, and no effect on treatment response observed (13).
Alternatively, functional effects may be only revealed in conjunction with polymorphisms of other genes entirely, such as has been described for 5-HTTLPR and IL-6 in an experimental model (53). One cannot rule out gene by gene interactions in the form of additive or synergistic effects of polymorphisms in the 5-HTTLPR with other candidate genes that have been studied in MDD such as HTR2A (54), GRIK4 (55) and FKBP5 (56). It is also interesting that the two of the studies that found the greatest effects on response rates (28,40) were performed in China and Taiwan, which may point to an ethnicity-moderated effect, although such an effect could not be identified here with formal statistical testing.
Furthermore genetic effects may only be evident in interaction with individual experiences such as adverse early life events (57). These questions remain unclear and cannot be resolved from the data conventionally reported in studies of overall associations of the 5-HTTLPR polymorphism and antidepressant response.
For remission rates, the picture is less clear. Pooled estimates indicate that the SS genotype is associated with remission rate, although with much less statistical certainty than appeared the case in the previous analysis (p=0.02 rather than p<0.0001). Indeed were we to correct for the multiple comparisons we performed for the remission data the effect observed for the SS genotype does not achieve conventional statistical significance.
A similar magnitude of estimate is obtained if analyses are limited to studies studying SSRIs alone, which suggests the inclusion of data from a variety of classes of antidepressant is not obscuring any effect in this subgroup. However, when analyses are restricted to exclusively unipolar cohorts, no effect on remission is seen, which raises the possibility that any effect might be driven by an effect on bipolar depression. This might be surprising given the disappointing treatment response to antidepressants in bipolar depression in recent studies (58).
Omitting individual study data from remission analyses does give rise to differing apparent magnitudes of effect. The effect of year of publication on estimates of effect in the SS vs LL comparison would be compatible with a common observation (8,59) that the first published study in a field often over-estimates the true effect. However, as the sensitivity analysis here indicates, the apparent effect of genotypes on remission rates does not simply depend on the inclusion of the first published data (11).
The asymmetry of funnel plots of this analysis on both visual inspection and statistical analysis suggests that this apparent effect of 5-HTTLPR genotype on remission rate may well be due to publication bias rather than a true genotype effect.
Funnel plots – plots of estimates of effect on the horizontal axis against study precision on the vertical – are a conventional approach to identifying publication bias (60). In the absence of bias, regardless of the overall direction and magnitude of effect, they should have the appearance of a symmetrical inverted funnel since treatment estimates should vary more widely between smaller studies than between larger trials that should more precisely estimate effects. The asymmetry shown for remission rates comparing SS to (SL and LL) or LL alone suggests that there are unreported data (e.g. unpublished studies, or studies where the necessary data for analysis are not available) that if included would further reduce the pooled estimate of effect. Differences in effect between smaller and larger studies, leading to funnel plot asymmetry, may be observed for other reasons, such as systematic differences in methodological quality or patient population exaggerating effect sizes (61). However as noted above, the studies included in the present analysis were highly consistent in such respects which indicates publication bias as a likely cause. This meta-analysis already includes further unpublished details from a number of studies (23,34,38,40,41,44) to reduce such bias, but it is likely that if remission rates were available from further studies, yielding a less biased estimate, the association would no longer be significant, as was found when trim and fill correction was applied.
On current evidence, there does not appear to be an overall effect of the biallelic 5-HTTLPR polymorphism on rates of antidepressant response and the evidence on remission is weak at best. Future studies should focus on clarifying interactions of this polymorphism and other factors that underlie the heterogeneity of effect seen.
Acknowledgements
Dr Taylor is funded by a UK NIHR Clinical Lectureship. Supported in part by a NARSAD Young Investigator Award (ZB), the NIH (K23-MH077914; ZB), Clinical and Translational Science Award Grant UL1 RR024139 from the National Center for Research Resources to Yale University (Z.B.). We acknowledge support from the Clinical Neuroscience Research Unit at the Connecticut Mental Health Center and the Department of Mental Health and Addictions Services, Connecticut.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis. Arch Gen Psychiatry. 2006;63:1217–1223. doi: 10.1001/archpsyc.63.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 3.Holsboer F. How can we realize the promise of personalized antidepressant medicines? Nat Rev Neurosci. 2008;9:638–646. doi: 10.1038/nrn2453. [DOI] [PubMed] [Google Scholar]
- 4.Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 5.Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv. 2004;4:109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Leon S, Janssens AC, Gonzalez-Zuloeta Ladd AM, Del-Favero J, Claes SJ, Oostra BA, et al. Meta-analyses of genetic studies on major depressive disorder. Mol Psychiatry. 2008;13:772–785. doi: 10.1038/sj.mp.4002088. [DOI] [PubMed] [Google Scholar]
- 7.Munafò MR, Freimer NB, Ng W, Ophoff R, Veijola J, Miettunen J, et al. 5-HTTLPR genotype and anxiety-related personality traits: a meta-analysis and new data. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:271–281. doi: 10.1002/ajmg.b.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. Jama. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jennings KA, Sheward WJ, Harmar AJ, Sharp T. Evidence that genetic variation in 5-HT transporter expression is linked to changes in 5-HT2A receptor function. Neuropharmacology. 2008;54:776–783. doi: 10.1016/j.neuropharm.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Smeraldi E, Zanardi R, Benedetti F, Di Bella D, Perez J, Catalano M. Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry. 1998;3:508–511. doi: 10.1038/sj.mp.4000425. [DOI] [PubMed] [Google Scholar]
- 12.Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- 13.Hu XZ, Rush AJ, Charney D, Wilson AF, Sorant AJ, Papanicolaou GJ, et al. Association between a functional serotonin transporter promoter polymorphism and citalopram treatment in adult outpatients with major depression. Arch Gen Psychiatry. 2007;64:783–792. doi: 10.1001/archpsyc.64.7.783. [DOI] [PubMed] [Google Scholar]
- 14.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: 2007. [Google Scholar]
- 15.Schwarzer G. meta: Meta-analysis. R package version 0.9–19. 2009 [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 18.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 19.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 20.Everitt BS, Hothorn T. A Handbook of Statistical Analyses Using R. Boca Raton, Florida: Chapman & Hall; 2006. [Google Scholar]
- 21.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 22.Kim DK, Lim SW, Lee S, Sohn SE, Kim S, Hahn CG, et al. Serotonin transporter gene polymorphism and antidepressant response. Neuroreport. 2000;11:215–219. doi: 10.1097/00001756-200001170-00042. [DOI] [PubMed] [Google Scholar]
- 23.Pollock BG, Ferrell RE, Mulsant BH, Mazumdar S, Miller M, Sweet RA, et al. Allelic variation in the serotonin transporter promoter affects onset of paroxetine treatment response in late-life depression. Neuropsychopharmacology. 2000;23:587–590. doi: 10.1016/S0893-133X(00)00132-9. [DOI] [PubMed] [Google Scholar]
- 24.Zanardi R, Benedetti F, Di Bella D, Catalano M, Smeraldi E. Efficacy of paroxetine in depression is influenced by a functional polymorphism within the promoter of the serotonin transporter gene. J Clin Psychopharmacol. 2000;20:105–107. doi: 10.1097/00004714-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Zanardi R, Serretti A, Rossini D, Franchini L, Cusin C, Lattuada E, et al. Factors affecting fluvoxamine antidepressant activity: influence of pindolol and 5-HTTLPR in delusional and nondelusional depression. Biol Psychiatry. 2001;50:323–330. doi: 10.1016/s0006-3223(01)01118-0. [DOI] [PubMed] [Google Scholar]
- 26.Rausch JL, Johnson ME, Fei YJ, Li JQ, Shendarkar N, Hobby HM, et al. Initial conditions of serotonin transporter kinetics and genotype: influence on SSRI treatment trial outcome. Biol Psychiatry. 2002;51:723–732. doi: 10.1016/s0006-3223(01)01283-5. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida K, Ito K, Sato K, Takahashi H, Kamata M, Higuchi H, et al. Influence of the serotonin transporter gene-linked polymorphic region on the antidepressant response to fluvoxamine in Japanese depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:383–386. doi: 10.1016/s0278-5846(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 28.Yu YW, Tsai SJ, Chen TJ, Lin CH, Hong CJ. Association study of the serotonin transporter promoter polymorphism and symptomatology and antidepressant response in major depressive disorders. Mol Psychiatry. 2002;7:1115–1119. doi: 10.1038/sj.mp.4001141. [DOI] [PubMed] [Google Scholar]
- 29.Arias B, Catalan R, Gasto C, Gutierrez B, Fananas L. 5-HTTLPR polymorphism of the serotonin transporter gene predicts non-remission in major depression patients treated with citalopram in a 12-weeks follow up study. J Clin Psychopharmacol. 2003;23:563–567. doi: 10.1097/01.jcp.0000095350.32154.73. [DOI] [PubMed] [Google Scholar]
- 30.Joyce PR, Mulder RT, Luty SE, McKenzie JM, Miller AL, Rogers GR, et al. Age-dependent antidepressant pharmacogenomics: polymorphisms of the serotonin transporter and G protein beta3 subunit as predictors of response to fluoxetine and nortriptyline. Int J Neuropsychopharmacol. 2003;6:339–346. doi: 10.1017/S1461145703003663. [DOI] [PubMed] [Google Scholar]
- 31.Durham LK, Webb SM, Milos PM, Clary CM, Seymour AB. The serotonin transporter polymorphism, 5HTTLPR, is associated with a faster response time to sertraline in an elderly population with major depressive disorder. Psychopharmacology (Berl) 2004;174:525–529. doi: 10.1007/s00213-003-1562-3. [DOI] [PubMed] [Google Scholar]
- 32.Lee MS, Lee HY, Lee HJ, Ryu SH. Serotonin transporter promoter gene polymorphism and long-term outcome of antidepressant treatment. Psychiatr Genet. 2004;14:111–115. doi: 10.1097/01.ypg.0000107928.32051.11. [DOI] [PubMed] [Google Scholar]
- 33.Serretti A, Zanardi R, Franchini L, Artioli P, Dotoli D, Pirovano A, et al. Pharmacogenetics of selective serotonin reuptake inhibitor response: a 6-month follow-up. Pharmacogenetics. 2004;14:607–613. doi: 10.1097/00008571-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Kraft JB, Slager SL, McGrath PJ, Hamilton SP. Sequence analysis of the serotonin transporter and associations with antidepressant response. Biol Psychiatry. 2005;58:374–381. doi: 10.1016/j.biopsych.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 35.Kato M, Fukuda T, Wakeno M, Fukuda K, Okugawa G, Ikenaga Y, et al. Effects of the serotonin type 2A, 3A and 3B receptor and the serotonin transporter genes on paroxetine and fluvoxamine efficacy and adverse drug reactions in depressed Japanese patients. Neuropsychobiology. 2006;53:186–195. doi: 10.1159/000094727. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, Lim SW, Kim S, Kim JW, Chang YH, Carroll BJ, et al. Monoamine transporter gene polymorphisms and antidepressant response in koreans with late-life depression. Jama. 2006;296:1609–1618. doi: 10.1001/jama.296.13.1609. [DOI] [PubMed] [Google Scholar]
- 37.Ng CH, Easteal S, Tan S, Schweitzer I, Ho BK, Aziz S. Serotonin transporter polymorphisms and clinical response to sertraline across ethnicities. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:953–957. doi: 10.1016/j.pnpbp.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Popp J, Leucht S, Heres S, Steimer W. Serotonin transporter polymorphisms and side effects in antidepressant therapy--a pilot study. Pharmacogenomics. 2006;7:159–166. doi: 10.2217/14622416.7.2.159. [DOI] [PubMed] [Google Scholar]
- 39.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong CJ, Chen TJ, Yu YW, Tsai SJ. Response to fluoxetine and serotonin 1A receptor (C-1019G) polymorphism in Taiwan Chinese major depressive disorder. Pharmacogenomics J. 2006;6:27–33. doi: 10.1038/sj.tpj.6500340. [DOI] [PubMed] [Google Scholar]
- 41.Kirchheiner J, Nickchen K, Sasse J, Bauer M, Roots I, Brockmoller J. A 40-basepair VNTR polymorphism in the dopamine transporter (DAT1) gene and the rapid response to antidepressant treatment. Pharmacogenomics J. 2007;7:48–55. doi: 10.1038/sj.tpj.6500398. [DOI] [PubMed] [Google Scholar]
- 42.Bozina N, Peles AM, Sagud M, Bilusic H, Jakovljevic M. Association study of paroxetine therapeutic response with SERT gene polymorphisms in patients with major depressive disorder. World J Biol Psychiatry. 2007:1–8. doi: 10.1080/15622970701308397. [DOI] [PubMed] [Google Scholar]
- 43.Kang RH, Wong ML, Choi MJ, Paik JW, Lee MS. Association study of the serotonin transporter promoter polymorphism and mirtazapine antidepressant response in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1317–1321. doi: 10.1016/j.pnpbp.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 44.Dogan O, Yuksel N, Ergun MA, Yilmaz A, Ilhan MN, Karslioglu HE, et al. Serotonin transporter gene polymorphisms and sertraline response in major depression patients. Genet Test. 2008;12:225–231. doi: 10.1089/gte.2007.0089. [DOI] [PubMed] [Google Scholar]
- 45.Wilkie MJ, Smith G, Day RK, Matthews K, Smith D, Blackwood D, et al. Polymorphisms in the SLC6A4 and HTR2A genes influence treatment outcome following antidepressant therapy. Pharmacogenomics J. 2008 doi: 10.1038/sj.tpj.6500491. [DOI] [PubMed] [Google Scholar]
- 46.Ruhe HG, Ooteman W, Booij J, Michel MC, Moeton M, Baas F, et al. Serotonin transporter gene promoter polymorphisms modify the association between paroxetine serotonin transporter occupancy and clinical response in major depressive disorder. Pharmacogenet Genomics. 2009;19:67–76. doi: 10.1097/FPC.0b013e32831a6a3a. [DOI] [PubMed] [Google Scholar]
- 47.Maron E, Tammiste A, Kallassalu K, Eller T, Vasar V, Nutt DJ, et al. Serotonin transporter promoter region polymorphisms do not influence treatment response to escitalopram in patients with major depression. Eur Neuropsychopharmacol. 2009;19:451–456. doi: 10.1016/j.euroneuro.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Huezo-Diaz P, Uher R, Smith R, Rietschel M, Henigsberg N, Marusic A, et al. Moderation of antidepressant response by the serotonin transporter gene. Br J Psychiatry. 2009;195:30–38. doi: 10.1192/bjp.bp.108.062521. [DOI] [PubMed] [Google Scholar]
- 49.Munafò MR, Durrant C, Lewis G, Flint J. Gene ×ronment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Smits KM, Smits LJ, Peeters FP, Schouten JS, Janssen RG, Smeets HJ, et al. The influence of 5-HTTLPR and STin2 polymorphisms in the serotonin transporter gene on treatment effect of selective serotonin reuptake inhibitors in depressive patients. Psychiatr Genet. 2008;18:184–190. doi: 10.1097/YPG.0b013e3283050aca. [DOI] [PubMed] [Google Scholar]
- 51.Jennings KA, Loder MK, Sheward WJ, Pei Q, Deacon RM, Benson MA, et al. Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J Neurosci. 2006;26:8955–8964. doi: 10.1523/JNEUROSCI.5356-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shioe K, Ichimiya T, Suhara T, Takano A, Sudo Y, Yasuno F, et al. No association between genotype of the promoter region of serotonin transporter gene and serotonin transporter binding in human brain measured by PET. Synapse. 2003;48:184–188. doi: 10.1002/syn.10204. [DOI] [PubMed] [Google Scholar]
- 53.Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, et al. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMahon FJ, Buervenich S, Charney D, Lipsky R, Rush AJ, Wilson AF, et al. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am J Hum Genet. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paddock S, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, et al. Association of GRIK4 with outcome of antidepressant treatment in the STAR*D cohort. The American journal of psychiatry. 2007;164:1181–1188. doi: 10.1176/appi.ajp.2007.06111790. [DOI] [PubMed] [Google Scholar]
- 56.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nature genetics. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 57.Mandelli L, Marino E, Pirovano A, Calati R, Zanardi R, Colombo C, et al. Interaction between SERTPR and stressful life events on response to antidepressant treatment. Eur Neuropsychopharmacol. 2009;19:64–67. doi: 10.1016/j.euroneuro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Sachs GS, Nierenberg AA, Calabrese JR, Marangell LB, Wisniewski SR, Gyulai L, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356:1711–1722. doi: 10.1056/NEJMoa064135. [DOI] [PubMed] [Google Scholar]
- 59.Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- 60.Light RJ, Pillemer DB. Summing up. The science of reviewing research. Cambridge, MA: Harvard University Press; 1984. [Google Scholar]
- 61.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]